Abstract
Entamoeba histolytica is the causative agent of dysentery and liver abscess and is prevalent in developing countries. Adhesion to the host is critical to infection and is mediated by amoebic surface receptors. One such receptor, the Gal/GalNAc lectin, binds to galactose or N-acetylgalactosamine residues on host components and consists of heavy (Hgl), light (Lgl) and intermediate subunits. The mechanism by which the lectin assembles into a functional complex is not known. The parasite also relies on cholesterol-rich domains (lipid rafts) for adhesion. Therefore, it is conceivable that rafts regulate the assembly or function of the lectin. To test this, amoebae were loaded with cholesterol and lipid rafts were purified and characterized. Western blotting showed that cholesterol loading resulted in co-compartmentalization of all three subunits in rafts. This co-compartmentalization was accompanied by an increase in the ability of the amoebae to bind to host cells in a galactose-specific manner, suggesting that there is a correlation between location and function of the Gal/GalNAc lectin. Cholesterol loading did not increase the surface levels of the lectin subunits. Therefore, the cholesterol-induced increase in adhesion was not the result of externalization of an internal pool of subunits. A mutant cell line that modestly responded to cholesterol with a slight increase in adhesion exhibited only a slight enrichment of Hgl and Lgl in rafts. This supports the connection between location and function of the Gal/GalNAc lectin. Actin can also influence the interaction of proteins with rafts. Therefore, the sub-membrane distribution of the lectin subunits was also assessed after treatment with an actin depolymerizing agent, cytochalasin D (CytoD). CytoD-treatment had no effect on the submembrane distribution of the subunits, suggesting that actin does not prevent the association of lectin subunits with rafts in this system. Together, these data provide insight into the molecular mechanisms regulating the location and function of this adhesin.
Keywords: Actin, Adhesion, Cholesterol, Entamoeba histolytica, Gal/GalNAc lectin, Lipid raft
1. Introduction
Entamoeba histolytica is a protozoan parasite and the causative agent of amoebic dysentery and amoebic liver abscess (ALA; reviewed in Laughlin and Temesvari, 2005). Infection is acquired by the ingestion of the cyst form of the parasite. Excystation occurs in the small intestine after which the newly emerged amoeboid trophozoites translocate to and colonize the bowel lumen. Colonization can result in two non-mutually exclusive outcomes: non-invasive disease or invasive disease. In non-invasive disease, the production and release of newly formed cysts facilitate disease spread. In invasive disease, amoebae invade the gut mucous barrier and bind to and damage colonic epithelial cells. Invasion may allow the parasite to cross the epithelial layer, enter the circulatory system and establish extraintestinal infections, the most common of which is ALA.
Adhesion of the parasite to host cells and host extracellular matrix components is considered an important virulence function, especially during invasive disease, and several amoebic cell surface receptors that participate in parasite-host interactions have been identified (reviewed in Laughlin and Temesvari, 2005; Boettner et al., 2008: Buss et al., 2010). The best characterized of these receptors is a multimeric galactose/N-acetylgalactosamine (Gal/GalNAc)-inhibitable lectin (reviewed in Petri et al., 1989, 2002). This adhesin binds to galactose and N-acetylgalactosamine residues on host components and is proposed to be a major virulence factor. It includes a transmembrane heavy subunit (Hgl) which may be disulfide-linked to a glycosylphosphatidylinisotol (GPI)-anchored light subunit (Lgl). The extracellular portion of Hgl possesses the carbohydrate recognition domain and the cytoplasmic tail of Hgl interacts with actin (McCoy and Mann, 2005) and exhibits sequence homology to signaling domains of β2 and β7 integrins (Vines et al., 1998; Dodson et al., 1999). This suggests that the Gal/GalNAc lectin might also have a signaling function. Over-expression of the cytoplasmic domain of Hgl has a dominant negative effect on parasite-host adhesion, supporting the importance of this domain as a signaling platform (Vines et al., 1998).
Immunoprecipitation studies suggest that the heterodimer associates non-covalently with a GPI-anchored intermediate subunit (Igl) (Cheng et al., 1998, 2001). Immunofluorescence microscopy demonstrates that all three subunits co-localize at the plasma membranes of trophozoites adhered to laminin-coated surfaces (Cheng et al., 2001). These findings suggest that the heterotrimer is the functional adhesin; however, little is known about what regulates the assembly of the subunits into an active complex.
In other systems membrane microdomains, known as lipid rafts, can regulate the assembly of adhesion and signaling molecules into functional multimers (reviewed in Brown, 2006). These membrane domains are characterized by high levels of cholesterol and sphingolipid. Biochemical purification of these domains is initiated by extraction with cold non-ionic detergents, which results in the isolation of detergent-resistant rafts and detergent-resistant actin-rich membrane. Further separation of these two membrane types can be achieved by sucrose gradient density centrifugation. Lipid rafts may be enriched in GPI-anchored and transmembrane proteins; changes in membrane cholesterol (Reversi et al., 2006), binding to ligand (Valensin et al., 2002), and/or interaction with the cellular cytoskeleton (Hao and August, 2005), thereby influencing the association of such proteins with raft microdomains.
Previously, it was shown that E. histolytica trophozoites possess lipid raft-like domains (Laughlin et al., 2004). Further, it was shown that treatment with raft-disrupting agents inhibits adhesion of the parasite to host cells (Laughlin et al., 2004) and to host extracellular matrix (Mittal et al., 2008). Biochemical purification of detergent-resistant rafts from trophozoites revealed that the subunits of the Gal/GalNAc lectin localize to both lipid rafts and actin rich-membranes (Laughlin et al., 2004). Thus, it is conceivable that the assembly and function of the Gal/GalNAc lectin subunits may be regulated by their association with various membrane microdomains.
The aim of this study was to explore the relationship between rafts and Gal/GalNAc lectin function by loading trophozoites with cholesterol and then examining the sub-membrane distribution of Hgl, Igl and Lgl, and measuring the parasite’s ability to bind to host cells. We further explored the relationship between lipid rafts and Gal/GalNAc function by characterizing rafts and adhesion in an E. histolytica mutant cell line with a known defect in Gal/GalNAc function. Finally, given that actin can influence the association of proteins with various microdomains (Hao and August, 2005), we explored the role of the actin cytoskeleton in Gal/GalNAc lectin-membrane microdomain interactions.
2. Materials and methods
2.1. Strains and culture conditions
Entamoeba histolytica trophozoites (strain HM-1:IMSS) were cultured axenically in TYI-S-33 media (Diamond et al., 1978) in 15 ml glass screw cap tubes at 37 °C. The generation of an E. histolytica cell line conditionally over-expressing a mutant version of EhRabA is described elsewhere (Welter and Temesvari, 2009). This mutant cell line was maintained in TYI-S-33 supplemented with 5 µg/ml of G418 and 15 µg/ml of hygromycin in the culture medium. Mutant EhRabA expression was induced by the addition of 5 µg/ml of tetracycline to the culture medium 24 h prior to performing assays. Chinese hamster ovary (CHO) cells were grown at 37 °C in DMEM supplemented with 10% (v/v) FBS, 1 mM of HEPES and a PenStrep mixture (50 units/ml of penicillin/50µg/ml of streptomycin) in 96-well plates.
2.2. Cholesterol loading and depolymerization of actin
Trophozoites (3.5 × 106) were incubated in serum-free (TYI-33) medium at 37 °C for 30 min and then incubated with 3 mg/ml of cholesterol as lipoprotein-cholesterol concentrate (LCC) (MP Biomedicals, Solon, OH, USA) in the presence or absence of a sterol carrier, 50 mM methyl-β-cyclodextrin (MβCD). Exposure to the lipid and carrier was carried out for 60 min at 37 °C.
To disrupt the actin cytoskeleton, 3 × 105 trophozoites were pre-treated with an actin disrupting agent, cytochalasin D (CytoD, Sigma-Aldrich, St. Louis, MO, USA 10 µM), or an equivalent volume of CytoD diluent (DMSO), for 60 min at 37 °C prior to isolation and characterization of detergent-resistant membrane (DRM).
2.3. Measurement of adhesion to host epithelial cells
To assess parasite-host adhesion we used a previously developed fluorescence-based assay (Powell et al., 2006). Briefly, CHO cells were grown to confluency in 96- well plates and fixed with 4% paraformaldehyde (to prevent parasite-mediated lysis of the epithelial cells during the assay). The fixed monolayers were washed twice with PBS, incubated with 250 mM glycine (to inactivate residual paraformaldehyde) and washed twice with PBS. Control and treated E. histolytica cells were stained with the fluorescent vital dye, calcein AM (5 µg/ml, Invitrogen, Carlsbad, CA, USA) in serum-free medium (37 °C, 30 min). Stained cells were added to the wells containing fixed CHO monolayers and incubated (37 °C, 30 min). The wells were then washed twice with warm PBS and the number of parasites adhering to CHO cells was determined by measuring fluorescence with a fluorimeter/plate reader (Model FLX800, BioTek Instruments, Winooski, VT, USA).
2.4. Lipid raft isolation and characterization
For control and treated cells, isolation of Triton-X-100-resistant membrane, resolution of DRM by sucrose gradient density centrifugation and characterization of gradient fractions by SDS-PAGE and western blot were carried out as previously described (Laughlin et al., 2004). Densitometric analyses of immunoblots were conducted using ImageJ Software (Version 1.42q; U.S. National Institutes of Health, Bethesda, MD, USA).
2.5. Measurement of cholesterol
Whole cells were lysed by incubation in 1% (v/v) Triton-X-100 in PBS at room temperature. Alternatively, DRM was isolated by cold-Triton-X-100 extraction as described above for lipid raft isolation followed by treatment with 1% (v/v) Triton-X-100 in PBS at room temperature. Cholesterol was quantified using the fluorescence-based Amplex Red Cholesterol Assay Kit (Invitrogen Corp., Carlsbad, CA, USA) according the manufacturer’s protocol.
