Abstract
In this study, apoptosis was induced by new type gosling viral enteritis virus (NGVEV) in experimentally infected goslings is reported in detail for the first time. After 3-day-old goslings were orally inoculated with a NGVEV-CN strain suspension, the time course of NGVEV effects on apoptotic morphological changes of the internal tissues was evaluated. These changes were observed by histological analysis with light microscopy and ultrastructural analysis with transmission electron microscopy. DNA fragmentation was assessed with a terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay and DNA ladder analysis. A series of characteristic apoptotic morphological changes including chromatin condensation and margination, cytoplasmic shrinkage, plasma membrane blebbing, and formation of apoptotic bodies were noted. Apoptosis was readily observed in the lymphoid and gastrointestinal organs, and sporadically occurred in other organs after 3 days post-infection (PI). The presence and quantity of TUNEL-positive cells increased with infection time until 9 days PI. DNA extracted from the NGVEV-infected gosling cells displayed characteristic 180~200 bp ladders. Apoptotic cells were ubiquitously distributed, especially among lymphocytes, macrophages, monocytes, and epithelial and intestinal cells. Necrosis was subsequently detected during the late NGVEV-infection phase, which was characterized by cell swelling, plasma membrane collapse, and rapidly lysis. Our results suggested that apoptosis may play an important role in the pathogenesis of NGVE disease.
Keywords: apoptosis, gosling, in vivo, NGVEV, viral enteritis
Introduction
Apoptosis, a cell suicide mechanism that helps eliminate redundant, damaged, or infected cells, was first observed in metazoan organisms by Kerr et al. [25]. Apoptosis is a physiological process characterized by a number of distinct morphological features and biochemical processes [16,41,42], including cell shrinkage and partial detachment from the substratum, plasma membrane blebbing, chromatin condensation, and intra-nucleosomal cleavage [28]. This form of physiological cell death is morphologically distinct from necrosis, in which the cell swells and disintegrates in a disorderly manner and eventually results in the destruction of the cellular organelles, rupture of the plasma membrane, and leakage of the cell content [39]. Apoptosis is recognized as an important process in different biological systems including embryonic development, cell turnover, and immune response against tumorigenic or virus-infected cells [21,29,37]. An increasing number of viruses or viral gene products have been reported to induce apoptosis [17,22,26,30].
New type gosling viral enteritis (NGVE) is an infectious disease that affects goslings less than 30 days of age, and is caused by an adenovirus called the NGVE virus (NGVEV) [4,5,12,13,15,27,40]. In gosling-producing areas, NGVE has resulted in significant economic losses due to the high mortality of the offspring of breeder geese [12,27]. This disease is clinically characterized by digestive, respiratory, and neurological symptoms along with sudden death [14,15]. Catarrhal haemorrhagic fibrinonecrotic enteritis in the small intestine and intestinal obstruction of the middle-lower portion of the intestine are typical pathological changes observed in NGVEV-infected goslings [15]. Previous immunofluorescence assay and immunohistochemical staining studies indicated that the principal sites of NGVEV antigen localization are the digestive organs and lymphoid organs [9,10]. Furthermore, other studies showed that apoptosis can be induced by NGVEV in monolayer primary duck embryo fibroblasts and the host cells of duck embryos [5-8]. According to our recent research results, NGVEV can induce apoptosis in infected gosling; however, detailed information about this process is still unavailable [11]. To investigate apoptosis evoked by NGVEV infection in vivo, apoptotic morphological changes were observed by light microscopy and transmission electron microscopy (TEM). DNA fragmentation was also monitored with a terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay and DNA ladder analysis.
In general, goose adenovirus caused neither clinical symptoms nor pathological lesions [43]. Although NGVE was identified in 1998 [12,13,15], the pathogenesis of this disease is not well understood. Furthermore, little is known about apoptosis induced by goose adenovirus, especially for pathogenic goose adenovirus. The results from the present study may shed light on the important role of apoptosis in the pathogenesis of NGVE disease, and provide new insights into mechanisms underlying infection with pathogenic goose adenovirus.
Materials and Methods
Experimental goslings and virus strain
The study was conducted with 3-day-old Sichuan White goslings (Anser cygnoides Linn. var domestica) that had not been vaccinated against NGVE and were negative for antibody against NGVEV as determined by ELISA prior to the viral challenge. One hundred and forty six goslings were randomly divided into an experimental, observation and control group.
