Abstract
In our previous review of the phenotypic features of ventricular septal defects, we concentrated on the perimembranous variant, showing how its distinguishing feature, as viewed from the right ventricle, was fibrous continuity in its postero-inferior rim between the leaflets of the aortic and tricuspid valves. In this second review, we focus on the morphology of those defects which have exclusively muscular rims when viewed from their right side, and the variant with the phenotypic feature of fibrous continuity between the leaflets of the two arterial valves. As with the defects described as being perimembranous, once they have been characterised, it is the position of the defect relative to the components of the morphologically right ventricle that is the primary determinant of the options and strategies for treatment. Therefore, clarification of the morphology is the key to establishing the related risks for each particular defect.
MeSH: Heart septal defects, ventricular, Heart defects, congenital, Heart ventricle/pathology, Heart ventricles/abnormalities
Introduction
In our first review,1 we emphasised the phenotypic feature of the type of ventricular septal defect which is defined as being perimembranous within the classification now established by the Association for European Paediatric Cardiology.2–3 At the same time, we highlighted the potential anatomic discrepancies that exist between this classification and the one promoted by The Society of Thoracic Surgeons.4 The defect characterised by fibrous continuity between the leaflets of the arterial and atrioventricular valves, however, although the type most frequently encountered during surgical repair, is just one of the anatomical variants of the holes which can be found between the ventricles. In this review, we describe the phenotypic features of the other two anatomic possibilities. These are defects which have an exclusively muscular rim when viewed from their right ventricular aspect, and those defects which have fibrous continuity between the leaflets of the aortic and pulmonary valves. As with the perimembranous defect, understanding the system of classification is greatly facilitated by knowledge of the structure of the normal ventricular septum. Our review will commence, therefore, by revisiting the normal morphology, paying particular reference to the features that are of importance in appreciating defects with exclusively muscular rims, and those roofed by fibrous continuity between the leaflets of the arterial valves.
The normal ventricular septum
The ventricular septum in the normal heart is mostly muscular. Only a small part, which is integrated into the aortic root, is made of fibrous tissue. This fibrous component is the so-called membranous septum.5 It is located inferiorly to the inter-leaflet triangle that fills the space between the ascending hinges of the non-coronary and right coronary leaflets of the aortic valve (Fig.1).
Figure 1.
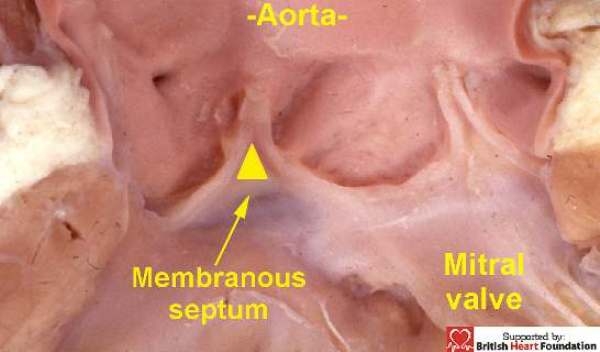
The membranous septum is found at the base of the inter-leaflet triangle which interposes between the right coronary and non-coronary leaflets of the aortic valve
When transilluminated from the right atrioventricular aspect, it can be seen, in most cases, to be crossed by the hinge of the tricuspid valve, thus dividing it into atrioventricular and interventricular components. It is then the position of the hinge which determines the proportions which are atrioventricular as opposed to interventricular6 (Fig.2).
Figure 2.
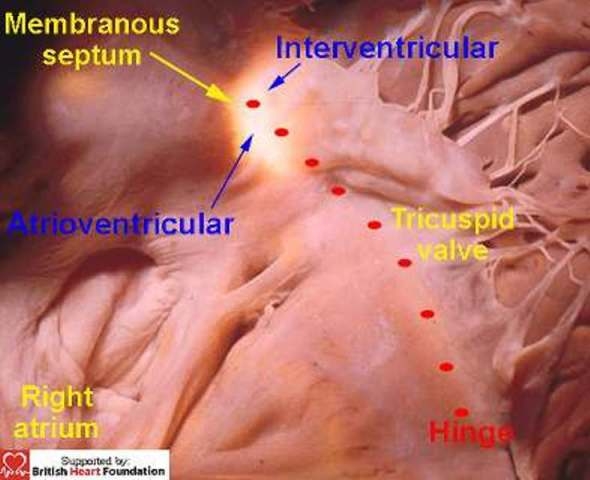
Trans-illumination from the left ventricle shows how the right atrioventricular aspect of the membranous septum is crossed by the hinge of the tricuspid valve. This divides it into an atrial and ventricular components. The conduction axis penetrates through the atrioventricular part of the septum.
The atrioventricular component of the septum is itself pierced by the atrioventricular conduction axis as it passes from the apex of the triangle of Koch to reach the crest of the muscular septum.5. Our first review emphasised that the normal ventricular septum “came apart” between its muscular and fibrous components in the presence of a defect which had fibrous continuity between the leaflets of the arterial and atrioventricular valves. With the exception of hearts with straddling and overriding of the tricuspid valve, this feature explains why the conduction axis is always found in postero-inferior position when these perimembranous defects are found in the setting of concordant atrioventricular connections (Fig.3).
Figure 3.
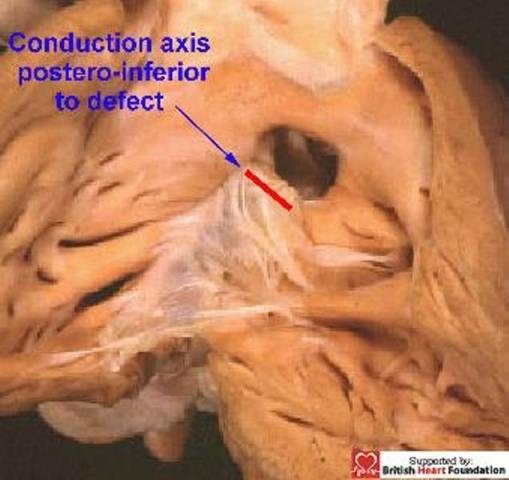
A perimembranous septal defect, showing the fibrous postero-inferior rim of the defect penetrated by the conduction axis
In terms of the variants seen with this particular lesion, the arrangement of the muscular septum, and particularly its relationship with the free-standing muscular subpulmonary infundibulum, are of crucial importance, as deficiencies of the muscular septum can be found opening to different parts of the right ventricle. To differentiate between these various defects, therefore, we find it helpful to relate their location relative to the components of the right ventricle, namely its inlet, outlet, and apical trabecular parts. As we will see, defects with exclusively muscular rims can also open into any of these components. In the normal heart, however, it is incorrect to imagine that the subpulmonary outlet component is separated from the subaortic outlet of the left ventricle by a discrete muscular septum, albeit that this is the impression given in some earlier works.7 In reality, in the normal heart, the leaflets of the pulmonary valve are supported by a sleeve of free-standing muscular infundibulum (Fig.4).
