Abstract
The present study determined the genetic relationships between 41 Staphyloccocus (S.) aureus isolates from bovines, humans, and food using a single enzyme amplified fragment length polymorphism (AFLP) technique. We evaluated the prevalence of staphylococcal enterotoxin (SE) genes and other virulence gene determinants by PCR. The identification of S. aureus was based on culturing and biochemical tests, and by amplifying a specific section of the 23S rRNA gene. PCR amplification of the SE genes (sea, seb, sec, see, seg, seh, and sei) singly or in combination was observed. Most isolates of bovine origin harbored hla (84%) and cap5 (74%), while most isolates from humans harbored hla (73%), cap8 (91%), and fnbA (100%). Strains from food sources were positive for hla (100%), cap5 (100%), and cap8 (64%) unlike isolates from humans or bovines. A single enzyme AFLP analysis revealed a correlation between AFLP clusters of some strains and the source of the isolates The genotypic results of the present study might help to better understand the distribution of prevalent S. aureus clones among humans, bovines, and food and will help control S. aureus infections in Indonesia.
Keywords: amplified fragment length polymorphism, enterotoxin, genetic determinant, Staphylococcus aureus
Introduction
Staphylococcus (S.) aureus is recognized worldwide as a major pathogen that causes food poisoning and various infections in animals and humans. The primary habitat of S. aureus is the nasal passage of humans, and on the skin and hair of warm-blooded animals. Hence, the sources of this organism found on food are mostly human or animal [24]. In dairy cows, S. aureus causes subclinical intramammary infections [7]. The main reservoir of S. aureus is an infected quarter of udder, and transmission between cows usually occurs during milking [7]. S. aureus is also an important food-borne pathogen because of its ability to produce a wide range of extracellular toxin proteins and virulence factors that contribute to the pathogenicity of the organism.
Molecular typing of S. aureus and determining the clonal relationships between isolates has proved useful for epidemiological studies. One of the most recent and promising of these genotyping techniques is amplified fragment length polymorphism (AFLP) analysis [4]. The objective of the present study was to determine the genetic relationship of S. aureus isolated from humans, bovines, and food. The S. aureus strains we isolated were also analyzed to evaluate the distributions of genes encoding enterotoxin and other virulence factors.
Materials and Methods
Bacterial isolates
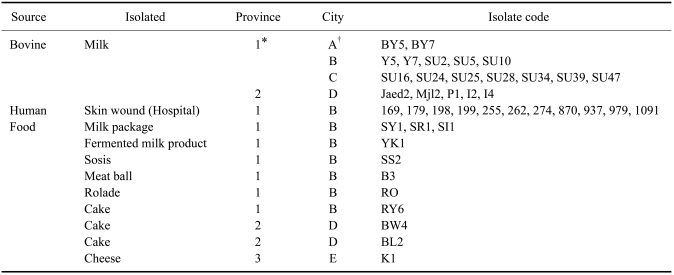
Samples of S. aureus were obtained from the mastitic milk of 19 dairy cattle from 19 farms located in Central and West Java, Indonesia. Eleven samples of S. aureus from humans were obtained from skin infections of patients at Sardjito Hospital, Indonesia. The strains were kindly provided by Dr. Hera Nirwati, Microbiology Laboratory, Faculty of Medicine, Gadjah Mada University, Indonesia. In addition, 11 samples of S. aureus were obtained from cooked foods in Central, West, and East Java, Indonesia. The foods were collected from several supermarkets stored in refrigerator. The origins of the strains are summarized in Table 1.
Table 1.
Staphylococcus aureus strains investigated in the present study
*1: Central Java, 2: West Java, 3: East Java; †A: Boyolali, B: Yogyakarta, C: Surakarta, D: Sumedang, E: Surabaya.