2.6. Cell surface biotinylation
Control and treated cells (3.5 × 106) were surface biotinylated and purified by avidin affinity chromatography using the Pierce Cell Surface Protein Isolation Kit (Pierce Biotechnology, Rockford, IL, USA) according to manufacturer’s specifications. Whole cell lysates and biotinylated surface proteins that were captured by avidin affinity chromatography were resolved by SDS-PAGE and analyzed for Hgl, Lgl, Igl and actin by western blotting and densitometry¨
2.7. Fluorescence microscopy
Actin staining using the fluorescent actin probe, Alexa Fluor 488-phalloidin (Invitrogen), was carried out as previously described (Welter et al., 2005). Stained cells were viewed by confocal laser scanning microscopy using an LSM510 microscope (Carl Zeiss MicroImaging, Thornwood, NY, USA).
2.8. Statistical analyses
All values are given as means ± S.D. Statistical analyses were performed using GraphPad Instat V.3. Comparisons of three or more groups were carried out with one-way ANOVA and a Tukey multiple comparison test. Comparisons of two groups were carried out using an unpaired T-test with Welch correction. P values less than 0.05 were considered statistically significant. P values less than 0.01 or 0.001 were considered highly statistically significant.
3. Results
3.1. Cholesterol loading alters the sub-membrane distribution of Gal/GalNAc lectin subunits
In other systems, cholesterol can regulate the location, assembly and/or function of raft-resident proteins (Pang et al., 1999; Eroglu et al., 2003; Pucadyil and Chattopadhyay, 2004; Reversi et al., 2006; Oh et al., 2009). Therefore, we determined whether cholesterol loading could influence the sub-membrane distribution of Gal/GalNAc lectin subunits. Since E. histolytica cells cannot use free cholesterol (Mata-Cardenas et al., 2000), cholesterol loading was carried out by exposing cells to a LCC as the source of cholesterol and a sterol carrier, MβCD (LCC + MβCD). In the absence of extracellular cholesterol, MβCD can remove cholesterol from cellular membranes. However, in the presence of extracellular cholesterol, this reagent facilitates the uptake of the sterol into cells (Atger et al., 1997). In contrast to treatment with LCC alone, treatment with LCC + MβCD resulted in a significant increase in cellular cholesterol in both whole cell lysates and in purified DRM (Fig. 1).
Fig. 1.
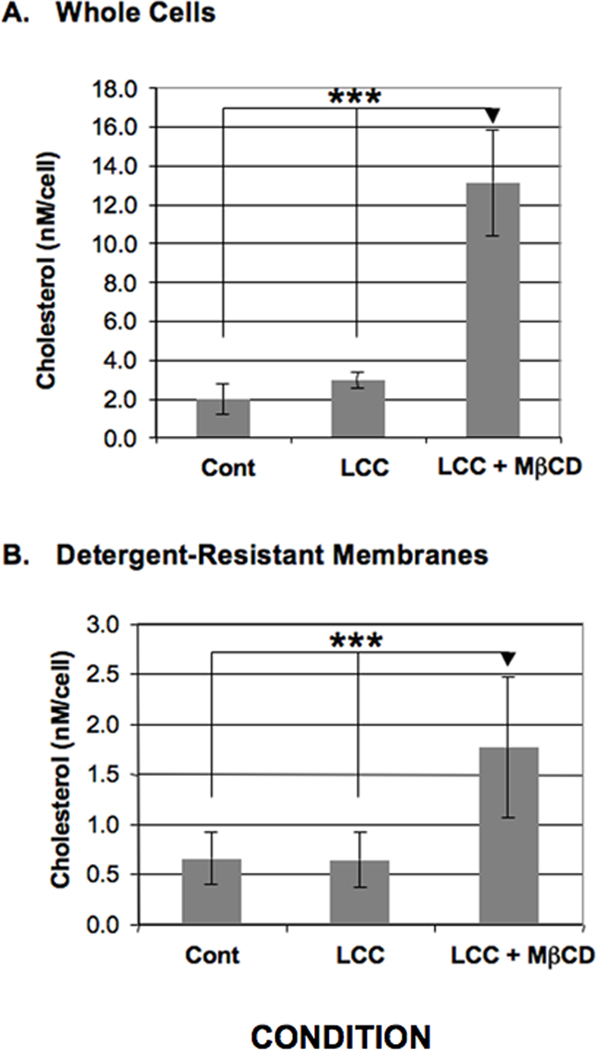
Cholesterol levels in Entamoeba histolytica whole cells or detergent-resistant membranes. Entamoeba histolytica trophozoites were treated with a cholesterol source, lipoprotein-cholesterol concentrate (LCC), in the absence (LCC) or presence of the cholesterol carrier, methyl-β-cyclodextrin (MβCD) (LCC+MβCD). Cholesterol levels were measured fluorometrically in whole cell lysates (A) or in detergent-resistant membranes isolated by cold Triton-X-100 extraction (B) in untreated control (Cont) or treated cells. The data are presented as the nanomolar quantity of cholesterol normalized to starting cell number. Treatment with a cholesterol source and a carrier (LCC+MβCD) significantly increased cholesterol levels (***P < 0.001) compared with control or treatment with the cholesterol source alone (LCC). The data represent the mean ± S.D. of five trials.
To investigate the sub-membrane compartmentalization of the lectin subunits under conditions of cholesterol loading, we purified DRM from untreated control cells and cells treated with LCC or LCC + MβCD by extraction with cold Triton-X-100. The total DRM fraction and the triton-soluble supernatant (TSS) were characterized by western blotting using antibodies specific for the Gal/GalNAc lectin subunits or actin and analyzed using densitometry. In untreated control cells the majority of Hgl, Lgl and Igl was found in the TSS (Fig. 2A–C). Treatment with LCC alone resulted in a slight enrichment of the Gal/GalNAc lectin subunits in the DRM fraction (Fig. 2A–C). However, treatment with LCC + MβCD gave rise to a marked enrichment of Hgl, Lgl and Igl in the DRM fraction (Fig. 2A–C); nearly 100% of each of the subunits was associated with DRM after cholesterol loading. This suggests that the sub-membrane location of the Gal/GalNAc lectin is sensitive to cholesterol-loading. Enrichment of actin in the DRM fraction was also observed after treatment with LCC + MβCD; however, under all conditions the proportion of actin in the TSS was always higher than that in the DRM (Fig. 2D).
Fig. 2.
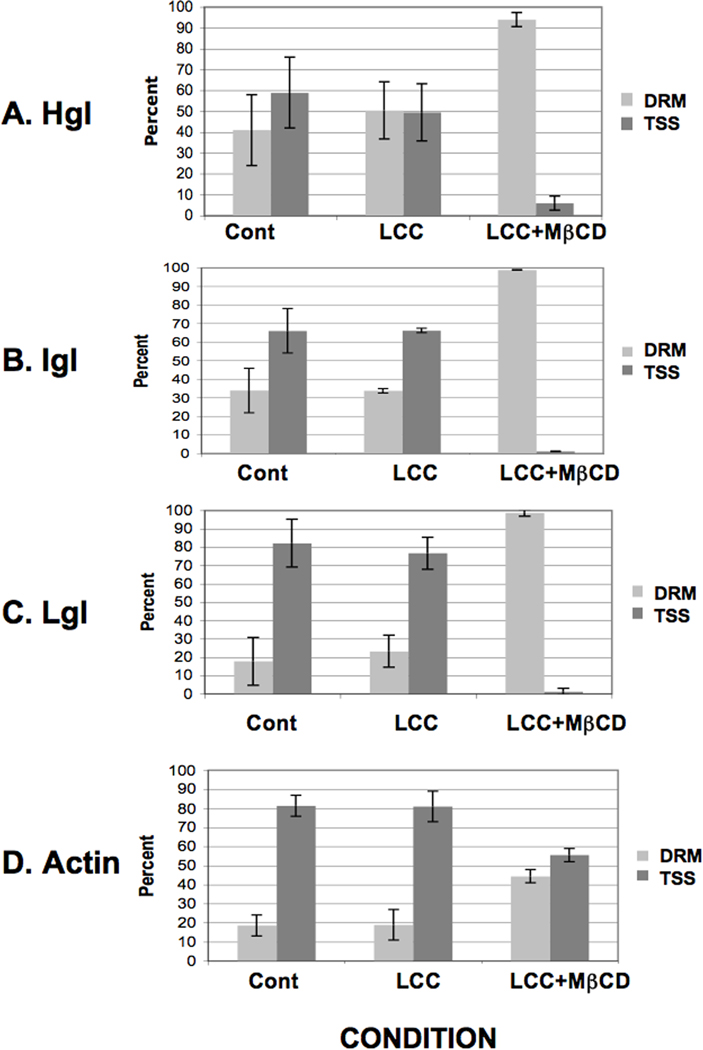
Cholesterol loading induces enrichment of the lectin subunits and actin in detergent-resistant membrane of Entamoeba histolytica. Entamoeba histolytica trophozoites were treated with a cholesterol source, lipoprotein-cholesterol concentrate (LCC), in the absence (LCC) or presence of the cholesterol carrier, methyl-β-cyclodextrin (MβCD) (LCC+MβCD). Detergent-resistant membrane (DRM) was isolated by triton extraction. SDS-PAGE and densitometry of western blots were used to quantify heavy subunit (Hgl) (A), intermediate subunit (Igl) (B), light subunit (Lgl) (C) and actin (D) in both DRM and the triton-soluble supernatant (TSS). Mean values ± S.D. of densitometric scans (n ≥ 3) are reported as a percentage of the total (DRM + TSS). Treatment with LCC alone induced a slight increase in the relative level of Hgl, Lgl, Igl and actin in DRM. However, cholesterol loading (LCC +MβCD) induced a marked increase in the relative levels of Hgl, Lgl, Igl and actin in DRM.
Since the DRM consists of both raft and actin-rich membrane, we resolved these two membrane types by sucrose density gradient centrifugation and characterized the fractions from the gradient by western blotting and densitometry as described in Section 2.4. Consistent with our previous report (Laughlin et al., 2004), Igl was enriched in the central portion of the gradient (fractions 9–14) in untreated control cells (Fig. 3C). These fractions were previously shown to possess high levels of cholesterol and, thus, are considered to contain lipid rafts. Also consistent with our previous report (Laughlin et al., 2004), in untreated control cells the relative levels of Hgl and Lgl were low to moderate in the raft fractions and increased steadily through higher density actin-rich fractions (Fig. 3A,B). Treatment with LCC alone had no effect on the relative sub-membrane distribution of Hgl, Lgl Igl or actin (Fig. 3A – D). However, LCC + MβCD-treatment resulted in enrichment of Hgl and Lgl in lipid raft fractions (Fig. 3A,B) that already contained the majority of Igl. Therefore, not only does cholesterol loading result in increased Hgl and Lgl in DRM, it specifically results in increased levels of these subunits in detergent-resistant rafts.