Figure 4.
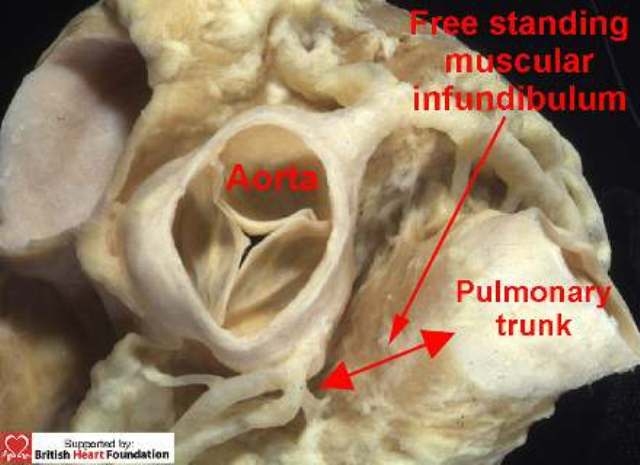
A normal heart showing the relationship of the great arteries. The pulmonary trunk is lifted away from the base of the heart by a free-standing muscular infundibulum
This sleeve lifts the leaflets of the pulmonary valve away from the base of the ventricular mass. A discrete extracardiac tissue plane is to be found between the offset hinges of the two arterial valves which contains fibroadipose tissue, and which separates the posterior aspect of the subpulmonary infundibulum from the aortic valvar sinuses. This arrangement continues to be seen in anomalies such as tetralogy of Fallot (Fig.5).
Figure 5.
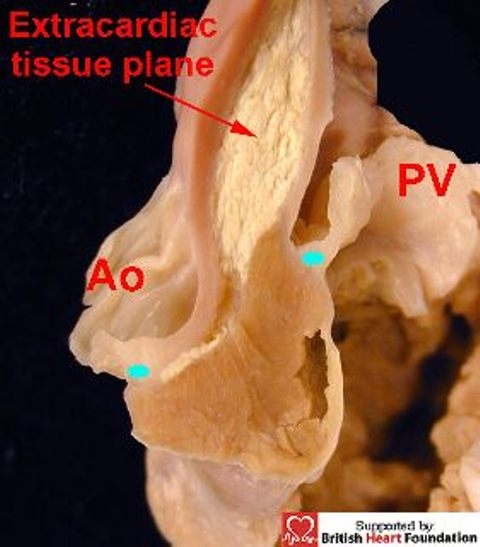
The attachment of the hinge points (blue dots) of the aortic (Ao), and the pulmonary valves (PV), are off-set, with an extracardiac tissue plane separating the two arterial trunks. The situation is illustrated here in the setting of tetralogy of Fallot
Appreciation of this arrangement is particularly important for those seeking to understand the structure of the defect which is roofed by fibrous continuity between the leaflets of the two arterial valves. In fact, this defect, which we describe as being doubly committed and juxtaarterial, cannot exist in the setting of the normal heart. It has far more in common with hearts possessing a common arterial trunk than it does with those having normal outflow components.8
Overall classification of ventricular septal deficiencies
The key feature when distinguishing between defects, as we emphasised in our first review,1 is to identify the right ventricular borders of the septal deficiency.9 We choose this viewpoint since this is now the most frequent approach taken by the surgeon during surgical correction, albeit often seen through the tricuspid valve. On this basis, any hole between the ventricles can be placed into one of three categories. The majority possess, as their hallmark, fibrous continuity between the leaflets of the aortic and tricuspid valves in the postero-inferior rim of the defect (Fig.6).
Figure 6.
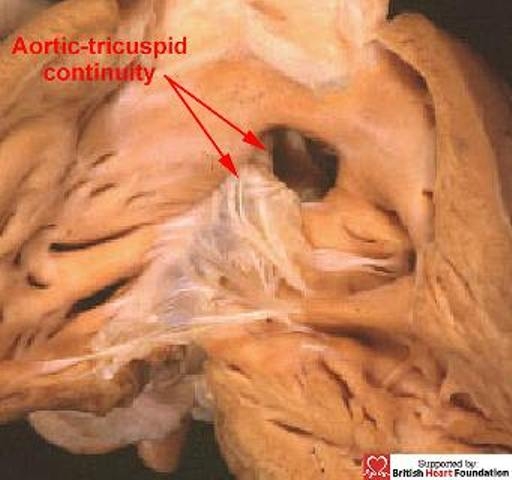
It is continuity between the leaflet of the aortic and tricuspid valves in the postero-inferior margin which is the hallmark of a perimembranous ventricular septal defect
These are the defects we describe as being perimembranous. The second group has in common the fact that the defects within it have entirely muscular borders, regardless of their position relative to the components of the right ventricle. The third group of defects is characterised by fibrous continuity between the leaflets of the two arterial valves, or else the presence of a common truncal valve. With such defects, which we call doubly committed and juxtaarterial, there is usually a muscular bar postero-inferiorly that separates the hinges of the leaflets of the aortic and tricuspid valves, or else interposes between the truncal and tricuspid valves. An important variation seen with these doubly committed defects, however, is for the septal deficiency to extend postero-inferiorly to abut on the area of fibrous continuity between the arterial and atrioventricular valves, thus making the defect also perimembranous.
Characteristics of muscular ventricular septal defects
Muscular defects can exist at any location within the septum (Fig.7). They can be multiple, or can co-exist with perimembranous or doubly committed defects, and their size can vary considerably. Their phenotypic feature, nonetheless, is the presence of exclusive muscular boundaries as seen from the right ventricle, even when there is overriding of the leaflets of an arterial valve (Fig.8).
Figure 7.
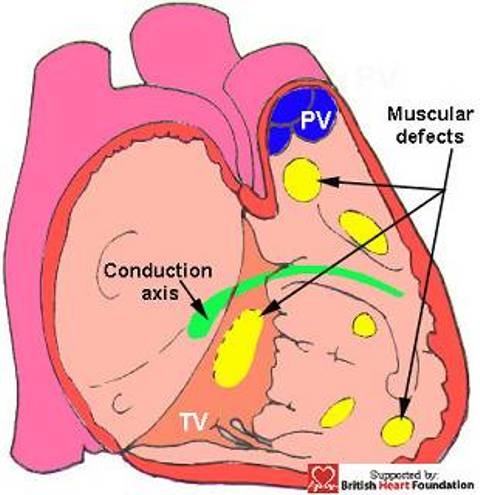
This diagrammatic representation shows the possible locations of muscular ventricular septal defects, and their relationship to the conduction axis.(TV= tricuspid valve, PV= pulmonary valve)
Figure 8.
