Abstract
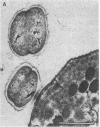
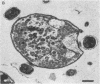
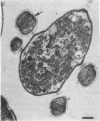
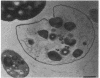
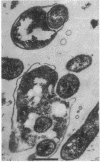
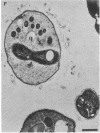
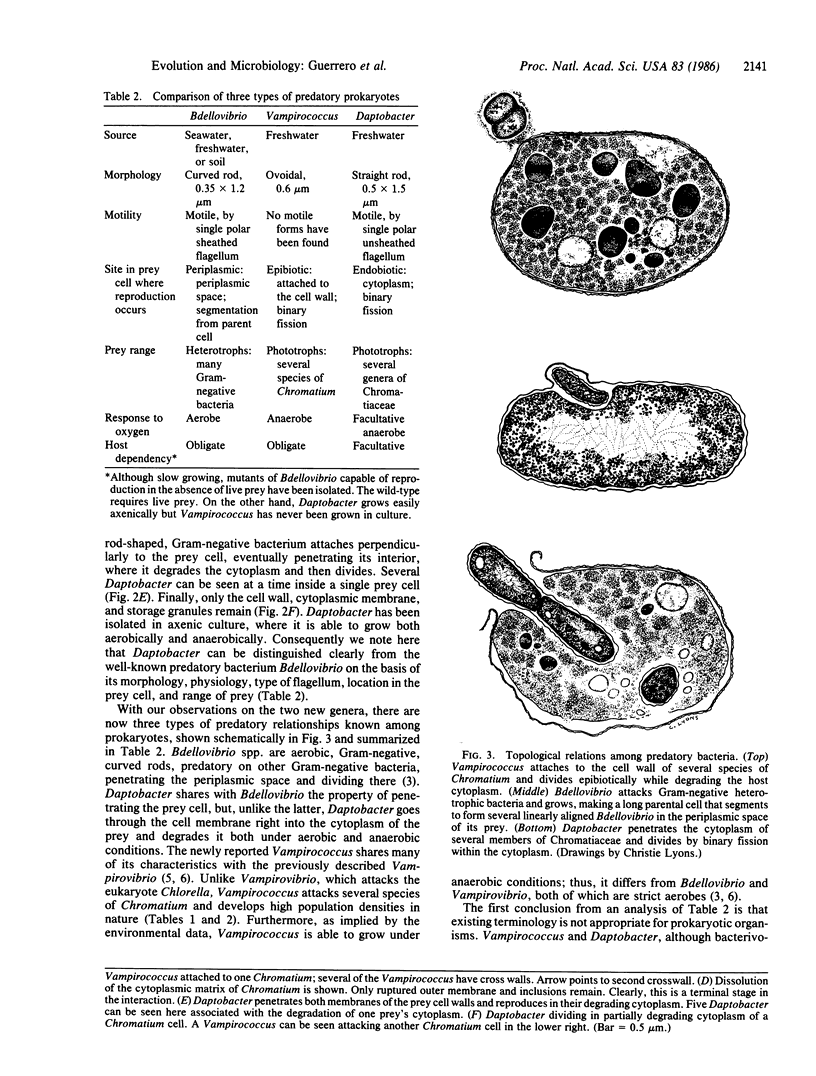
Two kinds of predatory bacteria have been observed and characterized by light and electron microscopy in samples from freshwater sulfurous lakes in northeastern Spain. The first bacterium, named Vampirococcus, is Gram-negative and ovoidal (0.6 μm wide). An anaerobic epibiont, it adheres to the surface of phototrophic bacteria (Chromatium spp.) by specific attachment structures and, as it grows and divides by fission, destroys its prey. An important in situ predatory role can be inferred for Vampirococcus from direct counts in natural samples. The second bacterium, named Daptobacter, is a Gram-negative, facultatively anaerobic straight rod (0.5 × 1.5 μm) with a single polar flagellum, which collides, penetrates, and grows inside the cytoplasm of its prey (several genera of Chromatiaceae). Considering also the well-known case of Bdellovibrio, a Gram-negative, aerobic curved rod that penetrates and divides in the periplasmic space of many chemotrophic Gram-negative bacteria, there are three types of predatory prokaryotes presently known (epibiotic, cytoplasmic, and periplasmic). Thus, we conclude that antagonistic relationships such as primary consumption, predation, and scavenging had already evolved in microbial ecosystems prior to the appearance of eukaryotes. Furthermore, because they represent methods by which prokaryotes can penetrate other prokaryotes in the absence of phagocytosis, these associations can be considered preadaptations for the origin of intracellular organelles.
Keywords: microbial ecology, microbial evolution, Chromatium, Daptobacter, Vampirococcus
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. Why microbial predators and parasites do not eliminate their prey and hosts. Annu Rev Microbiol. 1981;35:113–133. doi: 10.1146/annurev.mi.35.100181.000553. [DOI] [PubMed] [Google Scholar]
- Fallon R. D., Brock T. D. Lytic organisms and photooxidative effects: influence on blue-green algae (cyanobacteria) in lake mendota, wisconsin. Appl Environ Microbiol. 1979 Sep;38(3):499–505. doi: 10.1128/aem.38.3.499-505.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromov B. V., Mamkaeva K. A. elektronnomikroskopicheskoe izuchenie parazitizma bakterii Bdellovibrio chlorellavorus na kletkakh zelenoi vodorosli Chlorella vulgaris. Tsitologiia. 1972 Feb;14(2):256–260. [PubMed] [Google Scholar]
- Margulis L., Chase D., Guerrero R. Microbial communities. Bioscience. 1986 Mar;36(3):160–170. [PubMed] [Google Scholar]
- Pedrós-Alió C., Montesinos E., Guerrero R. Factors determining annual changes in bacterial photosynthetic pigments in holomictic lake cisó, Spain. Appl Environ Microbiol. 1983 Nov;46(5):999–1006. doi: 10.1128/aem.46.5.999-1006.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To L. P., Margulis L., Chase D., Nutting W. L. The symbiotic microbial community of the Sonoran Desert termite: Pterotermes occidentis. Biosystems. 1980;13(1-2):109–137. doi: 10.1016/0303-2647(80)90007-6. [DOI] [PubMed] [Google Scholar]
- Torrella F., Guerrero R., Seidler R. J. Further taxonomic characterization of the genus Bdellovibrio. Can J Microbiol. 1978 Nov;24(11):1387–1394. doi: 10.1139/m78-222. [DOI] [PubMed] [Google Scholar]
- Torrella F., Morita R. Y. Evidence by electron micrographs for a high incidence of bacteriophage particles in the waters of Yaquina Bay, oregon: ecological and taxonomical implications. Appl Environ Microbiol. 1979 Apr;37(4):774–778. doi: 10.1128/aem.37.4.774-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]