Abstract
For some time now, the research on gastric motility and function has fallen behind in the amount of research on gastric endocrine, exocrine secretion, and gastric morphology. In this paper, a noninvasive method to study gastric motility was developed, taking bioimpedance measurements over the gastric area simultaneously with the electrogastrography (EGG). This is based on the concept of observing and analyzing simultaneously the intrinsic electrical gastric activity (basic electric rhythm) and the mechanical gastric activity. Additionally, preliminary clinical studies of healthy subjects and subjects with functional dyspepsia (FD) and gastritis were carried out. The impedance gastric motility (IGM) measurements of the healthy and FD subjects were compared, along with the studies of the FD subjects before treatment and after one week and three weeks of treatment. We also compared IGM measurements of healthy subjects and subjects with erosive gastritis, along with the studies of the subjects with erosive gastritis before treatment and after one week of treatment. Results show that FD subjects have poor gastric motility (P<0.01). After a week of treatment, the gastric motility of FD subjects was not yet improved although the EGG had returned to normal by this time. By three weeks of treatment, the regular IGM rhythm returned in FD subjects. There was a significant difference of IGM parameters between the gastritis and healthy subjects (P<0.05). The EGG rhythm of the gastritis subjects returned to normal at one week post-treatment, while IGM parameters showed a trend to improvement (P>0.05), These results suggest the possibility of clinic application of the proposed method.
Keywords: Gastric motility, Electrical bioimpedance, Electrogastrography, Functional dyspepsia, Gastritis
1. Introduction
Gastric motility is one of the most critical physiological functions of the human body. Without coordinated gastric motility, digestion and absorption of dietary nutrients cannot take place. Impairment in gastric motility results in delayed emptying of the stomach and symptoms, such as nausea, vomiting, and abdominal pain or discomfort (Chen et al., 2000). For some time now, the research on gastric motility and function has fallen behind in the amount of research on gastric endocrine, exocrine secretion, and gastric morphology. One of the important reasons is an absence of convenient and effective measurement methods (Zhou and Ke, 2005).
Electrical bioimpedance measurements are dependent on the electrical properties of tissues and organs, and include morphological and functional information. The method has the outstanding advantages of being noninvasive, convenient, and providing considerable functional information. It can be considered a powerful tool in clinical diagnosis and medical research (Gajre et al., 2006). There are many applications using bioimpedance signals for different pathological conditions, but their use in gastric motility assessment needs to be explored in detail (Hadi et al., 2002).
Sutton and Thompson (1985) reported their research on extracting gastric movement signal by the electrical impedance method, and a curve reflecting gastric emptying was obtained. From the curve, gastric peristaltic information with a rhythm 2–4 cycles per min (cpm), which is in accord with gastric contraction, was extracted. Familoni et al. (1987) presented a technique to monitor gastric electrical activity (GEA) and mechanical activity as an aid in assessing gastric motor function. Kothapalli (1992) established a three-dimensional (3D) abdomen model to study the origin of changes in the epigastric signal, and analyzed the relationship between gastric impedance signal and food capacity, resistivity of the test meal, and gastric contraction when the excitation electrode and measurement electrode were located at different positions.
Early research with the impedance method to measure digestion was mainly concentrated on gastric emptying measurement (Chaw et al., 2001; Giouvanoudi et al., 2003; Huerta-Franco et al., 2009). There is little research on extraction of gastric motility information (Garay et al., 2006; Soulsby et al., 2006; Giouvanoudi and Spyrou, 2008). One of the primary reasons is that the rhythm of gastric motility is much lower, about 3 cpm. It is more difficult to extract the gastric motility signal and eliminate respiration interference. Chen and Wan (1992b) reported their work on obtaining the electric impedance signal to reflect gastric contraction, and measurement devices were developed (Chen and Wan, 1992a). However, the impedance signals obtained by the devices are all similar sine waveforms for both healthy and diseased subjects, because of incorrect filter processing. It has been difficult to differentiate normal or abnormal conditions from the signals. We have proposed a noninvasive electrical impedance method for gastric motility measurement and evaluation previously (Li et al., 2007). Multi-resolution analysis of the wavelet is adopted to separate impedance gastric motility (IGM) signal from the mixed impedance signal obtained on the body surface (Li and Ren, 2008). In this paper, we take bioimpedance measurements with simultaneous electrogastrography (EGG) to study gastric motility.