The NGVEV-CN strain (a high virulence field isolate) was provided by the Avian Diseases Research Centre of the Sichuan Agricultural University (China). The minimal lethal dose of the virus suspension (LD50) was 10-6.5±1.3/0.5 mL. This strain has been previously described [4-15].
Experimental NGVEV infection and sampling
The experimental group (n = 100) and observation group (n = 30) were orally inoculated with 0.2 mL of a NGVEV-CN strain suspension containing 1,000 LD50, while the control group (n = 16) received oral doses of sterile 0.2 mL PBS (0.15 M, pH 7.2) instead of the viral suspension. After infection, the virally-challenged goslings were monitored for clinical signs of disease. The experimental, observation, and control groups were maintained in different isolation units in a bio-secure animal housing building (Sichuan Agricultural University, China) and fed a commercial diet ad libitum. The research was conducted in accordance with the internationally accepted criteria for the care and use of laboratory animal as outlined in the National Institutes of Health guidelines, USA.
At intervals of 0.5, 1, 2, 6, 12, and 24 h, and 2, 3, 4, 6, 9, 12, 15, 20, 25, and 30 days post-infection (PI), three NGVEV-infected and one control goslings per time point were euthanatized. Tissue samples were collected from the Harderian gland, thymus, bursa of Fabricius (BF), proventriculus, gizzard, small intestine, cecum, rectum, myocardium, liver, spleen, kidney, pancreas, lung, and cerebrum. For the observation group, clinical symptoms, pathological changes, and mortality were monitored daily for 30 days. Dying or deceased goslings in the infected and observation groups were necropsied and the tissues were sampled.
Histological analysis
The histological analysis was performed according to a previously described method [15]. Tissue samples were fixed in 4% paraformaldehyde for 24 h, dehydrated through graded alcohols, and embedded in paraffin. The paraffin-embedded sections (4-µm thick) were stained with hematoxylin and eosin, and observed under a light microscope (80i; Nikon, Japan).
Ultrastructural changes analysis
The ultrastructural analysis was performed as follows [5,7]. Briefly, the tissue samples were fixed in 2.5% glutaraldehyde, post-fixed in 1.0% aqueous OsO4. After stepwise dehydration in ethanol, the samples were embedded in epoxy resin 618 and polymerized at 80℃ for 3 days. In addition, 50-nm slices were stained with uranyl acetate and lead citrate at room temperature for subsequent examination with TEM (H-600; Hitachi, Japan).
In situ detection of apoptosis
Tissue samples were fixed in 4% paraformaldehyde for 24 h, dehydrated through graded alcohols, and embedded in paraffin using a conventional manner. The paraffin-embedded tissues were cut into 5-µm thick sections. TUNEL analysis was carried out using the In Situ Cell Death Detection Kit (POD; Roche, Germany) according to the manufacturer's directions. The color reaction was developed with diaminobenzidine (Sigma, USA) and the sections were counterstained with 1% methyl green (Sigma, USA) prior to analysis by light microscopy according to the conventional protocol with some modifications [17,24,25]. Samples from the control gosling were processed in the same way. For the positive control, cells were incubated, fixed, and permeabilized with recombinant DNase I (Roche, Germany) for 10 min at 25℃ to induce DNA strand breaks prior to labeling. For the negative control, cells were incubated, fixed, and permeabilized in 50 µL/well of Label Solution (without terminal transferase) instead of the TUNEL reaction mixture. The number of apoptotic cells was counted by counting chamber (cells per 0.1 mm2 field) under a light microscope (80i; Nikon, Japan). Data were presented as the average result of three sections per tissue per gosling.
DNA ladder analysis
Tissue samples were dissected and lysed in 0.5 mL of extraction buffer (0.5% Triton X-100, 5 mM Tris, pH 7.5; 20 mM EDTA, and 100 µg/mL proteinase K) for 20 min on ice. DNA was extracted twice with phenol followed by chloroform/isoamyl alcohol extraction to remove proteins and residual traces of phenol. The DNA was then precipitated for 24 h in two volumes of ethanol at -20℃, resuspended in Tris-EDTA (pH 8.0) containing 20 µg/mL RNase (Takara, China), and incubated at 37℃ for 1 h. The DNA fragments were separated by electrophoresis on a 1.5% agarose gel and visualized under UV light after staining with GlodenView (SBS Genetech, China) staining. The fragments were compared to a 100-bp DNA ladder marker (Tiangen, China).
