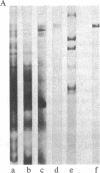
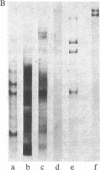
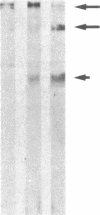
Abstract
Previous work has shown that high molecular weight polypeptides sharing epitopes with myelin-associated glycoprotein are transiently expressed in developing human central nervous system. We describe here the isolation of two of these polypeptides (150 and 225 kDa), their biochemical characterization, and their immunochemical localization. They are both glycosylated and 15 kDa of the apparent molecular mass of both polypeptides is contributed by N-linked carbohydrate moiety(ies). Both have multimeric forms held together by disulfide bonds. Immunochemical and immunohistochemical studies failed to show these polypeptides in myelin. However, in developing human central nervous system, one or both of them are present in the perikaryal cytoplasm and processes of cells with morphology consistent with actively myelinating oligodendrocytes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brackenbury R., Thiery J. P., Rutishauser U., Edelman G. M. Adhesion among neural cells of the chick embryo. I. An immunological assay for molecules involved in cell-cell binding. J Biol Chem. 1977 Oct 10;252(19):6835–6840. [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S. Characterization of the structural determinants required for the high affinity interaction of asparagine-linked oligosaccharides with immobilized Phaseolus vulgaris leukoagglutinating and erythroagglutinating lectins. J Biol Chem. 1982 Oct 10;257(19):11230–11234. [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion and the molecular processes of morphogenesis. Annu Rev Biochem. 1985;54:135–169. doi: 10.1146/annurev.bi.54.070185.001031. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Grumet M., Hoffman S., Edelman G. M. Two antigenically related neuronal cell adhesion molecules of different specificities mediate neuron-neuron and neuron-glia adhesion. Proc Natl Acad Sci U S A. 1984 Jan;81(1):267–271. doi: 10.1073/pnas.81.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirn M., Pierres M., Deagostini-Bazin H., Hirsch M., Goridis C. Monoclonal antibody against cell surface glycoprotein of neurons. Brain Res. 1981 Jun 15;214(2):433–439. doi: 10.1016/0006-8993(81)91208-7. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L. Protein A, avidin, and biotin in immunohistochemistry. J Histochem Cytochem. 1981 Nov;29(11):1349–1353. doi: 10.1177/29.11.6172466. [DOI] [PubMed] [Google Scholar]
- Kruse J., Keilhauer G., Faissner A., Timpl R., Schachner M. The J1 glycoprotein--a novel nervous system cell adhesion molecule of the L2/HNK-1 family. Nature. 1985 Jul 11;316(6024):146–148. doi: 10.1038/316146a0. [DOI] [PubMed] [Google Scholar]
- Kruse J., Mailhammer R., Wernecke H., Faissner A., Sommer I., Goridis C., Schachner M. Neural cell adhesion molecules and myelin-associated glycoprotein share a common carbohydrate moiety recognized by monoclonal antibodies L2 and HNK-1. Nature. 1984 Sep 13;311(5982):153–155. doi: 10.1038/311153a0. [DOI] [PubMed] [Google Scholar]
- Marton L. S., Stefansson K. Developmental alterations in molecular weights of proteins in the human central nervous system that react with antibodies against myelin-associated glycoprotein. J Cell Biol. 1984 Nov;99(5):1642–1646. doi: 10.1083/jcb.99.5.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry R. C., Helfand S. L., Quarles R. H., Roder J. C. Recognition of myelin-associated glycoprotein by the monoclonal antibody HNK-1. Nature. 1983 Nov 24;306(5941):376–378. doi: 10.1038/306376a0. [DOI] [PubMed] [Google Scholar]
- Noble M., Albrechtsen M., Møller C., Lyles J., Bock E., Goridis C., Watanabe M., Rutishauser U. Glial cells express N-CAM/D2-CAM-like polypeptides in vitro. Nature. 1985 Aug 22;316(6030):725–728. doi: 10.1038/316725a0. [DOI] [PubMed] [Google Scholar]
- Norton W. T., Poduslo S. E. Myelination in rat brain: method of myelin isolation. J Neurochem. 1973 Oct;21(4):749–757. doi: 10.1111/j.1471-4159.1973.tb07519.x. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Rathjen F. G., Schachner M. Immunocytological and biochemical characterization of a new neuronal cell surface component (L1 antigen) which is involved in cell adhesion. EMBO J. 1984 Jan;3(1):1–10. doi: 10.1002/j.1460-2075.1984.tb01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson K., Marton L., Antel J. P., Wollmann R. L., Roos R. P., Chejfec G., Arnason B. G. Neuropathy accompanying IgM lambda monoclonal gammopathy. Acta Neuropathol. 1983;59(4):255–261. doi: 10.1007/BF00691490. [DOI] [PubMed] [Google Scholar]
- Sternberger N. H., Quarles R. H., Itoyama Y., Webster H. D. Myelin-associated glycoprotein demonstrated immunocytochemically in myelin and myelin-forming cells of developing rat. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1510–1514. doi: 10.1073/pnas.76.3.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery J. P., Brackenbury R., Rutishauser U., Edelman G. M. Adhesion among neural cells of the chick embryo. II. Purification and characterization of a cell adhesion molecule from neural retina. J Biol Chem. 1977 Oct 10;252(19):6841–6845. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp B. D., Quarles R. H., Suzuki K. Immunocytochemical studies of quaking mice support a role for the myelin-associated glycoprotein in forming and maintaining the periaxonal space and periaxonal cytoplasmic collar of myelinating Schwann cells. J Cell Biol. 1984 Aug;99(2):594–606. doi: 10.1083/jcb.99.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]