Abstract
The seroprevalence of Japanese encephalitis virus (JEV) among equines was evaluated from January 2006 to December 2009 in 13 different states of India by hemagglutination inhibition (HI) test and virus neutralization test (VNT). Antibodies against JEV were detected in 327 out of 3,286 (10%) equines with a maximum prevalence reported in the state of Manipur (91.7%) followed by Gujarat (18.5%), Madhya Pradesh (14.4%), and Uttar Pradesh (11.6%). Evidence of JEV infection was observed in equines in Indore (Madhya Pradesh) where a 4-fold or higher rise in antibody titer was observed in 21 out of 34 horses in November 2007 to October 2006. In March 2008, seven of these horses had a subsequent 4-fold rise in JEV antibody titers while this titer decreased in nine animals. JEV-positive horse sera had a JEV/WNV (West Nile virus) ratio over 2.0 according to the HI and/or VNT. These results indicated that JEV is endemic among equines in India.
Keywords: equines, haemagglutination inhibition, Japanese encephalitis virus, serosurveillance, virus neutralization
Introduction
Japanese encephalitis (JE) is a mosquito-transmitted viral disease caused by the JE virus (JEV) belonging to the genus Flavivirus and family Flaviviridae. JEV is an enveloped virus with plus-sense, single-stranded RNA about 11 kb in length with a single open reading frame (ORF) encoding a polyprotein of approximately 3,400 amino acids. This peptide is subsequently cleaved into three structural and seven non-structural proteins [2]. JEV causes disease in equines, pigs, and humans. It is prevalent in eastern and southern Asia, and has spread to Indonesia, northern Australia, Papua New Guinea, and Pakistan [18]. Approximately 3 billion people live in JEV-endemic areas where 50,000 clinical cases and 15,000 human deaths due to JE are reported annually [8]. The virus may infect a number of other domestic animals including cattle, sheep, goat, dogs and cats, although these infections are typically asymptomatic.
Equines infected with JEV may develop clinical signs such as fever, anorexia, depression, impaired locomotion, impaired vision, paresis, and paralysis. The affected horse may collapse, fall in a coma and eventually die. Rates of mortality due to JE range between 5% and 30%. Sporadic cases of JE in horses have been reported in various countries, including Japan [13,25], Hong Kong [14], and Taiwan [15]. In addition, JE seropositivity among equines has been reported in Nepal [19] and Indonesia [24]. According to the 2007 Livestock Census, there are 1,186,000 equines including horses, ponies, mules and donkeys in India [5]. Since equines are not vaccinated against JE in India, these animals are potentially at risk, especially in states where JE outbreaks in humans have been reported [6]. There have been a few isolated reports describing JEV seropositivity in equines in India [3,7,10,16,21-23].
With global warming, changing agricultural practices and increases in vector populations, there has been rise in JE incidence among humans in India during the last decade. This disease has been reported in human populations in the states of Andhra Pradesh, Assam, Bihar, Goa, Haryana, Karnataka, Kerala, Mahrashtra, Manipur, Tamil Nadu, Uttar Pradesh, West Bengal, and Nagaland [6,20]. However, prevalence of JE among equines in India has not been reported during the last decade. This paper describes the serological evidence of JEV infection among equines in different states of India.
Materials and Methods
Collection of samples
For the seroprevalence study, blood samples from indigenous equines in various states of India were collected between January 2006 and December 2009. None of the horses included in this study had been vaccinated against JEV. Blood samples were stored at 4℃ after collection until they were transported to the laboratory. Serum was separated by centrifugation at 1,000 × g for 5 min and stored at -30℃ until further use. Equine serum samples collected from horses in Manipur during March 2003 and April 2005 and available in the Serum Bank of National Research Centre on Equines (Hisar, India) were also included in the study.