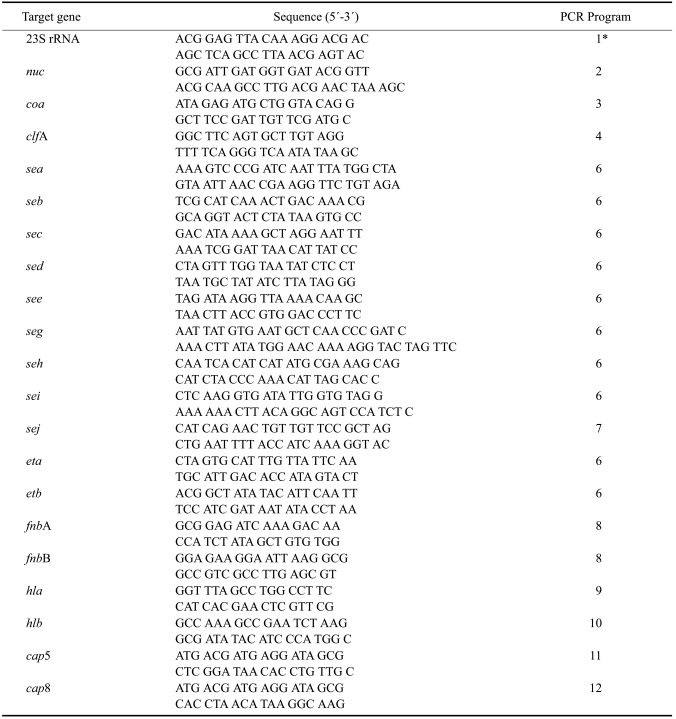
The strains were identified as S. aureus by their properties in culture, Gram staining, and biochemical tests. The biochemical tests included ones that measured catalase, coagulase, and clumping factor reactions as described previously [7]. The catalase test was done by placing a drop of hydrogen peroxide on a microscope slide. A small amount of bacterial isolate was added to hydrogen peroxide, bubbles of oxygen was observed for catalase-positive. The coagulase test was performed by cultivation of the bacteria in the tube coagulase test (Bactident-Coagulase; Merck, Germany). The presence of coagulation was observed at 6 and 24th hours. The clumping factor test was observed by the presence of agglutination reaction of the bacteria with rabbit plasma on a microscope slide. Furthermore, the strains were identified by PCR amplification of the thermonuclease nuc gene [6] and 23S rRNA gene [25]. The reaction mixture (30 µL) contained 1 µL primer 1 (10 pmol), 1 µL primer 2 (10 pmol; Invitrogen, USA), 14 µL PCR mix containing Taq DNA polymerase, MgCl2, and dNTPs (Roche, Germany), 5 µL of DNA template, and 9 µL distilled water. The DNA of the isolates was prepared with the QIAamp DNA mini kit (Qiagen, Germany) as described by the manufacturer. The amplification of the genes was carried out with a thermal cycler (Mastercycler; Eppendorf, Germany). The oligonucleotide primers and the thermal cycler programs are shown in Table 2.
Table 2.
Oligonucleotide primers and PCR programs used for amplifying the genes encoding 23S rRNA along with various other staphylococcal proteins including toxins and adhesive molecules
*1: 37 cycles of 94℃ for 40 sec, 64℃ for 60 sec, and 72℃ for 75 sec; 2: 37 cycles of 94℃ for 60 sec, 55℃ for 30 sec, and 72℃ for 30 sec; 3: 30 cycles of 94℃ for 60 sec, 58℃ for 60 sec, and 72℃ for 60 sec; 4: 35 cycles of 94℃ for 60 sec, 57℃ for 60 sec, and 72℃ for 60 sec; 5: 30 cycles of 94℃ for 60 sec, 60℃ for 60 sec, and 72℃ for 60 sec; 6: 30 cycles of 94℃ for 120 sec, 55℃ for 120 sec, and 72℃ for 60 sec; 7: 30 cycles of 94℃ for 60 sec, 62℃ for 60 sec, and 72℃ for 60 sec; 8: 30 cycles of 94℃ for 30 sec, 50℃ for 30 sec, and 72℃ for 60 sec; 9: 20 cycles of 94℃ for 10 sec, 53℃ for 10 sec, and 72℃ for 30 sec; 10: 20 cycles of 94℃ for 10 sec, 62℃ for 10 sec, and 72℃ for 30 sec; 11: 20 cycles of 94℃ for 15 sec, 57℃ for 15 sec, and 72℃ for 30 sec; 12: 20 cycles of 94℃ for 15 sec, 52℃ for 15 sec, and 72℃ for 30 sec.