Results
Clinical signs and pathological changes
Goslings in the observation group began to die after 3 days PI. The number of dead goslings peaked during 6~10 days PI, and 26 (26/30) goslings died before the 20 days PI; two (2/30) goslings died after this time. The infected goslings suffered from typical symptoms of acute NGVE disease including somnolence, loss of appetite, yellow or whitish-yellow diarrhea, spasmodic prostration and convulsions, upward spasmodic kicking, and sudden death.
Pathological changes of the intestine were observed. Obvious hyperaemia and heavy hemorrhaging of the small intestine along with swollen intestinal mucosa were observed in the infected or dead goslings during early NGVEV infection (after 3 days PI). During the late infection phase (after 9 days PI), fibrinonecrotic enteritis further developed with increased mucosal necrosis and inflammatory exudation of the small intestine. Intestinal obstructions formed in the middle-lower part of small intestine, which was considered to be a typical pathological change associated with NGVE disease. None of the control goslings died, developed any clinical symptoms, or displayed any pathological changes during the entire experiment.
Histological findings
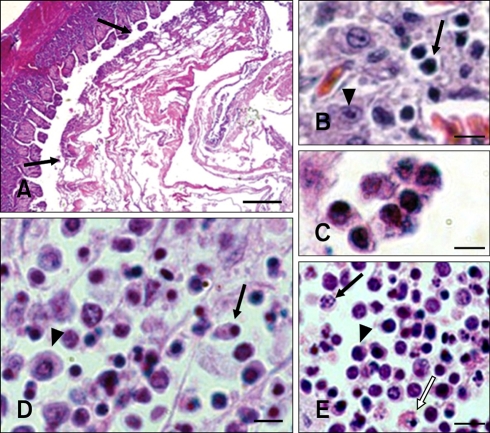
Catarrhal haemorrhagic fibrinonecrotic enteritis of the small intestine was observed in the infected or dead goslings. These lesions were composed of apoptotic cells, apoptotic bodies, necrotic cells, and inflammatory cells as well as fibrin (Fig. 1A). Few apoptotic cells were detected during the early NGVEV infection phase (1 to 48 h PI). Some apoptotic lymphocytes and macrophages were observed in the lymphoid organs from 48 to 72 h PI. During the late NGVEV infection phase (3 to 9 days PI), apoptosis was readily observed in lymphocytes, macrophages, monocytes, and epithelial and intestinal cells. Apoptosis was primarily detected in the BF, Harderian gland, thymus, and intestinal tract (small intestine, cecum, and rectum) of the NGVEV-infected goslings (Figs. 1B~E). This was characterized by the formation of small and spherical cytoplasmic fragments, a few of which contained pyknotic nuclear remnants, crimson coloration in the cytoplasm, and were isolated from the neighboring cells. However, the number of necrotic cells gradually increased while the number of apoptotic cells decreased during the late infection phase (after 9 days PI). The necrotic host cells were characterized by swelling, plasma membrane collapsed, and rapid lysis (Fig. 1E). Additionally, moderate numbers of inflammatory cells were found. Few apoptotic cells were observed in the samples from the control birds.
Fig. 1.
Histopathological microphotographs of apoptosis in new type gosling viral enteritis virus (NGVEV)-infected gosling. (A) Catarrhal hemorrhagic fibrinonecrotic enteritis with coagulative obstruction (arrows) was observed in the intestine tract of NGVEV-infected gosling that died 20 days post-infection (PI). (B) Apoptotic lymphocytes (arrow) and intestinal cells (arrowhead) were found in the small intestine. (C) Apoptotic cells and apoptotic bodies were identified in the intestinal. (D) Pyknosis (arrow) and necrosis (arrowhead) of the bursa of Fabricius (BF) lymphocytes. (E) Karyorrhexis (arrow), pyknosis (arrowshead), and necrosis (white arrow) of thymic lymphocytes were observed. H&E stain. Scale bars = 200 µm (A), 7 µm (B~D), 6 µm (E).