Fig. 3.
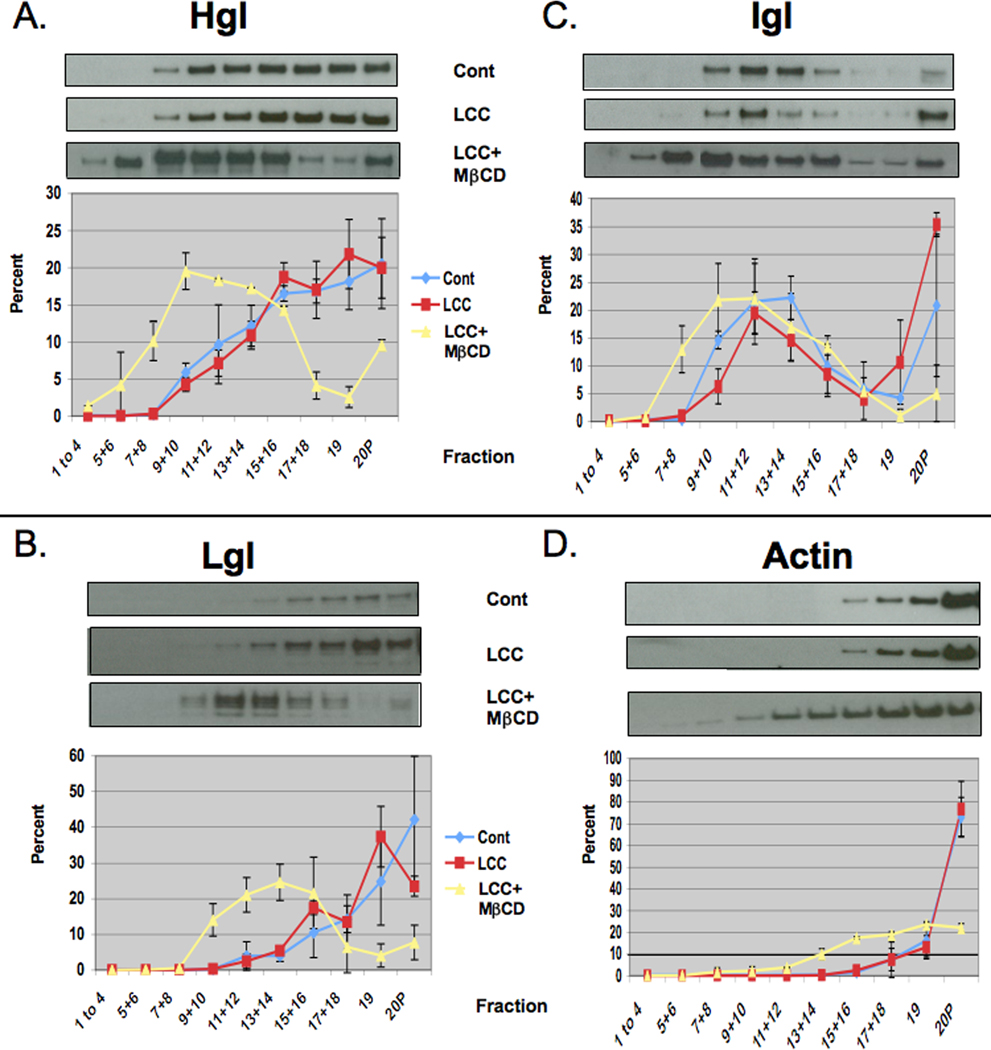
Cholesterol loading induces enrichment of the heavy (Hgl) and light (Lgl) subunits of the Gal/GalNAc lectins in rafts of Entamoeba histolytica. Entamoeba histolytica trophozoites were treated with a cholesterol source, lipoprotein-cholesterol concentrate (LCC), in the absence (LCC) or presence of the cholesterol carrier, methyl-β-cyclodextrin (MβCD) (LCC+MβCD). Triton-insoluble membranes were isolated and resolved by sucrose gradient density centrifugation. Nineteen fractions and the pellet (20P) were collected and subjected to western blot analyses using antibodies specific for Hgl (A), Lgl (B), intermediate subunit (Igl) (C) or actin (D). Mean values of densitometric scans (n ≥ 2), reported as a percentage of total detergent-resistant membrane (DRM)-associated protein, are shown for each subunit and actin. Representative western blots are shown above each panel. In both control (Cont) and treated cells the Igl remained localized to central fractions previously identified as lipid rafts (C). In untreated (blue line) or LCC-treated cells (red line), Hgl (A) and Lgl (B) predominantly localized to high density actin-rich (D) fractions. Upon cholesterol loading with LCC and a cholesterol carrier (LCC+MβCD) (yellow line), Hgl (A) and Lgl (B) were enriched in low density lipid rafts.
Importantly, cholesterol loading had little effect on the buoyancy of Igl (Fig. 3C) and only slightly increased the buoyancy of actin (Fig. 3D). This indicated that the cholesterol-induced enrichment of Hgl and Lgl into low density lipid rafts was specific. These data support the notion that cholesterol levels influence the sub-membrane location of the Gal/GalNAc lectin.
3.2. Cholesterol loading enhances galactose-sensitive adhesion to host cells
The co-localization of Hgl, Lgl and Igl in rafts after cholesterol loading may regulate the function of the Gal/GalNAc lectin. Therefore, we assessed the ability of control and cholesterol-loaded trophozoites to adhere to fixed epithelial monolayers using a published protocol (Powell and Temesvari, 2004). Assessing adhesion to fixed host monolayers, instead of live cell targets such as erythrocytes, ensured that any measurable changes in adhesion were due to the effects of cholesterol on parasite membranes and not on host cell membranes. Fixation also prevented host cell lysis by the parasite. Previously, it was shown that treatment with MβCD alone inhibited adhesion to host cells (Laughlin et al., 2004). Treatment with LCC alone resulted in a slight increase in adhesion (Fig. 4). On the other hand, treatment with LCC + MβCD resulted in a statistically significant increase in the ability of the parasite to bind to a model host epithelium (Fig. 4). This finding is interesting because it is, to our knowledge, the first to show a correlation between adhesion and the co-localization of the all three Gal/GalNAc lectin subunits in low density lipid rafts.
Fig. 4.
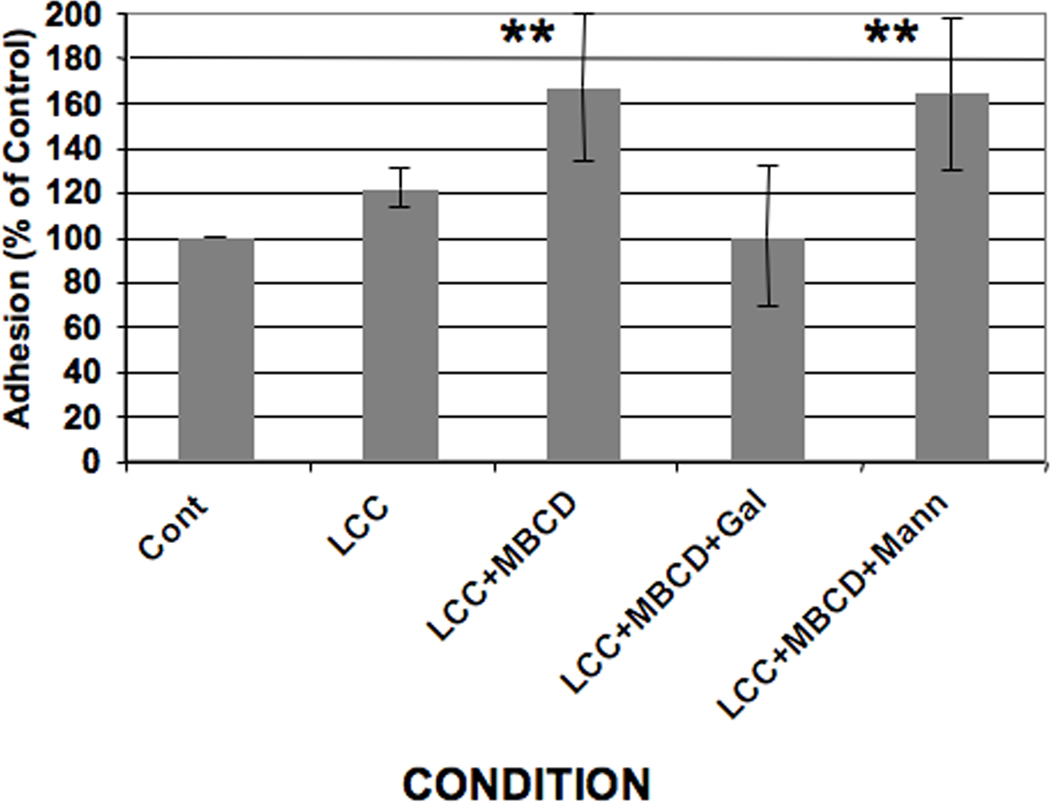
Effect of cholesterol loading on Entamoeba histolytica adhesion to host cells. Entamoeba histolytica trophozoites were pre-treated with a cholesterol source, lipoprotein-cholesterol concentrate (LCC), in the absence (LCC) or presence of the cholesterol carrier, methyl-β-cyclodextrin (MβCD) (LCC+MβCD). A standard adhesion assay was performed using control (Cont) and treated E. histolytica cells in the absence or presence of 10 mM galactose (+Gal) or 10 mM mannose (+Mann). Data are presented as a percentage of untreated control which was arbitrarily set to 100%. Treatment with a cholesterol source and a carrier (LCC+MβCD) significantly enhanced adhesion (**P < 0.01). Galactose abrogated LCC + MβCD-induced increases in adhesion whereas mannose had no effect. The data represent the mean ± S.D. of six trials.
