Abstract
Objective: The roles of gonadal hormones and nitric oxide (NO) on the analgesic effects of morphine, tolerance to morphine, and their interactions have been widely investigated. In the present study, the effect of L-arginine (an NO precursor) on morphine tolerance in sham and ovariectomized (OVX) female mice was investigated. Methods: Forty mice were divided into sham and OVX groups. On the first day, a hot plate test ((55±0.2) °C; cut-off 30 s) was carried out as a base record 15 min before injection of morphine (10 mg/kg, subcutaneously (s.c.)) and was repeated every 15 min after injection. The sham group was then divided into two subgroups: sham-tolerance-L-arginine (Sham-Tol-LA) and sham-tolerance-saline (Sham-Tol-Sal) which received either L-arginine 50 mg/kg (intraperitoneally (i.p.)) or saline 10 ml/kg (i.p.), respectively, three times in a day for three consecutive days. Morphine tolerance was induced in animals by injecting 30 mg/kg morphine (s.c.) three times/day for three days. This treatment was also used for OVX subgroups. On the fifth day, the hot plate test was repeated. The analgesic effect of morphine was calculated as the maximal percent effect (MPE). The results were compared using repeated measure analysis of variance (ANOVA). Results: There was no significant difference in MPE between the OVX and sham groups. The MPEs in both the Sham-Tol-Sal and OVX-Tol-Sal groups were lower than those in both the sham and OVX groups (P<0.01). The MPE in the OVX-Tol-Sal group was greater than that in the Sham-Tol-Sal group (P<0.01). The MPE in the Sham-Tol-LA group was higher than that in the Sham-Tol-Sal group (P<0.01). However, there was no significant difference between the Sham-Tol-LA and sham groups or between the OVX-Tol-LA and OVX-Tol-Sal groups. Conclusions: The results of the present study showed that repeated administration of morphine causes tolerance to the analgesic effect of morphine. L-Arginine could prevent tolerance to morphine but its effect was different in the presence of ovarian hormones.
Keywords: L-Arginine, Ovariectomized mice, Morphine, Tolerance
1. Introduction
Pain is an unpleasant sensation which results from exposure of the skin or other tissue to an injurious or potentially-injurious stimulus (Fatehi-Hassanabad et al., 2005). It has been suggested that sociocultural, psychological and biological conditions affect pain sensitivity (Wiesenfeld-Hallin, 2005; Gatchel et al., 2007). There is now strong evidence for gender-dependent differences in pain perception and analgesia (Craft et al., 2004; Loyd et al., 2007). The prevalence of disorders such as migraine and fibromyalgia, which are accompanied by chronic pain, is higher in women than in men (Craft, 2007). These sex-dependent differences, as well as the presence of estrogen receptors in areas of the nervous system which contribute to pain perception, suggest that gonadal hormones such as estradiol and testosterone may affect pain perception or analgesia (Shughrue et al., 1997; Craft et al., 2004). The high localization of estrogen receptors in peri-aqueductal gray (PAG) matter also suggests that estrogen may affect the descending analgesia system (Loyd and Murphy, 2008). Modulation of neurotransmitters such as γ-amino butyric acid (GABA), serotonin and calcitonin gene-related peptide (CGRP) by estrogen, may be further evidence for its involvement in pain modulation (Saleh and Connell, 2003). It has been documented that some endocrine systems including gonadal hormones may change the development of tolerance to or dependence on analgesic drugs. Some studies have shown that estradiol administration can lead to tolerance prevention (Cataldo et al., 2005). Other results showed that gonadal hormone utilization facilitates tolerance to morphine (Shekunova and Bespalov, 2006).
Nitric oxide (NO) is a major neurotransmitter synthesized from L-arginine by NO synthase (NOS) (Pan et al., 2005; Li et al., 2006) and is involved in nociception processes (Janicki and Jeske-Janicka, 1998; Hosseini et al., 2011). It has been reported that NO donors affect the release of neuropeptides, such as the substance P and CGRP, which contribute to pain transmission (Garry et al., 1994). It has also been suggested that NO inhibition potentiates carrageenan-induced hyperalgesia (Budzinski et al., 1999). The role of NO in morphine tolerance has also been reported. According to some reports, tolerance to morphine or its withdrawal syndrome is likely prevented by inhibition of NO synthesis (Toda et al., 2009). However, some studies have shown that morphine tolerance is decreased by enhancement of NO synthesis (Kolesnikov et al., 1992). Inhibition of NO synthesis and blocking of morphine tolerance result in an enhancement in morphine antinociception, which suggests a selective function for NO contributing to µ-receptor-mediated tolerance and dependence (Heinzen et al., 2005).