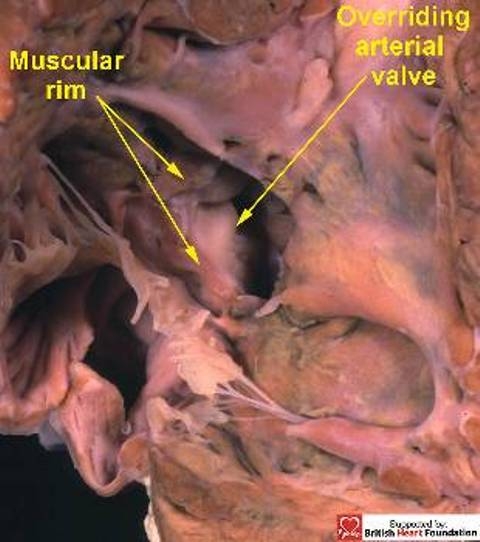
When viewed from the right ventricle, this ventricular septal defect has a complete muscular rim, despite the overriding of the aortic valve
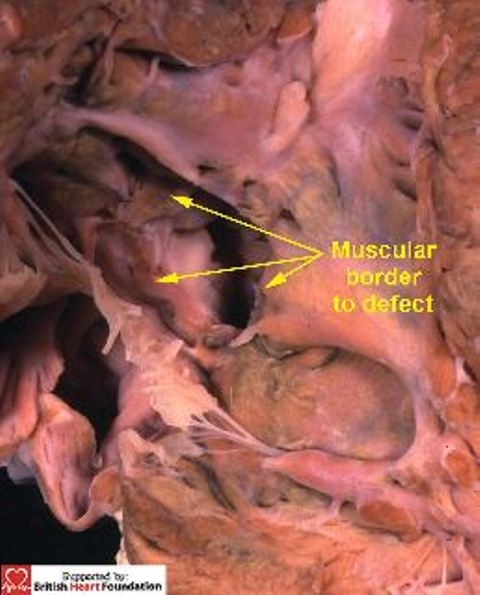
Defects that open to the inlet of the ventricle are closely related to the septal leaflet of the tricuspid valve (Fig. 9–10).
Figure 9.
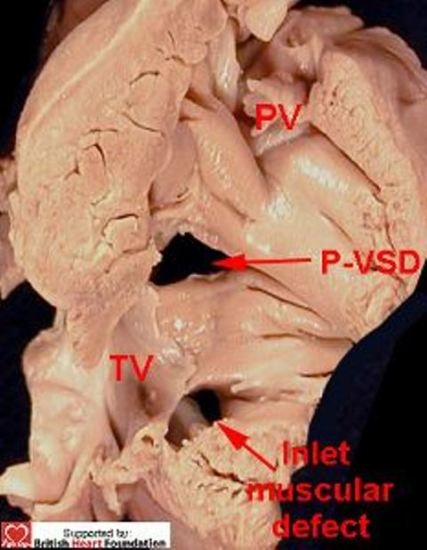
Both of these ventricular septal defects are closely related to the tricuspid valve. This shows the right ventricular aspect. (PV= pulmonary valve, P-VSD= perimembranous ventricular septal defect)
Figure 10.
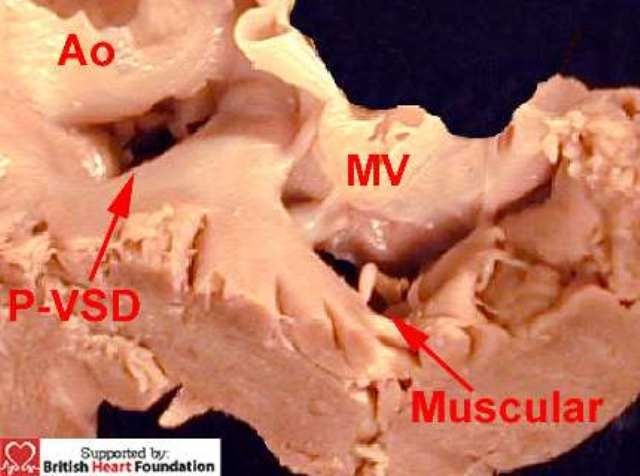
From the left ventricular aspect, the defect under the mitral valve (MV) is seen to have a muscular rim. This other defect is perimembranous ventricular septal defect (P-VSD) being roofed by fibrous continuity between the aortic valve (Ao) and the atrioventricular valves
Those opening between the apical trabecular components of the ventricles can take various forms and guises. The apical muscular septum itself is covered on its right ventricular aspect by a coarse meshwork of apical trabeculations. These include the moderator band, which arises as one of a series of septoparietal trabeculations from the prominent septomarginal trabeculation (Fig.11).
Figure 11.
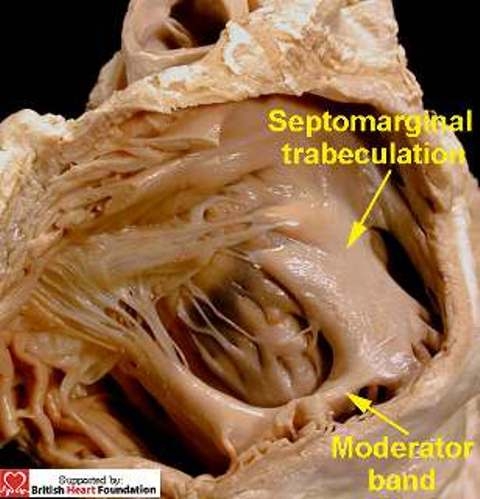
This view of the right ventricular aspect of the normal heart shows the septomarginal trabeculation, which gives rise to the moderator band
The presence of these trabeculations influences markedly the nature of deficiencies within the apical muscular septum. Those defects which are large and solitary are usually seen in mid-ventricular position, located either immediately posterior to (Fig.12) or anterior (Fig.13) to the body of the septomarginal trabeculation.
Figure 12.
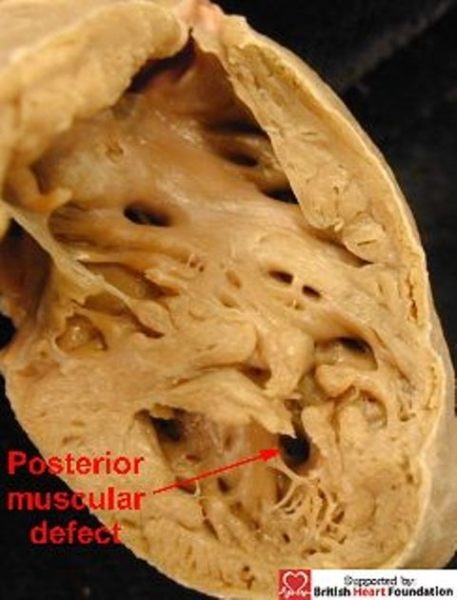
This muscular ventricular septal defect is posterior to the septomarginal trabeculation
Figure 13.