DNA isolation and purification
A QIAmp DNA mini kit (Qiagen, Germany) was used to purify the DNA from S. aureus according to the manufacturer's protocol. The bacterial strains were cultivated on blood agar base (Oxoid, Germany) containing 5% defibrinated sheep blood for 24 h at 37℃. A total of 5~10 S. aureus colonies were suspended with 180 µL TE buffer (10 mM Tris-HCl and 1 mM EDTA [pH 8]) containing 5 µL lysostaphin (1.8 U/µL; Sigma, USA) in 2-mL microfuge tubes. The suspension was incubated for 1 h at 37℃, and 25 µL of proteinase K (14.8 mg/mL; Sigma, USA) and 200 µL of AL buffer (containing reagents AL1 and AL2; Qiagen, Germany) were then added. The suspensions were incubated for 30 min at 56℃, and then for 10 min at 95℃ before being spun at 6,000 × g for a few seconds. A total of 420 µL ethanol was added to each sample and placed in a spin QIAmp column. After centrifugation at 6,000 × g for 1 min, the spin columns were placed in a clean collection tube and the sample was washed twice with 500 µL of AW buffer (Qiagen, Germany). After the second wash and a centrifugation at 6,000 × g for 3 min, the QIAamp spin columns were placed in a clean 2-mL microfuge tube, and the DNA was eluted twice with 200 µL and 100 µL of AE buffer (Qiagen, Germany). DNA was stored at -20℃.
Genotype characterization
A PCR method was used to identify the genetic determinants of various virulence factors. The oligonucleotide primers used amplified the genes encoding clumping factor (clfA) [23]; coagulase (coa) [11]; staphylococcal enterotoxins (SE) (sea) [26], (seb, sec, sed, and see) [13], (seg, seh, and sei) [12], (sej) [16]; exfoliative toxin A (eta) and B (etb) [13]; fibronectin binding protein A (fnbA) and B (fnbB) [14]; alpha-(hla) and beta-hemolysin (hlb) [5]; and capsular polysaccharide 5 (cap5) and 8 (cap8) [17]. The sequences of the primers and PCR conditions are shown in Table 2.
AFLP analysis
An AFLP analysis was performed according to Boerema et al. [4]. Genomic DNA (5 µL) was digested overnight (16 h) at 37℃ with 10 U of HindIII (Invitrogen, USA) and 5 mM spermidine trihydrochloride (Sigma, USA) added to a final volume of 20 µL. The DNA of the isolates was prepared with the QIAamp DNA mini kit (Qiagen, Germany) as described by the manufacturer's protocol. The 5 µL of digested DNA was added to a ligation reaction containing 0.2 µg of each adapter oligonucleotide (ADH-1 ACG GTA TGC GAC AG and ADH-2 AGC TCT GTC GCA TAC CGT GAG) (Invitrogen, USA) and 1 U of T4 DNA ligase (Invitrogen, USA) in a final volume of 20 µL, and incubated for 4 h at room temperature (approximately 20℃). The ligated DNA samples were heated for 10 min at 80℃ to inactivate the T4 ligase and then diluted 1 : 5 in sterile water.
PCR were performed in a total volume of 50 µL containing 2.5 µL of template DNA, 200 µM of dNTPs (Roche, Germany), 1 µL of HI-X primer (GGT ATG CGA CAG AGC TTX, where X = A, T, G or C; 100 pmol/µL; Invitrogen, USA), and 1 µL (5 U) of Taq DNA polymerase (Roche, Germany) in 1 × PCR buffer provided by the manufacturer (Roche, Germany). Each HI-X primer was used in four separate PCR reactions. Amplification was performed in a thermal cycler (Eppendorf, Germany). After initial denaturation for 4 min at 94℃, target gene fragments were amplified for 33 cycles. Each cycle consisted of a denaturation step for 1 min at 94℃, an annealing step for 1 min at 60℃, and an extension for 2.5 min at 72℃. Each adapter oligonucleotide incorporated an additional base pair in the restriction site in order to eliminate it after the ligation reaction. The PCR products were separated by gel electrophoresis in a 1.5% (w/v) agarose gel (Roth, Germany) in 0.5 × TBE buffer (containing a mixture of Tris base, boric acid and EDTA). A 1-kb Plus DNA ladder (Invitrogen, USA) was used as a size marker. The resulting bands were visualized using ethidium bromide staining under UV transillumination. The gel images were subsequently evaluated visually. AFLP patterns were analyzed using BioNumerics software (ver. 6.01; Applied Maths, Belgium). Dendrograms were obtained with the average linkage method (unweight pair group average method, UGPMA) using Dice coefficient.