2. Methods
2.1. Composite course from electrical gastric activity to mechanical gastric activity
Gastric contraction is a mechanical behavior of the electrical activity occurring on the cell membrane surface of the smooth muscle. It begins from electric activity of the smooth muscle, followed by evoked contraction of the gastric corpus and antrum, and then transmits to the distal pylorus. It is a composite course from electrical activity to mechanical contraction, then to gastric peristalsis and transmission. Gastric contraction complies with the rhythm of electric activity, and is affected by amplitude, time limitation, transmission direction, and distance of the transmission contraction (Zhou and Ke, 2005). Gastric motility is a complex composite course from electrical gastric activity to mechanical gastric activity, and it is very important to measure and evaluate gastric motility according to the composite course (Ren et al., 2010).
There are two kinds of gastric myoelectrical activity to be observed, the slow wave and the spike potential. Gastric antrum contraction occurs only when the slow wave occurs with the spike potential. The spike potential appears during the slow wave phase, and the rhythm of gastric contraction may be determined by the slow wave (Ma et al., 2006). The EGG recorded from the body surface reflects the myoelectrical activity of different areas of the stomach, but corresponds to gastric slow wave accurately, and therefore can be used to investigate the rhythm of gastric contraction.
Stomach volume augments gradually when food is ingested. In the gastric active period, such as contraction and peristalsis after a meal, the content of the stomach changes greatly, as does the impedance of the stomach. Via the impedance measurement of the stomach during digestion, the information reflecting the stomach volume (gastric emptying) and gastric motility (contraction and peristalsis) can be extracted.
2.2. Experimental procedures of IGM and EGG
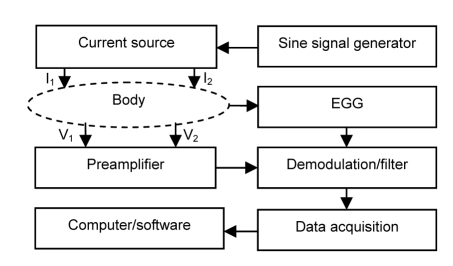
We have developed a study method of gastric motility based on electrical bioimpedance measurements and simultaneous EGG (Li et al., 2007; 2008; Deng et al., 2008). The block diagram of the measurement system is shown in Fig. 1.
Fig. 1.
Diagram of gastric motility measurement system
I1, I2: excitation electrodes; V1, V2: measurement electrodes
The measurement system consists of a sine signal generator, current source, electrodes (I1, I2, V1, V2), preamplifier, demodulation/filter circuit, data acquisition system, and a computer. A measurement current of 50 kHz, 2 mA provided by the current source goes into the abdomen zone of the measured subject via excitation electrodes I1 and I2 (Fig. 2). The impedance signal picked up from measurement electrodes V1 and V2 is fed into preamplifier, then the demodulation/filter, and goes into data acquisition system where analog-to-digital (A/D) conversion and digitization processing are carried out. The digital data then is sent to a computer where the proprietary software is in charge of the IGM and EGG information extraction, analyzing, and parameter calculations. Gastric emptying measurement and IGM and EGG spectral analyses are also executed by the proprietary software in the computer.
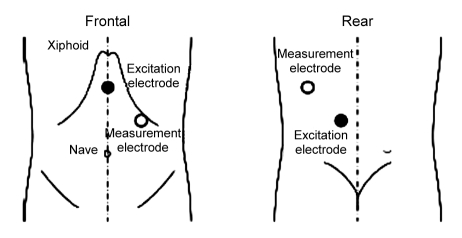
Fig. 2.
