Abstract
The Association for European Paediatric Cardiology, the Society of Thoracic Surgeons, and the European Association for Cardiothoracic Surgery, have recently published detailed hierarchical listings for the description of ventricular septal defects. This review details the anatomic basis for the European codes, illustrating the phenotypic features of the various holes that can be described as perimembranous ventricular septal defects.
MeSH: Heart septal defects, ventricular, Heart defects, congenital, Heart ventricle/pathology, Heart ventricles/abnormalities
Introduction
There is currently a significant new impetus in the drive to create a generally acceptable nomenclature to underscore the creation of databases used for the collection and validation of results in the treatment of congenital cardiac malformations. Thus, the Association for European Paediatric Cardiology has published its short and long lists, along with accompanying commentaries.1–3 The Society of Thoracic Surgeons, together with the European Association for Cardiothoracic Surgery, have now published an extensive supplement, containing a short surgical code and companion of detailed hierarchical listings.4 To move forward, and to achieve the international consensus desired by all, it is essential that the potential discrepancies between these listings be resolved. Most of the differences are semantic, and can be overcome by the simple process of cross-mapping. But some differences are more profound, and potentially reflect varying interpretations of the morphology of malformations. It is possible that interventricular communications fall into this latter category. If we are to resolve such potential scientific disagreements, then it is essential that those offering definitions do so on the basis of clear anatomic descriptions for the phenotypes of the different holes considered to require distinction. In this review, and a subsequent article, we will illustrate the features which are used to distinguish the entities categorised within the European classification,2–3 emphasising those differences which might exist in comparison to the surgical hierarchies.4 In this initial discussion, we will concentrate on the defect we define as being perimembranous. But first of all, we must explain what we perceive to be the defect.
What is a ventricular septal defect?
The problems which exist cannot be resolved without first deciding on the boundaries of the space to be defined as the “septal defect”. When there is a simple hole within the muscular septum separating the right and left ventricular cavities (Fig. 1), then there is no problem.
Figure 1.
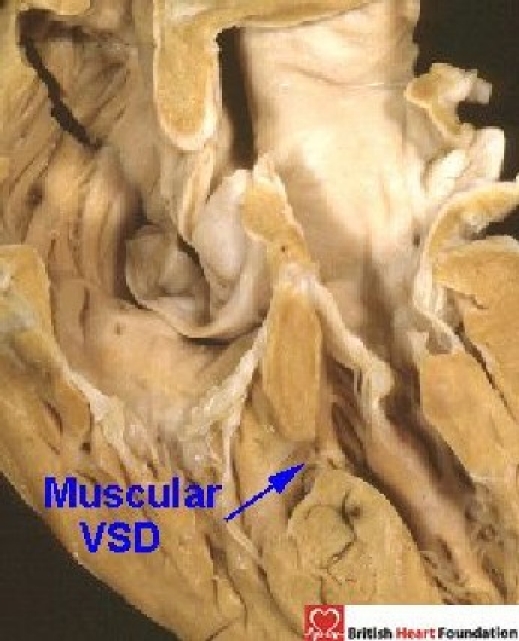
Four-chamber section showing communication between the two ventricles through a muscular ventricular septal defect (VSD)
Difficulties arise when the orifice of an arterial valve overrides the crest of the muscular ventricular septum (Fig. 2).
Figure 2.
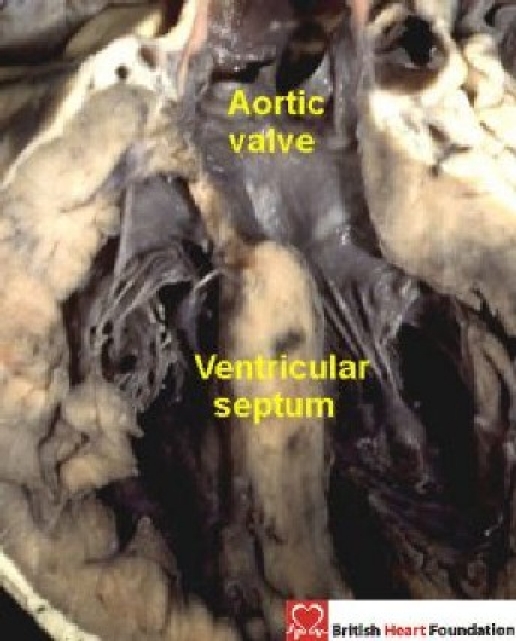
Overriding of the muscular ventricular septum by the orifice of the aortic valve
In this setting, some might define the upward extension of the plane of the long axis of the ventricular septum as representing the septal defect (Fig. 3).
Figure 3.
