Abstract
Unilateral “absence” of the pulmonary artery is an uncommon malformation frequently associated with congenital heart disease. In fact, the pulmonary artery is typically discontinuous from the main pulmonary artery, not absent. We report a case of right pulmonary artery discontinuity not associated with congenital heart disease and review the embryogenesis and treatment of this rare congenital cardiac anomaly.
MeSH: Heart defects, congenital, Pulmonary artery, Catheterization
Introduction
Unilateral absence of the pulmonary artery is a rare congenital malformation frequently associated with congenital heart disease. Less commonly, absence of the pulmonary artery is found in an otherwise normal heart. Frequently, the distal segments of the “absent” pulmonary artery remain present, receiving blood flow from collateral arteries or from a persistently patent ductus arteriosus.1,2 In this situation, the pulmonary artery is not absent, but rather interrupted or discontinuous from the main pulmonary artery. The distal segment often can be demonstrated at cardiac catheterization or surgery. Resorption of the proximal sixth aortic arch during cardiac development may result in pulmonary artery discontinuity. We report a case of isolated right pulmonary artery (RPA) discontinuity and review the embryogenesis and treatment of this uncommon disorder.
Case report
A healthy, 11 year-old African American male was evaluated in the Emergency Department for rib pain after minor trauma. A chest roentgenogram obtained to exclude rib fracture revealed rightward displacement of the heart and mediastinum, decreased right lung volume, left lung hyperinflation, and herniation of the left upper lobe across the midline (fig 1). Further history revealed no previous episodes of chest pain, shortness of breath, palpitations, exercise intolerance, or syncope. Parents described the child as an active participant in age-appropriate athletic activities. Past medical history was negative for chronic respiratory infections and hemoptysis. Family history was negative for congenital heart disease.
Figure 1.
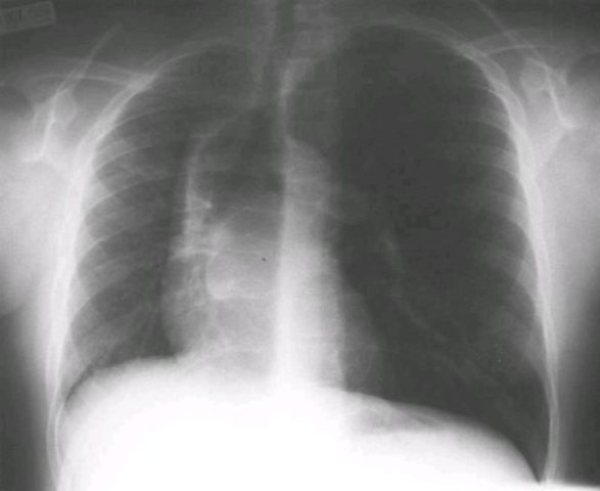
Chest roentgenogram (postero-anterior view) showing rightward displacement of the heart and mediastinum, decreased right lung volume, left lung hyperinflation, and herniation of the left upper lobe across the midline
Physical examination revealed a mildly obese male in no distress. Pulse, respiratory rate, and right arm blood pressure were normal. There was no jugular venous distention. The chest was symmetric and the lungs were clear with equal breath sounds. The point of maximal impulse was displaced medially. The first and second heart sounds were normal. A grade 1/6 soft, low-pitched, systolic ejection murmur was heard at the mid left sternal border with radiation throughout the left chest. Diastole was clear. The liver was not enlarged. Pulses and perfusion were normal. There was no cyanosis, clubbing, or peripheral edema.
An electrocardiogram was normal. An echocardiogram revealed normal ventricular size and function and normal intracardiac anatomy. The proximal RPA was not visualized. Suprasternal images suggested an aortopulmonary window. Right ventricular pressures could not be estimated by Doppler.
At cardiac catheterization, oximetry data showed no evidence of intracardiac shunting. Right and left ventricular pressures (systolic/end-diastolic) were 22/4 and 95/8, respectively. Pressures in the main pulmonary artery and left pulmonary artery (LPA) were normal. Cardiac output was 6.6 liters/minute (Fick), and the pulmonary-to-systemic flow ratio was 1. Pulmonary and systemic vascular resistances were normal. Aortic root angiography showed no evidence of an aortopulmonary window. The aortic arch was left-sided with a prominent right innominate artery diverticulum (fig 2).
Figure 2.