It has been well documented that estrogen affects endothelial NOS (eNOS) activity and expression as well as the release of NO in both peripheral and nervous tissues (Hishikawa et al., 1995; Russell et al., 2000; Stefano et al., 2000; Farsetti et al., 2009; Liu et al., 2009; Zhang et al., 2011). Estrogen also affects neuronal NOS (nNOS) mRNA, the number of nitergic neurons, and the production of NO in the brain (Grohé et al., 2004). Interactions of both sex hormones and NO with other neurotransmitters, such as GABA, acetylcholine, serotonin, and dopamine, have been widely documented (Kugaya et al., 2003; Mitsushima et al., 2009). In particular, it has been suggested that the interaction between estrogen and the NO system may be responsible for morphine tolerance.
Therefore, the aim of the present study was to clarify various effects of L-arginine (an NOS precursor) on morphine tolerance in ovariectomized and sham-operated female mice.
2. Materials and methods
2.1. Animals and drugs
Forty female Balb/c mice ((9±1) weeks of age, and (3±8) g in weight) were used. The animals were housed in ten standard cages, at room temperature ((22±1) °C) on a 12-h light/dark cycle. Food and water were available continuously. Animal handling and all related procedures were in accordance with the standards of animal care approved by the Ethical Committee Acts, Mashhad University of Medical Sciences. The drugs used were L-arginine (Sigma, Chemical Co., St. Louis, MO, USA) and morphine powder (TEMAD Ltd., Teheran, Iran) dissolved in saline.
2.2. Nociceptive test
To assess nociceptive responses, a hot plate method was used. In this method, the mice were placed on a hot plate with the temperature setting controlled at (55±0.2) °C. The cut-off time was 30 s. A nociceptive response was defined as the licking of forepaws or the moving of hind paws. The time duration between placing the animals on the hot plate and the licking of forepaws or the moving of hind paws was considered as the reaction time. The hot plate test was performed as a base record 15 min before injection of morphine (10 mg/kg, subcutaneously (s.c.)) (Ojewole, 2008) and was repeated five times, every 15 min after injection.
2.3. Surgery
Mice were ovariectomized under ketamine anesthesia (50 mg/kg, intraperitoneally (i.p.)). Anesthesia was confirmed by reduced respiratory rate and no response to gentle pinching of the foot pad. The surgery was performed as the method described by Saffarzadeh et al. (2010). Briefly, the skin was incised in the flank region and the ovaries were isolated by ligation of the most proximal portion of the oviduct before removal. In the sham group, the ovaries were not removed.
2.4. Tolerance induction
Morphine tolerance was induced in animals by injecting 30 mg/kg morphine (s.c.) three times a day for three consecutive days, as described by Meng et al. (2008).
2.5. Experimental design
Forty mice were divided into two groups: the sham and ovariectomized (OVX) groups. During surgery, a few mice were omitted from the experiment. Six weeks after surgery, the hot plate test ((55±0.2) °C; cut-off 30 s) was carried out as a base record 15 min before injection of morphine (10 mg/kg, s.c.) and was repeated every 15 min after injection on the first day. The sham group was divided into two subgroups: (1) sham-tolerance-L-arginine (Sham-Tol-LA), and (2) sham-tolerance-saline (Sham-Tol-Sal), which received L-arginine 50 mg/kg (i.p.) or saline 10 ml/kg (i.p.), respectively, three times a day for three consecutive days. Morphine tolerance was induced in animals by injecting 30 mg/kg morphine (s.c.) three times a day for three days. The animals of the OVX group were also divided into two subgroups: (1) ovariectomized-tolerance-L-arginine (OVX-Tol-LA) and (2) ovariectomized-tolerance-saline (OVX-Tol-Sal), and were given the same treatments as the sham subgroups. On the fifth day, the hot plate test was repeated.
2.6. Statistical analysis
The analgesic effect of morphine was calculated as the maximal possible effect [MPE=(t t−t b)/(t c−t b)×100%, where t t is test response time, t b is basal response time, and t c is cut-off time] (Moghaddam et al., 2004; Sepehri and Shafeiee, 2006). All data are presented as the mean±standard error of the mean (SEM) of the MPE. Statistical comparisons of base reaction latency time between groups were made using one-way analysis of variance (ANOVA) and post-hoc Tukey’s test or Student’s t-test. A repeated measure ANOVA followed by post-hoc Tukey’s test was used for comparison of MPE after injection of morphine. Differences were considered statistically significant when P<0.05. Table 1 shows a summary of the groups, subgroups, treatments, and tests.
Table 1.