Ultrastructural changes
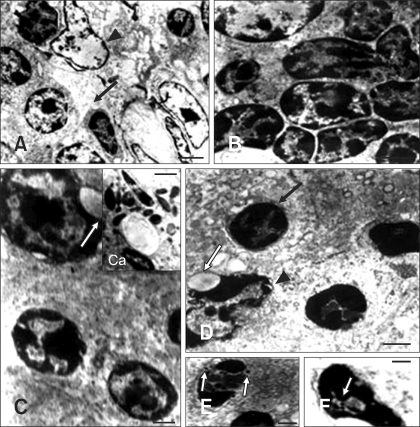
Tissues from NGVEV-infected and control goslings were carefully examined with TEM. Three distinct phases of apoptotic morphological changes were observed following NGVEV infection. Few apoptotic cells were observed in NGVEV-infected samples during first 48 h PI. However, apoptotic cells were widely observed in the BF, thymus, intestinal tract (small intestine, cecum, and rectum), spleen, liver, and cerebrum during the later phase (3 to 6 days PI). This was characterized by the cell dehydration and detachment from the substratum, a loss of desmosome in adjacent cells, nuclear chromatin condensation, and endoplasmic reticulum swelling (Fig. 2). Meanwhile, the cells shrank and the electron density of the cytoplasmic contents increased. During the last apoptotic phase (6 to 9 days PI), further morphological changes were observed. At this time, chromatin clumped or broke up into pieces, the cytoplasm condensed, and some vacuoles appeared on the cytoplasm membrane or in the cytoplasm. Subsequently, nuclear fragments and cytoplasm constituents were packaged into apoptotic bodies (Fig. 2C). The target cells included lymphocytes, macrophages, monocytes, epithelial cells, and endotheliocytes. Many mature NGVEV particles and some viral nucleocapsids were readily observed in the cytoplasm and nucleus of apoptotic lymphocytes and epithelia (Figs. 2E and F). Nevertheless, apoptotic cells were rarely observed in the control samples during the entire experiment.
Fig. 2.
Electron microphotographs of apoptosis in NGVEV-infected gosling. (A) Apoptotic lymphocytes (arrow) of the Bursa of Fabricius showed reduced nuclear size along with increased electron density and margination of the nuclear content. Necrotic lymphocytes (arrowhead) showed expansion of the cell nucleus, cell membrane lysis, and the release of intracellular contents. (B) The nucleus of apoptotic thymic lymphocytes showed increased electron density, shrinkage, and margination. The cytoplasm of these cells was also reduced. (C) Nuclear shrinkage, nuclear membrane blebbing (arrow) was observed in apoptotic intestinal cells. The chromatin clumped and marginated, or broke up into pieces and formed apoptotic bodies (Ca). (D) Apoptotic lymphocytes (arrow) and epithelial cells (arrowhead) of the spleen showed cell nuclear shrinkage and nuclear membrane blebbing (white arrow). And chromatin condensation and margination were also observed. (E and F) NGVEV particles (white arrows) were observed in the nuclei of apoptotic cells. Electron stain. Scale bars = 1 µm (A); 1.2 µm (B); 500 mm (C); 400 mm (Ca, E); 600 mm (D); 300 mm (F).
TUNEL assay
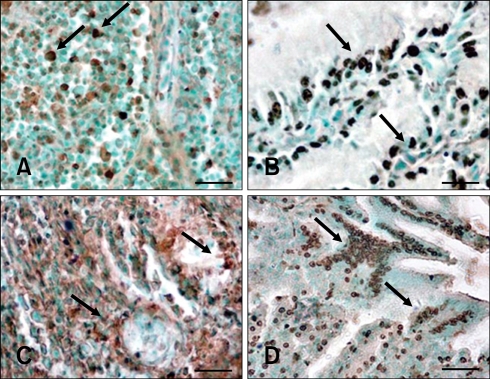
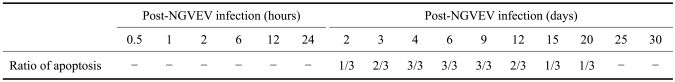
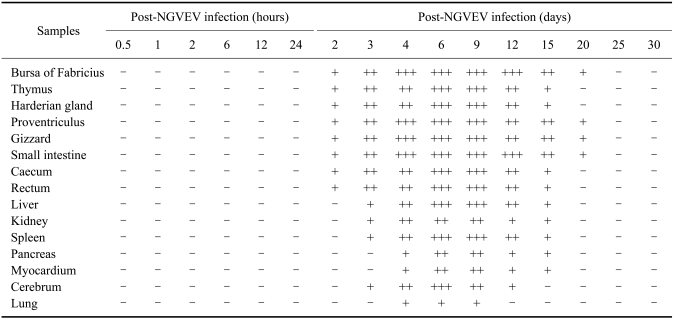
Additional evidence of apoptosis in tissues from NGVEV-infected goslings was obtained by a TUNEL assay using a digoxigenin-labeled antibody that recognized dUTP attached to the free 3'-OH ends of DNA molecules. TUNEL-specific signals (brown staining) appeared in the BF, thymus, Harderian gland, proventriculus, gizzard, and intestine tract as early as 2 days PI. This was predominantly seen in the digestive organs and lymphoid organs, especially after 4 days PI (Fig. 3, Tables 1 and 2). Results from the TUNEL assay showed that the presence and quantity of TUNEL-positive cells increased with infection times up to 9 days PI (Tables 1 and 2). Hardly any positive signal was detected in samples from the control birds.