Position of the excitation and measurement electrodes
The impedance signal acquired from the abdomen surface is a mixed signal. It contains not only IGM, but also the components of impedance blood flow, breath, and some other disturbances. The normal rhythm of IGM is about 3 cpm and the breath signal is about 12 cpm. Both IGM and the breath rhythm belong to the ultra-low frequency signal and the amplitude of the breath signal is usually much higher than that of IGM. It is a challenge to extract IGM information effectively from the mixed signal. A low-pass filter may eliminate the influences of high-frequency noise and heart activity interference; however, it is difficult to eliminate the influence of respiration and separate IGM signal from the mixed signal. Thus, one uses a narrow band-pass filter and the high-order active low-pass filter. In the gastric motility measurement system, the wavelet transform is introduced and then IGM signal is separated successfully from the impedance signals of breath and blood flow among the mixed signal (Li et al., 2007).
The rhythm of the IGM signal is classified. The rhythm of 2–4 cpm is the normal rhythm, while that below 2 cpm is bradygastria, and that above 4 cpm is tachygastria. Based on this classification, we carried out analyses of frequency spectra, energy spectra, dynamic spectra, running spectra, frequency instability coefficient (FIC), power instability coefficient (PIC), percentage of normal frequency (PNF), and percentage of normal power (PNP), for both IGM and EGG.
The definitions of FIC and PIC are as below (Li and Ren, 2008):
| FIC=SDF/DFAv, |
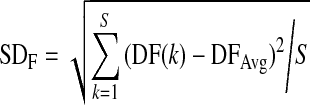 , ,
|
where DFAv is the average of the dominant frequency within 2–4 cpm among the signal segments analyzed, SDF is the standard deviation of the dominant frequency, DF(k) is the dominant frequency for the kth signal segment analyzed, DFAvg is the average of the dominant frequency for all signal segments analyzed, and S is the number of all the segments.
| PIC=SDP/DPAv, |
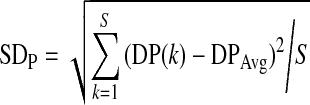 , ,
|
where DPAv is average of the dominant power within 2–4 cpm among the signal segments analyzed, SDP is the standard deviation of the dominant power, DP(k) is the dominant power for the kth signal segment analyzed, DPAvg is the average of the dominant power for all signal segments analyzed, and S is the number of all the segments.
2.3. Body measurement and statistical analysis
The measurement method of gastric motility used in the study is a noninvasive method. The study was approved by the ethical committee, and all subjects in the study signed a consent form.
In preparation for the measurement, the subject was calm and seated on a chair. The signals of IGM and EGG were recorded continually for 30–40 min after a test meal. The meal consists of milk (200 ml) and bread (100 g), 1 300 and 2 850 kJ, respectively.
The statistical software SPSS 13.0 was used to analyze the data. The data are expressed as mean±SD. Variance analysis between the study and control groups was undertaken, and the significant difference was accepted when P<0.05.
3. Results
In order to validate the feasibility and show the potential applications of the proposed method in this paper, some preliminary studies of gastric motility measurements in healthy volunteers, subjects with functional dyspepsia (FD), and subjects with gastritis have been carried out (Li and Ren, 2009; Liu et al., 2009).
3.1. Gastric motility measurement of the healthy subjects
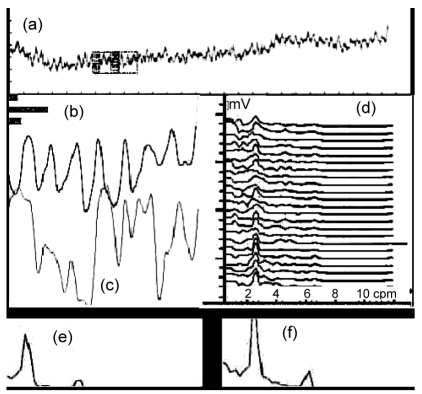
The raw IGM signal and the spectral analyses of the IGM and EGG signals for a healthy volunteer are illustrated in Fig. 3. The dynamic spectrum in Fig. 3d shows that the dominant frequency of IGM signals focuses on 2.8 cpm.