Summary of groups, subgroups, treatments and tests in our experiment
| Group and subgroup | Treatment and test |
| Sham | 1. Hot plate test: base record 15 min before injection; |
| 2. Morphine injection: single dose (10 mg/kg); | |
| 3. Hot plate test: 5 times (15 min interval after injection), MPE calculation. | |
| Sham-Tol-Sal | 1. Morphine injection for inducing tolerance: 30 mg/kg, 3 times/d, for 3 d; |
| 2. Co-treatment with morphine: saline (10 ml/kg), 3 times/d, for 3 d; | |
| 3. Hot plate test: base record 15 min before injection; | |
| 4. Morphine injection: single dose (10 mg/kg); | |
| 5. Hot plate test: 5 times (15 min interval after injection), MPE calculation. | |
| Sham-Tol-LA | 1. Morphine injection for inducing tolerance: 30 mg/kg, 3 times/d, for 3 d; |
| 2. Co-treatment with morphine: L-arginine (50 mg/kg), 3 times/d, for 3 d; | |
| 3. Hot plate test: base record 15 min before injection; | |
| 4. Morphine injection: single dose (10 mg/kg); | |
| 5. Hot plate test: 5 times (15 min interval after injection), MPE calculation. | |
| OVX | 1. Hot plate test: base record 15 min before injection; |
| 2. Morphine injection: single dose (10 mg/kg); | |
| 3. Hot plate test: 5 times (15 min interval), MPE calculation. | |
| OVX-Tol-Sal | 1. Morphine injection for inducing tolerance: 30 mg/kg, 3 times/d, for 3 d; |
| 2. Co-treatment with morphine: saline (10 ml/kg), 3 times/d, for 3 d; | |
| 3. Hot plate test: base record 15 min before injection; | |
| 4. Morphine injection: single dose (10 mg/kg); | |
| 5. Hot plate test: 5 times (15 min interval after injection), MPE calculation. | |
| OVX-Tol-LA | 1. Morphine injection for inducing tolerance: 30 mg/kg, 3 times/d, for 3 d; |
| 2. Co-treatment with morphine: L-arginine (50 mg/kg), 3 times/d, for 3 d; | |
| 3. Hot plate test: base record 15 min before injection; | |
| 4. Morphine injection: single dose (10 mg/kg); | |
| 5. Hot plate test: 5 times (15 min interval after injection), MPE calculation. | |
3. Results
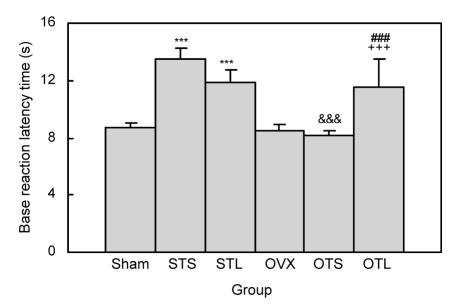
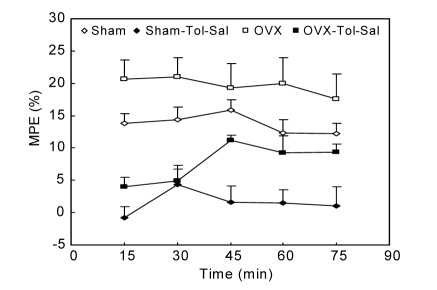
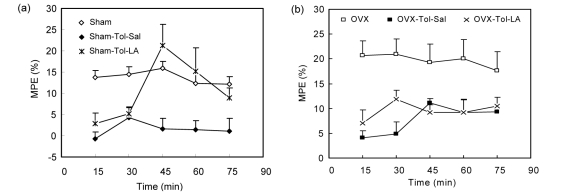
There was no significant difference in base reaction latency time between the sham and OVX groups (Fig. 1). The base reaction latency time in the OVX-Tol-Sal group was lower than that in the Sham-Tol-Sal group (P<0.001; Fig. 1). The animals of the Sham-Tol-LA group had a higher base reaction latency time than those of the sham group (P<0.001; Fig. 1). However, there was no significant difference between the Sham-Tol-LA and Sham-Tol-Sal groups in base reaction latency time (Fig. 1). The base reaction latency time in the OVX-Tol-LA group was higher than that in either the OVX-Tol-Sal or OVX group (P<0.001; Fig. 1). There was no significant difference in MPE between the OVX and sham groups (Fig. 2). The MPE in the OVX-Tol-Sal group was significantly higher than that in the Sham-Tol-Sal group (P<0.01; Fig. 2). The MPEs in both the Sham-Tol-Sal and OVX-Tol-Sal groups were lower than those in both the sham and OVX groups (P<0.01; Fig. 2). The MPE in the Sham-Tol-LA group was higher than that in the Sham-Tol-Sal group (P<0.01; Fig. 3a). However, there was no significant difference between the Sham-Tol-LA and sham groups. There was no significant difference between the OVX-Tol-LA and OVX-Tol-Sal groups. However, the MPE of the OVX-Tol-LA group was lower than that of the OVX group (P<0.01; Fig. 3b).