Viruses and cells
JEV (strain P20778) and West Nile virus (WNV; strain G22886) were obtained from the National Institute of Virology, Pune (India). The viruses were propagated in Swiss albino suckling mice via intra-cerebral inoculation and porcine stable kidney (PS) cells procured from National Centre for Cell Sciences, India. The PS cells were grown at 37℃ in Eagle's minimal essential medium (EMEM) supplemented with 10% fetal bovine serum (FBS), 100 IU/mL penicillin, 100 µg/mL streptomycin, and 0.25 µg/mL amphotericin-B (Sigma-Aldrich, USA).
Hemagglutination inhibition (HI) test
HI test was carried out in duplicate in 96-well microplates using 8 hemagglutinating antigen (HA) units/25 µL of sucrose-acetone extracted antigen prepared from the brains of suckling mice infected with JEV or WNV as described previously [4]. Serum samples were treated with cold acetone to remove non-specific HA inhibitors and were absorbed with goose erythrocytes [4]. Hyper-immune serum to both JEV and WNV previously raised in rabbits by inoculating inactivated purified viruses, were included in each test as positive controls. HI titers were expressed as the reciprocal of the highest dilution of the serum that completely inhibited haemagglutination. An HI titer of 20 and above was considered positive.
Virus neutralization test (VNT)
Serial two-fold dilutions of JEV and WNV (four wells per dilution per virus) in 100 µL volume of EMEM supplemented with 2% FBS were made in 96-well culture plates. To each well, 100 µL of PS cells (2 × 105 cells/mL in EMEM supplemented with 10% FBS) were added and incubated for 5 days at 37℃ in 5% CO2. The wells showing cytopathic effects (CPE) were recorded to calculate 50% tissue culture infectivity dose (TCID50) of both the viruses. VNT was performed in 96-well tissue culture plates using PS cells. Serum samples were heat inactivated at 56℃ for 30 min and serial two-fold dilutions of the serum in a 50 µL volume was mixed with an equal volume of 300 TCID50 of the JEV or WNV. After incubating the virus-serum mixture at 37℃ for 1 h, 100 µL of a PS cell suspension (2 × 105 cells/mL) was added to each well. The plates were incubated at 37℃ in 5% CO2 for 5 days and CPE in each well was recorded. The neutralization titers were expressed as the reciprocal of the highest serum dilution that completely inhibited CPE in the wells. Titers of 4 and above were considered positive.
Statistical analysis
Chi-squared test was used to analyze differences in JEV seroprevalence between states and years. A p-value less than 0.05 was considered to be statistically significant.
Results
JEV seroprevalence
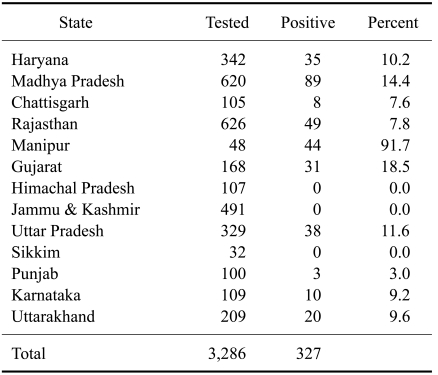
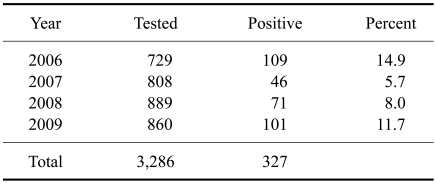
We conducted a JEV serosurveillance study in equines in 13 states of India including Jammu & Kashmir (J&K), Himachal Pradesh, Punjab, Haryana, Rajasthan, Gujarat, Uttarakhand, Uttar Pradesh, Madhya Pradesh, Chhattisgarh, Karnataka, Sikkim and Manipur. Out of 3,286 equine serum samples tested, 327 (10%) were positive for JEV antibodies according to both HI and VNT results (Table 1). No significant differences in JEV sero-prevalence were observed from year to year between 2006 and 2009 (Table 2). However, differences (p < 0.05) in JEV positivity among different states were observed (Table 1). None of the equines from the northern hill states of J&K, Himachal Pradesh, or Sikkim were positive for JEV antibodies. The maximum JEV seroprevalence was recorded in Manipur (a north-eastern state of India) where 91.7% (44 out of 48) equines were positive, followed by Gujarat (18%) and Madhya Pradesh (14.4%).