To determine whether cholesterol-enhanced adhesion depended on functional Gal/GalNAc lectin, we quantified binding of LCC + MβCD-treated cells to host cells in the presence of galactose, which is a competitive inhibitor of Gal/GalNAc lectin-ligand binding (Ravdin and Guerrant, 1981). Although galactose did not completely eliminate adhesion to monolayers, suggesting the involvement of other receptors, galactose abrogated the ability of LCC + MβCD treatment to increase adhesion (Fig. 4). This observation suggests that the specific cholesterol-induced increase in adhesion relied on Gal/GalNAc lectin function. A control sugar, mannose, was not able to prevent the cholesterol-induced increase in adhesion (Fig. 4), supporting the authenticity of our observation.
3.3. Cholesterol loading does not alter surface levels of the Gal/GalNAc lectin subunits
Augmentation of membrane cholesterol could have altered the trafficking of the Gal/GalNAc lectin leading to increased levels of receptor on the cell surface. This could account for increased galactose-sensitive adhesion after cholesterol loading. Therefore, we quantified surface levels of Hgl, Lgl and Igl before and after cholesterol loading. First, to determine whether biotin labeling and avidin affinity chromatography could be used to measure surface levels of the lectin subunits, wild-type (WT) cells were treated with and without sulfo-NHS-SS-biotin to label surface proteins. The cells were lysed and lysates were subjected to avidin-agarose affinity chromatography. Equivalent fractions of the starting cell lysate and affinity purified protein were analyzed for the lectin subunits or actin (control) by SDS-PAGE, western blotting and densitometry. Western blotting demonstrated that biotinylation did not alter total levels of the subunits or actin (Fig. 5A). Furthermore, biotinylation allowed for the recovery of Hgl, Lgl and Igl by avidin affinity chromatography (Fig. 5A). Actin, a cytoplasmic protein, was not recovered by avidin affinity chromatography, confirming that intracellular proteins were not labeled (Fig. 5A). These data suggest that biotin labeling can be used to assess the surface levels of the lectin subunits.
Fig. 5.
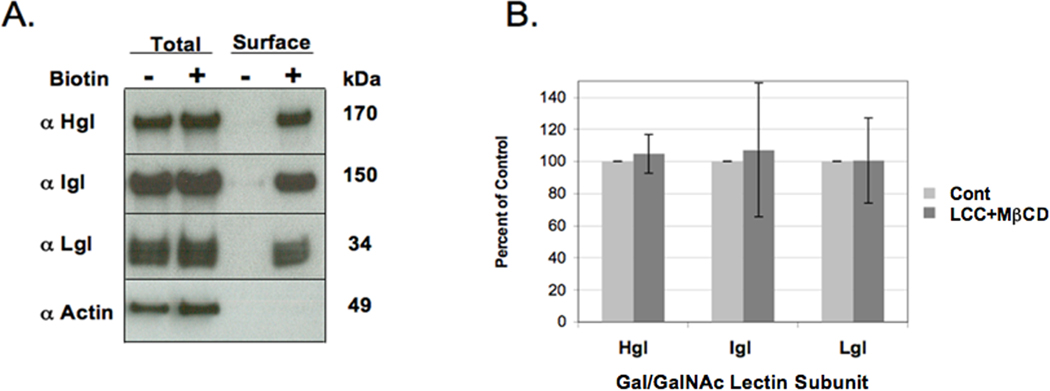
Assessment of cell surface levels of the lectin subunits after cholesterol loading of Entamoeba histolytica. (A) Wildtype E. histolytica cells were incubated in the absence (−) or presence (+) of sulfo-NHS-SS-biotin and lysed. Lysates were subjected to avidin affinity chromatography. Whole cell lysates (Total) and affinity purified protein (Surface) were analyzed by SDS-PAGE and western blotting. Biotinylation did not alter the total levels of heavy (Hgl), light (Lgl), intermediate subunit (Igl) or actin (Total, compare first and second lanes). Only Hgl, Igl, and Lgl were affinity purified after biotinylation (Surface, compare third and fourth lanes). Actin, a control cytoplasmic protein, could not be recovered by affinity chromatography; therefore, it was not biotinylated (Surface, compare third and fourth lanes). (B) Control (Cont) and lipoprotein-cholesterol concentrate (LCC) + methyl-β-cyclodextrin (MβCD)-treated cells were biotinylated and processed as described in Section 2.6. Surface levels of Hgl, Lgl and Igl were quantified by densitometric scans of western blots. The data are presented as a percentage of control cells which was arbitrarily set to 100% for each subunit. The data represent the mean ± S.D. of four trials. Cholesterol loading did not alter the steady state surface levels of the lectin subunits.
Control and cholesterol-loaded cells were subsequently biotinylated and processed.. The levels of surface Hgl, Lgl and Igl were determined by densitometric scanning of western blots. Cholesterol loading had no effect on the steady state surface levels of the lectin (Fig. 5B). These findings suggest that the cholesterol-induced increase in adhesion occurred by a mechanism that does not involve marked externalization of an internal pool of subunits. We cannot rule out the possibility that cholesterol loading increased both externalization and internalization of receptor; however our observation suggests that the specific membrane domain in which the lectin resides may influence its function.
3.4. Depolymerization of actin is not sufficient to alter the buoyancy of Gal/GalNAc lectin subunits
Although the relative level of actin in total DRM increased after cholesterol loading (Fig. 2), sucrose gradient fractionation of DRM showed that the amount of actin remained low in the lipid raft fractions compared with high density fractions (Fig. 3). A large body of evidence supports a role for a dynamic actin cytoskeleton in E. histolytica-host cell interactions (Lopez-Revilla and Cano-Mancera, 1982; Bracha and Mirelman, 1983; Bailey et al., 1990; Arhets et al., 1998; McCoy and Mann, 2005). Thus, it was surprising that Hgl and Lgl were enriched in this low-actin membrane after cholesterol loading and raised the possibility that actin is a negative regulator of Hgl- and/or Lgl-raft interactions. Therefore, we determined whether depolymerization of actin could affect the sub-membrane distribution of the lectin subunits.
We employed CytoD, an actin depolymerizing agent, to reduce the level of F-actin in trophozoites. FITC-phalloidin-staining and fluorescence microscopy of CytoD-treated trophozoites revealed a substantial loss of polymerized actin, supporting the utility of this reagent in this system (Fig. 6). DRM was isolated and characterized from control and CytoD-treated amoebae as described in Section 3.1. After treatment with CytoD, actin was minimally detected in buoyant fractions (Fig. 7D), further supporting the ability of CytoD to disrupt cytoskeletal-membrane interactions in E. histolytica. However, CytoD-treatment, and thus loss of polymerized actin, failed to induce redistribution of any of the lectin subunits (Fig. 7A – C). Therefore, simple loss of actin is not sufficient to permit the association of Hgl and Lgl with lipid rafts or the loss of Igl from lipid rafts.
Fig. 6.
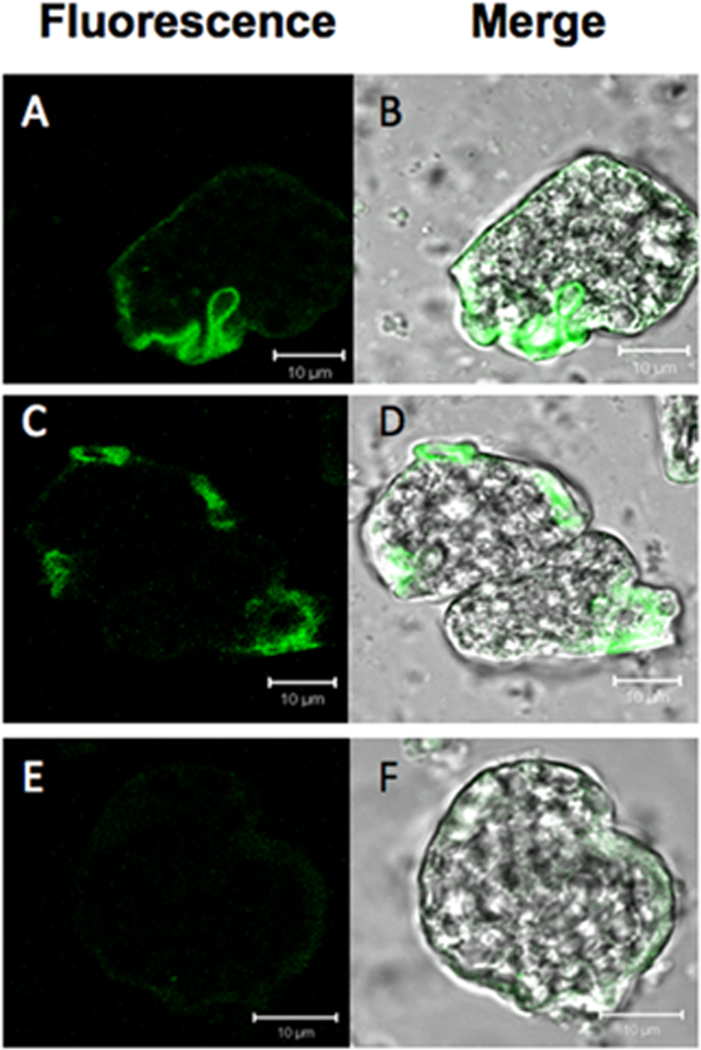
Actin stain of cytochalasin D (CytoD)-treated Entamoeba histolytica trophozoites and untreated control trophozoites. Untreated control amoebae (A,B), amoebae exposed to CytoD diluent (DMSO) (C,D) or 10 µM CytoD (E,F) were stained with Alexa 488 (green)-conjugated phalloidin and visualized by confocal scanning fluorescence microscopy. Both fluorescence (A,C,E) and merged differential interference contrast (DIC) images (B,D,F) are shown. Actin is minimally detected in cells treated with CytoD, indicating that CytoD can affect the depolymerization of actin in E. histolytica. The scale bars represent 10 µm.
Fig. 7.
Actin depolymerization has no effect on the sub-membrane distribution of the Gal/GalNAc lectin subunits. Entamoeba histolytica trophozoites were treated with 10 µM cytochalasin D (CytoD) or an equal volume of diluent control (DMSO). Triton-insoluble membranes were isolated and resolved by sucrose gradient centrifugation. Nineteen fractions and the pellet (20P) were collected and subjected to western blot analyses using antibodies specific for heavy (Hgl) (A), light (Lgl) (B), intermediate subunit (Igl) (C) or actin (D). Mean values of densitometric scans (n = 2), reported as a percentage of total detergent-resistant membrane (DRM)-associated protein, are shown for each subunit and actin. Representative western blots are shown above each panel. In treated cells, actin is only found in the non-buoyant pellet (D), indicating disruption of the cytoskeleton by CytoD. Hgl (A), Lgl (B) and Igl (C) exhibit identical sub-membrane distributions in control (blue line) and treated (red line) cells.