Results
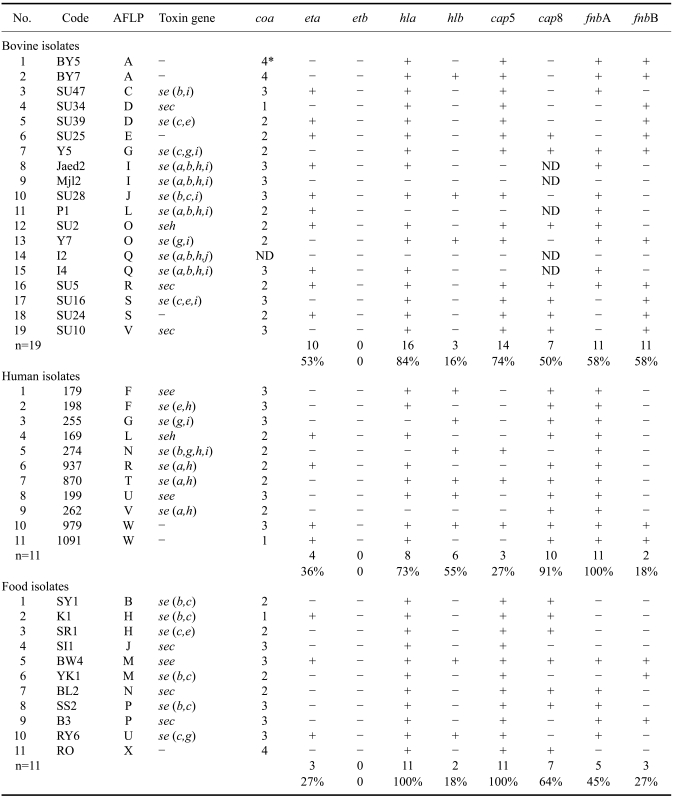
According to the results of cultural and biochemical properties, along with amplification of the nuc and 23S rRNA specific to S. aureus, all 41 isolates examined in the present investigation were identified as S. aureus. All 41 cultures investigated were Gram positive, positive for catalase, coagulase, and clumping factor reaction on microscope slides. PCR amplification of the clumping factor gene (clfA) revealed that all isolates had a single amplicon approximately 1,000 bp in size (Table 3). Amplification of the coa gene revealed four different PCR products of 540, 600, 680, and 850 bp for 1, 8, 7, and 2 of the S. aureus isolated from bovines, and for 1, 4, 5, and 1 of the food isolates, respectively. Three different PCR products with sizes of 540, 600, and 680 bp were found for 1, 5, and 5 of the S. aureus strains isolated from human, respectively. The distribution of each isolate for coa gene of S. aureus is shown in Table 3.
Table 3.
Summary of the genotypic properties of the Staphylococcus aureus strains isolated from bovines, humans,and food based on the AFLP analysis along with the distribution of the staphylococcal enterotoxin genes and various virulence determinants
AFLP: amplified fragment length polymorphism. *Size of the coa genes: 1 (540 bp), 2 (600 bp), 3 (680 bp), 4 (850 bp); -: negative, ND: not detected, n: number of strains.
By PCR amplification, one or more staphylococcal enterotoxin gene was observed either singly (sec, see, and seh), or in a combination of two genes [se(a,h), se(e,h), se(g,i), se(c,g), se(b,c), and se(c,e)], three genes [se(b,c,i), se(c,e,i), se(c,g,i)], or four genes [se(a,b,h,i), Fig. 1; se(a, b,h,j), and se(b,g,h,i)]. No strain harbored sed, sej, or etb. The eta gene was found in 10 out of the 19 S. aureus strains isolated from bovines, four of the 11 strains collected from humans, and three of the 11 strains from food. The hla gene was found almost in all S. aureus isolates including 16 isolates from bovines, eight isolates from humans, and all 11 isolates from food. The hlb gene was observed in three isolates from bovines, six isolates from humans, and two isolates from food. It was noted that most of the S. aureus isolated from bovines harbored the gene cap5 (14 isolates) while most strains isolated from humans contained the cap8 (10 isolates) and fnbA (11 isolates) genes. Both cap5 (11 isolates) and cap8 genes (7 isolates) were predominantly found in strains from food. Distribution of the various genes among the S. aureus cultures investigated in the present study is shown in Table 3.