Table 1.
Seroprevalence of Japanese encephalitis virus in equines from 2006 to 2009 according to states
Table 2.
Seroprevalence of Japanese encephalitis virus in equines from 2006 to 2009 according to years
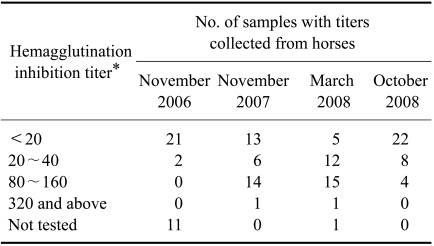
Serum samples from two adjoining equine farms in Indore (Madhya Pradesh) with a total of 34 horses have been collected annually since 2005. In 2005, all horses from these farms tested negative for JEV antibodies while only two horses in November 2006 were positive with an HI titer of 40 and a VNT titer of 4. However, in November 2007, 21 horses from these two farms were significantly positive with HI titers of 20~40 (n = 6), 80~160 (n = 14), or 320 or above (n = 1). There were 28 positive horses in March 2008. Out of these, seven showed a 4-fold rise and nine showed a 4-fold decline in JEV antibody titers according to the HI test and VNT compared to the results from November 2007. JEV antibody titers declined in the positive horses by October 2008 (Table 3). Some of these animals were anorectic and pyretic in October 2007 (rectal temperature around 38.9℃) for 2~3 days although no neurological signs or mortality were reported in these farms.
Table 3.
Seroprevalence of Japanese encephalitis virus among horses (n = 34) from Indore (Madhya Pradesh) between 2006 and 2008
*Titers are expressed as the reciprocal of the highest dilution of serum. Titers for haemagglutination inhibition 20 and above were considered positive.
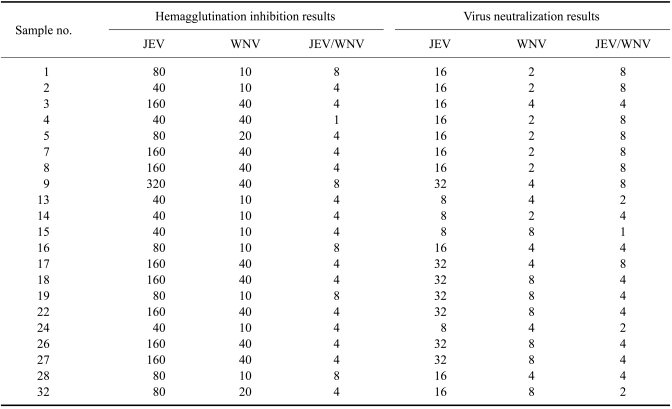
Cross-reactivity of the JEV-positive equine sera from Indore (Madhya Pradesh) with WNV was evaluated. All the 21 positive equine samples collected from Indore in November 2007 had JEV HI titers between 40 and 320. Twelve of these also had WNV HI titers between 20 and 40 (Table 4). Similarly, the 21 equine samples had JEV VN titers between 8 and 32. Out of these, 12 horses had neutralizing antibodies against WNV with VN titers between 2 and 8. However, the JEV titer/WNV titer ratios were 4 and above for 20 out of 21 horses according to the HI test, while the VNT showed that 17 out of the 21 horses had JEV/WNV ratios of 4 and above (Table 4).
Table 4.