Fig. 3.
IGM measurement and spectral analysis result for a healthy volunteer
(a) Original mixed impedance signal which comes from the abdomen surface; (b) IGM signal extracted from the mixed signal; (c) Synchronous EGG; (d) The dynamic spectra of IGM (the interval of each spectrum line is 1 min); (e, f) Power spectra before and after the test meal, respectively
3.2. Gastric motility measurement of FD patients
Gastric motility measurements of 30 healthy volunteers (control group) and 28 FD subjects (study group) were carried out. The volunteers were university teachers with ages of (45.2±12.3) years. Subjects with FD (aged (40.9±9.7) years) came from the First Affiliated Hospital of Chongqing University of Medical Sciences, and were diagnosed according to the Rome III classification for FD. Results of the IGM measurement are shown in Table 1. It can be seen from Table 1 that the PNF in the study group (FD subjects) is obviously lower than that in the control group (P<0.01), and the FIC is higher than that in the control group (P<0.01). The PNP and PIC between the two groups are also different (P<0.01). This indicates that the FD subjects have a poor rhythm of gastric motility. The results are in accordance to gastric physiology and pathology principles for FD.
Table 1.
IGM measurement results of healthy and FD subjects
| Group | Case number | PNF (%) | PNP (%) | FIC | PIC |
| Control | 30 | 68.47±26.43 | 60.13±3.34 | 1.36±0.08 | 0.18±0.16 |
| Study | 28 | 28.32±16.92 | 50.79±9.90 | 2.08±0.55 | 0.23±0.05 |
| P | <0.01 | <0.01 | <0.01 | <0.01 | |
Data are expressed as mean±SD. PNF: percentage of normal frequency; PNP: percentage of normal power; FIC: frequency instability coefficient; PIC: power instability coefficient
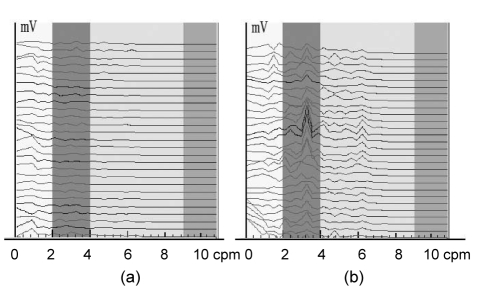
Fig. 4 shows the EGG dynamic spectra of FD subjects before treatment and in a week treatment (domperidone tablets, 10 mg, three times per day for oral administration, half an hour before meals), and the interval of each spectrum line is 1 min. In Fig. 4, EGG of FD patient is weak and the rhythm is disordered before the treatment (Fig. 4a); then in a week treatment, the EGG enhances and the rhythm gets back to 2–4 cpm (Fig. 4b). This suggests that the gastric electric activity of FD patients tended towards normal after a week treatment.
Fig. 4.
EGG dynamic spectra of FD patients before treatment (a) and in a week treatment (b)
The interval of each spectrum line is 1 min
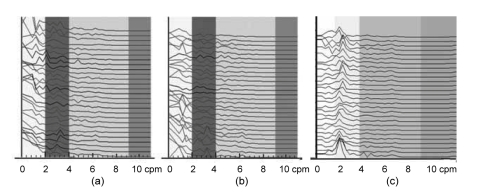
Fig. 5 shows the IGM signal spectra of FD subjects before treatment, after one week of treatment, and after three weeks of treatment. Compared with the EGG dynamic spectra in Fig. 4, the IGM in Fig. 5 shows little change after one week of treatment, and the rhythm also is disordered; until after three weeks of treatment, the IGM rhythm appears back to 2–4 cpm.
Fig. 5.
