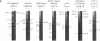Abstract
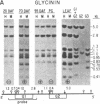
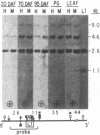
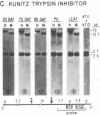
We investigated soybean seed protein gene transcription during development. We found that seed protein genes are transcriptionally activated and then repressed during embryogenesis and that these genes are either inactive or transcribed at low levels in the mature plant. We further observed that genes encoding mRNAs with vastly different prevalences are transcribed at similar rates. DNA gel blot studies showed that transcriptionally active and inactive seed protein genes have indistinguishable methylation patterns. We conclude that both transcriptional and posttranscriptional processes regulate seed protein mRNA levels in the absence of detectable DNA methylation changes.
Keywords: soybean, gene regulation, transcription, seed proteins
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. L., Burdon R. H. DNA methylation in eukaryotes. CRC Crit Rev Biochem. 1982;13(4):349–384. doi: 10.3109/10409238209108714. [DOI] [PubMed] [Google Scholar]
- BONNER J., HUANG R. C., GILDEN R. V. CHROMOSOMALLY DIRECTED PROTEIN SYNTHESIS. Proc Natl Acad Sci U S A. 1963 Nov;50:893–900. doi: 10.1073/pnas.50.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach L. R., Spencer D., Randall P. J., Higgins T. J. Transcriptional and post-transcriptional regulation of storage protein gene expression in sulfur-deficient pea seeds. Nucleic Acids Res. 1985 Feb 11;13(3):999–1013. doi: 10.1093/nar/13.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. E., Takio K., Titani K., Ryan C. A. Wound-induced trypsin inhibitor in alfalfa leaves: identity as a member of the Bowman-Birk inhibitor family. Biochemistry. 1985 Apr 23;24(9):2105–2108. doi: 10.1021/bi00330a002. [DOI] [PubMed] [Google Scholar]
- Cabrera C. V., Lee J. J., Ellison J. W., Britten R. J., Davidson E. H. Regulation of cytoplasmic mRNA prevalence in sea urchin embryos. Rates of appearance and turnover for specific sequences. J Mol Biol. 1984 Mar 25;174(1):85–111. doi: 10.1016/0022-2836(84)90366-8. [DOI] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Fischer R. L., Goldberg R. B. Structure and flanking regions of soybean seed protein genes. Cell. 1982 Jun;29(2):651–660. doi: 10.1016/0092-8674(82)90181-7. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Goldberg R. B., Hoschek G., Ditta G. S., Breidenbach R. W. Developmental regulation of cloned superabundant embryo mRNAs in soybean. Dev Biol. 1981 Apr 30;83(2):218–231. doi: 10.1016/0012-1606(81)90468-1. [DOI] [PubMed] [Google Scholar]
- Goldberg R. B., Hoschek G., Kamalay J. C., Timberlake W. E. Sequence complexity of nuclear and polysomal RNA in leaves of the tobacco plant. Cell. 1978 May;14(1):123–131. doi: 10.1016/0092-8674(78)90307-0. [DOI] [PubMed] [Google Scholar]
- Goldberg R. B., Hoschek G., Tam S. H., Ditta G. S., Breidenbach R. W. Abundance, diversity, and regulation of mRNA sequence sets in soybean embryogenesis. Dev Biol. 1981 Apr 30;83(2):201–217. doi: 10.1016/0012-1606(81)90467-x. [DOI] [PubMed] [Google Scholar]
- Goldberg R. B., Hoschek G., Vodkin L. O. An insertion sequence blocks the expression of a soybean lectin gene. Cell. 1983 Jun;33(2):465–475. doi: 10.1016/0092-8674(83)90428-2. [DOI] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R. W., Foard D. E., Larkins B. A. Molecular cloning and analysis of a gene coding for the Bowman-Birk protease inhibitor in soybean. J Biol Chem. 1984 Aug 10;259(15):9883–9890. [PubMed] [Google Scholar]
- Hill J. E., Breidenbach R. W. Proteins of soybean seeds: I. Isolation and characterization of the major components. Plant Physiol. 1974 May;53(5):742–746. doi: 10.1104/pp.53.5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer E., Darnell J. E., Jr The primary transcription unit of the mouse beta-major globin gene. Cell. 1981 Feb;23(2):585–593. doi: 10.1016/0092-8674(81)90154-9. [DOI] [PubMed] [Google Scholar]
- Hofer E., Hofer-Warbinek R., Darnell J. E., Jr Globin RNA transcription: a possible termination site and demonstration of transcriptional control correlated with altered chromatin structure. Cell. 1982 Jul;29(3):887–893. doi: 10.1016/0092-8674(82)90450-0. [DOI] [PubMed] [Google Scholar]
- Kamalay J. C., Goldberg R. B. Organ-specific nuclear RNAs in tobacco. Proc Natl Acad Sci U S A. 1984 May;81(9):2801–2805. doi: 10.1073/pnas.81.9.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamalay J. C., Goldberg R. B. Regulation of structural gene expression in tobacco. Cell. 1980 Apr;19(4):935–946. doi: 10.1016/0092-8674(80)90085-9. [DOI] [PubMed] [Google Scholar]
- Langridge J., Langridge P., Bergquist P. L. Extraction of nucleic acids from agarose gels. Anal Biochem. 1980 Apr;103(2):264–271. doi: 10.1016/0003-2697(80)90266-3. [DOI] [PubMed] [Google Scholar]
- Luthe D. S., Quatrano R. S. Transcription in Isolated Wheat Nuclei: I. ISOLATION OF NUCLEI AND ELIMINATION OF ENDOGENOUS RIBONUCLEASE ACTIVITY. Plant Physiol. 1980 Feb;65(2):305–308. doi: 10.1104/pp.65.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthe D. S., Quatrano R. S. Transcription in Isolated Wheat Nuclei: II. CHARACTERIZATION OF RNA SYNTHESIZED IN VITRO. Plant Physiol. 1980 Feb;65(2):309–313. doi: 10.1104/pp.65.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R. Active transepithelial potassium transport in frog skin via specific potassium channels in the apical membrane. Acta Physiol Scand. 1984 Feb;120(2):287–296. doi: 10.1111/j.1748-1716.1984.tb00136.x. [DOI] [PubMed] [Google Scholar]
- Polacco J. C., Thomas A. L., Bledsoe P. J. A soybean seed urease-null produces urease in cell culture. Plant Physiol. 1982 May;69(5):1233–1240. doi: 10.1104/pp.69.5.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacco J. C., Winkler R. G. Soybean leaf urease: a seed enzyme? Plant Physiol. 1984 Apr;74(4):800–803. doi: 10.1104/pp.74.4.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sengupta-Gopalan C., Reichert N. A., Barker R. F., Hall T. C., Kemp J. D. Developmentally regulated expression of the bean beta-phaseolin gene in tobacco seed. Proc Natl Acad Sci U S A. 1985 May;82(10):3320–3324. doi: 10.1073/pnas.82.10.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverthorne J., Tobin E. M. Demonstration of transcriptional regulation of specific genes by phytochrome action. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1112–1116. doi: 10.1073/pnas.81.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin A. J. Evaluating the contribution of posttranscriptional processing to differential gene expression. Dev Biol. 1979 Jan;68(1):47–58. doi: 10.1016/0012-1606(79)90242-2. [DOI] [PubMed] [Google Scholar]
- Vodkin L. O., Rhodes P. R., Goldberg R. B. cA lectin gene insertion has the structural features of a transposable element. Cell. 1983 Oct;34(3):1023–1031. doi: 10.1016/0092-8674(83)90560-3. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]