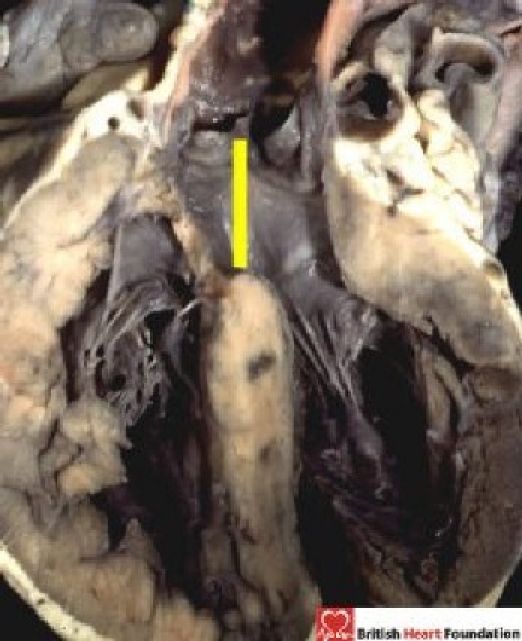
The plane of the long axis of the muscular ventricular septum extends up into the arterial outflow tract, and reaches to the leaflets of the aortic valve
Such a plane certainly represents the location of the interventricular communication but, in the heart illustrated, the leaflets of the aortic valve form the roof of the defined defect. No surgeon would place a patch on this location to restore ventricular septal integrity. In fact, in the heart illustrated, the surgeon would place the patch so as to reconnect the aorta with the left ventricle. Thus, for the surgeon, it is the right ventricular margin of the cone of space subtended by the leaflets of the overriding valve that is the locus of interest (Fig. 4).
Figure 4.
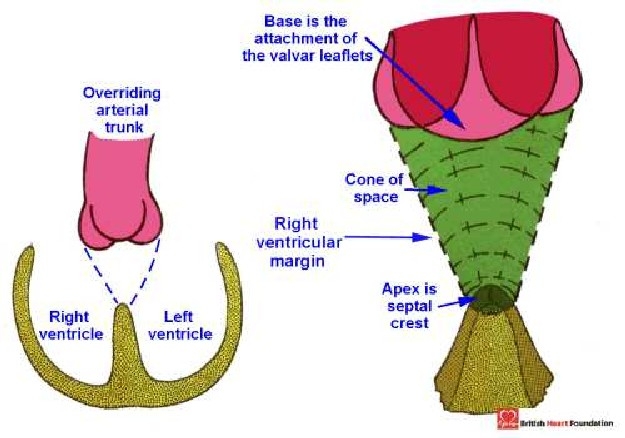
Diagrammatic representation of the cone of space found between the crest of the muscular ventricular septum and the leaflets of the overriding aortic valve
Because of the surgical focus on this plane, this is the space that we define as THE septal defect. At the same time, we recognise that the left ventricular margin of the cone of space is also important as the outflow tract from the left ventricle (Fig. 5.)
Figure 5.
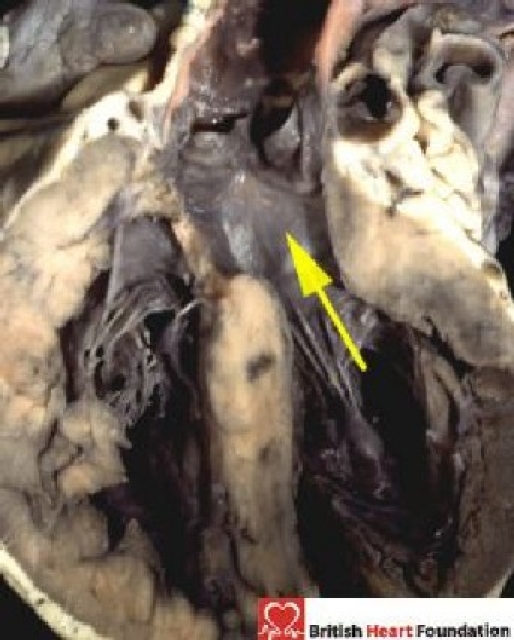
The leftward margin of the interventricular cone of space is the outflow tract of the left ventricle
These differences in definition of the hole chosen to represent the defect have important consequences for categorisation. Consider again the situation in which the extension of the long axis of the ventricular septum is taken as the defect. The postero-inferior rim of this particular plane is made up of fibrous continuity between the leaflets of the aortic and mitral valves (Fig. 6 below). As we will see, it is fibrous continuity between the aortic valve and an atrioventricular valve that we use as our defining feature for a certain set of septal defects.
Figure 6.
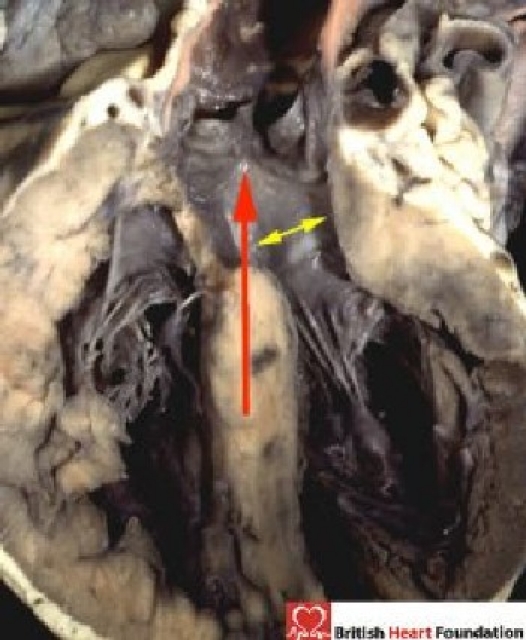
The outflow tract of the left ventricle is roofed by fibrous continuity between the leaflets of the mitral and aortic valves (yellow arrow). The long axis of the ventricular septum extends up into the aortic outflow tract (red arrow)
But, for us, the significant atrioventricular valve for the purposes of categorisation is the tricuspid valve. The reason we choose the tricuspid valve is shown when we study again our illustrated heart with overriding of the aortic valve. In the chosen specimen (Fig. 7), a muscular bar interposes between the aortic and tricuspid valves. The presence of this muscular ledge between the edge of the defect and the fibrous membranous septum protects the specialised muscular axis responsible for atrioventricular conduction (See below). Consequently, we make our categorisation of ventricular septal defects by viewing the margins of the plane chosen to represent the defect from the aspect of the surgeon approaching from the right side.