IGM dynamic spectra of FD patients before (a), after one week (b) and three weeks (c) of treatment
The interval of each spectrum line is 1 min
3.3. Gastric motility evaluation of gastritis patients
We conducted IGM parameter measurement of healthy volunteers and erosive gastritis subjects, and compared the IGM and EGG parameters for the gastritis subjects before and after one week of treatment (sodium rabeprazole tablets, 10 mg, one time per day for oral administration in the morning). Thirty healthy volunteers (control group) were university teachers with ages of (45.2±12.3) years. Thirty subjects with erosive gastritis (study group; aged (50.5±13.0) years) came from the First Affiliated Hospital of Chongqing University of Medical Sciences and were diagnosed by gastroscope examination. The statistic results of the studies are shown in Tables 2, 3, and 4.
Table 2.
IGM parameters for 30 gastritis subjects and 30 healthy subjects
| Group | PNF (%) | PNP (%) | FIC | PIC |
| Control | 68.5±26.5 | 60.1±3.3 | 1.36±0.08 | 0.18±0.16 |
| Study | 36.1±21.8 | 44.6±4.8 | 2.23±0.55 | 0.24±0.05 |
| P | <0.05 | <0.05 | <0.05 | <0.05 |
Data are expressed as mean±SD. PNF: percentage of normal frequency; PNP: percentage of normal power; FIC: frequency instability coefficient; PIC: power instability coefficient
Table 3.
EGG parameters for 30 gastritis subjects before and after one week of treatment
| Treatment | PP (%) |
FIC | PIC | ||
| 0–2 cpm | 2–4 cpm | >4 cpm | |||
| Before | 24.0±5.6 | 51.5±11.1 | 24.4±5.5 | 2.22±0.43 | 0.34±0.03 |
| After one week | 22.7±3.4 | 54.3±6.7 | 23.1±3.3 | 1.77±0.19 | 0.23±0.02 |
| P | >0.05 | >0.05 | >0.05 | <0.05 | <0.05 |
Data are expressed as mean±SD. PP: percentage of power; FIC: frequency instability coefficient; PIC: power instability coefficient
Table 4.
IGM parameters for 30 gastritis subjects before and after one week of treatment
| Treatment | PP (%) |
FIC | PIC | ||
| 0–2 cpm | 2–4 cpm | >4 cpm | |||
| Before | 27.5±2.4 | 44.6±4.8 | 27.9±2.4 | 2.23±0.55 | 0.24±0.05 |
| After one week | 27.4±2.2 | 44.9±4.4 | 27.8±2.2 | 1.91±0.65 | 0.21±0.06 |
| P | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 |
Data are expressed as mean±SD. PP: percentage of power; FIC: frequency instability coefficient; PIC: power instability coefficient
Table 2 shows that there is a significant difference in the IGM parameters between the control and study groups. PNF and PNP of the study group are significantly lower than those of the control group (P<0.05). FIC and PIC of the study group are evidently higher than those of the control group (P<0.05). These results suggest that the gastric motility function of the gastritis subjects was reduced and the stomach peristalsis was disordered.
Table 3 indicates that the EGG power of normal rhythm (2–4 cpm) for the gastritis subjects was raised and the power of the abnormal rhythm (0–2 cpm and >4 cpm) declined after one week of treatment, although this was not statistically significant (P>0.05). It is important that FIC and PIC of the gastritis subjects were reduced (P<0.05). This suggests that the EGG of the gastritis subjects tended to be regular and stable after one week of treatment, after which the EGG rhythm improved.
The IGM parameters in Table 4 show that the power ratios of IGM signals for the gastritis subjects in all frequency bands had little change before and after treatment (P>0.05), although FIC and PIC showed a trend to decreasing (P>0.05).
4. Discussion
The results of this study indicate that the IGM of FD subjects is not yet improved with one week of treatment while the EGG is returned to normal. After three weeks of treatment, the regular IGM rhythm of FD subjects is normal and shows a recovery of contraction function of the stomach. The gastric motility of human body is regulated by a series of sensory signals from the nervous system and has a close relationship with the electric activity of the gastric smooth muscle (Zhou and Ke, 2005; Ma et al., 2006). While the EGG of FD subjects returned to normal after one week of treatment, this improvement may not yet couple with or transfer to the mechanical activity of the stomach. After three weeks of treatment, when the influence of the electric activity has already coupled with or transferred to mechanical activity of the stomach via the regulation mechanism of nerve and electric activities, the normal IGM rhythm is seen in the spectra, and suggests the recovery of contraction function of the stomach.