3.5. A genetic model supports a regulatory role for membrane microdomains in Gal/GalNAc function
Rab GTPases are small proteins that cycle between GTP- and GDP-bound states and regulate vesicle and protein trafficking events in eukaryotic cells. In other systems, Rabs can regulate the association of receptors (i.e., integrins) with rafts (Fabbri et al., 2005; Wang et al., 2010). We previously characterized an E. histolytica cell line that conditionally over-expressed a mutant form of a unique Rab, EhRabA, that is constitutively GTP-bound (Welter and Temesvari. 2009). In particular, this transgenic cell line exhibited a reduced ability to bind to unfixed host red blood cells in a galactose- specific manner. To determine whether this adhesion defect extended to this mutant cell line’s ability to respond to cholesterol, we treated cells with LCC or LCC + MβCD and assessed their ability to bind to host epithelial monolayers. Adhesion to host monolayers was not augmented by exposure to LCC and was increased by exposure to LCC + MβCD (Fig. 8A), albeit less dramatically than WT cells (Fig. 4). Importantly, cholesterol loading was efficient in the mutant cell line (Fig. 8B).
Fig. 8.
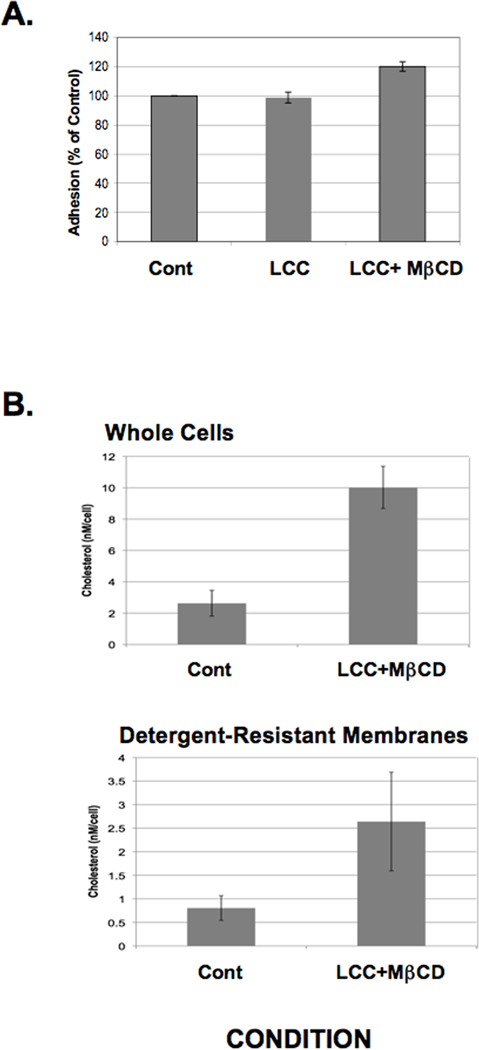
Adhesion of Entamoeba histolytica trophozoites expressing a mutant Rab GTPase is slightly enhanced by cholesterol loading. (A) The ability of lipoprotein-cholesterol concentrate (LCC)- or LCC + methyl-β-cyclodextrin (MβCD)-treated mutant E. histolytica cells to bind to host epithelial cells was assessed as described in Section 2.3. Data are presented as a percentage of untreated control (Cont) which was arbitrarily set to 100%. Cholesterol loading only slightly enhanced adhesion. The data represent the mean ± S.D. of three trials. (B) Trophozoites expressing mutant EhRabA were loaded with cholesterol as described in Section 2.2. Cholesterol levels were measured fluorometrically in whole cell lysates or in detergent-resistant membranes (DRM). The data are presented as the nanomolar quantity of cholesterol normalized to starting cell number. Treatment with a cholesterol source and a carrier (LCC+MβCD) increased cholesterol levels in both whole cell lysates and DRM of the mutant cell line. These data show that it is possible to cholesterol-load this mutant cell line. The data represent the mean ± S.D. of three trials.
We also characterized the sub-membrane distribution of the Gal/GalNAc lectin in untreated and cholesterol-loaded mutant cells. Again, in contrast to WT cells (Figs. 2 and 3), enrichment of lectin subunits in total DRM and enrichment of Hgl and Lgl in rafts of the cholesterol-loaded mutant cells were unremarkable (Fig. 9). These observations support the connection between submembrane localization and function of the Gal/GalNAc lectin. Notably, the mutant cell line was capable of trafficking proteins to rafts, since Igl was enriched in this compartment in the transgenic cell line (Fig. 9B).
Fig. 9.
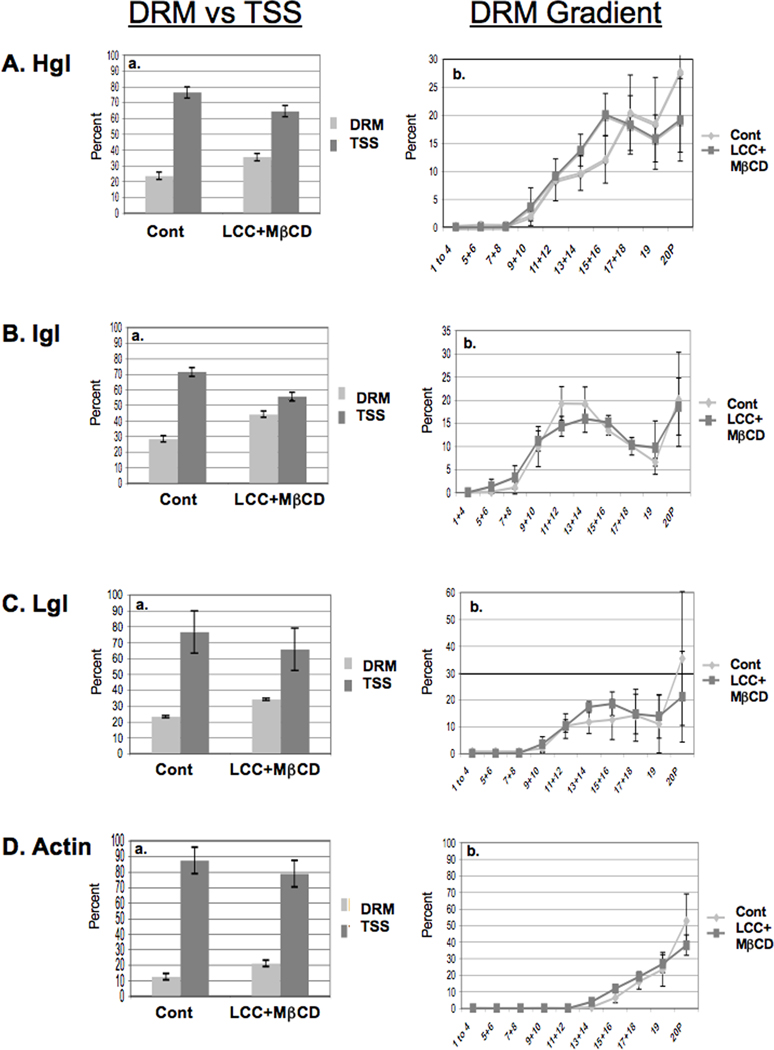
The sub-membrane distribution of lectin subunits are minimally altered by cholesterol loading in Entamoeba histolytica trophozoites expressing a mutant Rab GTPase. (Detergent resistant membrane (DRM) versus triton-soluble supernatant (TSS)) Trophozoites expressing mutant EhRabA were loaded with cholesterol as described in Section 2.2. DRM was isolated by triton extraction. SDS-PAGE and densitometry of western blots were used to quantify heavy (Hgl) (A), intermediate (Igl) (B), light subunit (Lgl) (C) and actin (D) in both DRM and the TSS (Aa, Ba, Ca, Da). Mean values ± S.D. of densitometric scans (n = 3) are reported as a percentage of the total (DRM + TSS). Subunits of the Gal/GalNAc lectin and actin were minimally enriched in DRM after cholesterol loading. DRM was resolved by sucrose gradient density centrifugation. Nineteen fractions and the pellet (20P) were collected and subjected to western blot analyses using antibodies specific for Hgl (Ab), Lgl (Bb), Igl (Cb) or actin (Db). Mean values of densitometric scans (n=3), reported as a percentage of total DRM-associated protein, are shown for each subunit and actin. Hgl and Lgl were minimally enriched in rafts after cholesterol-loading. Cont, control; LCC + MβCD, lipoprotein-cholesterol concentrate + methyl-β-cyclodextrin.
Previously, immunofluorescence microscopy showed that a fraction of Hgl and Lgl was mislocalized to an endoplasmic reticulum (ER)-like compartment in the mutant cell line (Welter and Temesvari, 2009). To obtain an accurate measure of the cell surface levels of the lectin subunits on the mutant cell line, we used biotinylation as described in Section 3.3. Despite adhesion defects (Welter and Temesvari, 2009; this study), the surface levels of the lectin subunits were significantly higher in the mutant cell line than those in the WT cells (Fig. 10).
Fig. 10.