Fig. 3.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) analysis of apoptosis in NGVEV-infected gosling. TUNEL-specific staining (brown, arrows) was widely observed in the Bursa of Fabricius (A), Harderian gland (B), small intestine (C), and proventriculus (D). Scale bars = 15 µm (A); 10 µm (B); 25 µm (C); 20 µm (D).
Table 1.
Time-course effect of new type gosling viral enteritis virus (NGVEV) on apoptosis in vivo
Positive was judged as the result was over 0.3 after subtracting the apoptotic ratio (per 0.1 mm2 field) of control gosling from that of the infection gosling. And the result less than 0.3 was considered negative. Three goslings were tested per time point. So, if there were N goslings judged positive, which was recorded as N/3.
Table 2.
Distribution and terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) staining intensity of apoptotic cells from NGVEV-infected goslings
The average numbers of apoptotic cells (cells per 0.1 mm2 field) were recorded as follows: no apoptosis (-), less than 10% apoptotic cells (+), 10~40% apoptotic cells (++), and more than 40% apoptotic cells (+++).
DNA ladder analysis
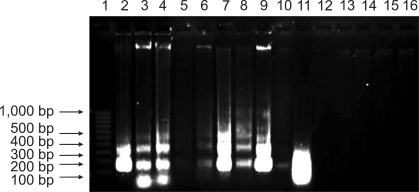
As showed in Fig. 4, DNA extracted from the proventriculus, gizzard, small intestine, cecum, rectum, Harderian gland, thymus, BF, and spleen of the infected goslings are fragmented into multiple fragments with lengths that are integer multiples of approximately 180~200 base pairs (Tables 1 and 2, Lanes 2 to 10). This result corresponded to the fragmentation of chromosomal DNA by endonucleases. DNA ladders could be seen after 3 days PI, and were clearly visible 6~9 days PI. No obvious DNA ladder patterns were observed in samples from the control birds during the entire experiment.
Fig. 4.
DNA ladder analysis of apoptotic cells from NGVEV-infected gosling by agarose gel electrophoresis. DNA extracted from the NGVEV-infected gosling 6 days PI formed characteristic 180~200 bp ladders. Lane 1: 100-bp ladder marker; Lanes 2 to 16: DNA from the proventriculus, gizzard, small intestine, cecum, rectum, Harderian gland, thymus, BF, spleen, liver, cerebrum, pancreas, kidney, myocardium, and lung, respectively.
Discussion
Many investigators have reported that adenoviruses can induce apoptosis both in vitro and in vivo [18,31,32,36], but few have examined goose adenovirus. NGVEV is considered to be goose adenovirus and scant information is available about apoptosis induced in vivo by this virus. Therefore, we conducted experiments to study apoptosis induced by NGVEV in the natural host experimentally infected with the virus. In our study, apoptosis and necrosis were detected in the early and late NGVEV-infection phase, respectively.
The induction of apoptosis was readily observed in the lymphoid and digestive organs, and sporadically appeared in other organs after 3 days PI. The presence and quantity of apoptotic cells increased with infection time until 9 days PI. Necrosis of the host cells eventually occurred, which was consistent with findings from our previous in vitro studies [7,8]. However, it must be noted that a number of apoptotic cells appeared in the liver, spleen, and cerebrum, which were also target organs, while few appeared in pancreas, kidney, myocardium, or lung.