Fig. 1.
Comparison of base reaction latency time between Sham, Sham-Tol-Sal (STS), Sham-Tol-LA (STL), OVX, OVX-Tol-Sal (OTS), and OVX-Tol-LA (OTL) groups
Data are shown as mean±SEM (n=18 in Sham, n=8 in Sham-Tol-Sal, n=10 in Sham-Tol-LA, n=15 in OVX, n=7 in OVX-Tol-Sal, and n=8 in OVX-Tol-LA). *** P<0.001 compared to the Sham group, +++ P<0.001 compared to the OVX group, and ### P<0.001 compared to the OVX-Tol-Sal group, &&& P<0.001 compared to Sham-Tol-Sal
Fig. 2.
Comparison of MPE between Sham, Sham-Tol-Sal, OVX, and OVX-Tol-Sal groups
Data are shown as mean±SEM (n=18 in Sham, n=8 in Sham-Tol-Sal, n=15 in OVX, n=7 in OVX-Tol-Sal). Using repeated measure ANOVA, there was no significant difference in MPE between the OVX and Sham groups. The MPEs in both the Sham-Tol-Sal and OVX-Tol-Sal groups were less than those of both the Sham and OVX groups (P<0.01). The MPE in the OVX-Tol-Sal group was significantly higher than that of the Sham-Tol-Sal group (P<0.01)
Fig. 3.
Comparisons of MPEs between Sham, Sham-Tol-Sal, and Sham-Tol-LA groups (a) and between OVX, OVX-Tol-Sal, and OVX-Tol-LA groups (b)
Data are shown as mean±SEM (n=18 in Sham, n=8 in Sham-Tol-Sal, n=10 in Sham-Tol-LA, n=15 in OVX, n=7 in OVX-Tol-Sal, and n=8 in OVX-Tol-LA). The MPE in the Sham-Tol-LA group was higher than that in the Sham-Tol-Sal group (P<0.01). There was no significant difference between the OVX-Tol-LA and OVX-Tol-Sal groups
4. Discussion
Gender-dependent differences in nociception and opioid tolerance have been well documented (Craft et al., 2004; Wiesenfeld-Hallin, 2005). It has been hypothesized that gonadal hormones may have a role in opioid tolerance (Terner et al., 2005). Interaction of gonadal hormones and NO with neurotransmitters involved in pain modulation has been widely suggested (Kugaya et al., 2003). It has been well documented that estrogen interacts with the NO system in both peripheral and nervous tissues (Nematbakhsh and Khazaei, 2004; Hosseini et al., 2009; 2010; Saffarzadeh et al., 2010; Azizi-Malekabadi et al., 2011). Therefore, some actions of estradiol are probably mediated by NO (Hishikawa et al., 1995; Patchev et al., 1999; Russell et al., 2000; Stefano et al., 2000; Grohé et al., 2004; Farsetti et al., 2009; Shih, 2009). However, the role of NO in sex hormone-dependent changes in behavioral responses of female mice and rats has also been reported (Sadeghipour et al., 2007; Hosseini et al., 2011). It was shown that NOS inhibition antagonized epinephrine-induced hyperalgesia in normal male but not female rats (Dina et al., 2001). We hypothesized that the NO signaling pathway may contribute to morphine tolerance differently in sham and OVX female mice. For this reason, the effect of L-arginine (the precursor of NO) on morphine-induced tolerance in sham and OVX female mice was investigated. The hot plate test used in the present study is a well known standard method for pain threshold evaluation after morphine administration (Langerman et al., 1995).
The results showed no difference in MPE between the OVX and sham groups (on the first day). There is evidence that steroid hormones modulate the analgesic effects of morphine (Russell et al., 2000; Cataldo et al., 2005). Changes in β-endorphin receptors in the nervous system, functional coupling of the µ-opioid and GABA receptors, and alteration in µ-opioid and GABA-mediated hyperpolarization of neurons by estradiol and progesterone (Kelly et al., 1992) confirm that gonadal hormones may affect the analgesic function of opioids. The association of high levels of estrogen with increased levels of proenkephalin gene expression has also been reported (Romano et al., 1988). In the present study, the MPEs of both the Sham-Tol-Sal and OVX-Tol-Sal groups (after tolerance) were lower than those of both the sham and OVX groups (before tolerance). Thus, repeated morphine administration can lead to tolerance in both OVX and sham-operated mice, as previously reported (Bourne, 2008; Hernández et al., 2009). There are many controversial reports regarding the effect of estradiol on morphine tolerance (Cataldo et al., 2005; Shekunova and Bespalov, 2006). The results of the present study confirmed a significant difference between the OVX-Tol-Sal and Sham-Tol-Sal groups in MPE.