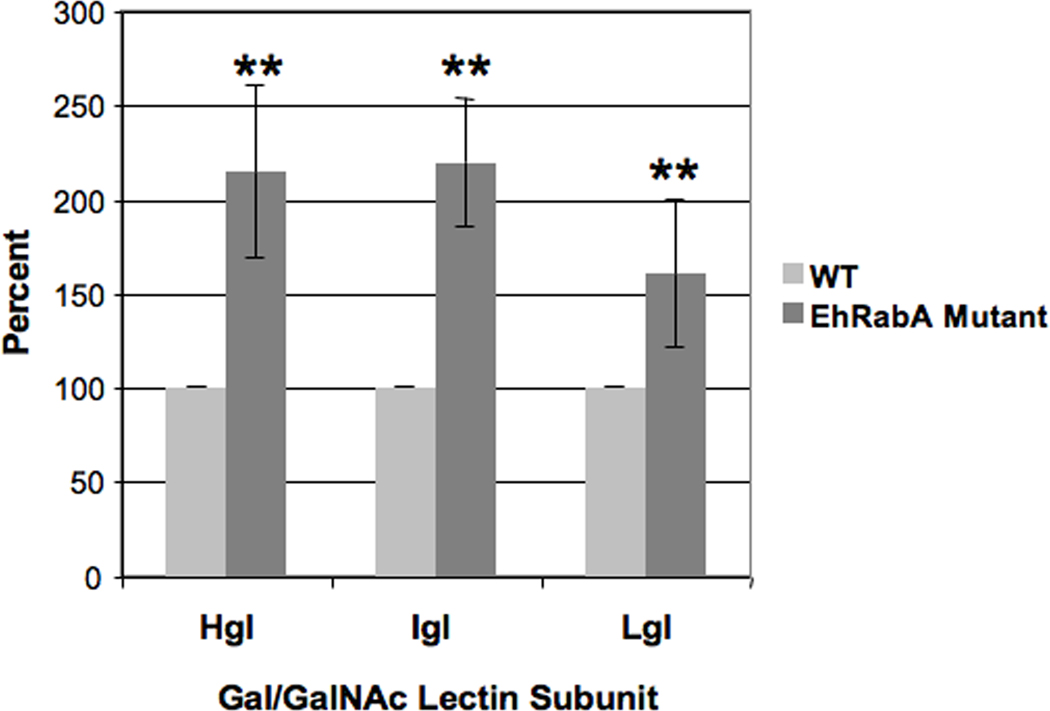
Assessment of cell surface levels of the lectin subunits on Entamoeba histolytica trophozoites expressing a mutant Rab GTPase. Wild-type E. histolytica cells (WT) and cells expressing GTP-bound EhRabA (EhRabA mutant) were incubated with sulfo-NHS-SS-biotin on ice, lysed and subjected to avidin affinity chromatography. Precipitated proteins were characterized by SDS-PAGE, western blot and densitometry. The data are presented as the amount of subunit on the cell surface as a percentage of WT control which was arbitrarily set to 100%. The mutant cell line exhibited significantly higher levels of heavy (Hgl), intermediate (Igl) or light subunit (Lgl) (**P < 0.01) (n = 3).
4. Discussion
The principal finding of this study was that cholesterol loading of E. histolytica trophozoites resulted in enrichment of the Hgl and Lgl of the Gal/GalNAc lectin in low density rafts possessing the Igl. Furthermore, the co-compartmentalization of the subunits in rafts was accompanied by an increase in the ability of the parasite to adhere to host cells in a galactose-sensitive manner. This correlation between adhesion and the sub-membrane distribution of the lectin subunits supports a role for membrane microdomains in regulating the function of this important virulence factor. Interestingly, a mutant cell line that was incapable of responding to cholesterol loading with a substantial increase in adhesion was also incapable of Hgl and Lgl raft enrichment under the same condition. This further supports the correlation between sub-membrane position and Gal/GalNAc function.
The importance of lateral heterogeneity in biological membranes has been highlighted in many systems and it is well-established that the lipids surrounding membrane proteins can modulate their function. For example, cholesterol-loaded neutrophils exhibit enhanced interaction with P-selectin coated surfaces and with endothelial monolayers (Oh et al., 2009). Furthermore, cholesterol can modulate the function of the human oxytocin receptor (Reversi et al., 2006), the human neuropeptide (galanin) receptor (Pang et al., 1999) and the bovine serotonin 1A receptor (Pucadyil and Chattopadhyay, 2004). The Drosophila metabotropic glutamate receptor (mGluR) exists as a low-affinity receptor when localized to non-raft membrane and a high-affinity receptor when localized to sterol-rich lipid rafts (Eroglu et al., 2003). Enrichment of membranes with cholesterol shifts the mGluR receptor into the high-affinity raft-associated state. Our data similarly show that an increase in sterol richness enhances raft-association of Hgl and Lgl and may act as a positive regulator of Gal/GalNAc lectin function.
The use of fixed cells as a model host epithelium guaranteed that the effects of cholesterol were restricted to parasite membranes. However, it has been established that fixation can expose phosphatidylserine (PS) on host cell membranes (Wong et al., 2006). PS has been shown to be one of the ligands used by E. histolytica to interact with the host (Boettner et al., 2005); however an E. histolytica PS receptor has not been identified (Sateriale and Huston, 2011). Our data suggest that the increase in adhesion that occurred after cholesterol loading relied substantially on the Gal/GalNAc lectin and not on a putative PS receptor, since this increase could be nearly completely abrogated in the presence of galactose.
Unlike Hgl and Lgl, the localization of Igl was not affected by cholesterol loading; the majority of the subunit remained constitutively associated with raft-like domains under all conditions. Although we cannot rule out that other conditions alter Igl-raft interaction, our observations show that the molecular mechanisms regulating the trafficking and localization of Igl differ from those regulating the localization of Hgl and Lgl. This is supported by another report demonstrating that expression of a constitutively GTP-bound Rab GTPase or treatment with Brefeldin A, a Golgi disruptor, alters the subcellular localization of Hgl and Lgl but not of Igl (Welter and Temesvari. 2009).
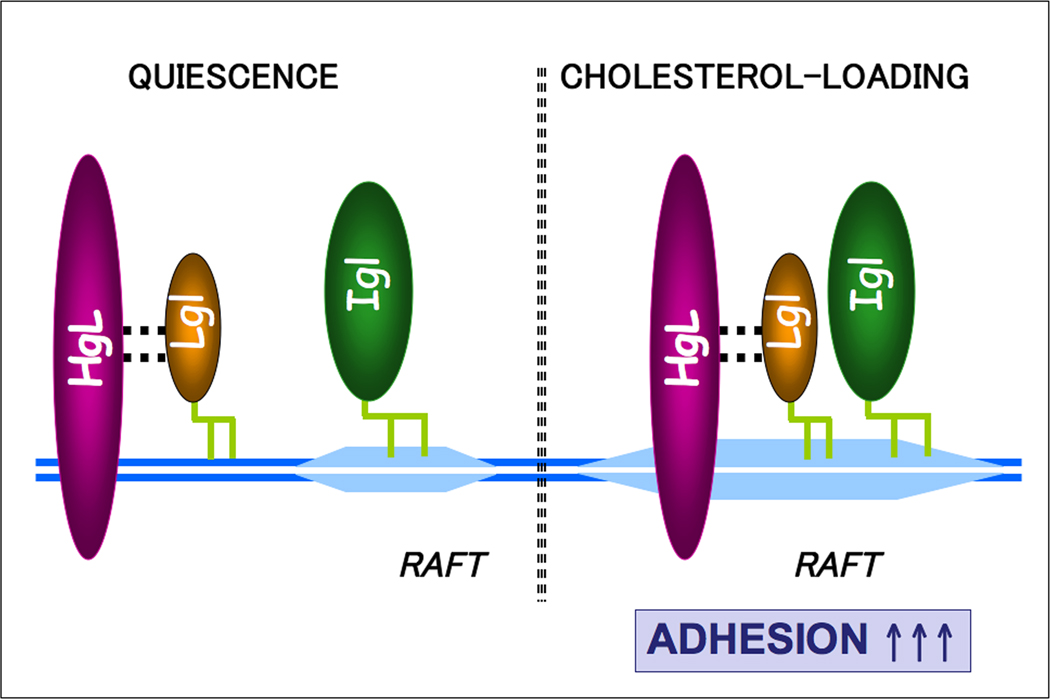
Based on our findings, an intriguing model of Gal/GalNAc function may be proposed. Perhaps in a quiescent state, GPI-anchored Igl subunits predominantly reside in raft-like domains whereas Hgl-Lgl dimers predominantly reside in a different sub-membrane compartment. Subsequent activation events (e.g., cholesterol loading) stimulate the recruitment of Hgl-Lgl dimers into raft-like domains (Fig. 11). Although our data do not indicate whether Igl interacts physically with Hgl-Lgl dimers in rafts, co-localization of the subunits in these domains may assist in transforming the lectin into a functional adhesin.
Fig. 11.
Model of Entamoeba histolytica Gal/GalNAc lectin assembly. Glycosylphosphatidylinisotol (GPI)-anchored intermediate subunits (Igl) predominantly reside in raft-like domains whereas the heavy-light subunit (Hgl-Lgl) dimer predominantly resides in different sub-membrane compartments. Subsequent activation events such as cholesterol loading stimulate the recruitment of Hgl-Lgl dimers into the raft-like domains. Co-localization of the three subunits in rafts may assist in transforming the lectin into a functional adhesin. This is supported by the observation that galactose-sensitive adhesion is increased after cholesterol loading.
Biotinylation of cell surface proteins revealed that there was no significant increase in the level of cell surface lectin after cholesterol loading. Thus, it is possible that Hgl and Lgl move laterally into rafts after exposure to cholesterol. Indeed, simple translocation of subunits between membrane domains on the cell surface would represent a rapid method of controlling Gal/GalNAc lectin function because it would not depend on vesicle trafficking, protein synthesis or protein turnover. Nonetheless, we cannot rule out that both externalization and internalization (by endocytosis) of the Gal/GalNAc lectin were increased by cholesterol loading. Comparable increases in both inside-out and outside-in trafficking would not have been detected by biotinylation which measures steady state levels of surface protein. Importantly, the data demonstrate that the parasite can achieve increased galactose-sensitive adhesion without increasing the net levels of the lectin subunits on the surface. Enrichment of Hgl and Lgl in rafts may be the mechanism by which this is accomplished.
The mutant cell line, which did not respond to cholesterol loading with a dramatic increase in adhesion or with Hgl- and Lgl-raft enrichment, exhibited significantly higher levels of the subunits on the surface compared with control cells. Previously, using immunofluorescence microscopy, it was shown that Hgl and Lgl were mislocalized to an ER-like compartment in the mutant cell line (Welter and Temesvari, 2009). Although this seems contradictory, it is possible that the high levels of subunits in the ER confounded previous microscopic interpretation of plasma membrane levels. This would not be a short-coming of the biotinylation technique used in the current study to specifically assess cell surface levels. The exact molecular defect in the transgenic cell line is unknown. EhRabA might directly facilitate plasma-membrane delivery of vesicles containing lectin subunits. Increased surface levels observed in the mutant cell line expressing a GTP-bound EhRabA (this study) support this notion. EhRabA might also facilitate the delivery of other raft components which, in turn, regulate the response of the subunits to cholesterol loading. In either case, our observations suggest that regardless of the amount of lectin subunit on the surface, enrichment in DRM and lipid rafts may be necessary for efficient binding to ligands. In other words, sub-membrane location might be more important than cell surface levels in the regulation of Gal/GalNAc lectin function.
