Abstract
The implantation of a mechanical circulatory device for end-stage ventricular failure is a possible therapeutic approach in adult and pediatric cardiac surgery and cardiology. The aim of this article is to present mechanical circulatory assist devices used in infants and children with special emphasis on extracorporeal membrane oxygenation, Berlin Heart assist device, centrifugal pump and Medos assist device. The success of long-term support with implantable ventricular assist devices in adults and children has led to their increasing use as a bridge to transplantation in patients with otherwise non-treatable left ventricular failure, by transforming a terminal phase heart condition into a treatable cardiopathy. Such therapy allows rehabilitation of patients before elective cardiac transplantation (by removing contraindications to transplantation mainly represented by organ impairment) or acting as a bridge to recovery of the native left ventricular function (depending on underlying cardiac disease). Treatment may also involve permanent device implantation when cardiac transplantation is contraindicated. Indications for the implantation of assisted circulation include all states of cardiac failure that are reversible within a variable period of time or that require heart transplantation. This article will address the current status of ventricular assist devices by examining historical aspects of its development, current technical issues and clinical features of pediatric ventricular assist devices, including indications and contraindications for support.
MeSH: Ventricular assist device, ECMO, Centrifugal pump, Heart failure, Medos, HeartMate, Berlin Heart, Thoratec, Jarvik 2000
Definition
A ventricular assist device is a pump that is attached between the heart and the aorta or pulmonary artery which helps to circulate a person's blood when the heart can no longer adequately support circulation. The aim of such a device is to unload the heart and to provide an adequate peripheral circulation with sufficient organ function. In reducing cardiac work and oxygen consumption the required energy for repair processes and synchronized heart performance can be spared and leads to a quicker recovery of “stunned” myocardial segments.1
There is great interest in implanting ventricular assist devices for circulatory support in children. Ventricular assist devices of various design and function principles have been used for temporary support of failing hearts aiming at myocardial recovery or keeping the patient alive until later transplantation. Despite improvements in operative techniques, management of cardiopulmonary bypass and myocardial protection, myocardial dysfunction can occur after operation for complex congenital heart disease with involvement of the left and right ventricle.2 Approximately 5 % of children undergoing open heart surgery need mechanical circulatory assistance.3 Reports of ventricular assist device support in smaller children (< 20 kg) remain limited.2 This is partly due to technical considerations, for example, low flow rates may be a risk for thromboembolic complications when adult-sized systems are applied in children.4 Moreover, left ventricular assist devices can only be inserted when intracardiac shunts are closed, and right ventricular assist devices require some kind of pulmonary valve.5
Owing to the lack of sufficiently small, commercially available valves, and the tendency for thrombus formation in small pumps, the attempt to scale down adult blood pumps for pediatric use remains difficult. However, the need for pediatric ventricular assist devices is increasing: first owing to the possibility of corrective operations for many previously inoperable forms of complex congenital heart disease in younger and smaller patients than before, and second, to the success of pediatric heart transplantations which leads to an increase of the number of transplant candidates and of the waiting time for the rare donor organs.
A surgical infrastructure consisiting of a well equipped neonatal and pediatric intensive care unit and a permanently accessible laboratory for monitoring coagulation status is needed to obtain satisfactory results.
History of ventricular assist devices in adults
To repair and replace a heart a medical dream. The first concept of circulatory support as a prosthesis that could replace the heart and sustain sufficient flow for peripheral circulation was probably postulated by the French physician Legallois in 1812.6,7 In 1849 Loebell began his work on an isolated kidney model and in 1854 Claude Bernard conducted the famous foie lavé experiment.8 Between 1848 and 1858 Brown-Séquard demonstrated the need to oxygenate the blood that was used as perfusion solution. In 1868 Ludwig and Schmidt achieved extracorporeal oxygenation, using venous blood-gurgle in a flask. Von Schroeder built the first steady-flow bubble oxygenator in 1882; Frey and Gruber developed the first film oxygenator in 1885. In 1916 McLean discovered heparin, which allowed the blood to circulate through artificial tubes for a longer time. Dale and Schuster in 1928 were the first to construct a diaphragm pump. The transatlantic flyer Lindbergh, spurred on by his sister-in-law's heart disease, together with Carrel, developed an oxygenating pump, and demonstrated the possibility of extracorporeal perfusion. In 1934 DeBakey introduced the concept of a continuous flow blood transfusion instrument that was a simple roller pump.9 In 1937 Gibbon described a heart-lung machine and reported on first animal experiments. In 1954 Gibbon carried out the first successful human open heart operation (atrium septal defect closure on a 18 year old girl) with the use of the heart-lung machine.10,11 In 1957 Akutsu and Kolff implanted two compact pumps into a dog's chest following cardiectomy. In 1961 Dennis performed a left heart bypass by inserting cannulae in the left atrium and returning blood through the femoral artery.12
In 1962 Kolff and Moulopoulos developed the intraaortic balloon pump and demonstrated their value in an animal model.13 In 1966 Kantrowitz performed its first clinical application in a 45-year-old woman in cardiogenic shock.14 In 1963 Spencer supported a 6-year old girl after an operation to repair a ventricular septal defect.15 In 1964 Liotta published results from the first clinical implantation of a pulsatile left ventricular assist device.9 In 1968 Raffert reported on a centrifugal pump for blood pumping. In 1969 Cooley implanted the first artificial heart, keeping the patient alive for over sixty hours. The Jarvik-7 artificial heart, named after its designer Robert K. Jarvik, an American physician, was first used during the early 1980s16 and in 1982 a team led by DeVries of the University of Utah implanted the Jarvik-7 for the first time. The patient survived with a Jarvik-7 for 112 days. At that time patients were anchored to large consoles with poor quality of life. The Jarvik-7 was further developed by Cardiowest and is nowadays known as CardioWest Artificial Heart.