NO is a potent biological messenger with a diversity of physiological functions (Moncada et al., 1991; Luo and Cizkova, 2000). Our results demonstrated that co-treatment of the animals with morphine and L-arginine affects morphine-induced tolerance; the MPE in the Sham-Tol-LA group was higher than that of the Sham-Tol-Sal group. However, there was no significant difference between the OVX-Tol-LA and OVX-Tol-Sal groups. It seems that L-arginine is able to diminish tolerance in the sham group, but it does not show the ability to decrease morphine tolerance in the OVX group. There are contradictory reports regarding the role of NO in tolerance to morphine and other opioids (Kolesnikov et al., 1992; Toda et al., 2009). Recently, it has been reported that morphine tolerance or its withdrawal syndrome is likely to be prevented by inhibition of NO synthesis (Toda et al., 2009). However, some reports claimed that morphine tolerance is decreased by the enhancement of NO synthesis (Kolesnikov et al., 1992). It has also been proved that NO acutely enhances the analgesic effects of opioids (Pataki and Telegdy, 1998; Heinzen and Pollack, 2004). All of these reports show that NO has a role in morphine-induced tolerance. Therefore, the results of the present study may imply that L-arginine antagonizes the effect of morphine when it is used in combination with morphine during tolerance induction in the Sham-Tol-LA group but not in the OVX-Tol-LA group. This finding may suggest that the effect of NO on the nervous system is dependent on gonadal hormones. It has been documented that ovariectomy reduces nNOS and eNOS expression and Ca2+-dependent NOS activity, which are reversed by 17-β-estradiol (Grohé et al., 2004; Ceylan-Isik et al., 2009). In the present study, NOS activity reduction in OVX rats may explain why co-administration of L-arginine (the substrate for NOS) with morphine did not affect tolerance. However, further investigation including, for example, the determination of NOS activity, is needed to elucidate the exact mechanism(s) involved.
5. Conclusions
It is concluded that there are potential levels of interaction between sex hormones and the NO signaling systems in the regulation of morphine tolerance.
Footnotes
Project supported by the Vice Presidency of Research of Mashhad University of Medical Sciences, Iran
References
- 1.Azizi-Malekabadi H, Hosseini M, Saffarzadeh F, Karami R, Khodabandehloo F. Chronic treatment with the nitric oxide synthase inhibitor, L-NAME, attenuates estradiol-mediated improvement of learning and memory in ovariectomized rats. Clinics. 2011;66(4):673–679. doi: 10.1590/S1807-59322011000400024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bourne N. Managing acute pain in opioid tolerant patients. J Perioper Pract. 2008;18(11):498–503. doi: 10.1177/175045890801801105. [DOI] [PubMed] [Google Scholar]
- 3.Budzinski M, Misterek K, Gumulka W, Dorociak A. Inhibition of inducible nitric oxide synthase in persistent pain. Life Sci. 1999;66(4):301–305. doi: 10.1016/S0024-3205(99)00421-X. [DOI] [PubMed] [Google Scholar]
- 4.Cataldo G, Bernal S, Markowitz A, Ogawa S, Ragnauth A, Pfaff DW, Bodnar RJ. Organizational manipulation of gonadal hormones and systemic morphine analgesia in female rats: effects of adult ovariectomy and estradiol replacement. Brain Res. 2005;1059(1):13–19. doi: 10.1016/j.brainres.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Ceylan-Isik AF, Erdogan-Tulmac OB, Ari N, Ozansoy G, Ren J. Effect of 17β-oestradiol replacement on vascular responsiveness in ovariectomized diabetic rats. Clin Exp Pharmacol Physiol. 2009;36(11):e65–e71. doi: 10.1111/j.1440-1681.2009.05255.x. [DOI] [PubMed] [Google Scholar]
- 6.Craft RM. Modulation of pain by estrogens. Pain. 2007;132(S1):S3–S12. doi: 10.1016/j.pain.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 7.Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain. 2004;8(5):397–411. doi: 10.1016/j.ejpain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Dina OA, Aley KO, Isenberg W, Messing RO, Levine JD. Sex hormones regulate the contribution of PKCepsilon and PKA signalling in inflammatory pain in the rat. Eur J Neurosci. 2001;13(12):2227–2233. doi: 10.1046/j.0953-816x.2001.01614.x. [DOI] [PubMed] [Google Scholar]