Others have shown that it is possible to alter parasite adhesiveness without altering the surface levels of the Gal/GalNAc lectin subunits. For example, over-expression of the intracellular (C-terminal) domain of Hgl isoforms, Hgl2 (Tavares et al., 2005) and Hgl3 (Vines et al., 1998), can inhibit adhesion to host cells. Trophozoites expressing the Hgl2 C-terminal peptide also exhibit altered motility (Coudrier et al., 2005). When used to infect hamster livers, this same transgenic cell line induces a different host pathophysiology characterized by a reduced host inflammatory response (Blazquez et al., 2007). The dominant negative activity of these C-terminally-derived peptides reveals the importance of downstream signaling by Hgl or downstream interactions of Hgl with other proteins. It is possible that proper localization to raft domains facilitate these downstream activities. Monitoring the submembrane localization of the lectin subunits in cells expressing Hgl dominant-negative acting peptides will provide insight into this question.
The relationship between cholesterol levels and virulence in E. histolytica is intriguing. First, the most common site of extra-intestinal infection is the liver, an organ rich in cholesterol. Second, patients who are cyst passers have lower levels of serum cholesterol than those with ALA (Bansal et al., 2005). Third, the virulence of laboratory grown amoebae can be maintained by periodic passage through hamster livers (Neal and Vincent, 1956) or by axenic culture in cholesterol-supplemented media (Sharma, 1959; Singh et al., 1971; Das and Ghoshal, 1976; Bos and van de Griend, 1977; Katiyar et al., 1987; Serrano-Luna et al., 2010). For example, a recent study by Serrano-Luna et al. (2010) showed that long-term culture in media supplemented with liposomes consisting of phosphatidylcholine and cholesterol preserved virulence as measured by ALA formation. Serrano-Luna et al. (2010) and others (Saxena et al., 1986; Katiyar et al., 1987) have also shown that exposure to cholesterol can enhance phagocytosis, an important virulence function that relies on Gal/GalNAc function (Vines et al., 1998). It is not our intention to oversimplify the role of cholesterol in E. histolytica virulence; however, control of lectin-raft interaction by cholesterol may represent a cellular mechanism that contributes to virulence.
In T cells, integrins such as lymphocyte associated antigen 1 (LFA-1) and α4β1, are restricted from associating with rafts by the cytoskeleton; depolymerization of F-actin by treatment with CytoD permits their movement into these microdomains (Leitinger and Hogg, 2002). On the other hand, depolymerization of actin in myoblasts prevents the association of N-cadherin, another adhesion molecule, with detergent-resistant rafts (Causeret et al., 2005). Therefore, actin can serve as a negative or positive regulator of protein-raft interaction in a cell-specific manner. In the current study, depolymerization of actin was not sufficient to increase the association of Hgl or Lgl with raft-like fractions. Thus, intact actin does not appear to behave as a negative regulator of Hgl or Lgl raft engagement in E. histolytica. Importantly, our data do not rule out the possibility that actin is a positive regulator lectin-membrane interactions.
Since Hgl and Lgl are thought to be covalently attached through disulfide bridges (Petri et al., 2002), it was not surprising that both subunits were found in lipid rafts after cholesterol loading. In other systems, both transmembrane and GPI-anchored proteins localize to rafts. In E. histolytica, it is not known what regulates the affinity of the Gal/GalNAc subunits for rafts and it remains to be seen whether ligand binding by the lectin also influences the submembrane distribution of the Hgl and Lgl. It is also not known how cholesterol or lipid rafts modulate lectin function. It is possible that localization to rafts induces conformational changes in Hgl and/or Lgl which positively regulates function. It is also possible that lipid and other protein constituents of rafts exercise a stimulatory effect on the resident lectin subunits. In either case, mutagenic studies of the lectin subunits will be necessary to discover specific protein domains or specific post-translational modifications regulating function and the affinity of the lectin for various membrane domains under various conditions.
In conclusion, we believe our findings are the first known to demonstrate a correlation between the function of the Gal/GalNAc lectin and the sub-membrane localization of its subunits. Therefore, this study provides significant insight into the molecular mechanisms regulating this important adhesion and signaling molecule, and thus provides insight into pathogenicity of this global pathogen.
Acknowledgments
The authors thank W. A. Petri, Jr. (University of Virginia Health Sciences Center, Charlottesville, VA, USA), for antibodies specific to the Gal/GalNAc lectin subunits. The authors acknowledge the assistance of the Jordan Hall Imaging Facility (JHIF) of Clemson University, USA for fluorescence microscopy. The authors acknowledge Mr. David Welter (Auburn University, Auburn, AL, USA) for artwork associated with the graphical abstract. The project described was supported by grant no. R01AI046414 from the National Institute of Allergy and Infectious Diseases, USA to L.A.T. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This material is based upon work supported by the National Institute of Food and Agriculture/United States Department of Agriculture (NIFA/USDA), under project number SC-1700312 (Technical Contribution No. 5844 of the Clemson University Experiment Station). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, or the USDA. The authors thank the members of the Temesvari laboratory for critical reading of the manuscript and helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arhets P, Olivo JC, Gounon P, Sansonetti P, Guillen N. Virulence and functions of myosin II are inhibited by overexpression of light meromyosin in Entamoeba histolytica. Mol. Biol. Cell. 1998;9:1537–1547. doi: 10.1091/mbc.9.6.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atger VM, de la Llera Moya M, Stoudt GW, Rodrigueza WV, Phillips MC, Rothblat GH. Cyclodextrins as catalysts for the removal of cholesterol from macrophage foam cells. J. Clin. Invest. 1997;99:773–780. doi: 10.1172/JCI119223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey GB, Nudelman ED, Day DB, Harper CF, Gilmour JR. Specificity of glycosphingolipid recognition by Entamoeba histolytica trophozoites. Infect. Immun. 1990;58:43–47. doi: 10.1128/iai.58.1.43-47.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal D, Bhatti HS, Sehgal R. Altered lipid parameters in patients infected with Entamoeba histolytica, Entamoeba dispar and Giardia lamblia. Br. J. Biomed. Sci. 2005;62:63–65. doi: 10.1080/09674845.2005.11732686. [DOI] [PubMed] [Google Scholar]
- Boettner DR, Huston CD, Linford AS, Buss SN, Houpt E, Sherman NE, Petri WA., Jr Entamoeba histolytica phagocytosis of human erythrocytes involves PATMK, a member of the transmembrane kinase family. PLoS Pathog. 2008;4:e8. doi: 10.1371/journal.ppat.0040008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettner DR, Huston CD, Sullivan JA, Petri WA., Jr Entamoeba histolytica and Entamoeba dispar utilize externalized phosphatidylserine for recognition and phagocytosis of erythrocytes. Infect. Immun. 2005;73:3422–3430. doi: 10.1128/IAI.73.6.3422-3430.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos HJ, van de Griend RJ. Virulence and toxicity of axenic Entamoeba histolytica. Nature. 1977;265:341–343. doi: 10.1038/265341a0. [DOI] [PubMed] [Google Scholar]
- Bracha R, Mirelman D. Adherence and ingestion of Escherichia coli serotype 055 by trophozoites of Entamoeba histolytica. Infect. Immun. 1983;40:882–887. doi: 10.1128/iai.40.3.882-887.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazquez S, Rigothier M-C, Huerre M, Guillén N. Initiation of inflammation and cell death during liver abscess formation by Entamoeba histolytica depends on activity of the galactose/N-acetyl-D-galactosamine lectin. Int. J. Parasitol. 2007;37:425–433. doi: 10.1016/j.ijpara.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Brown DA. Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology. 2006;21:430–439. doi: 10.1152/physiol.00032.2006. [DOI] [PubMed] [Google Scholar]
- Buss SN, Hamano S, Vidrich A, Evans C, Zhang Y, Crasta OR, Sobral BW, Gilchrist CA, Petri WA., Jr Members of the Entamoeba histolytica transmembrane kinase family play non-redundant roles in growth and phagocytosis. Int. J. Parasitol. 2010;40:833–843. doi: 10.1016/j.ijpara.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causeret M, Taulet N, Comunale F, Favard C, Gauthier-Rouviere C. N-cadherin association with lipid rafts regulates its dynamic assembly at cell-cell junctions in C2C12 myoblasts. Mol. Biol. Cell. 2005;16:2168–2180. doi: 10.1091/mbc.E04-09-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng XJ, Hughes MA, Huston CD, Loftus B, Gilchrist CA, Lockhart LA, Ghosh S, Miller-Sims V, Mann BJ, Petri WA, Jr, Tachibana H. Intermediate subunit of the Gal/GalNAc lectin of Entamoeba histolytica is a member of a gene family containing multiple CXXC sequence motifs. Infect. Immun. 2001;69:5892–5898. doi: 10.1128/IAI.69.9.5892-5898.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng XJ, Tsukamoto H, Kaneda Y, Tachibana H. Identification of the 150-kDa surface antigen of Entamoeba histolytica as a galactose- and N-acetyl-D-galactosamine-inhibitable lectin. Parasitol. Res. 1998;84:632–639. doi: 10.1007/s004360050462. [DOI] [PubMed] [Google Scholar]