Figure 7.
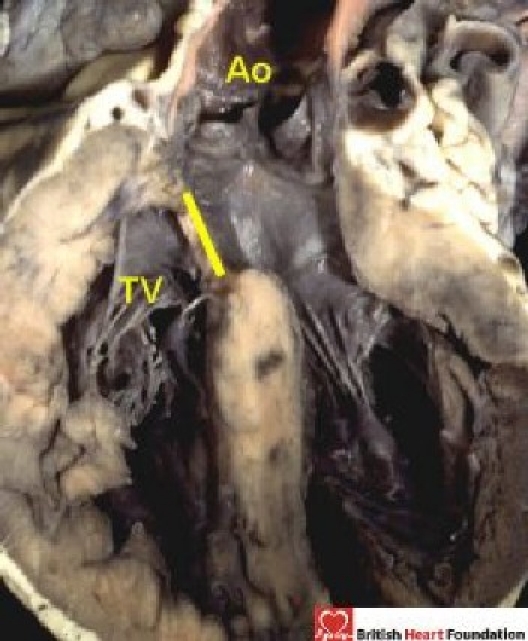
The rightward margin of the interventricular cone of space has a muscular ledge (yellow line) that separates the aortic (Ao) from the tricuspid valve (TV)
What is the significance of atrioventricular junction morphology?
Perhaps the major scientific difference between the proposed classifications for holes between the ventricles is the continuing problem of the “atrioventricular canal ventricular septal defect”. This particular problem is resolved by paying attention to the morphology of the atrioventricular junctions. Thus, there is no question but that hearts can exist with a common atrioventricular junction, and with shunting confined at ventricular level. These hearts are fundamentally different nevertheless, from extensive holes between the ventricles which abut on the membranous part of the septum, and which open primarily into the inlet component of the right ventricle, being shielded by the septal leaflet of the tricuspid valve. Within the European code, it is only the hearts with a common atrioventricular junction (Fig. 8) which are considered to represent “atrioventricular canal” defects, irrespective of the level of shunting across the defect.
Figure 8.
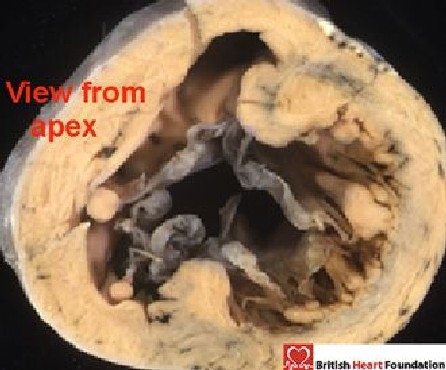
Viewed from the apex, the common valve connects to both ventricles and guards a common atrioventricular junction
This is because, in these hearts, the left atrioventricular valve is half of an essentially common atrioventricular valve, even if the valvar orifice is divided into separate parts for the right and left ventricles, as seen in the “ostium primum” defect. This left valve guarding half of the common junction possesses three leaflets, and closes in trifoliate fashion. Hearts with junctional morphology of this type do exist with shunting possible only at ventricular level because the bridging leaflets of the common atrioventricular valve are firmly attached to the leading edge of the atrial septum (Fig. 9).
Figure 9.
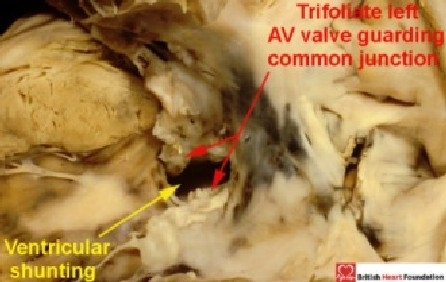
In some hearts with a common atrioventricular valve, as seen here from the left ventricle, the leaflets of the common valve are attached to the leading edge of the atrial septum. In this case it results in shunting only at ventricular level
When such hearts are examined from the right side, the bridging leaflets are seen as they cross through the septal defect (Fig. 10).
Figure 10.
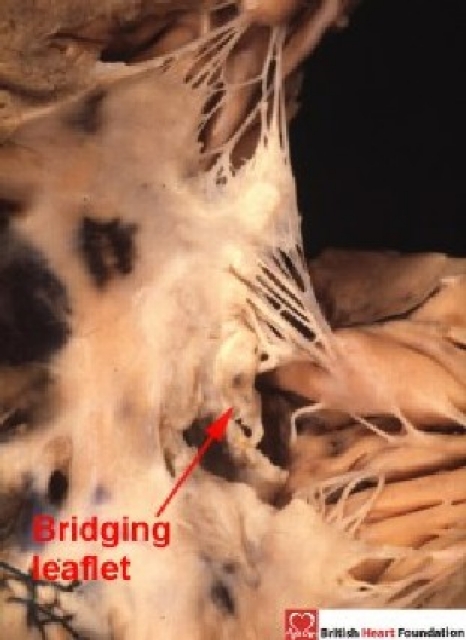
From the right ventricular aspect, the bridging leaflets cross through the septal defect
This arrangement is fundamentally different from a ventricular septal defect that opens primarily to the inlet of the right ventricle in hearts with separate right and left atrioventricular junctions. In these latter malformations, the left atrioventricular valve is morphologically a mitral valve and, like the normal mitral valve, has but one zone of apposition between its paired leaflets (Fig. 11).