In the present study, apoptotic lymphocytes (especially in the lymphoid organs) were firstly detected in the NGVEV-infected osling, while necrosis was later detected in the epithelia and some lymphocytes. In addition, some viral particles were observed in apoptotic intestinal epithelia and lymphocytes by TEM. Our previous studies indicated that the lymphoid organs of the infected goslings serve as the principal sites for NGVEV localization [9,10]. It has been suggested that NGVEV infection induced the host cells of BF, Harderian gland, and thymus to undergo apoptosis. Subsequently, theapoptotic epithelia and lymphocytes disrupted the lymphocytic microenvironment. Therefore, apoptotic depletion of the lymphocytes by NGVEV may be partially mediated by this mechanism which is similar to the one proposed for HIV-related thymocyte depletion [34,35]. Furthermore, apoptosis facilitates persistent viral infection in host cells and enables progeny viral dissemination [2,33]. Apoptotic bodies are consumed by neighboring cells via phagocytosis and provide a means for releasing virions into the extracellular fluid without inducing an inflammatory response [22]. It has been hypothesized that virus-induced lymphocytic apoptosis is involved in the pathogenesis of many viral infections [1,3,20,23,38]. Due to apoptosis and necrosis of host lymphocytes, cellular and humoral immune functions are markedly depressed in the central as well as peripheral immune organs after NGVEV infection. It is therefore easy to accelerate the promotion of death by secondary infection.
Virus infection and replication are usually associated with apoptosis; these are likely to be responsible for the pathologic symptoms that accompanies infectious diseases [19,32,33]. Apoptosis and necrosis were widely detected in the digestive organs (especially in the intestine tract). Characteristic pathological changes associated with NGVE included duodenal fibrinonecrotic enteritis and intestinal obstruction of the middle-lower part of the small intestine, which may be caused by apoptosis or necrosis of intestine glandular cells and epithelia. It was presumed that apoptotic cells, apoptotic bodies, necrotic cells, and inflammatory cells combined with fibrin filled the intestine lumen to form coagulative obstructions. Apoptosis of the digestive organs contributed to a series of functional disorders of the digestive system which interfered with cells function, resulting in a series of secondary pathological changes and clinical symptoms.
The intracellular events that trigger the apoptotic response following NGVEV infection require further investigation. Based on the results of our study, it is possible to speculate that apoptosis of host cells in vivo may play an important role in the pathogenesis of NGVE. The findings from the present work could be useful for achieving a better understanding of the mechanisms involved in NGVEV infection.
Acknowledgments
This work was supported by grants Changjiang Scholars and Innovative Research Team in University (PCSIRT0848) and China Agricultural Research System (CARS-43-8), and the Culture Fund for Excellent Doctoral Dissertations of Sichuan Agriculture University, China (2008scybpy-1).
References
- 1.Adair BM. Immunopathogenesis of chicken anemia virus infection. Dev Comp Immunol. 2000;24:247–255. doi: 10.1016/s0145-305x(99)00076-2. [DOI] [PubMed] [Google Scholar]
- 2.Barber GN. Host defense, viruses and apoptosis. Cell Death Differ. 2001;8:113–126. doi: 10.1038/sj.cdd.4400823. [DOI] [PubMed] [Google Scholar]
- 3.Bruschke CJM, Hulst MM, Moormann RJM, van Rijn PA, van Oirschot JT. Glycoprotein Erns of pestiviruses induces apoptosis in lymphocytes of several species. J Virol. 1997;71:6692–6696. doi: 10.1128/jvi.71.9.6692-6696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S, Cheng AC, Wang MS. Studies on adaptation of NGVEV in duck embryo fibroblasts and multiplication characteristic. Vet Sci China. 2006;36:773–778. [Google Scholar]