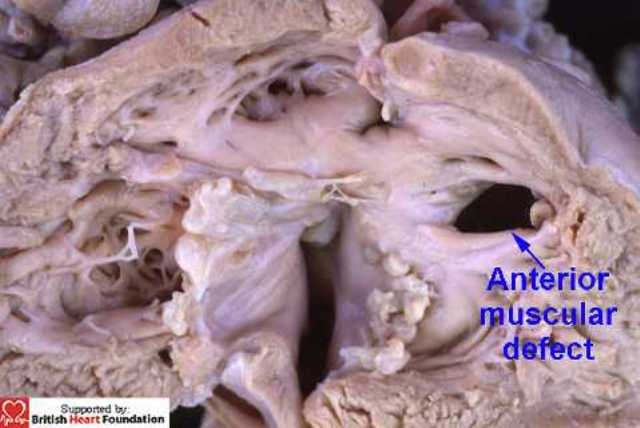
This muscular ventricular septal defect is anterior to the septomarginal trabeculation
Defects opening to the right ventricle anterior to the septomarginal trabeculation, but located closer towards the ventricular apex, however, are often crossed by the septoparietal trabeculations. In other instances, an apical defect can be crossed by the septomarginal trabeculation itself. Because of this, the obviously solitary defect as seen from the left ventricle (Fig.14) can have multiple openings as viewed from the right ventricle (Fig.15).
Figure 14.
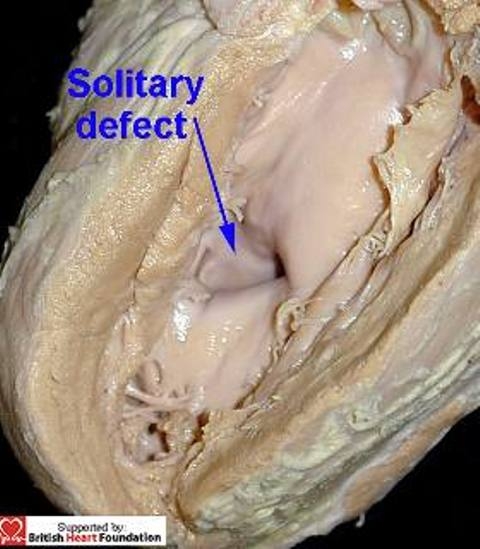
This muscular ventricular septal defect, viewed from its left ventricular aspect, is seen to be a solitary defect
Figure 15.
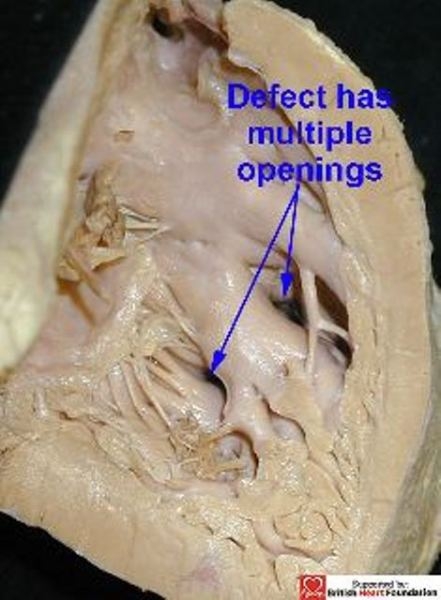
From the right ventricular aspect, the defect is crossed by the septomarginal trabeculations giving the appearance of multiple defects
The precise nature of these types of defect has been called into question recently by suggestions that the hole extends between the apex of the left ventricle and the infundibulum of the right ventricle.10,11 This argument depends entirely on the definition used for the infundibulum of the right ventricle. In our opinion, the infundibulum is simply the free-standing muscular sleeve supporting the leaflets of the pulmonary valve, seen even in anomalous situations such as tetralogy of Fallot (Fig.16).
Figure 16.
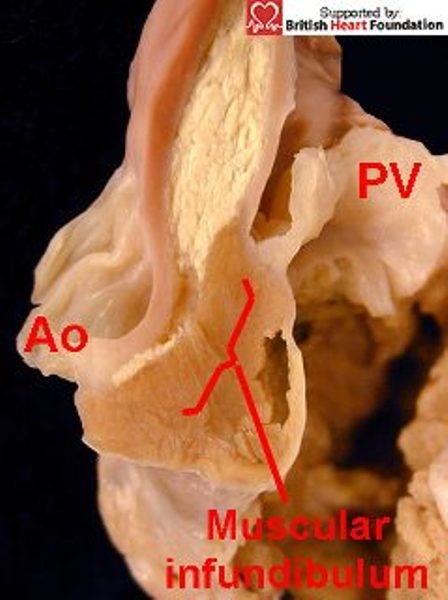
Muscular infundibulum raises the attachment of the pulmonary valve (PV) away from that of the aorta (Ao)
This is the structure that is removed by the surgeon when preparing the pulmonary autograft for use in the Ross procedure. The very fact that the pulmonary valve can be removed in this manner (Fig.17) shows clearly that the infundibulum has no relationship with the apical component of the septum. Although we differ with regard to the definition of the “infundibulum”, the points of clinical significance made with relation to these defects are of considerable importance. Thus, unless the presence of multiple right-sided orifices, but with a solitary left-sided opening, is appreciated, then complete surgical repair can be difficult, with the potential for leaving residual defects.11
Figure 17.
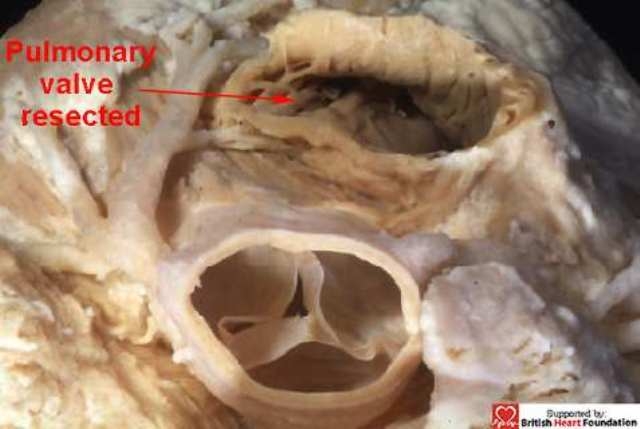
The pulmonary trunk has been resected in this normal heart, along with the free-standing muscular infundibulum. The dissection has not encroached upon the cavity of the left ventricle
Although in this case the defect is strictly a solitary hole, other variants, particularly towards the apex can exist. The apical muscular septum can be sponge-like, and truly multiple defects produce the so-called “swiss-cheese” septum. This represents failure of compaction of the apical septum, such that multiple tracks now persist between the ventricles. As a result these defects can also pose significant problems for complete surgical repair. The final type of muscular defect is the one that opens to the right ventricular outlet. This can take the form of a defect, usually small, which opens immediately beneath the free-standing muscular subpulmonary infundibulum (Fig.18). Alternatively, muscular outlet defects can be associated with overriding of the leaflets of the aortic valve, either in the setting of tetralogy of Fallot (Fig.19), or with an unobstructed subpulmonary outlet (Fig.20).