Figure 11.
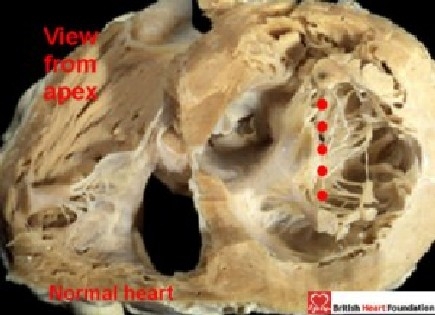
Viewed from the apex in a normal heart, the aortic and mural leaflets of the mitral valve close along a solitary zone of apposition (red dots)
The tricuspid valve in this setting is then located exclusively within the separate right atrioventricular junction, with no straddling of its valvar leaflets. The criterion concerning the arrangement of the atrioventricular junctions, however, serves also to distinguish those hearts with inlet ventricular septal defects in which there is straddling and overriding of the tricuspid valve. In these hearts, the right atrioventricular junction is shared to varying extent between the ventricles. The ventricular septal defect seen with such straddling tricuspid valves is also frequently categorised as representing an “atrioventricular canal defect”5–6. But, although the right atrioventricular junction overrides the crest of the muscular ventricular septum, it is not part of a common junction. Indeed, the separate left atrioventricular junction (Fig. 12) is guarded by a morphologically mitral valve, distinguishing the hearts from those with a common atrioventricular junction. It is the common junction, therefore, which should be the hallmark for distinction of the “atrioventricular canal malformation”. Only those hearts with exclusively ventricular shunting in the setting of such a common junction (Fig. 9 and 10 should be named as “atrioventricular canal VSD's”.
Figure 12.
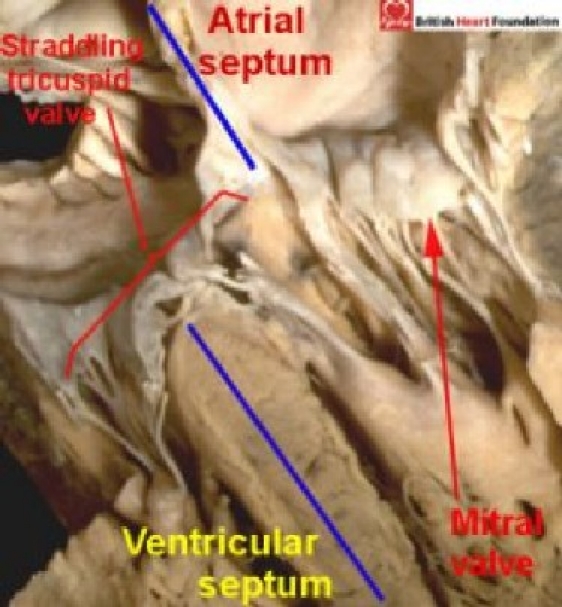
Four-chamber section highlighting the malalignment of the atrial and ventricular septums. In the setting of straddling and overriding of the tricuspid valve. The mitral valve guards a separate left atrioventricular connections
How then, do we Categorise Holes between the Ventricles?
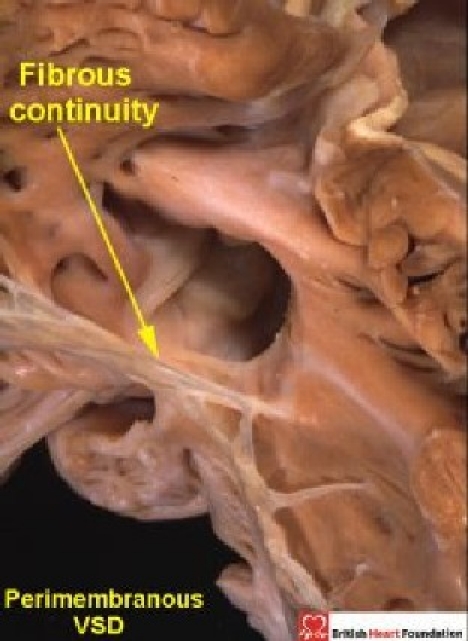
On the basis of the features considered above, our observations suggest that, when the surgeon views the boundaries of holes between the ventricles from the right side, these holes can be placed into one of three categories. The largest category seen in the autopsy room, and at surgery, is that where part of the border of the defined defect is composed of fibrous continuity between the leaflets of the aortic and tricuspid valves (Fig. 13).
Figure 13.