Hemagglutination inhibition and virus neutralization titersa gainst Japanese encephalitis virus (JEV) and West Nile virus (WNV) in equine serum samples collected from Indore (Madhya Pradesh) in November 2007
*Titers are expressed as the reciprocal of the highest dilution of serum. Titers for hemagglutination inhibition 20 and above along with virus neutralization titers of 4 and above were considered positive.
Since maximum sero-positivity was observed in equines from Manipur, serum samples from equines in the state of Manipur collected in March 2003 (n = 71) and April 2005 (n = 35) and archived at the Serum Bank of National Research Centre on Equines were also tested for JEV antibodies. Only two out of 71 (2.8%) equines in March 2003 and 14 out of 35 (35%) equines in April 2005 were positive for JEV antibodies. However, 91.7% of equines in Manipur were positive in March 2007.
Discussion
JE remains endemo-epidemic in several Asian countries. Several outbreaks among humans and domestic animals are reported every year in different regions of the world. Sporadic cases of JE in horses have been observed in Japan [12,25], Hong Kong [14], and Korea [15] despite the use of JEV vaccinations in these countries. JEV outbreaks have been reported in humans from 24 states/Union Territories of India. In northern India, large epidemics of JE occur during summer whereas in the southern region JE tends to be endemic throughout the year [17]. Out of 3,286 equines in 13 states in India, 10% were positive for JEV antibodies according to our sero-surveillance. In India, vaccination against JEV is not performed in equines. There have been a few isolated reports describing sero-positive equines in India [3,7,10,16,21-23]; however, the present study is the first report of JEV sero-surveillance of equines from 13 different states.
Based on seroconversion, it was apparent that JEV infection occurred in two farms in Indore (Madhya Pradesh) during October 2007 when some horses were reported to be anorectic and pyretic for 2~3 days although no neurological signs or mortality were observed. Attempted virus isolation and RT-PCR detection of viral RNA in serum samples from these horses were not successful (data not shown). In horses, most JEV infections remain sub-clinical and the only symptom may be fever and a short period of lethargy [17].
Serodiagnosis is primarily used for JEV surveillance because virus isolation from JEV-infected horses is difficult due to transient viremia and low virus titers [9,16,17]. HI test, VNT, and ELISA are commonly used for sero-diagnoses of JEV. However, cross-reactivity with related flaviviruses, including WNV, has been observed in these assays. WNV infection in horses has not been documented in India although it is circulating among humans and animals in this country [1]. Therefore, it is critical to distinguish between JEV and WNV in areas where both viruses are endemic. When sera are tested for both antigens, an animal is considered positive for the virus that shows a 2-4-fold higher antibody titer. JEV infection in horses from Indore (Madhya Pradesh) was confirmed by demonstration of a JEV titer/WNV titer ratio that was 4 and above according to either the HI test or VNT, indicating that these animals were in fact infected with JEV and not with WNV [11].
In the present study, more than 90% of equines tested in the north-eastern state of Manipur were JEV-positive in March 2007. Manipur has an indigenous breed of equine, called Manipuri ponies, which are used for playing polo. Our retrospective study of archived sera revealed that only 2% of the equines in Manipur were sero-positive for JEV in March 2003. However, the rate of sero-positivity rose to 35% in April 2005 and 90% in 2007. It should be mentioned that human JEV infection is endemic in the North-Eastern states of India and human deaths due to JEV have been reported from the state of Manipur during last 5 years. Our findings indicate that these animals were sub-clinically infected after 2003, making them seropositive, since no history of neurological illness was reported for these animals.
Due to changing agricultural practices (increased irrigation facilities that provide mosquito breeding sites), expanding ranges for migratory birds (like cattle egrets), and increased animal husbandry (pig rearing), JEV infection has been reported during last decade in newer areas of India, Nepal, and Australia. The JEV seroprevalence in horses we observed may have potential implications for the future spread of JEV in equine populations, resulting in increased morbidity and mortality in these animals. In addition, JEV may also spread via mosquito vectors to humans and other animal population in 10 out of the 13 states where JEV sero-positivity in horses has been reported in the present study [6,17,20].