We also found a significant difference in IGM parameters between the gastritis subjects and the healthy controls. After one week of treatment, the EGG rhythm of the gastritis subjects returned to normal, while some IGM parameters showed only a trend to improvement. This suggests that the influence of gastric electric activity may not have coupled with mechanical contraction of the stomach after only one week of treatment. From the clinical point of view, although the subjects in the study had felt some alleviation after a week of treatment, the symptoms of the gastritis were not completely relieved. The fact coincided with the results of Tables 3 and 4, and the suggestion is that continued treatment should be offered.
EGG reflects the gastric electric activity of the stomach and is more sensitive to the regulation mechanisms of nerve and electric activities. The improvement of the EGG after treatment is only the beginning of the improvement of gastric motility function, and it does not indicate the cure of gastric disorder or the recovery of gastric motility. IGM is a veritable measure of gastric contraction and peristalsis, and reflects the gastric motility function more effectively. Gastric study based on IGM measurement and simultaneous EGG is a noninvasive, convenient, and effective method.
Footnotes
Project (Nos. 60471041 and 60901045) supported by the National Natural Science Foundation of China
References
- 1.Chaw CS, Yazaki E, Evans DF. The effect of pH change on the gastric emptying of liquids measured by electrical impedance tomography and pH-sensitive radiotelemetry capsule. Int J Pharm. 2001;227(1-2):167–175. doi: 10.1016/S0378-5173(01)00795-5. [DOI] [PubMed] [Google Scholar]
- 2.Chen JD, Lin XM, Abo M. Multi-channel Gastric Electrical Stimulation for the Acceleration of Gastric Emptying. First International Conference on Advances in Medical Signal and Information Processing; Bristol, UK. 2000. pp. 60–65. [DOI] [Google Scholar]
- 3.Chen RX, Wan DR. Abnormal impedance gastrogram and upper gastrointestinal symptom. Med Biol Eng Comput. 1992;29(Suppl.):14–15. [Google Scholar]
- 4.Chen RX, Wan DR. Further Investigation of Reliability on Impedance Gastrograph for Continuous Measurement of Human Gastric Contractile Activity; Proceedings of the 8th International Conference on Electrical Bio-impedance; July; Kupio, Finland. 1992. pp. 151–152. [Google Scholar]
- 5.Deng J, Zhao S, Sha H, et al. Electrical Bioimpedance Method to Measure Gastric Motility Function; Proceedings of the 2nd International Conference on Bioinformatics and Biomedical Engineering; Shanghai, China. 2008. pp. 616–619. [DOI] [Google Scholar]
- 6.Familoni BO, Kingma YJ, Bowes KL. Noninvasive assessment of human gastric motor function. IEEE Trans Biomed Eng. 1987;34(1):30–36. doi: 10.1109/TBME.1987.326012. [DOI] [PubMed] [Google Scholar]
- 7.Gajre SS, Anand S, Singh U, et al. Novel Method of Using Dynamic Electrical Impedance Signals for Noninvasive Diagnosis of Knee Osteoarthritis. Engineering in Medicine and Biology Society. 28th Annual International Conference of the IEEE; New York, NY. 2006. pp. 2207–2210. [DOI] [PubMed] [Google Scholar]