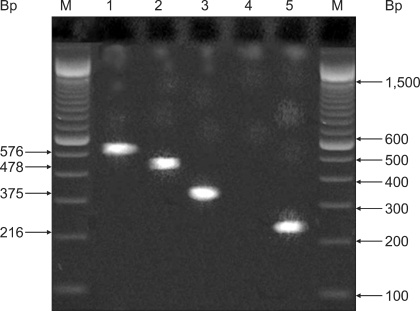
Fig. 1.
Agarose gel electrophoresis of se gene PCR products including sei (Lane 1, 576 bp), seh (Lane 2, 478 bp), seb (Lane 3, 375 bp), a negative enterotoxine strain (Lane 4), and sea (Lane 5, 216 bp). M: marker = 100 bp DNA ladder.
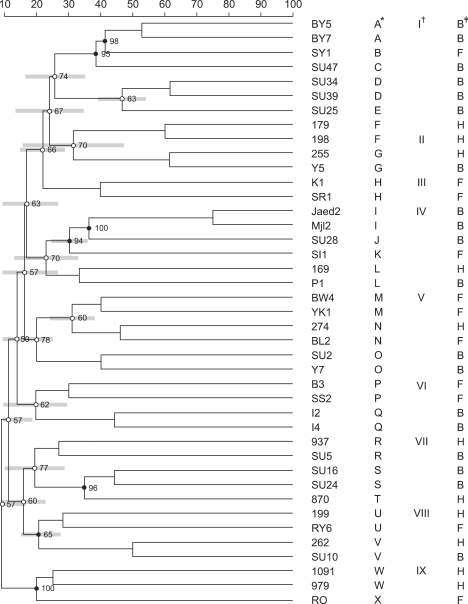
To evaluate the relationships between the S. aureus strains isolated from humans, bovines, and food, AFLP was performed with primer G (with a G base at the 3' end) using a single specific restriction enzyme. AFLP types were defined on the basis of 5 to 12 bands in the gel. This allowed us to differentiate 23 AFLP types (A to X). A dendrogram was generated with the AFLP data using BioNumerics software with the Dice coefficient and cluster analysis with UPGMA (Fig. 2). Using a cutoff value of 0.25, all S. aureus patterns could be grouped into nine clusters (I, II, III, IV, V, VI, VII, VIII, and IX). Results of this analysis presented in Fig. 2 revealed a correlation between some AFLP strain clusters and the origins of the strains. Pattern types A, B, C, D, and E formed one cluster (I), and six out of seven of the strains associated with this cluster were of bovine origin. Similarly, three out of four strains from the second cluster (II) formed by patterns F and G were of human origin while one strain was derived from food. A third cluster (III) was formed by pattern H and originated from food. By cutting point of 0.25, the other strains could be grouped into smaller clusters.
Fig. 2.
Dendrogram of amplified fragment length polymorphism (AFLP) patterns showing the relatedness of the 41 Staphylococcus aureus strains examined in this study. Degrees of similarity were calculated using BioNumerics software with the Dice coefficient and cluster analysis with UPGMA. *AFLP patterns A to X (23 types). †Clusters of strains identified by a cutoff value of 0.25 (I to IX). ‡Strain designations: B; bovine source, F; food source, H; human source.
Discussion
According to phenotypic and genotypic properties, all 41 isolates investigated in the present study were identified as S. aureus. Molecular identification and characterization were performed by PCR amplification of genes encoding 23S rRNA, clumping factors, nuclease, and coagulase. Gene polymorphisms of the coagulase gene have been commonly used for molecular typing of S. aureus [2]. A comparable PCR-based system for identifying S. aureus isolated from various sources had already been used by numerous authors [1,2,23,25].
According to Nishi et al. [19], S. aureus genotyping by determining expression patterns of different toxins might be useful for comparing epidemiologically related strains. Evaluating the expression of different toxin genes in the S. aureus isolates revealed different combinations of sea, seb, sec, see, seg, seh, sei and sej gene expression in some of the S. aureus strains we investigated. Different combinations of these genes in clinical S. aureus isolates have also been described by Zhang et al. [27] and Jarraud et al. [12]. The seg and sei genes were present in most strains associated with staphylococcal toxic shock syndrome and staphylococcal scarlet fever [12]; this could be explained by a shared location of these genes on a pathogenicity island [3,15]. The seh gene was found in the strains isolated from humans in this study. Staphylococcal enterotoxin H has been described as having the highest affinity ever measured for an enterotoxin specific for MHC class II molecules [18]. The importance of toxin production by S. aureus isolated from animals with bovine mastitis associated with udder-associated pathogenesis remains unclear. According to Ferens et al. [8], superantigenic enterotoxins seem to induce immunosuppression in dairy animals. In contrast to eta, etb was not found in this study. Hayakawa et al. [10] reported that the production of exfoliative toxins by S. aureus isolates from cattle with bovine mastitis seems to be rare.