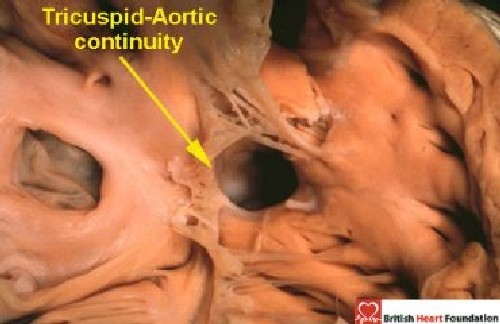
The heart illustrated has tricuspid-aortic valvar continuity, this being the diagnostic feature of a perimembranous ventricular septal defect
We call these holes perimembranous defects. This is because they abut directly on the atrioventricular component of the membranous septum. In addition, frequently they possess a remnant of the interventricular membranous septum reinforcing their postero-inferior rim. The remainder of this initial review will be devoted to these perimembranous defects. The second category includes all holes which, when viewed from their right side, have exclusively muscular borders. As already discussed, there is general agreement that these are muscular ventricular septal defects (Fig. 1). The third category is the one in which the defining feature is fibrous continuity between the leaflets of the aortic and pulmonary valves, or else the presence of a common truncal valve. We call these defects doubly committed and juxtaarterial. Our second review will detail the phenotypic features of the muscular and doubly committed defects. We use this categorisation into three basic patterns in all hearts, irrespective of their segmental interconnections. We also recognise the defect in which there is a common atrioventricular junction and shunting exclusively at ventricular level (Fig. 9–10). But, as explained above, because of the significance of the structure of the trifoliate left atrioventricular valve, and because the hearts lack atrioventricular septal structures, we prefer to categorise them as atrioventricular septal defects, albeit with only ventricular shunting across the defect.
The Philosophy of Categorisation
When categorising holes between the ventricles, therefore, we use a system of description which accounts not only for “isolated” ventricular septal defects, but also for those seen in the presence of other associated malformations, such as tetralogy of Fallot, double outlet ventricle, tricuspid atresia, double inlet ventricle, and so on.If all these holes are accurately categorised, then the description chosen confers important information regarding their relationship to structures such as the conduction tissue axis and the arterial valves. If, in addition, their size and potential for closure are defined, these features having important consequences upon the haemodynamics of ventricular shunting, then the strategy for subsequent treatment can properly be determined.
The Membranous Septum and Perimembranous Septal Defects
The normal membranous septum is usually a small structure (Fig. 14).
Figure 14.
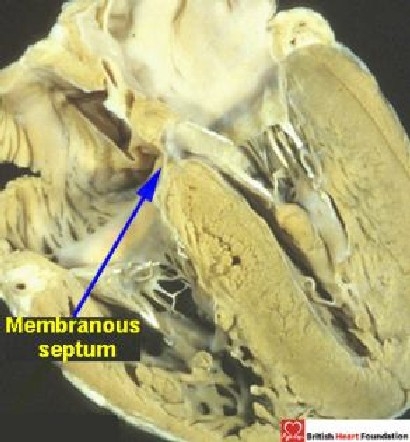
Four-chamber section showing the normal location of the small membranous septum
It is an integral part of the aortic root, where it is confluent with the right fibrous trigone, forming the base of the fibrous triangle between the right and the non-coronary leaflets of the aortic valve (Fig. 15).
Figure 15.
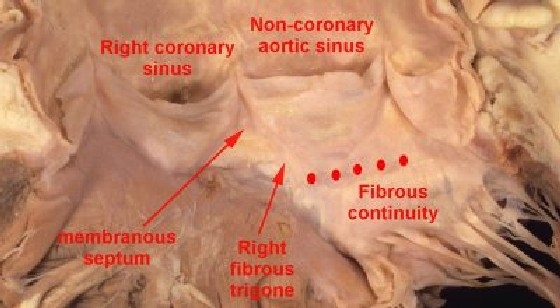
This figure shows the close relationship of the membranous septum to the right fibrous trigone, which is adjacent to the area of fibrous continuity between the aortic and mitral valve leaflets (red dots)
The atrioventricular conduction axis penetrates through the atrioventricular part of this septum, this being demarcated by the hinge of the septal leaflet of the tricuspid valve attached to the right side of the septum. The bundle itself is then sandwiched between the interventricular component of the septum and the muscular septum (Fig. 16).
Figure 16.
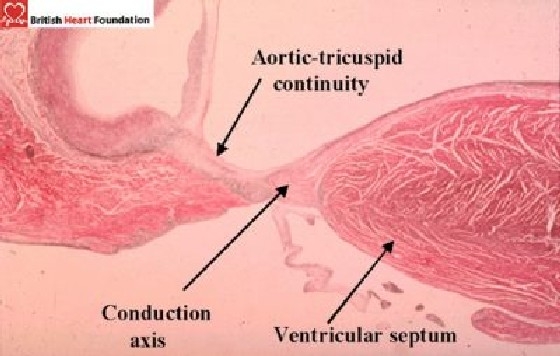
Histology section showing the conduction tissue on the crest of the ventricular septum, along with its relationship to the aortic and tricuspid valves
When the septum “comes apart” in the setting of perimembranous septal defects, it does so between its fibrous and muscular components. The conduction bundle stays on the crest of the muscular ventricular septum. Such perimembranous defects can then be sub-classified according to the direction in which they open to the right ventricle, but the location of the conduction axis stays constant. To account for the variations, the right ventricle itself can be divided into three regions, inlet, outlet, and apical. These parts correspond to their relationship with the tricuspid valve, the sub-pulmonary infundibulum, and the apical trabeculated portion of the morphologically right ventricle, respectively (Fig. 17).
Figure 17.