Along with a 'One Health' approach for collectively combating JEV infection in human-animal interface, additional surveys of animal, human, and vector populations are necessary to understand the effects of climate changes on JEV distribution and disease transmission via vectors. This will provide better assessment of the risks posed by JEV which will, in turn, help to establish strategies for effective control and management of this virus.
Acknowledgments
We thank the Indian Council of Agricultural Research, New Delhi for providing necessary financial assistance. The authors also acknowledge the assistance provided by various state animal husbandry departments for our equine survey and the technical assistance of Mr. P. P. Choudhury, National Research Centre on Equines, Hisar, India.
References
- 1.Bondre VP, Jadi RS, Mishra AC, Yergolkar PN, Arankalle VA. West Nile virus isolates from India: evidence for a distinct genetic lineage. J Gen Virol. 2007;88:875–884. doi: 10.1099/vir.0.82403-0. [DOI] [PubMed] [Google Scholar]
- 2.Burke DS, Monath TP. Flaviviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 4th ed. Philadelphia: Lippincott; 2001. pp. 1043–1125. [Google Scholar]
- 3.Chakravarti SK, Sardana DN, Mukherjee KK, Mitra AC, Chakrabarty MS. Serological evidence of Infection with Japanese encephalitis virus in Mules of Eastern Himalayan region. Indian J Med Res. 1981;73:4–7. [PubMed] [Google Scholar]
- 4.Clarke DH, Casals J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958;7:561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- 5.Department of Animal Husbandry, Dairying and Fisheries. Basic Animal Husbandry Statistics-2010 (AHS Series-12) New Delhi: Department of Animal Husbandry, Dairying and Fisheries, Minister of Agriculture, Government of India; 2011. pp. 45–46. [Google Scholar]
- 6.Dhillon GP, Raina VK. Epidemiology of Japanese encephalitis in context with Indian scenario. J Indian Med Assoc. 2008;106:660–663. [PubMed] [Google Scholar]
- 7.D'Souza MB, Nagarkatti S, Rao KM. Serological evidence for arboviral infection among horses - HI test by filter paper disc method. Indian J Med Res. 1978;67:708–712. [PubMed] [Google Scholar]
- 8.Ghosh D, Basu A. Japanese encephalitis-a pathological and clinical perspective. PLoS Negl Trop Dis. 2009;3:e437. doi: 10.1371/journal.pntd.0000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gould DJ, Byrne RJ, Hayes DE. Experimental infection of horses with Japanese encephalitis virus by mosquito bits. Am J Trop Med Hyg. 1964;13:742–746. doi: 10.4269/ajtmh.1964.13.742. [DOI] [PubMed] [Google Scholar]
- 10.Gowal D, Singh G, Gowal KN, Bhau LNR, Saxena SN. Serological evidence of Japanese encephalitis virus infection in equines of North Himalayan region. Indian Vet J. 1990;67:398–401. [Google Scholar]
- 11.Hirota J, Nishi H, Matsuda H, Tsunemitsu H, Shimiz S. Cross-reactivity of Japanese encephalitis virus-vaccinated horse sera in serodiagnosis of west nile virus. J Vet Med Sci. 2010;72:369–372. doi: 10.1292/jvms.09-0311. [DOI] [PubMed] [Google Scholar]
- 12.Ihara T, Kano R, Nakajima Y, Sugiura T, Imagawa H, Izuchi T, Samejima T. Detection of antibody to Japanese encephalitis virus (JEV) by enzyme-linked immunosorbent assay (ELISA) J Equine Sci. 1997;8:25–28. [Google Scholar]