PCR analysis of genetic determinants has been suggested as an important tool for defining the pathogenicity of infectious S. aureus. Various staphylococcal surface determinants related to bacteria adherence to human epithelial cells have been reported [22]. Staphylococcal cell wall teichoic acid, lipoteichoic acid, type 5 and type 8 capsular polysaccharides, and fibronectin binding proteins had been proposed as major ligands [21,22]. Jönsson et al. [14] reported that two S. aureus fibronectin binding proteins, and the corresponding fnbA and fnbB genes, show a high degree of sequence similarity. In the present study, fnbA was detected in all human isolates. Booth et al. [5] observed that 89.7% of clinical isolates of S. aureus possess fnbA whereas only 20.1% harbor fnbB. It was interesting that most of the S. aureus isolated from bovines in our study harbored the hla (84%) and cap5 (74%) genes, and most of strains isolated from humans contained the hla (73%), cap8 (91%), and fnbA (100%) genes. The hla (100%) gene and both cap5 (100%) and cap8 (64%) genes were predominantly found in the S. aureus strains isolated from food.
To determine the clonal relationships between the isolates, a single-enzyme AFLP technique was used. Boerema et al. [4] reported that single-enzyme AFLP has a lower discriminatory power than pulsed field gel electrophoresis (PFGE), but is a useful typing method for investigating clonal relationships between different S. aureus strains. An AFLP procedure is easy to perform, and the results can be obtained within 24 h compared to the approximately 3~4 days required to acquire PFGE results. This makes AFLP more suited for routine use or analyzing a large number of isolates.
In the present study, single-enzyme AFLP analysis revealed a correlation between some AFLP clusters and the origins of the strains. In correlation with the AFLP clusters, some genes seem to be more closely associated with strains from food (hla and cap5) and humans (cap8 and fnbA) compared to ones from bovine. This findings indicated that cluster 1 was associated with bovine isolates (hla and cap5). Surprisingly, one isolate from food belonged to bovine cluster group 1. However, this food isolate (hla and cap5) was associated to the S. aureus of the bovine group. Additionally, cluster 2 was found to primarily contain human isolates (cap8 and fnbA) with one strain derived from bovine origin. The bovine isolate carried the cap8 and fnbA genes and was associated to the human isolates. The food isolate in cluster 1 was probably of bovine origin, and the bovine isolate in cluster 2 was probably of human origin. Additional studies are required to further investigate these unique findings.
The food isolates (hla and cap5) were grouped in cluster 3. The other clusters were formed by a variety of bovine, human, and food isolates. However, the geographical origin of the isolates resulted in a high level of diversity. Griffith et al. [9] reported that genetic variability in a population might be caused by genetic mutation, recombination, and movement of an individual or a group of population from one region to another. It is possible that these patterns were influenced by movement of dairy cattle from one area to another. Cross contamination between humans and food might occur via humans with skin lesions or nasal discharge [20], probably while handling food or during food processing.
At present, there is still very little information about the relationship between specific genetic determinants and S. aureus isolates from bovine, human and food sources in Indonesia. The results of the present study identified specific virulence traits of S. aureus pathogenicity and provided a better understanding about the distribution of prevalent S. aureus clones among bovines, food, and humans. This will help to determine the source and transmission routes of infective S. aureus strains.
Acknowledgments
This work was supported by the Ministry of Education, Indonesia through a Competence Award 2008~2010. We would like to thank Ms. Nathalie Tack at Applied Maths NV (Belgium) for providing guidance for using BioNumerics software.