- 5.Chen S, Cheng AC, Wang MS. Morphologic observations of new type gosling viral enteritis virus (NGVEV) virulent isolate in infected duck embryo fibroblasts. Avian Dis. 2008;52:173–178. doi: 10.1637/8080-072107-ResNote. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Cheng AC, Wang MS. Morphogenesis of new gosling type viral enteritis virus and ultrastructural pathology of tissues in experimentally infected duck embryo. Act Vet Zootech Sinica. 2008;39:182–188. [Google Scholar]
- 7.Chen S, Cheng AC, Wang MS, Chen XY. Dynamic changes of apoptosis in duck embryo fibroblasts induced by new type gosling viral enteritis virus. Prog Nat Sci. 2008;18:239–244. [Google Scholar]
- 8.Chen S, Cheng AC, Wang MS, Peng X. Detection of apoptosis induced by new type gosling viral enteritis virus in vitro through fluorescein annexin V-FITC/PI double labeling. World J Gastroenterol. 2008;14:2174–2178. doi: 10.3748/wjg.14.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S, Cheng AC, Wang MS, Zhu DK, Luo Q, Liu F, Chen XY. Immunohistochemical detection and localization of new type gosling viral enteritis virus in paraformaldehyde-fixed paraffin-embedded tissue. Vet Immunol Immunopathol. 2009;130:226–235. doi: 10.1016/j.vetimm.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Chen S, Cheng AC, Wang MS, Zhu DK, Luo Q, Liu F, Chen XY. Detection and localization of a goose adenovirus in paraffin-embedded experimentally infected gosling tissue sections using indirect immunofluorescent assay. Avian Pathol. 2009;38:167–174. doi: 10.1080/03079450902737854. [DOI] [PubMed] [Google Scholar]
- 11.Chen S, Cheng AC, Wang MS, Zhu DK, Jia RY, Luo QH, Liu F, Chen XY, Yang JL. Humoral and cellular immune responses in adult geese induced by an inactivated vaccine against new type gosling viral enteritis virus. Poult Sci. 2010;89:2410–2418. doi: 10.3382/ps.2010-00958. [DOI] [PubMed] [Google Scholar]
- 12.Cheng AC. Research on a new infectious disease of goslings. Vet Sci China. 1998;28:3–6. [Google Scholar]
- 13.Cheng AC. Isolation, identification and properties of goslings new type viral enteritis virus. Act Vet Zootech Sinica. 2000;31:548–556. [Google Scholar]
- 14.Cheng AC, Wang MS. Morphogenesis of gosling new type viral enteritis virus and the ultrastructural changes of tissues of gosling artificially infected with the virus. Chin J Virol. 2002;18:348–354. [Google Scholar]
- 15.Cheng AC, Wang MS, Chen XY, Guo YF, Liu ZY, Fang PF. Pathogenic and pathological characteristic of new type gosling viral enteritis first observed in China. World J Gastroenterol. 2001;7:678–684. doi: 10.3748/wjg.v7.i5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cobb JP, Hotchkiss RS, Karl IE, Buchman TG. Mechanisms of cell injury and death. Br J Anaesth. 1996;77:3–10. doi: 10.1093/bja/77.1.3. [DOI] [PubMed] [Google Scholar]
- 17.Eleouet JF, Chilmonczyk S, Besnardeau L, Laude H. Transmissible gastroenteritis coronavirus induces programmed cell death in infected cells through a caspase-dependent pathway. J Virol. 1998;72:4918–4924. doi: 10.1128/jvi.72.6.4918-4924.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ezoe H, Fatt RB, Mak S. Degradation of intracellular DNA in KB cells infected with cyt mutants of human adenovirus type 12. J Virol. 1981;40:20–27. doi: 10.1128/jvi.40.1.20-27.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garrido-Fariña GI, Cornejo-Cortés MA, Martínez-Rodríguez A, Reyes-Esparza J, Alba-Hurtado F, Tórtora-Pérez J. A study of the process of apoptosis in animals infected with the contagious ecthyma virus. Vet Microbiol. 2008;129:28–39. doi: 10.1016/j.vetmic.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Hanon E, Lambot M, Hoornaert S, Lyaku J, Pastoret PP. Bovine herpesvirus 1-induced apoptosis: phenotypic characterization of susceptible peripheral blood mononuclear cells. Arch Virol. 1998;143:441–452. doi: 10.1007/s007050050301. [DOI] [PubMed] [Google Scholar]
- 21.Hardwick JM. Viral interference with apoptosis. Semin Cell Dev Biol. 1998;9:339–349. doi: 10.1006/scdb.1998.0243. [DOI] [PubMed] [Google Scholar]