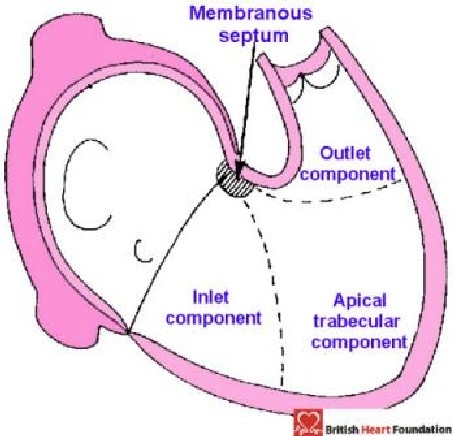
Diagrammatic representation showing how the right ventricle can be described in terms of three components
Should the defect open into more than one of these regions, then it is described as a confluent defect. Previously, we have considered the muscular septum itself as having inlet, apical, and outlet components.7 We have revised this description. Because of the wedged location of the normal subaortic outflow tract, the inlet of the right ventricle is mostly separated by the muscular septum from the outlet of the left ventricle (Fig. 11). Thus, the septum we considered to be “inlet” is, in reality, an inlet/outlet septal structure. Furthermore, the larger part of the right ventricular outlet is made up of the free-standing sleeve of subpulmonary infundibulum. The “outlet septum”, therefore, is not nearly as extensive as was initially believed. In fact, it is not possible with any certainty to distinguish the precise location of the small outlet septal component of the normal heart.8 These facts call into question the wisdom of dividing the ventricular septum in tripartite fashion. It remains entirely accurate and simple, nonetheless, to describe the inlet, apical, and outlet components of the right ventricle (Fig. 17).
Perimembranous defects opening into the inlet of the right ventricle are seen beneath the septal leaflet of the tricuspid valve (Fig. 18). Here, there is fibrous continuity in the roof of the defect between the leaflets of the tricuspid and the mitral valves, and this fibrous tissue forms the postero-inferior border of the defect. The hole between the ventricles can partially be blocked by the septal leaflet of the tricuspid valve, by aneurysms of the membranous septum, or by accessory tissue tags derived from the tricuspid valve. The superior border is made up of the remnant of the membranous septum and the fibrous tissue of the aortic root.
Figure 18.
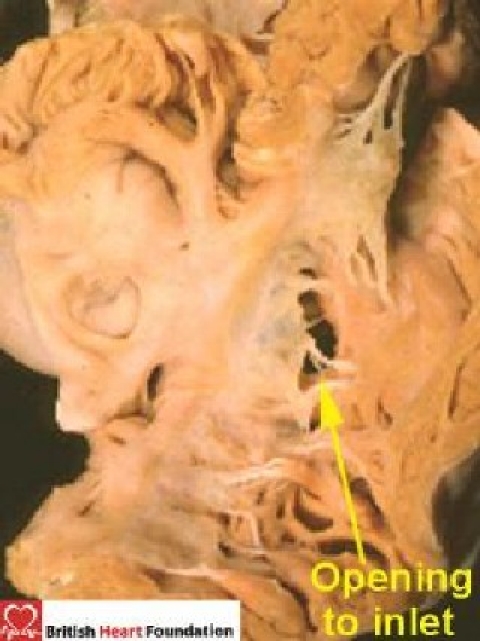
From the right ventricle, the perimembranous defect opening to the inlet is seen under the septal leaflet of the tricuspid valve
Pure outlet defects, without malalignment of the septal structures (Fig. 19), are rare. This is because, as emphasised, the outlet part of the muscular septum is very small in the normal heart. Much more commonly, outlet perimembranous defects are associated with malalignment of the muscular outlet septum and overriding of the aortic valve. When seen, they are usually, but not always, associated with obstructive lesions of the ventricular outflow tracts (see below).
Figure 19.
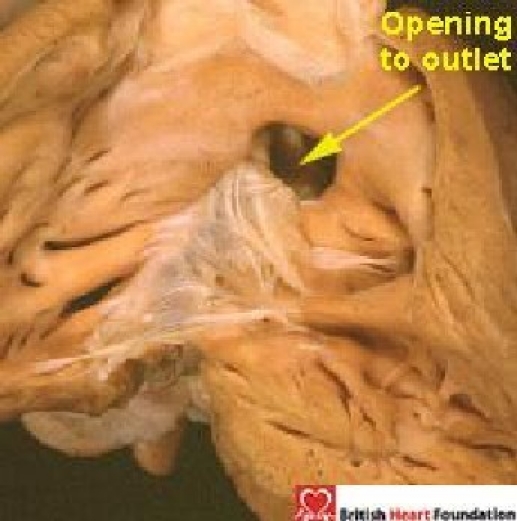
Perimembranous ventricular septal defect opening to the outlet of the right ventricle
When the outlet septum is maligned in antero-cephalad fashion, but there is no stenosis of the outflow tract, the hearts are classified as “Eisenmenger” ventricular septal defects.9 In this setting, it is an easy matter for the blood from the right ventricle to be directed through the ventricular septal defect to the aorta when the pulmonary resistance exceeds the systemic, and they are typically seen in the setting of pulmonary hypertension. More frequently, the defect is found with antero-cephalad deviation of the muscular outlet septum in the setting of tetralogy of Fallot. The hallmark of this defect is obstruction of the subpulmonary outflow tract produced by the “squeeze” between the deviated outlet septum and the hypertropied septoparietal trabeculations.10 This is the ventricular septal defect termed by some a "conoventricular defect". Significantly, although the defect is most usually perimembranous (Fig. 20), it can also be found with a muscular postero-inferior rim (Fig. 21).