References
- 1.Akineden Ö, Annemüller C, Hassan AA, Lämmler C, Wolter W, Zschöck M. Toxin genes and other characteristics of Staphylococcus aureus isolates from milk of cows with mastitis. Clin Diagn Lab Immunol. 2001;8:959–964. doi: 10.1128/CDLI.8.5.959-964.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Annemüller C, Lämmler C, Zschöck M. Genotyping of Staphylococcus aureus isolated from bovine mastitis. Vet Microbiol. 1999;69:217–224. doi: 10.1016/s0378-1135(99)00117-0. [DOI] [PubMed] [Google Scholar]
- 3.Baba T, Takeuchi F, Kuroda M, Yuzawa H, Aoki K, Oguchi A, Nagai Y, Iwama N, Asano K, Naimi T, Kuroda H, Cui L, Yamamoto K, Hiramatsu K. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet. 2002;359:1819–1827. doi: 10.1016/s0140-6736(02)08713-5. [DOI] [PubMed] [Google Scholar]
- 4.Boerema JA, Clemens R, Brightwell G. Evaluation of molecular methods to determine enterotoxigenic status and molecular genotype of bovine, ovine, human and food isolates of Staphylococcus aureus. Int J Food Microbiol. 2006;107:192–201. doi: 10.1016/j.ijfoodmicro.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Booth MC, Pence LM, Mahasreshti P, Callegan MC, Gilmore MS. Clonal associations among Staphylococcus aureus isolates from various sites of infection. Infect Immun. 2001;69:345–352. doi: 10.1128/IAI.69.1.345-352.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brakstad OG, Aasbakk K, Maeland JA. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J Clin Microbiol. 1992;30:1654–1660. doi: 10.1128/jcm.30.7.1654-1660.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brückler J, Schwarz S, Untermann F. Staphylokokken-Infektionen und Enterotoxine band. II/I. In: Blobel H, Schlieβer T, editors. Handbuch der bakteriellen Infektionen bei Tieren, 2. Auflage. Stuttgart: Gustav Fischer Verlag Jena; 1994. [Google Scholar]
- 8.Ferens WA, Davis WC, Hamilton MJ, Park YH, Deobald CF, Fox L, Bohach G. Activation of bovine lymphocyte subpopulations by Staphylococcal enterotoxin C. Infect Immun. 1998;66:573–580. doi: 10.1128/iai.66.2.573-580.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffiths AJF, Wessler SR, Lewontin RC, Gelbart WM, Suzuki DT, Miller JH. An Introduction to Genetic Analysis. 8th ed. New York: Freeman WH; 2004. p. 782. [Google Scholar]
- 10.Hayakawa Y, Hayashi M, Shimano T, Komae H, Takeuchi K, Endau M, Igarashi H, Hashimoto N, Takeuchi S. Production of exfoliative toxin A by Staphylococcus aureus isolated from mastitic cow's milk and farm bulk milk. J Vet Med Sci. 1998;60:1281–1283. doi: 10.1292/jvms.60.1281. [DOI] [PubMed] [Google Scholar]
- 11.Hookey JV, Richardson JF, Cookson BD. Molecular typing of Staphylococcus aureus based on PCR restriction fragment length polymorphism and DNA sequence analysis of the coagulase gene. J Clin Microbiol. 1998;36:1083–1089. doi: 10.1128/jcm.36.4.1083-1089.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarraud S, Cozon G, Vandenesch F, Bes M, Etienne J, Lina G. Involvement of enterotoxins G and I in staphylococcal toxic shock syndrome and staphylococcal scarlet fever. J Clin Microbiol. 1999;37:2446–2449. doi: 10.1128/jcm.37.8.2446-2449.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson WM, Tyler SD, Ewan EP, Ashton FE, Pollard DR, Rozee KR. Detection of genes for enterotoxins, exfoliative toxins, and toxic shock syndrome toxin 1 in Staphylococcus aureus by the polymerase chain reaction. J Clin Microbiol. 1991;29:426–430. doi: 10.1128/jcm.29.3.426-430.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jönsson K, Signäs C, Müller HP, Lindberg M. Two different genes encode fibronectin binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene. Eur J Biochem. 1991;202:1041–1048. doi: 10.1111/j.1432-1033.1991.tb16468.x. [DOI] [PubMed] [Google Scholar]