- Coudrier E, Amblard F, Zimmer C, Roux P, Olive-Marin J-C, Rigothier M-C, Guillén N. Myosin II and the Gal-GalNAc lectin play a crucial role in tissue invasion by Entamoeba histolytica. Cell. Microbiol. 2005;7:19–27. doi: 10.1111/j.1462-5822.2004.00426.x. [DOI] [PubMed] [Google Scholar]
- Das SR, Ghoshal S. Restoration of virulence to rat of axenically grown Entamoeba histolytica by cholesterol and hamster liver passage. Ann. Trop. Med. Parasitol. 1976;70:439–443. doi: 10.1080/00034983.1976.11687144. [DOI] [PubMed] [Google Scholar]
- Diamond LS, Harlow DR, Cunnick CC. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans. R. Soc. Trop. Med. Hyg. 1978;72:431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- Dodson JM, Lenkowski PW, Jr, Eubanks AC, Jackson TF, Napodano J, Lyerly DM, Lockhart LA, Mann BJ, Petri WA., Jr Infection and immunity mediated by the carbohydrate recognition domain of the Entamoeba histolytica Gal/GalNAc lectin. J. Infect. Dis. 1999;179:460–466. doi: 10.1086/314610. [DOI] [PubMed] [Google Scholar]
- Eroglu C, Brugger B, Wieland F, Sinning I. Glutamate-binding affinity of Drosophila metabotropic glutamate receptor is modulated by association with lipid rafts. Proc. Natl. Acad. Sci. U.S.A. 2003;100:10219–10224. doi: 10.1073/pnas.1737042100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri M, Di Meglio S, Gagliani MC, Consonni E, Molteni R, Bender JR, Tacchetti C, Pardi R. Dynamic partitioning into lipid rafts controls the endo- exocytic cycle of the αL/β2 integrin, LFA-1, during leukocyte chemotaxis. Mol. Biol. Cell. 2005;16:5793–5803. doi: 10.1091/mbc.E05-05-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S, August A. Actin depolymerization transduces the strength of B-cell receptor stimulation. Mol. Biol. Cell. 2005;16:2275–2284. doi: 10.1091/mbc.E04-10-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar SK, Prasad AK, Ghoshal S, Das SR, Sagar P. Cholesterol induced changes in glucose-6-phosphate generating enzymes, concanavalin A agglutinability and haemolytic activity of axenic Entamoeba histolytica. Ann. Trop. Med. Parasitol. 1987;81:201–205. doi: 10.1080/00034983.1987.11812113. [DOI] [PubMed] [Google Scholar]
- Laughlin RC, McGugan GC, Powell RR, Welter BH, Temesvari LA. Involvement of raft-like plasma membrane domains of Entamoeba histolytica in pinocytosis and adhesion. Infect. Immun. 2004;72:5349–5357. doi: 10.1128/IAI.72.9.5349-5357.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin RC, Temesvari LA. Cellular and molecular mechanisms that underlie Entamoeba histolytica pathogenesis: prospects for intervention. Expert Rev. Mol. Med. 2005;7:1–19. doi: 10.1017/S1462399405009622. [DOI] [PubMed] [Google Scholar]
- Leitinger B, Hogg N. The involvement of lipid rafts in the regulation of integrin function. J. Cell. Sci. 2002;115:963–972. doi: 10.1242/jcs.115.5.963. [DOI] [PubMed] [Google Scholar]
- Lopez-Revilla R, Cano-Mancera R. Adhesion of Entamoeba histolytica trophozoites to human erythrocytes. Infect. Immun. 1982;37:281–285. doi: 10.1128/iai.37.1.281-285.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata-Cardenas BD, Vargas-Villarreal J, Gonzalez-Salazar F, Martinez-Rodriguez H, Morales-Vallarta M, Said-Fernandez S. Entamoeba histolytica is unable to use free cholesterol, phospholipids, and fatty acids under axenic cultivation conditions. Arch. Med. Res. 2000;31:S212–S213. doi: 10.1016/s0188-4409(00)00192-2. [DOI] [PubMed] [Google Scholar]
- McCoy JJ, Mann BJ. Proteomic analysis of Gal/GalNAc lectin-associated proteins in Entamoeba histolytica. Exp. Parasitol. 2005;110:220–225. doi: 10.1016/j.exppara.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Mittal K, Welter BH, Temesvari LA. Entamoeba histolytica: Lipid rafts are involved in adhesion of trophozoites to host extracellular matrix components. Exp. Parasitol. 2008;120:127–153. doi: 10.1016/j.exppara.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal RA, Vincent P. Strain variation in Entamoeba histolytica. II. The effect of serial liver passage on the virulence. Parasitology. 1956;46:173–182. [PubMed] [Google Scholar]
- Oh H, Mohler ER, 3rd, Tian A, Baumgart T, Diamond SL. Membrane cholesterol is a biomechanical regulator of neutrophil adhesion. Arterioscler. Thromb. Vasc. Biol. 2009;29:1290–1297. doi: 10.1161/ATVBAHA.109.189571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang L, Graziano M, Wang S. Membrane cholesterol modulates galanin-GalR2 interaction. Biochemistry. 1999;38:12003–12011. doi: 10.1021/bi990227a. [DOI] [PubMed] [Google Scholar]
- Petri WA, Jr, Chapman MD, Snodgrass T, Mann BJ, Broman J, Ravdin JI. Subunit structure of the galactose and N-acetyl-D-galactosamine-inhibitable adherence lectin of Entamoeba histolytica. J. Biol. Chem. 1989;264:3007–3012. [PubMed] [Google Scholar]
- Petri WA, Jr, Haque R, Mann BJ. The bittersweet interface of parasite and host: lectin-carbohydrate interactions during human invasion by the parasite Entamoeba histolytica. Annu. Rev. Microbiol. 2002;56:39–64. doi: 10.1146/annurev.micro.56.012302.160959. [DOI] [PubMed] [Google Scholar]
- Powell RR, Temesvari LA. Involvement of a Rab8-like protein of Dictyostelium discoideum, Sas1, in the formation of membrane extensions, secretion and adhesion during development. Microbiology. 2004;150:2513–2525. doi: 10.1099/mic.0.27073-0. [DOI] [PubMed] [Google Scholar]
- Powell RR, Welter BH, Hwu R, Bowersox B, Attaway C, Temesvari LA. Entamoeba histolytica: FYVE-finger domains, phosphatidylinositol 3-phosphate biosensors, associate with phagosomes but not fluid filled endosomes. Exp. Parasitol. 2006;112:221–231. doi: 10.1016/j.exppara.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Pucadyil TJ, Chattopadhyay A. Cholesterol modulates ligand binding and G- protein coupling to serotonin(1A) receptors from bovine hippocampus. Biochim. Biophys. Acta. 2004;1663:188–200. doi: 10.1016/j.bbamem.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Ravdin JI, Guerrant RL. Role of adherence in cytopathogenic mechanisms of Entamoeba histolytica. Study with mammalian tissue culture cells and human erythrocytes. J. Clin. Invest. 1981;68:1305–1313. doi: 10.1172/JCI110377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reversi A, Rimoldi V, Brambillasca S, Chini B. Effects of cholesterol manipulation on the signaling of the human oxytocin receptor. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R861–R869. doi: 10.1152/ajpregu.00333.2006. [DOI] [PubMed] [Google Scholar]
- Sateriale A, Huston CD. A Sequential Model of Host Cell Killing and Phagocytosis by Entamoeba histolytica. J. Parasitol. Res. 2011;2011:926706. doi: 10.1155/2011/926706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena A, Kaul D, Vinayak VK. Amoebic erythrophagocytosis: signficance of membrane cholesterol to phospholipid ratio. Int. Res. Commun. Syst. Med. Sci. 1986;14:330–331. [Google Scholar]
- Serrano-Luna J, Gutierrez-Meza M, Mejia-Zepeda R, Galindo-Gomez S, Tsutsumi V, Shibayama M. Effect of phosphatidylcholine-cholesterol liposomes on Entamoeba histolytica virulence. Can. J. Microbiol. 2010;56:987–995. doi: 10.1139/W10-088. [DOI] [PubMed] [Google Scholar]
- Sharma R. Effect of cholesterol on the growth and virulence of Entamoeba histolytica. Trans. R. Soc. Trop. Med. Hyg. 1959;53:278–281. doi: 10.1016/0035-9203(59)90008-2. [DOI] [PubMed] [Google Scholar]
- Singh BN, Srivastava RV, Dutta GP. Virulence of strains of Entamoeba histolytica to rats and the effect of cholesterol, rat caecal and hamster liver passage on the virulence of non-invasive strains. Indian. J. Exp. Biol. 1971;9:21–27. [PubMed] [Google Scholar]
- Tavares P, Rigothier M-C, Khun H, Roux P, Huerre M, Guillén N. Roles of cell adhesion and cytoskeleton activity in Entamoeba histolytica pathogenesis: a delicate balance. Infect. Immun. 2005;73:1771–1778. doi: 10.1128/IAI.73.3.1771-1778.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valensin S, Paccani SR, Ulivieri C, Mercati D, Pacini S, Patrussi L, Hirst T, Lupetti P, Baldari CT. F-actin dynamics control segregation of the TCR signaling cascade to clustered lipid rafts. Eur. J. Immunol. 2002;32:435–446. doi: 10.1002/1521-4141(200202)32:2<435::AID-IMMU435>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Vines RR, Ramakrishnan G, Rogers JB, Lockhart LA, Mann BJ, Petri WA., Jr Regulation of adherence and virulence by the Entamoeba histolytica lectin cytoplasmic domain, which contains a β2 integrin motif. Mol. Biol. Cell. 1998;9:2069–2079. doi: 10.1091/mbc.9.8.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Yoo Y, Fan H, Kim E, Guan KL, Guan JL. Regulation of Integrin β1 recycling to lipid rafts by Rab1a to promote cell migration. J. Biol. Chem. 2010;285:29398–29405. doi: 10.1074/jbc.M110.141440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welter BH, Powell RR, Leo M, Smith CM, Temesvari LA. A unique Rab GTPase, EhRabA, is involved in motility and polarization of Entamoeba histolytica cells. Mol. Biochem. Parasitol. 2005;140:161–173. doi: 10.1016/j.molbiopara.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Welter BH, Temesvari LA. Overexpression of a mutant form of EhRabA, a unique Rab GTPase of Entamoeba histolytica, alters endoplasmic reticulum morphology and localization of the Gal/GalNAc adherence lectin. Eukaryot. Cell. 2009;8:1014–1026. doi: 10.1128/EC.00030-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K, Li X, Ma Y. Paraformaldehyde induces elevation of intracellular calcium and phosphatidylserine externalization in platelets. Thromb. Res. 2006;117:537–542. doi: 10.1016/j.thromres.2005.04.030. [DOI] [PubMed] [Google Scholar]