Figure 20.
In tetralogy of Fallot, the ventricular septal defect (VSD) usually has a perimembranous postero-inferior margin
Figure 21.
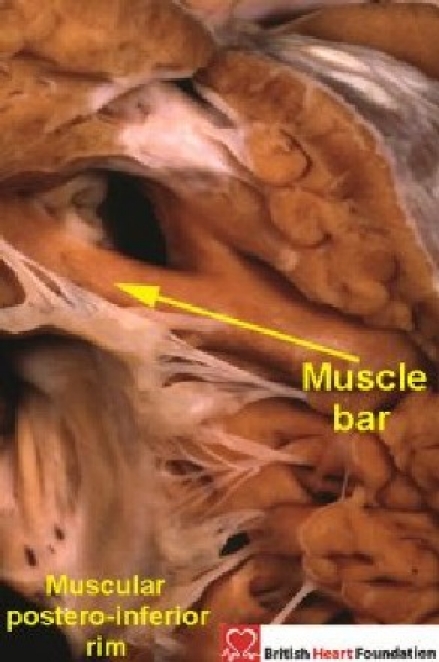
In this case of tetralogy of Fallot, a muscular bar separates the aortic valvar leaflets from the defect, producing a muscular postero-inferior rim
It is the precise morphology of this postero-inferior margin which dictates the vulnerability of the atrioventricular conduction axis. The European classification provides system of categorisation that differentiates between these features.2–3 Defects opening exclusively to the apical component of the ventricle are relatively rare compared to those opening to either inlet or outlet, or those which are confluent. The defects opening towards the apex and remaining perimembranous are small, frequently being slit-like, with the medial papillary muscle marking the apical extent of the defect. The conduction bundle is typically closely adjacent to the postero-inferior margins of the defect in these cases, but a fan-like right bundle branch originating at the same level from the main axis as the left bundle branch has also been described.11 Oftentimes the septal leaflet of the tricuspid valve is cleft. Much more common are the larger defects opening towards the apex which are confluent with either inlet or outlet perimembranous extensions.
The Conduction Axis in Perimembranous Septal Defects
Defining the margins of the defect as we have suggested provides an accurate guide to the route taken by the conduction axis.12 In perimembranous septal defects, the conduction tissue is always found postero-inferior to the defect, the only variation being seen in association with straddling and overriding of the tricuspid valve. In the typical situation, the atrioventricular node is found in its expected location at the apex of the triangle of Koch. The conduction bundle then penetrates through the myocardium from the apex of the triangle of Koch towards the left ventricular outflow tract (Fig. 22).
Figure 22.
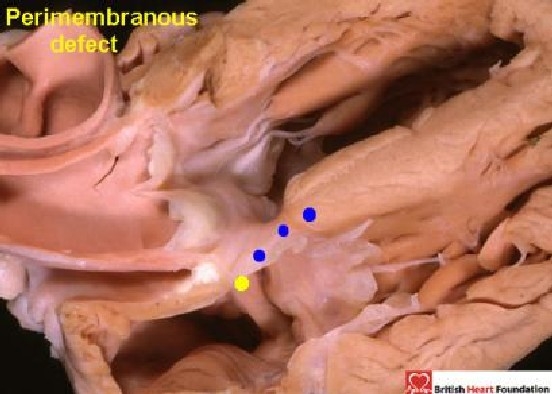
The conduction axis passes from the atrioventricular node in the right atrium (yellow dot), towards the crest of the ventricular septum (blue dots), and is positioned postero-inferiorly relative to the perimembranous ventricular septal defect
The conduction axis then branches on the crest of the septum, with the right bundle branch emerging beneath the medial papillary muscle (Fig. 23).
Figure 23.
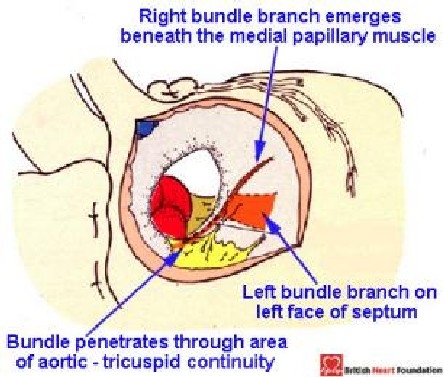
Diagrammatic representation of the direction of the conduction axis as seen from the right atrial aspect in a perimembranous ventricular septal defect, seen here in the setting of tetralogy of Fallot
The area for the surgeon to avoid is along the right ventricular border, and is demarcated by a line drawn from the apex of the triangle of Koch to the medial papillary muscle. The phenotypic feature in cases with straddling and overriding of the tricuspid valve is the malalignment between the atrial and ventricular septums (Fig. 12). Because of this, the atrioventricular node is no longer found at the apex of the triangle of Koch, but is abnormally positioned, being formed at the point where the muscular ventricular septum meets the atrioventricular junction (Fig. 24).
Figure 24.