- 9.Farsetti A, Grasselli A, Bacchetti S, Gaetano C, Capogrossi MC. The telomerase tale in vascular aging: regulation by estrogens and nitric oxide signaling. J Appl Physiol. 2009;106(1):333–337. doi: 10.1152/japplphysiol.91360.2008. [DOI] [PubMed] [Google Scholar]
- 10.Fatehi-Hassanabad Z, Jafarzadeh M, Fatehi M, Razavi-Tossi M. Sex affects the feeling of pain in the mice, possible involvement of nitric oxide. Daru. 2005;13(3):116–119. [Google Scholar]
- 11.Garry MG, Richardson JD, Hargreaves KM. Sodium nitroprusside evokes the release of immunoreactive calcitonin gene-related peptide and substance P from dorsal horn slices via nitric oxide-dependent and nitric oxide-independent mechanisms. J Neurosci. 1994;14(7):4329–4337. doi: 10.1523/JNEUROSCI.14-07-04329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133(4):581. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- 13.Grohé C, Kann S, Fink L, Djoufack PC, Paehr M, van Eickels M, Vetter H, Meyer R, Fink KB. 17β-estradiol regulates nNOS and eNOS activity in the hippocampus. Neuroreport. 2004;15(1):89–93. doi: 10.1097/00001756-200401190-00018. [DOI] [PubMed] [Google Scholar]
- 14.Heinzen EL, Pollack GM. Pharmacodynamics of morphine-induced neuronal nitric oxide production and antinociceptive tolerance development. Brain Res. 2004;1023(2):175–184. doi: 10.1016/j.brainres.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Heinzen EL, Booth RG, Pollack GM. Neuronal nitric oxide modulates morphine antinociceptive tolerance by enhancing constitutive activity of the mu-opioid receptor. Biochem Pharmacol. 2005;69(4):679–688. doi: 10.1016/j.bcp.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Hernández L, Romero A, Almela P, Garcia-Nogales P, Laorden ML, Puig MM. Tolerance to the antinociceptive effects of peripherally administered opioids: expression of β-arrestins. Brain Res. 2009;1248:31–39. doi: 10.1016/j.brainres.2008.10.065. [DOI] [PubMed] [Google Scholar]
- 17.Hishikawa K, Nakaki T, Marumo T, Suzuki H, Kato R, Saruta T. Up-regulation of nitric oxide synthase by estradiol in human aortic endothelial cells. FEBS Lett. 1995;360(3):291–293. doi: 10.1016/0014-5793(95)00124-R. [DOI] [PubMed] [Google Scholar]
- 18.Hosseini M, Sadeghnia HR, Salehabadi S, Alavi H, Gorji A. The effect of L-arginine and L-NAME on pentylenetetrazole induced seizures in ovariectomized rats, an in vivo study. Seizure. 2009;18(10):695–698. doi: 10.1016/j.seizure.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Hosseini M, Headari R, Oryan S, Hadjzadeh MA, Saffarzadeh F, Khazaei M. The effect of chronic administration of L-arginine on the learning and memory of estradiol-treated ovariectomized rats tested in the morris water maze. Clinics. 2010;65(8):803–807. doi: 10.1590/S1807-59322010000800011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosseini M, Taiarani Z, Hadjzadeh MA, Salehabadi S, Tehranipour M, Alaei HA. Different responses of nitric oxide synthase inhibition on morphine-induced antinociception in male and female rats. Pathophysiology. 2011;18(2):143–149. doi: 10.1016/j.pathophys.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Janicki P, Jeske-Janicka M. Relevance of nitric oxide in pain mechanisms and pain management. Curr Pain Headache Rep. 1998;2(4):211–216. doi: 10.1007/s11916-998-0022-5. [DOI] [Google Scholar]
- 22.Kelly MJ, Loose MD, Ronnekleiv OK. Estrogen suppresses mu-opioid- and GABAB-mediated hyperpolarization of hypothalamic arcuate neurons. J Neurosci. 1992;12(7):2745–2750. doi: 10.1523/JNEUROSCI.12-07-02745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolesnikov YA, Pick CG, Pasternak GW. NG-nitro-L-arginine prevents morphine tolerance. Eur J Pharmacol. 1992;221(2-3):399–400. doi: 10.1016/0014-2999(92)90732-J. [DOI] [PubMed] [Google Scholar]