- 15.Kuroda M, Ohta T, Uchiyama I, Baba T, Yuzawa H, Kobayashi I, Cui L, Oguchi A, Aoki K, Nagai Y, Kian J, Ito T, Kanamori M, Matsumaru H, Maruyama A, Murakami H, Hosoyama A, Mizutani-Ui Y, Takahashi NK, Sawano T, Inoue R, Kaito C, Sekimizu K, Hirakawa H, Kuhara S, Goto S, Yabuzaki J, Kanehisa M, Yamashita A, Oshima K, Furuya K, Yoshino C, Shiba T, Hattori M, Ogasawara N, Hayashi H, Hiramatsu K. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet. 2001;357:1225–1240. doi: 10.1016/s0140-6736(00)04403-2. [DOI] [PubMed] [Google Scholar]
- 16.Monday SR, Bohach GA. Use of multiplex PCR to detect classical and newly described pyrogenic toxin genes in staphylococcal isolates. J Clin Microbiol. 1999;37:3411–3414. doi: 10.1128/jcm.37.10.3411-3414.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore PCL, Lindsay JA. Genetic variation among hospital isolates of methicillin-sensitive Staphylococcus aureus: evidence for horizontal transfer of virulence genes. J Clin Microbiol. 2001;39:2760–2767. doi: 10.1128/JCM.39.8.2760-2767.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nilsson H, Björk P, Dohlsten M, Antonsson P. Staphylococcal enterotoxin H displays unique MHC class II-binding properties. J Immunol. 1999;163:6686–6693. [PubMed] [Google Scholar]
- 19.Nishi J, Yoshinaga M, Miyanohara H, Kawahara M, Kawabata M, Motoya T, Owaki T, Oiso S, Kawakami M, Kamewari S, Koyama Y, Wakimoto N, Tokuda K, Manago K, Maruyama I. An epidemiologic survey of methicillin-resistant Staphylococcus aureus by combined use of mec-HVR genotyping and toxin genotyping in a university hospital in Japan. Infect Control Hosp Epidemiol. 2002;23:506–510. doi: 10.1086/502097. [DOI] [PubMed] [Google Scholar]
- 20.Sandel MK, McKillip JL. Virulence and recovery of Staphylococcus aureus relevant to the food industry using improvements on traditional approaches. Food Control. 2004;15:5–10. [Google Scholar]
- 21.Sheagren JN. Staphylococcus aureus: the persistent pathogen. N Engl J Med. 1984;310:1368–1373. doi: 10.1056/NEJM198405243102107. [DOI] [PubMed] [Google Scholar]
- 22.Soell M, Diab M, Haan-Archipoff G, Beretz A, Herbelin C, Poutrel B, Klein JP. Capsular polysaccharide types 5 and 8 of Staphylococcus aureus bind specifically to human epithelial (KB) cells, endothelial cells, and monocytes and induce release of cytokines. Infect Immun. 1995;63:1380–1386. doi: 10.1128/iai.63.4.1380-1386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephan R, Annemüller C, Hassan AA, Lämmler C. Characterization of enterotoxigenic Staphylococcus aureus strains isolated from bovine mastitis in north-east Switzerland. Vet Microbiol. 2001;78:373–382. doi: 10.1016/s0378-1135(00)00341-2. [DOI] [PubMed] [Google Scholar]
- 24.Stewart CM. Staphylococcus aureus and Staphylococcal Enterotoxins. In: Hocking AD, editor. Foodborne Microorganisms of Public Health Importance. 6th ed. Sydney: The Food Microbiology Group of the Australian Institute of Food Science and Technology Inc.; 2003. pp. 359–379. [Google Scholar]
- 25.Straub JA, Hertel C, Hammes WP. A 23S rDNA-targeted polymerase chain reaction-based system for detection of Staphylococcus aureus in meat starter cultures and dairy products. J Food Prot. 1999;62:1150–1156. doi: 10.4315/0362-028x-62.10.1150. [DOI] [PubMed] [Google Scholar]
- 26.Tsen HY, Chen TR. Use of the polymerase chain reaction for specific detection of type A, D and E enterotoxigenic Staphylococcus aureus in foods. Appl Microbiol Biotechnol. 1992;37:685–690. doi: 10.1007/BF00240750. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S, Iandolo JJ, Stewart GC. The enterotoxin D plasmid of Staphylococcus aureus encodes a second enterotoxin determinant (sej) FEMS Microbiol Lett. 1998;168:227–233. doi: 10.1111/j.1574-6968.1998.tb13278.x. [DOI] [PubMed] [Google Scholar]