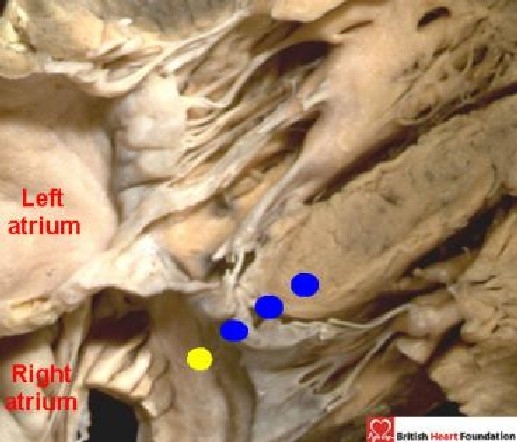
In this figure, which shows deviation of the ventricular septum, the location of the atrioventricular node is not at the apex of the triangle of Koch, but is found where the ventricular septum meets the right atrium (yellow dot). The conduction axis then passes towards the crest of the ventricular septum (blue dots)
Even when seen in isolation, the ventricular septal defect is a common congenital cardiac malformation. Consensus is needed to clarify description of the defect, and this requires agreement on the fundamental features that underscore definition. The first step in this process is to agree the plane of space which is to be defined as “the” defect. Further steps towards consensus can then be taken by providing a coherent definition of the “atrioventricular canal ventricular septal defect”, and agreeing on what precisely is meant by the “conoventricular” defect. The field of diagnosis is becoming ever more advanced with the advent of techniques such as 3-dimensional echocardiography and magnetic resonance imaging. These sophisticated diagnostic methods permit the defect to be viewed in the planes necessary to define precisely the postero-inferior margin of the defect, its size, and its location. This information then permits identification of the position of the conduction axis. The categorisation as suggested here concentrates on these features of clinical significance, and at the same time facilitates assessment of the probability of spontaneous closure of the hole, and the likelihood of developing aortic regurgitation. Use of these accurate definitions, therefore, coupled with exact descriptions of the defect, should help the diagnostic team, the surgeon, and ultimately the patient.
References
- 1.Franklin RCG, Anderson RH, Daniëls O, Elliott M, Gew illig MHML, Ghisla R, Krogman ON, Ulmer HE, Stocker FP. Report of the Coding Committee of the Association for European Paediatric Cardiology. Cardiol Young. 2000;10(Suppl 1):1–7. [PubMed] [Google Scholar]
- 2.Franklin RCG, Anderson RH, Daniëls O, Elliott M, Gewillig MHML, Ghisla R, Krogman ON, Ulmer HE, Stocker FP. The European Paediatric Cardiac Code – The Short List. Cardiol Young. 2000;10(Suppl 1):8–26. [PubMed] [Google Scholar]
- 3.Franklin RCG. The European Paediatric Cardiac Code Long List: structure and function. Cardiol Young. 2000;10(Suppl 1):27–146. doi: 10.1017/s1047951100007770. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs JP, Burke RP, Quintessenza JA, Mavroudis C. Congenital Heart Surgery Nomenclature and Database Project: ventricular septal defect. Ann Thorac Surg. 2000;69(4 Suppl):S25–35. doi: 10.1016/s0003-4975(99)01270-9. [DOI] [PubMed] [Google Scholar]
- 5.Neufeld HN, Titus JL, DuShane JW, Burchell HB, Edwards JE. Isolated ventricular septal defect of the persistant common atrioventricular type. Circulation. 1961;23:685–696. doi: 10.1161/01.cir.23.5.685. [DOI] [PubMed] [Google Scholar]
- 6.LaCorte MA, Fellows KE, Williams RG. Overriding tricuspid valve: echocardiographic and angiographic features. 8 cases of ventricular septal defect of atrioventricular canal type. Am J Cardiol. 1976;37:911–919. doi: 10.1016/0002-9149(76)90118-1. [DOI] [PubMed] [Google Scholar]
- 7.Soto B, Becker AE, Moulaert AJ, Lie JT, Anderson RH. Classification of ventricular septal defects. Br Heart J. 1980;43:332–343. doi: 10.1136/hrt.43.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merrick AF, Yacoub MH, Ho SY, Anderson RH. Anatomy of the muscular subpulmonary infundibulum with regard to the Ross procedure. Ann Thor Surg. 2000;69:556–61. doi: 10.1016/s0003-4975(99)01300-4. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda T, Suzuki T, Ito T. Clinical and morphologic features of perimembranous ventricular septal defect with overriding of the aorta - the so-called Eisenmenger ventricular septal defect. A study making comparisons with tetralogy of Fallot and perimembranous ventricular defect without aortic overriding. Cardiol Young. 2000;10:343–352. doi: 10.1017/s1047951100009641. [DOI] [PubMed] [Google Scholar]
- 10.Gatzoulis MA, Soukias N, Ho SY, Josen M, Anderson RH. Echocardiographic and morphological correlations in tetralogy of Fallot. Eur Heart J. 1999;20:221–231. doi: 10.1053/euhj.1998.1273. [DOI] [PubMed] [Google Scholar]
- 11.Ueda M, Becker AE. Morphological characteristics of perimembranous ventricular septal defects and their surgical significance. Int J Cardiol. 1985;8:149–157. doi: 10.1016/0167-5273(85)90282-7. [DOI] [PubMed] [Google Scholar]
- 12.Anderson RH, Wilcox BR. The surgical anatomy of ventricular septal defect. J Card Surg. 1992;7:17–35. doi: 10.1111/j.1540-8191.1992.tb00773.x. [DOI] [PubMed] [Google Scholar]