- 24.Kugaya A, Epperson CN, Zoghbi S, van Dyck CH, Hou Y, Fujita M, Staley JK, Garg PK, Seibyl JP, Innis RB. Increase in prefrontal cortex serotonin 2A receptors following estrogen treatment in postmenopausal women. Am J Psychiatry. 2003;160(8):1522–1524. doi: 10.1176/appi.ajp.160.8.1522. [DOI] [PubMed] [Google Scholar]
- 25.Langerman L, Zakowski M, Piskoun B, Grant G. Hot plate versus tail flick: evaluation of acute tolerance to continuous morphine infusion in the rat model. J Pharmacol Toxicol Methods. 1995;34(1):23–27. doi: 10.1016/1056-8719(94)00077-H. [DOI] [PubMed] [Google Scholar]
- 26.Li HC, Chen QZ, Ma Y, Zhou JF. Imbalanced free radicals and antioxidant defense systems in schizophrenia: a comparative study. J Zhejiang Univ-Sci B. 2006;7(12):981–986. doi: 10.1631/jzus.2006.B0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu SZ, Yan H, Hou WK, Xu P, Tian J, Tian LF, Zhu BF, Ma J, Lu SM. Relationships between endothelial nitric oxide synthase gene polymorphisms and osteoporosis in postmenopausal women. J Zhejiang Univ-Sci B. 2009;10(8):609–618. doi: 10.1631/jzus.B0920137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loyd DR, Murphy AZ. Androgen and estrogen (α) receptor localization on periaqueductal gray neurons projecting to the rostral ventromedial medulla in the male and female rat. J Chem Neuroanat. 2008;36(3-4):216–226. doi: 10.1016/j.jchemneu.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loyd DR, Morgan MM, Murphy AZ. Morphine preferentially activates the periaqueductal gray-rostral ventromedial medullary pathway in the male rat: a potential mechanism for sex differences in antinociception. Neuroscience. 2007;147(2):456–468. doi: 10.1016/j.neuroscience.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo ZD, Cizkova D. The role of nitric oxide in nociception. Curr Pain Headache Rep. 2000;4(6):459–466. doi: 10.1007/s11916-000-0070-y. [DOI] [PubMed] [Google Scholar]
- 31.Meng G, Wu N, Zhang C, Su RB, Lu XQ, Liu Y, Yun LH, Zheng JQ, Li J. Analgesic activity of ZC88, a novel N-type voltage-dependent calcium channel blocker, and its modulation of morphine analgesia, tolerance and dependence. Eur J Pharmacol. 2008;586(1-3):130–138. doi: 10.1016/j.ejphar.2008.02.066. [DOI] [PubMed] [Google Scholar]
- 32.Mitsushima D, Takase K, Takahashi T, Kimura F. Activational and organisational effects of gonadal steroids on sex-specific acetylcholine release in the dorsal hippocampus. J Neuroendocrinol. 2009;21(4):400–405. doi: 10.1111/j.1365-2826.2009.01848.x. [DOI] [PubMed] [Google Scholar]
- 33.Moghaddam HF, Kesmati M, Kargar HMP, Rezaei S. The effect of electrical lesion of paragigantocellularis lateralis nucleus on acute pain perception in presence of clonidine (α2 adrenergic agonist) in rat. Iran J Basic Med Sci. 2004;7(2):3. (in Persian) [Google Scholar]
- 34.Moncada S, Higgs E, Hodson H, Knowles R, López-Jaramillo P, McCall T, Palmer R, Radomski M, Rees D, Schulz R. The L-arginine: nitric oxide pathway. J Cardiovasc Pharmacol. 1991;17(s3):S1–S9. doi: 10.1097/00005344-199117003-00002. [DOI] [Google Scholar]
- 35.Nematbakhsh M, Khazaei M. The effect of estrogen on serum nitric oxide concentrations in normotensive and DOCA Salt hypertensive ovariectomized rats. Clin Chim Acta. 2004;344(1-2):53–57. doi: 10.1016/j.cccn.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 36.Ojewole JA. Analgesic, anti-inflammatory and hypoglycaemic effects of Securidaca longepedunculata (Fresen.) [Polygalaceae] root-bark aqueous extract. Inflammopharmacology. 2008;16(4):174–181. doi: 10.1007/s10787-007-0016-7. [DOI] [PubMed] [Google Scholar]
- 37.Pan JW, Zhan RY, Tong Y, Zhou YQ, Zhang M. Expression of endothelial nitric oxide synthase and vascular endothelial growth factor in association with neovascularization in human primary astrocytoma. J Zhejiang Univ-Sci B. 2005;6(7):693–698. doi: 10.1631/jzus.2005.B0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pataki I, Telegdy G. Further evidence that nitric oxide modifies acute and chronic morphine actions in mice. Eur J Pharmacol. 1998;357(2-3):157–162. doi: 10.1016/S0014-2999(98)00561-5. [DOI] [PubMed] [Google Scholar]