- 8.Garay L, Ramos EG, Cardiel E, Munoz R, Hernandez PR. In vivo and in situ measurement of electrical impedance for determination of distention in proximal stomach of rats. Med Eng Phys. 2006;28(7):648–655. doi: 10.1016/j.medengphy.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Giouvanoudi AC, Spyrou NM. Epigastric electrical impedance for the quantitative determination of the gastric acidity. Physiol Meas. 2008;29(11):1305–1317. doi: 10.1088/0967-3334/29/11/006. [DOI] [PubMed] [Google Scholar]
- 10.Giouvanoudi A, Amaee WB, Sutton JA, Horton P, Morton R, Hall W, Morgan L, Freedman MR, Spyrou NM. Physiological interpretation of electrical impedance epigastrography measurements. Physiol Meas. 2003;24(1):45–55. doi: 10.1088/0967-3334/24/1/304. [DOI] [PubMed] [Google Scholar]
- 11.Hadi NA, Giouvanoudi A, Morton R, Horton PW, Spyrou NM. Variations in gastric emptying times of three stomach regions for simple and complex meals using scintigraphy. IEEE Trans Nucl Sci. 2002;49(5):2328–2331. doi: 10.1109/TNS.2002.803785. [DOI] [Google Scholar]
- 12.Huerta-Franco R, Vargas-Luna M, Hernandez E, Capaccione K, Cordova T. Use of short-term bio-impedance for gastric motility assessment. Med Eng Phys. 2009;31(7):770–774. doi: 10.1016/j.medengphy.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Kothapalli B. Origin of changes in the epigastric impedance signal as determined by a three-dimensional model. IEEE Trans Biomed Eng. 1992;39(10):1005–1010. doi: 10.1109/10.161332. [DOI] [PubMed] [Google Scholar]
- 14.Li ZY, Ren CS. Gastric motility measurement and evaluation of functional dyspepsia by a bio-impedance method. Physiol Meas. 2008;29(6):S373–S382. doi: 10.1088/0967-3334/29/6/S31. [DOI] [PubMed] [Google Scholar]
- 15.Li ZY, Ren CS. Impedance gastric motility measurement to evaluate functional dyspepsia. Chin J Biomed Eng. 2009;28(3):372–376. (in Chinese) [Google Scholar]
- 16.Li ZY, Sha H, Wang Y, et al. A New Approach of Gastric Motility Measurement and Evaluation by Bioimpedance; Proceedings of 13th International Conference on Electrical Bio-Impedance & 8th Conference on Electrical Impedance Tomography; Aug. 29; Graz, Austria. 2007. pp. 691–694. [Google Scholar]
- 17.Li ZY, Sha H, Zhao S, Wang Y, Ren CS. Liquid gastric emptying measurement using an electrical bio-impedance method. Chin J Med Instrum. 2008;32(4):253–256. (in Chinese) [PubMed] [Google Scholar]
- 18.Liu SR, Li ZY, Ren CS, Liu CL, Fang XJ. Research on gastric motility for erosive gastritis. Chin J Med Phys. 2009;26(3):1224–1227. (in Chinese) [Google Scholar]
- 19.Ma DS, Zhou B, Fu B, et al. Gastrointestinal Motor and Clinical Aspects. Beijing: Military Medicine Science Press; 2006. pp. 37–44. (in Chinese) [Google Scholar]
- 20.Ren CS, Li ZY, Zhao S. Use of electrical bioimpedance for gastric motility measurement and evaluation. World Chin J Digestol. 2010;18(1):1–8. (in Chinese) [Google Scholar]
- 21.Soulsby CT, Khela M, Yazaki E, Evans DF, Hennessy E, Jeremy PT. Measurements of gastric emptying during continuous nasogastric infusion of liquid feed: electric impedance tomography versus gamma scintigraphy. Clin Nutr. 2006;25(4):671–680. doi: 10.1016/j.clnu.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Sutton JA, Thompson S. Measurement of gastric emptying rates by radioactive isotope scanning and epigastric impedance. The Lancet. 1985;325(8434):898–900. doi: 10.1016/S0140-6736(85)91674-5. [DOI] [PubMed] [Google Scholar]
- 23.Zhou L, Ke MY. Textbook of Neurogastroenterology and Motility, Basic and Clinical Aspects. Beijing: Science Press; 2005. pp. 501–521. (in Chinese) [Google Scholar]