Figure 18.
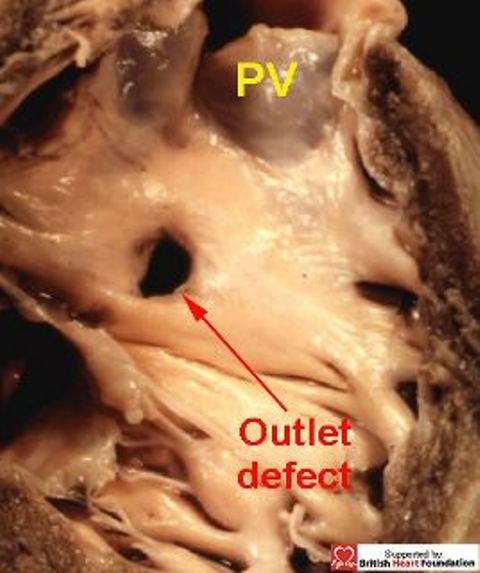
An outlet muscular defect, showing its relationship to the pulmonary valve (PV)
Figure 19.
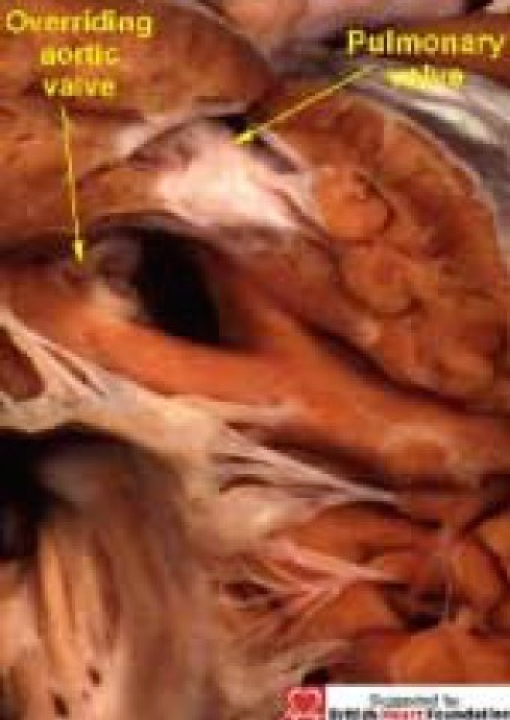
A heart with tetralogy of Fallot, with overriding of the aortic valve (Ao), which is connected to both ventricles, and separated from the tricuspid valve (TV) by a muscle bar of the septomarginal trabeculation (SMT)
Figure 20.
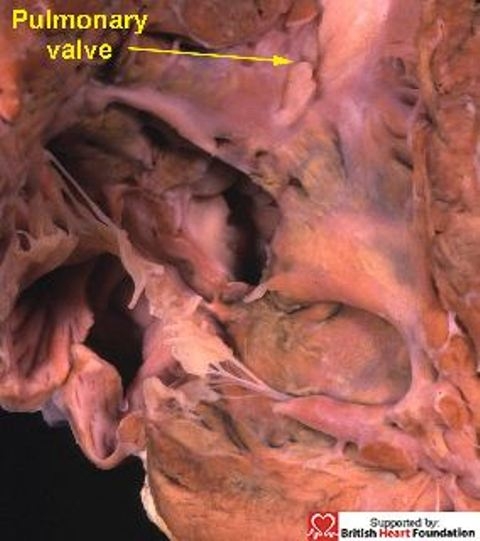
This specimen has deviation of the of the muscular outlet septum, but with an unobstructed pulmonary outflow tract
When categorising these defects, it is important to appreciate that, although the direct extension of the plane of the muscular ventricular septum transects the leaflets of the overriding arterial valve, with part of the aortic valve unequivocally supported by right ventricular structures, (Fig.21) the septal defect still has exclusively muscular borders when viewed from its right ventricular aspect (Fig.22). Significantly, a well-formed muscular bar interposes between the leaflets of the aortic and tricuspid valves, thus providing protection for the branching components of the atrioventricular conduction axis (Fig.23).
Figure 21.
Sectioning the heart shown in Fig.20 reveals that the aortic valve overrides the crest of the ventricular septum
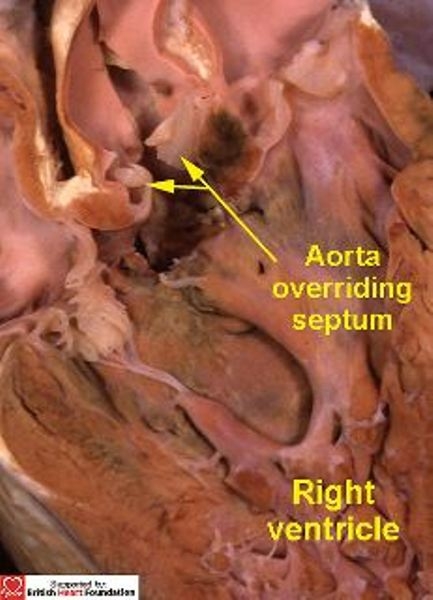
Figure 22.
Viewed from the right ventricular aspect, the defect, seen in Figs. 20 & 21, has a complete muscular border
Figure 23.