- 13.Konishi E, Shoda M, Ajiro N, Kondo T. Development and evaluation of an enzyme-linked immunosorbent assay for quantifying antibodies to Japanese encephalitis virus nonstructural 1 protein to detect subclinical infections in vaccinated horses. J Clin Microbiol. 2004;42:5087–5093. doi: 10.1128/JCM.42.11.5087-5093.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lam KHK, Ellis TM, Williams DT, Lunt RA, Daniels PW, Watkins KL, Riggs CM. Japanese encephalitis in a racing thoroughbred gelding in Hong Kong. Vet Rec. 2005;157:168–173. doi: 10.1136/vr.157.6.168. [DOI] [PubMed] [Google Scholar]
- 15.Lian WC, Liau MY, Mao CL. Diagnosis and genetic analysis of Japanese encephalitis virus infected in horses. J Vet Med B Infect Dis Vet Public Health. 2002;49:361–365. doi: 10.1046/j.1439-0450.2002.00509.x. [DOI] [PubMed] [Google Scholar]
- 16.Mall MP, Kumar A, Malik SV. Sero-positivity of domestic animals against Japanese encephalitis in Bareilly area, U.P. J Commun Dis. 1995;27:242–246. [PubMed] [Google Scholar]
- 17.Misra UK, Kalita J. Overview: Japanese encephalitis. Prog Neurobiol. 2010;91:108–120. doi: 10.1016/j.pneurobio.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Nga PT, del Carmen Parquet M, Cuong VD, Ma SP, Hasebe F, Inoue S, Makino Y, Takagi M, Nam VS, Morita K. Shift in Japanese encephalitis virus (JEV) genotype circulating in northern Vietnam: implications for frequent introductions of JEV from Southeast Asia to East Asia. J Gen Virol. 2004;85:1625–1631. doi: 10.1099/vir.0.79797-0. [DOI] [PubMed] [Google Scholar]
- 19.Pant GR. A serological survey of pigs, horses, and ducks in Nepal for evidence of infection with Japanese encephalitis virus. Ann N Y Acad Sci. 2006;1081:124–129. doi: 10.1196/annals.1373.013. [DOI] [PubMed] [Google Scholar]
- 20.Parida M, Dash PK, Tripathi NK, Ambuj, Sannarangaiah S, Saxena P, Agarwal S, Sahni AK, Singh SP, Rathi AK, Bhargava R, Abhyankar A, Verma SK, Rao PV, Sekhar K. Japanese encephalitis outbreak, India, 2005. Emerg Infect Dis. 2006;12:1427–1430. doi: 10.3201/eid1209.060200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rai GP, Tuteja U, Kumar P. Comparison of haemagglutination inhibition and indirect fluorescent antibody tests to detect certain flavivirus antibodies in equines. Acta Microbiol Hung. 1992;39:69–73. [PubMed] [Google Scholar]
- 22.Raut CG, Thakare JP, Padbidri VS, Sapkal GN, Mishra AC, Paramasivan R, Gokhale MD, Mourya DT, Shouche YS, Jayakumar PC. A focal outbreak of Japanese encephalitis among horses in Pune district, India. J Commun Dis. 2003;35:40–42. [PubMed] [Google Scholar]
- 23.Shankar H, Mall MP, Yadav MP. Seroprevalence of group-B arboviruses and equine herpes virus-1 infections in nervous disorders in equines. Indian J Anim Sci. 1988;58:1–5. [Google Scholar]
- 24.Widjaja S, Soekotjo W, Hartati S, Jenning GB, Corwin AL. Prevalence of hemagglutination-inhibition and neutralizing antibodies to arboviruses in horses of Java. Southeast Asian J Trop Med Public Health. 1995;26:109–113. [PubMed] [Google Scholar]
- 25.Yamanaka T, Tsujimura K, Kondo T, Yasuda W, Okada A, Noda K, Okumura T, Matsumura T. Isolation and genetic analysis of Japanese encephalitis virus from a diseased horse in Japan. J Vet Med Sci. 2006;68:293–295. doi: 10.1292/jvms.68.293. [DOI] [PubMed] [Google Scholar]