- 39.Patchev VK, Hayashi S, Orikasa C, Almeida OFX. Ontogeny of gender-specific responsiveness to stress and glucocorticoids in the rat and its determination by the neonatal gonadal steroid environment. Stress. 1999;3(1):41–54. doi: 10.3109/10253899909001111. [DOI] [PubMed] [Google Scholar]
- 40.Romano GJ, Harlan RE, Shivers BD, Howells RD, Pfaff DW. Estrogen increases proenkephalin messenger ribonucleic acid levels in the ventromedial hypothalamus of the rat. Mol Endocrinol. 1988;2(12):1320–1328. doi: 10.1210/mend-2-12-1320. [DOI] [PubMed] [Google Scholar]
- 41.Russell K, Haynes M, Caulin-Glaser T, Rosneck J, Sessa W, Bender J. Estrogen stimulates heat shock protein 90 binding to endothelial nitric oxide synthase in human vascular endothelial cells. J Biol Chem. 2000;275(7):5026. doi: 10.1074/jbc.275.7.5026. [DOI] [PubMed] [Google Scholar]
- 42.Sadeghipour H, Ghasemi M, Sadeghipour H, Riazi K, Soufiabadi M, Fallahi N, Dehpour A. Nitric oxide involvement in estrous cycle-dependent changes of the behavioral responses of female rats in the elevated plus-maze test. Behav Brain Res. 2007;178(1):10–17. doi: 10.1016/j.bbr.2006.11.045. [DOI] [PubMed] [Google Scholar]
- 43.Saffarzadeh F, Eslamizade MJ, Nemati Karimooy HA, Hadjzadeh MA, Khazaei M, Hosseini M. The effect of L-arginine on Morris water maze tasks of ovariectomized rats. Acta Physiol Hung. 2010;97(2):216–223. doi: 10.1556/APhysiol.97.2010.2.8. [DOI] [PubMed] [Google Scholar]
- 44.Saleh TM, Connell BJ. Estrogen-induced autonomic effects are mediated by NMDA and GABAA receptors in the parabrachial nucleus. Brain Res. 2003;973(2):161–170. doi: 10.1016/S0006-8993(03)02432-6. [DOI] [PubMed] [Google Scholar]
- 45.Sepehri G, Shafeiee MN. Effect of cuneiformis nucleus inactivation by lidocaine microinjection on the analgesic response of morphine in rats. Iran Biomed J. 2006;10(1):21–26. [Google Scholar]
- 46.Shekunova EV, Bespalov AY. Effects of memantine on estrogen-dependent acute tolerance to the morphine analgesia in female rats. Eur J Pharmacol. 2006;535(1-3):78–85. doi: 10.1016/j.ejphar.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 47.Shih CD. Activation of estrogen receptor-dependent nitric oxide signaling mediates the hypotensive effects of estrogen in the rostral ventrolateral medulla of anesthetized rats. J Biomed Sci. 2009;16(1):60. doi: 10.1186/1423-0127-16-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol. 1997;388(4):507–525. doi: 10.1002/(SICI)1096-9861(19971201)388:4<507::AID-CNE1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 49.Stefano GB, Prevot V, Beauvillain JC, Cadet P, Fimiani C, Welters I, Fricchione GL, Breton C, Lassalle P, Salzet M, et al. Cell-surface estrogen receptors mediate calcium-dependent nitric oxide release in human endothelia. Circulation. 2000;101(13):1594–1597. doi: 10.1161/01.cir.101.13.1594. [DOI] [PubMed] [Google Scholar]
- 50.Terner J, Lomas L, Picker M. Influence of estrous cycle and gonadal hormone depletion on nociception and opioid antinociception in female rats of four strains. J Pain. 2005;6(6):372–383. doi: 10.1016/j.jpain.2005.01.354. [DOI] [PubMed] [Google Scholar]
- 51.Toda N, Kishioka S, Hatano Y, Toda H. Modulation of opioid actions by nitric oxide signaling. Anesthesiology. 2009;110(1):166–181. doi: 10.1097/ALN.0b013e31819146a9. [DOI] [PubMed] [Google Scholar]
- 52.Wiesenfeld-Hallin Z. Sex differences in pain perception. Gend Med. 2005;2(3):137–145. doi: 10.1016/S1550-8579(05)80042-7. [DOI] [PubMed] [Google Scholar]
- 53.Zhang W, Wei QW, Wang ZC, Ding W, Wang W, Shi FX. Cell-specific expression and immunolocalization of nitric oxide synthase isoforms and the related nitric oxide/cyclic GMP signaling pathway in the ovaries of neonatal and immature rats. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2011;12(1):55–64. doi: 10.1631/jzus.B1000174. [DOI] [PMC free article] [PubMed] [Google Scholar]