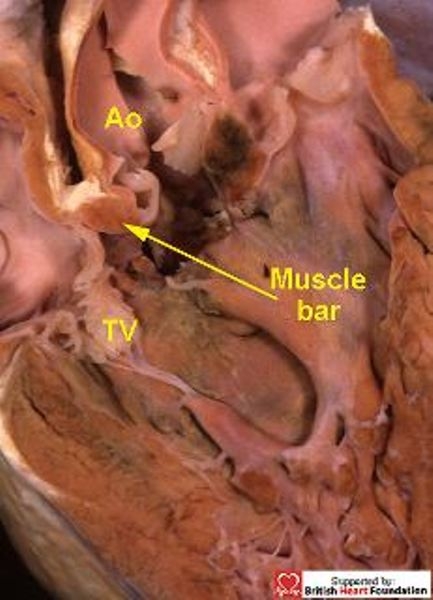
The section shows the muscular bar which separates the aorta (Ao) from the tricuspid valve (TV), thus protecting the conduction bundle
It is proper recognition of the location of the muscular defects relative to the septomarginal trabeculation that provides the information necessary to predict their relationship relative to the ventricular conduction tissues. Inlet defects are posterior and inferior to the conduction axis, but can be closely related to the conducting bundles if positioned adjacent to the medial papillary muscle. Apical defects posterior to the septomarginal trabeculation are also behind the right bundle branch, which descends within the substance of the septomarginal trabeculation. Those anterior to the trabeculation, and those opening to the right ventricular outlet, are in front of the bundle. Although such defects are well distant from the branching bundle, it is important to respect the integrity of the septomarginal trabeculation. This is because trauma to the right ventricular aspect can produce haemorrhage that can track along the insulating sheath surrounding the right bundle branch.12 Such haemorrhage and oedema can then infiltrate the more proximal segments of the conduction axis, with the potential to produce complete atrioventricular block. With defects that open to the ventricular outlet, it is the size of the muscular bar interposing between the leaflets of the aortic and tricuspid valves that is the key feature relative to the vulnerability of the conduction tissues. When substantial, sutures can be inserted within the musculature without fear of damaging the conducting bundles. If flimsy, however, then sutures could pass directly into the conduction axis and induce iatrogenic heart block.
Characteristics of doubly committed and juxtaarterial ventricular septal defects
As we have emphasised, in the right ventricle of the normal heart, the leaflets of the pulmonary valve are supported by the sleeve of free-standing subpulmonary infundibulum (Figs.4,17). This raises the valve away from the body of the right ventricle, and also separates it from the leaflets of the aortic valve (Fig.24). The essence of the doubly committed defect, however, is fibrous continuity between the leaflets of the aortic and pulmonary arterial valves, which are now at the same level in the roof of the defect (Figs.25). Due to the semilunar nature of their hinges, the leaflets can be offset to some degree if one of the aortic sinuses is wedged between two of the leaflets of the pulmonary valve (Fig.26).
Figure 24.
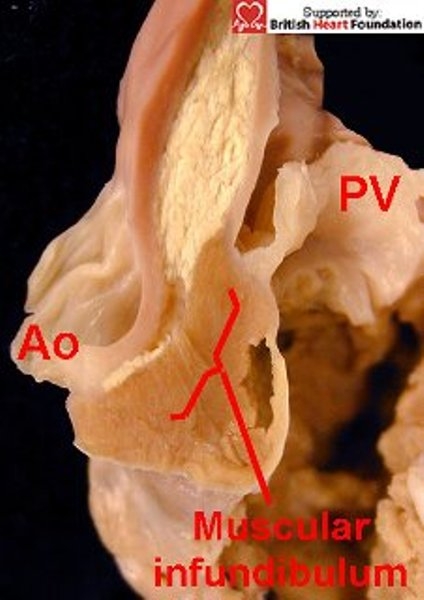
In the normal setting, and seen here in tetralogy of Fallot, the arterial valves are separated by a free standing muscular infundibulum, so the hinge-point attachment of the pulmonary valve (PV) is raised away from the aortic root.(Ao)
Figure 25.
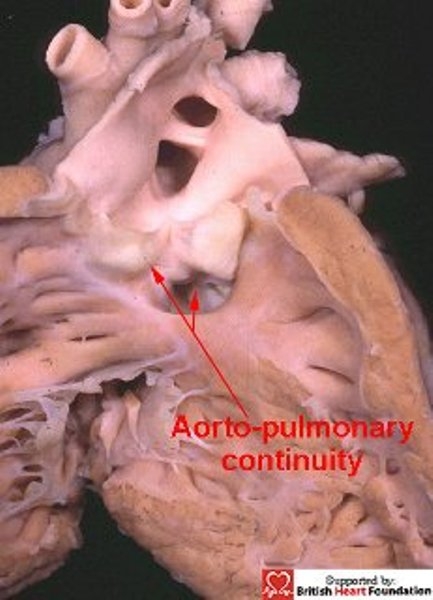
With doubly committed defects, the arterial valves are at the same level, with fibrous continuity between the leaflets of the aortic and pulmonary valves
Figure 26.
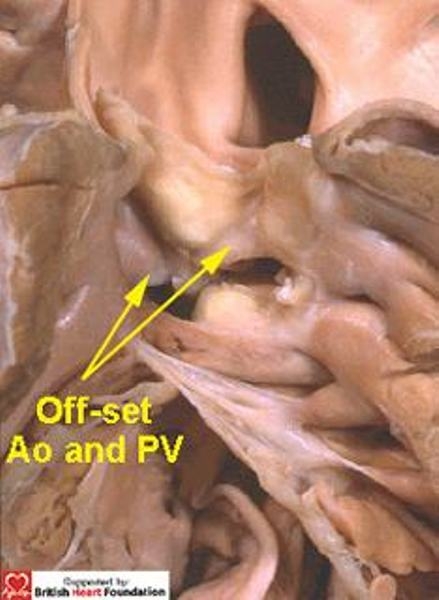
Although this defect is doubly committed, the arterial valves are still off-set
So the doubly committed defect, therefore, exists where there is absence of formation of a completely muscular infundibulum within the right ventricle. Because of this lack of infundibulum, the orifice of one (Fig.25), or sometimes both (Fig.26), of the arterial valves can override the crest of the ventricular septum.
This is the arrangement seen with common arterial trunk, in which the septal defect is again doubly committed and juxtaarterial (Fig.27).
Figure 27.
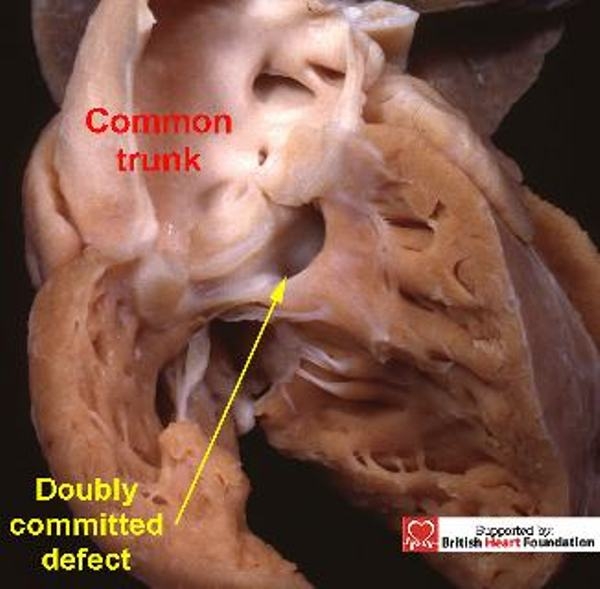
By its very nature, the defects seen with common arterial trunk, is doubly committed and juxtaarterial, and is seen here arising overriding the venticular septum
Typically, the common trunk arises astride the ventricular septum, with the septal defect having exclusively muscular rims as seen from its right ventricular aspect (Fig.28). With all these defects, the key to the vulnerability of the conduction axis is once more the nature of the postero-inferior rim. Usually this is muscular, and providing the muscle bundle is substantial (Figs.28,29), it will protect the conduction axis. Should the muscular tissue become attenuated, or disappear, so that the doubly committed defect extends to become perimembranous, (Fig.30) then the conduction axis is at risk in the postero-inferior margin.