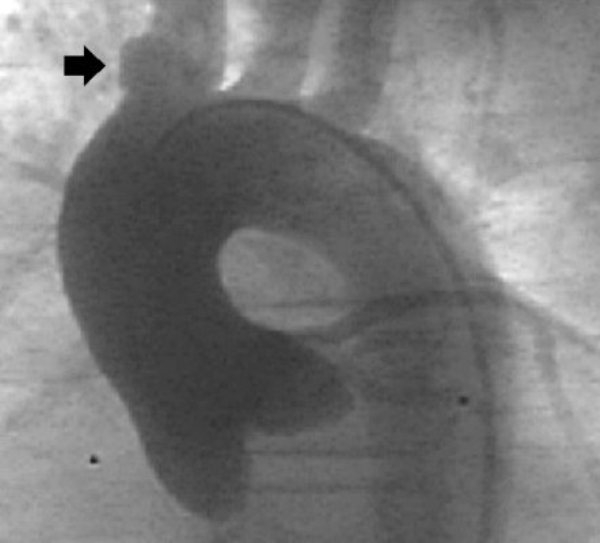
Ascending aortogram demonstrating a left aortic arch with normal branching and a diverticulum of the innominate artery (arrow)
Collateral vessels to the right lung arose from the right subclavian artery, right common carotid artery, and proximal descending aorta. There was no patent ductus arteriosus. A normal, left-sided ductal ampulla remnant was present distal to the left subclavian artery. A right ventriculogram showed normal ventricular systolic function, a dilated proximal LPA, and absence of the proximal RPA (fig 3).
Figure 3.
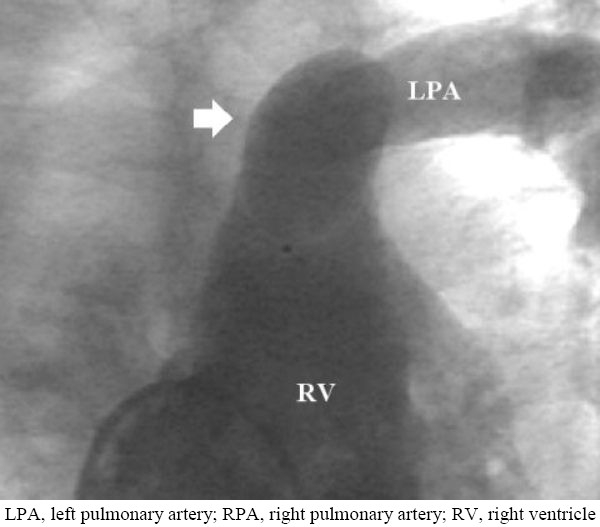
Right ventriculogram demonstrating absence of the proximal right pulmonary artery
Pulmonary vein wedge angiography demonstrated retrograde filling of a 7 mm in diameter distal RPA with right upper, middle, and lower lobe branches (fig 4). There was no right-sided pulmonary vein stenosis.
Figure 4.
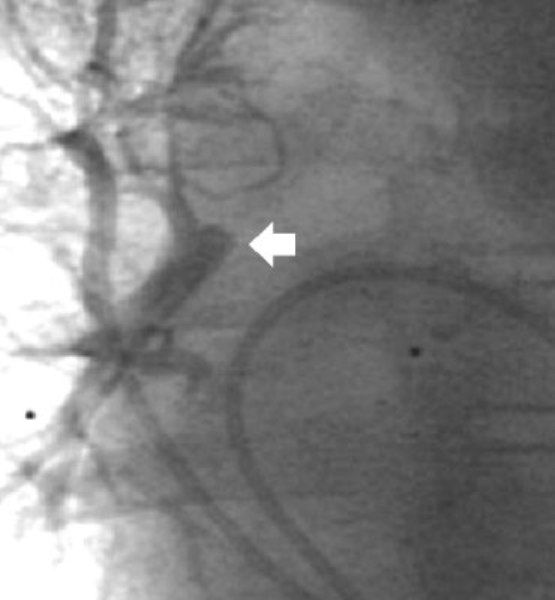
Right upper pulmonary vein wedge angiogram demonstrating retrograde filling of the distal right pulmonary artery (arrow) and right upper, middle, and lower lobe pulmonary artery branches
Discussion
Unilateral absence of the pulmonary artery is an uncommon malformation that was first described by Fraentzel in 1868.3 Anatomic studies1 and reports from the surgical literature2,4 have shown that the distal segment of an “absent” pulmonary artery may be demonstrated by angiography or at surgery or autopsy. Therefore, the terms “unilateral pulmonary artery discontinuity” or “unilateral nonconfluent pulmonary artery” more accurately describe this abnormality.
Affected individuals often have asymmetric chests and asymmetric development of the lung, and in interruption of the RPA, this may give a CXR appearance of dextrocardia. Congenital heart disease, including tetralogy of Fallot, right-sided aortic arch, atrial or ventricular septal defect, or persistence of the ductus arteriosus is more common with LPA discontinuity. RPA discontinuity, however, is usually an isolated finding in an asymptomatic patient with normal intracardiac anatomy. In most cases, the affected pulmonary artery is on the side opposite the aortic arch.4 A diverticulum of the innominate artery is frequently demonstrated angiographically (fig 2).
Pfefferkorn5 first reported the innominate artery diverticulum and suggested a possible developmental origin for this angiographic finding. During normal embryogenesis, the proximal portions of the sixth aortic arches form the proximal pulmonary arteries. During normal development of a left aortic arch, the distal right sixth arch disappears and the distal left sixth arch forms the ductus arteriosus.