- 22.Hay S, Kannourakis G. A time to kill: viral manipulation of the cell death program. J Gen Virol. 2002;83(Pt 7):1547–1564. doi: 10.1099/0022-1317-83-7-1547. [DOI] [PubMed] [Google Scholar]
- 23.Johnson CM, Benson NA, Papadi GP. Apoptosis and CD4+ lymphocyte depletion following feline immunodeficiency virus infection of a T-lymphocyte cell line. Vet Pathol. 1996;33:195–203. doi: 10.1177/030098589603300209. [DOI] [PubMed] [Google Scholar]
- 24.Jungmann A, Nieper H, Müller H. Apoptosis is induced by infectious bursal disease virus replication in productively infected cells as well as in antigen-negative cells in their vicinity. J Gen Virol. 2001;82(Pt 5):1107–1115. doi: 10.1099/0022-1317-82-5-1107. [DOI] [PubMed] [Google Scholar]
- 25.Kerr JF, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koyama AH. Induction of apoptotic DNA fragmentation by the infection of vesicular stomatitis virus. Virus Res. 1995;37:285–290. doi: 10.1016/0168-1702(95)00026-m. [DOI] [PubMed] [Google Scholar]
- 27.Liu YZ, Li Y, Wei XT, Zhang JF. Isolation and identification of goslings new type viral enteritis virus. Acta Agric Jiangxi. 2008;20:95–96. [Google Scholar]
- 28.Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- 29.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 30.Neilan JG, Lu Z, Kutish GF, Zsak L, Lewis TL, Rock DL. A conserved African swine fever virus IκB homolog, 5EL, is nonessential for growth in vitro and virulence in domestic swine. Virology. 1997;235:377–385. doi: 10.1006/viro.1997.8693. [DOI] [PubMed] [Google Scholar]
- 31.Rautenschlein S, Sharma JM. Immunopathogenesis of haemorrhagic enteritis virus (HEV) in turkeys. Dev Comp Immunol. 2000;24:237–246. doi: 10.1016/s0145-305x(99)00075-0. [DOI] [PubMed] [Google Scholar]
- 32.Rautenschlein S, Suresh M, Sharma JM. Pathogenic avian adenovirus type II induces apoptosis in turkey spleen cells. Arch Virol. 2000;145:1671–1683. doi: 10.1007/s007050070083. [DOI] [PubMed] [Google Scholar]
- 33.Roulston A, Marcellus RC, Branton PE. Viruses and apoptosis. Annu Rev Microbiol. 1999;53:577–628. doi: 10.1146/annurev.micro.53.1.577. [DOI] [PubMed] [Google Scholar]
- 34.Stanley SK, McCune JM, Kaneshima H, Justement JS, Sullivan M, Boone E, Baseler M, Adelsberger J, Bonyhadi M, Orenstein J, Fox CH, Fauci AS. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J Exp Med. 1993;178:1151–1163. doi: 10.1084/jem.178.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su L, Kaneshima H, Bonyhadi M, Salimi S, Kraft D, Rabin L, McCune JM. HIV-1-induced thymocyte depletion is associated with indirect cytopathogenicity and infection of progenitor cells in vivo. Immunity. 1995;2:25–36. doi: 10.1016/1074-7613(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 36.Takemori N, Riggs JL, Aldrich C. Genetic studies with tumorigenic adenoviruses. I. Isolation of cytocidal (cyt) mutants of adenovirus type 12. Virology. 1968;36:575–586. doi: 10.1016/0042-6822(68)90189-x. [DOI] [PubMed] [Google Scholar]
- 37.Teodoro JG, Branton PE. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thoulouze MI, Lafage M, Montano-Hirose JA, Lafon M. Rabies virus infects mouse and human lymphocytes and induces apoptosis. J Virol. 1997;71:7372–7380. doi: 10.1128/jvi.71.10.7372-7380.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trump BF, Berezesky IK, Chang SH, Phelps PC. The pathways of cell death: Oncosis, apoptosis, and necrosis. Toxicol Pathol. 1997;25:82–88. doi: 10.1177/019262339702500116. [DOI] [PubMed] [Google Scholar]
- 40.Woolcock PR. Viral infections of waterfowl. In: Saif YM, Fadly AM, Glisson JR, McDougald LR, Ńolan LK, Swayne DE, editors. Diseases of Poultry. 12th ed. Iowa: Wiley-Blackwell; 2008. p. 370. [Google Scholar]
- 41.Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 42.Young LS, Dawson CW, Eliopoulos AG. Viruses and apoptosis. Br Med Bull. 1997;53:509–521. doi: 10.1093/oxfordjournals.bmb.a011627. [DOI] [PubMed] [Google Scholar]
- 43.Zsák L, Kisary J. Characterization of adenoviruses isolated from geese. Avian Pathol. 1984;13:253–264. doi: 10.1080/03079458408418529. [DOI] [PubMed] [Google Scholar]