Figure 28.
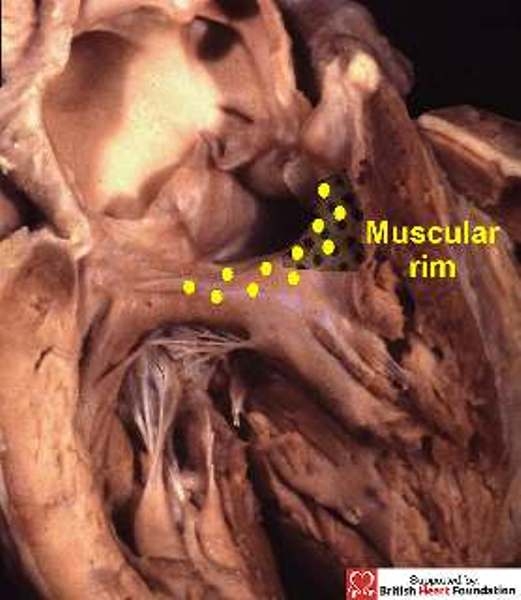
In this heart with common trunk, there is a muscular postero-inferior rim (yellow dots), separating the truncal valve from the tricuspid valve
Figure 29.
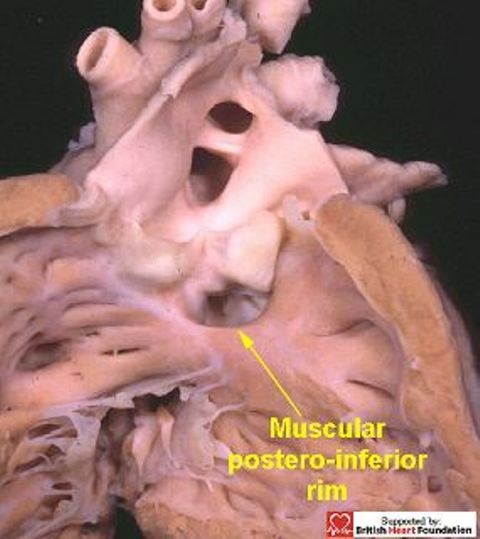
The postero-inferior rim of the defect is usually muscular in doubly committed defects
Figure 30.
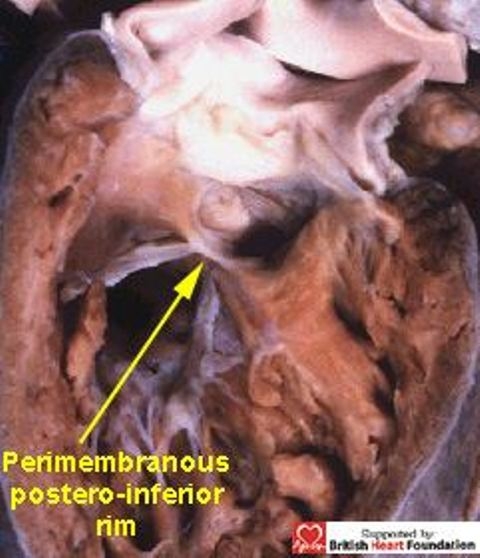
In contrast, the doubly committed defect in this heart extends to become perimembranous, with fibrous contunity between the leaflets of the aortic and tricuspid valves, as well as between the aortic and pulmonary valves
The doubly committed defect is frequently encountered in Asian countries.13 The crucial phenotypic feature of this defect is the altered morphology of the arterial trunks with respect to the normally structured heart. This defect can only exist in absence of the “septal” component of the free-standing infundibular sleeve which normally supports the pulmonary trunk, a trait seen in hearts with common arterial trunk. It is no coincidence, therefore, that both these lesions are found in the setting of 22q11 deletion, pointing to their similar genetic background which itself is more prevalent in far-Eastern populations.8,14,15
Associated features
The likelihood of spontaneous closure of any ventricular septal defect depends largely on its location. It is usually thought that the majority of defects that close are perimembranous, followed by muscular defects. It is also likely that many small muscular defects do not come to the attention of the cardiologist, the defects being present during fetal life, yet closing during the neonatal period16 and first year of life. Similarly, the classical murmur of the “maladie du Roger” is probably due to small, isolated, muscular defects that close with time. In contrast, defects sufficiently large enough to warrant referral to a tertiary centre, show no correlation between the probability of closure and the size of the defect. Thus, Du et al17 found solitary muscular defects were larger than multiple ones, yet the former were more likely to close spontaneously. Large defects found in the apical region of the septum are most likely to remain open. Conversely, spontaneous closure of doubly committed defects is not at all common.18 When it does occur, it is due to prolapse of the aortic valve leaflets, so it is not necessarily a good thing.
In this respect, the timing of surgical or interventional repair to prevent prolapse of the relatively unsupported leaflets of the arterial valves is of the essence. The intense mechanical stresses due to a high-pressure environment at the ventriculoarterial junction greatly increase the risk of prolapse of the leaflets of the aortic valve. The valvar tissues become exposed to increased velocities, and are then subject to progressive mechanical weakness. The uncertain integrity of the arterial valves, leads many centres to recommend early surgical treatment of the doubly committed defect.19–21 In the extensive study by Tohyama and colleagues,22 it was noted that seven-tenths of patients aged up to 35 years developed prolapse of the aortic valve, all involving the right coronary leaflet, and a third of the total developed overt aortic regurgitation. Early diagnosis and repair of the doubly committed defect has been suggested to prevent the onset of aortic regurgitation. Offsetting of the arterial valves has also been thought to be a factor in the formation of aneurysm of the right coronary sinus of Valsalva.
Conclusion
The most common congenital cardiac lesions are holes between the ventricles. These can exist at any location within the muscular septum, and can be viewed as opening into any part of the right ventricle. When attention is paid to the borders of the hole as viewed from the right ventricle, then all defects can be placed in one of three phenotypic groups. Early diagnosis for such defect using echocardiography is now routine, even in early fetal life.23 The phenotypic features we have suggested for diagnosis are just as valid in this setting as in postnatal life. With exact fetal diagnosis, it will almost certainly prove possible to identify those defects that are the most likely to close, as opposed to those which will require interventional or surgical treatment. For those requiring treatment, use of this information for anatomic categorisation permits an accurate assessment of any individual defect relative to the atrioventricular conduction axis, knowledge that is essential to the surgeon in order to achieve successful closure without fear of inducing atrioventricular block. Thus, proper diagnosis, particularly of the doubly committed defect, should now be the prelude to timely successful management, and hopefully a normal post-interventional or post-operative outcome.