Maldevelopment of the sixth aortic arch is one way to explain this patient's RPA discontinuity. Abnormal early resorption of the proximal right sixth arch obliterates the proximal RPA. Persistence of the distal right sixth arch links the distal RPA to the right dorsal aorta, the primitive innominate artery. Late closure of the distal right sixth arch, a vessel histologically identical to the contralateral ductus arteriosus, creates RPA discontinuity and the innominate artery diverticulum (fig 5).
Figure 5.
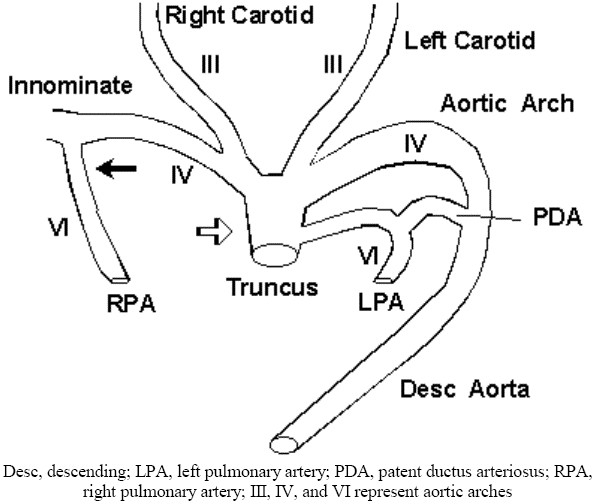
Resorption of the proximal right sixth aortic arch obliterates the proximal RPA (open arrow). Persistence of the distal right sixth arch (closed arrow) links the distal RPA to the proximal right dorsal aorta, the primitive innominate artery.
There is no consensus regarding the treatment for isolated pulmonary artery discontinuity. Most agree that treatment should be reserved for the small number of patients with hemoptysis, recurrent lower respiratory tract infection, or pulmonary hypertension.2,6–8 Treatment options for these patients include partial or total pneumonectomy, closure of selected collateral arteries not solely responsible for pulmonary blood flow, or a primary versus staged pulmonary artery anastomosis. Others advocate an early search for the occult pulmonary artery in all patients regardless of symptoms followed by a staged repair to promote distal pulmonary artery growth and lung development.4 Early reestablishment of pulmonary blood flow, either primarily or after a shunt procedure, may allow the affected lung to develop more normally.
In the asymptomatic patient with isolated pulmonary artery discontinuity, conservative management with close follow-up seems most appropriate. Careful auscultation of the second heart sound and echocardiographic estimation of right ventricular pressure should be performed regularly to detect early signs of pulmonary hypertension. If pulmonary hypertension is suspected, cardiac catheterization is mandatory to measure pulmonary artery pressures directly and to determine the source of pulmonary blood flow.
Conclusions
Unilateral absence of the pulmonary artery is frequently associated with congenital heart disease, but may be found occasionally as an isolated lesion. Asymptomatic patients with isolated pulmonary artery discontinuity deserve close, conservative management designed to detect the early development of pulmonary hypertension.
References
- 1.Sotomora RF, Edwards JE. Anatomic identification of so-called absent pulmonary artery. Circulation. 1978;57:624–633. doi: 10.1161/01.cir.57.3.624. [DOI] [PubMed] [Google Scholar]
- 2.Moreno-Cabral RJ, McNamara JJ, Reddy VJ, Caldwell P. Unilateral absent pulmonary artery: surgical repair with a new technique. J Thorac Cardiovasc Surg. 1991;102:463–465. [PubMed] [Google Scholar]
- 3.Fraentzel O. Ein Fall von Abnormer Communication der Aorta mit der Arteria Pulmonalis. Virchows Arch [A] 1868;43:420–426. [Google Scholar]
- 4.Presbitero P, Bull C, Haworth SG, deLeval MR. Absent or occult pulmonary artery. Br Heart J. 1984;52:178–185. doi: 10.1136/hrt.52.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfefferkorn JR, Löser H, Pech G, Toussaint R, Hilgenberg F. Absent pulmonary artery: a hint to its embryogenesis. Pediatr Cardiol. 1982;3:283–286. doi: 10.1007/BF02427028. [DOI] [PubMed] [Google Scholar]
- 6.Canver CC, Pigott JD, Mentzer RM. Neonatal pneumonectomy for isolated unilateral pulmonary artery agenesis. Ann Thorac Surg. 1991;52:294–295. doi: 10.1016/0003-4975(91)91356-z. [DOI] [PubMed] [Google Scholar]
- 7.Sreeram N, Asante-Korang A, Ladusans E. Distal ductal origin of the right pulmonary artery: prospective diagnosis and primary repair in infancy. Int J Cardiol. 1992;35:272–274. doi: 10.1016/0167-5273(92)90190-e. [DOI] [PubMed] [Google Scholar]
- 8.Ohta T. Congenital absence of the right pulmonary artery: a case report and review. Tokai J Exp Clin Med. 1983;8:97–104. [PubMed] [Google Scholar]