References
- 1.McCarthy KP, Ho SY, Anderson RH. Categorisation of ventricular septal defects: review of the perimembranous morphology. Images Paediatr Cardiol. 2000;3:21–30. http://www.magnet.mt/health/impaedcard . [PMC free article] [PubMed] [Google Scholar]
- 2.Franklin RCG, Anderson RH, Daniëls O, Elliott M, Gewillig MHML, Ghisla R, Krogman ON, Ulmer HE, Stocker FP. The European Paediatric Cardiac Code – The Short List. Cardiol Young. 2000;10(Suppl 1):8–26. [PubMed] [Google Scholar]
- 3.Franklin RCG. The European Paediatric Cardiac Code Long List: structure and function. Cardiol Young. 2000;10(Suppl 1):27–146. doi: 10.1017/s1047951100007770. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs JP, Burke RP, Quintessenza JA, Mavroudis C. Congenital Heart Surgery Nomenclature and Database Project: ventricular septal defect. Ann Thorac Surg. 2000;69(Suppl 4):S25–35. doi: 10.1016/s0003-4975(99)01270-9. [DOI] [PubMed] [Google Scholar]
- 5.Anderson RH, Ho SY, Becker AE. Anatomy of the human atrioventricular junctions revisited. Anatomical Record. 2000;260:81–91. doi: 10.1002/1097-0185(20000901)260:1<81::AID-AR90>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Allwork SP, Anderson RH. Developmental anatomy of the membranous part of the ventricular septum in the human heart. Br Heart J. 1979;41:275–280. doi: 10.1136/hrt.41.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soto B, Becker AE, Moulaert AJ, Lie JT, Anderson RH. Classification of ventricular septal defects. Br Heart J. 1980;43:332–343. doi: 10.1136/hrt.43.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McElhinney DB, Anderson RH. Developmental anomalies of the outflow tracts and aortic arch: towards an understanding of the role of deletions within the 22nd chromosome. Cardiol Young. 1999;9:451–457. doi: 10.1017/s1047951100005321. [DOI] [PubMed] [Google Scholar]
- 9.Gatzoulis MA, Li J, Ho SY. The echocardiographic anatomy of ventricular septal defects. Cardiol Young. 1997;7:471–484. [Google Scholar]
- 10.Kumar K, Lock JE, Geva T. Apical muscular ventricular septal defects between the left ventricle and the right ventricular infundibulum. Circulation. 1997;95:1207–1213. doi: 10.1161/01.cir.95.5.1207. [DOI] [PubMed] [Google Scholar]
- 11.Stellin G, Padalino M, Milanesi O, Rubino M, Lasarotto D, Van Praagh R, Van Praagh S. Surgical closure of the apical ventricular sepral defect through a right ventricular apical infundibulotomy. Ann Thorac Surg. 2000;69:597–601. doi: 10.1016/s0003-4975(99)01333-8. [DOI] [PubMed] [Google Scholar]
- 12.Latham RA, Anderson RH. Anatonical variations in atrioventricular conduction system with reference to ventricular septal defects. Br Heart J. 1972;34:185–190. doi: 10.1136/hrt.34.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Momma K, Toyama K, Takao A, Ando M, Nakazawa M, Hirosawa K, Imai Y. Natural history of subarterial infundibular ventricular septal defect. Am Heart J. 1984;108:1312–1317. doi: 10.1016/0002-8703(84)90759-2. [DOI] [PubMed] [Google Scholar]
- 14.Momma K, Ando M, Matsukova R, Soo K. Interruption of the aortic arch associated with deletion of chromosome 22q11 is associated with a subarterial and doubly committed ventricular septal defect in Japanese patients. Cardiol Young. 1999;9:463–467. doi: 10.1017/s1047951100005357. [DOI] [PubMed] [Google Scholar]
- 15.Momma K, Ando M, Matsukova R, Soo K. Truncus arteriosus communis associated with chromosome 22q11 deletion. J Am Coll Cardiol. 1997;30:1067–1071. doi: 10.1016/s0735-1097(97)00240-4. [DOI] [PubMed] [Google Scholar]
- 16.Orie J, Flotta D, Sherman FS. To be or not to be a VSD. Am J Cardiol. 1994;74:1284–1285. doi: 10.1016/0002-9149(94)90568-1. [DOI] [PubMed] [Google Scholar]
- 17.Du ZD, Roguin N, Wu X-J. Spontaneous closure of muscular ventricular septal defect identified by echocardiography. Cardiol Young. 1998;8:500–505. doi: 10.1017/s1047951100007174. [DOI] [PubMed] [Google Scholar]
- 18.Sim EKW, Grignani RT, Wong ML, Quek SC, Wong JCL, Yip WCL, Lee CN. Influence of surgery on aortic valve prolapse and aortic regurgitation in doubly committed subarterial ventricular septal defect. Am J Cardiol. 1999;84:1445–1448. doi: 10.1016/s0002-9149(99)00594-9. [DOI] [PubMed] [Google Scholar]
- 19.Santini F, Mazzucco A. Timing for surgical closure of subpulmonary ventricular septal defects in infancy. Am J Cardiol. 1997;80:976–977. [PubMed] [Google Scholar]
- 20.Komai H, Naito Y, Fujiwara K, Noguchi Y, Nishimura Y, Uemura S. Surgical strategy for doubly committed subarterial ventricular septal defect with aortic cusp prolapse. Ann Thorac Surg. 1997;64:1146–1149. doi: 10.1016/s0003-4975(97)00718-2. [DOI] [PubMed] [Google Scholar]
- 21.de Leval MR, Pozzi M, Starnes V, Sullivan ID, Stark J, Sommerville J, Anderson RH, Deanfield JE. Surgical management of doubly committed subarterial ventricular septal defects. (III-46).Circulation. 1988;78(suppl III):III–40. [PubMed] [Google Scholar]
- 22.Tohyama K, Satomi G, Momma K. Aortic valve prolapse and aortic regurgitation associated with subpulmonic ventricular septal defect. Am J Cardiol. 1997;79:1285–1289. doi: 10.1016/s0002-9149(97)00105-7. [DOI] [PubMed] [Google Scholar]
- 23.Carvalho JS, Moscoso G, Ville Y. First trimester transabdominal fetal echocardiography. Lancet. 1998;351:1023–1027. doi: 10.1016/S0140-6736(97)08406-7. [DOI] [PubMed] [Google Scholar]