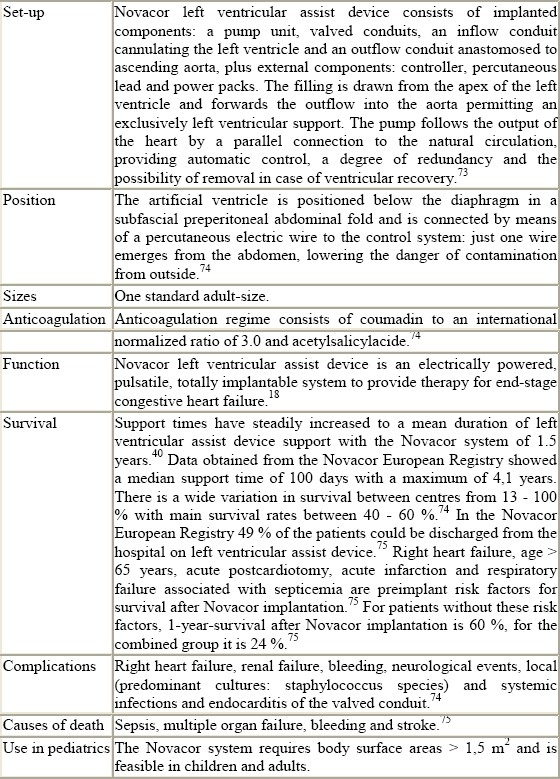
The Novacor LVAD (Baxter Corp., Oakland, CA), initially designed for permanent use, has been used as a bridge to transplantation since 1984. Unlike Jarvik this ventricle is placed heterotopically below the diaphragm allowing the natural heart to remain in place.17 Early systems required a large console but eventually, a wearable version, supported by a small electronic controller and batteries worn on a belt, was introduced in 1993.18–20
Since 1987 centrifugal Biomedicus pump started in clinical use. In 1988 “Berlin Heart” (Berlin Heart Institut, Mediport Kardiotechnik Berlin, Germany) pumps became available.21 The first HeartMate ventricular assist devices were implanted in 1992. The Medos ventricular assist device has been in clinical use since 1994 with the first implantation in an adult patient performed by Sievers. In 1994 Abiomed BVS 5000 ventricular assist device became available at Hahnemann University Hospital, Philadelphia, PA.22
History of ventricular assist devices in children
Despite the success of ventricular assist devices in adults, little has been done to develop similar devices for pediatric patients. Scientific and engineering efforts have all been focused on heart-lung machines and circulatory support systems for adults. Indeed, the first neonatal oxygenator was released in the 1990s.23 In 1973 Soeter described the use of extracorporeal life support in a 4-year-old girl with severe hypoxemia after repair of Tetralogy of Fallot.24 The system employed used a rotary pump with a membrane oxygenator and a heat exchanger. The patient was weaned from the support within 48 hours and was discharged on the 13th postoperative day. In 1974 Bartlett reported the use of extracorporeal membrane oxygenation in pediatric patients with respiratory failure or after repair of congenital heart disease.25,26 Hill and Pyle described two patients who had severe postcardiotomy dysfunction, and who both died despite the implantation of extracorporeal membrane oxygenation.27,28 In 1989 the Extracorporeal Life support Organization was founded as an organization to study the clinical use of extracorporeal membrane oxygenation and maintained the Extracorporeal Life support Organization registry which compiles data on the use of extracorporeal membrane oxygenation. These data show that pediatric cardiac support has accounted for approximately 13 % of total extracorporeal membrane oxygenation utilization.29
In 1980 Pollock reported the first use of an intraaortic balloon pump in children,30 when a 6 year old child was pumped following a cardiac operation. In 1983 Veasy reported the use of small balloon catheters in 15 children.31 Despite the commercial availability of pediatric-sized balloon catheters in the early 1980s, intraaortic balloon pump for children and infants has not become widespread.
The Thoratec ventricular assist devices (Thoratec Laboratories Corporation, Pleasanton, CA) have been available since the early 1980s, and can be implanted in children and adolescents.32 Ventricular assist devices using centrifugal pumps have been used for infants and children since the development of a pediatric centrifugal pump head by Medtronic Bio-Medicus (Eden Prairie, MN) in the late 1980s.33–35
In 1992, the “Berlin Heart” offered the first commercially available system with miniaturized paracorporeal pumps and cannulae.21 In the same year an 8-year-old child was supported with a Berlin Heart for 8 days in intractable circulatory failure, followed by a successful transplantation and an uneventful postoperative course.21 The first implantation of a Medos ventricular assist device as a bridge to transplant took place in 1994.36
Types of ventricular assist devices
The use of temporary ventricular assist devices in adults and children is now routine, and the development of permanent ventricular assist devices and total artificial hearts is the subject of ongoing research in the United States, Europe, Australia, Japan, China, Korea, Russia and other countries. A detailed description of all these devices is beyond the scope of this document and only those with significant design features or devices sufficiently developed to be nearing clinical trials will be discussed.
Blood pumps are divided into two main groups according to the nature of their flow: nonpulsatile and pulsatile pumps. Nonpulsatile pumps produce flat continuous flow in which there is no pulse pressure. These pumps include centrifugal pumps and axial flow pumps. As a bridge to cardiac transplantation in adults the most widely used devices are those with pulsatile flow (Abiomed, Thoratec, Novacor, Berlin Heart, Heart Mate, Medos). In these devices blood is forced by positive pressure produced by the pump, squeezing the artificial ventricle which is contained within a rigid shell. Overall survival rates range in non-pulsatile centrifugal pumps is between 25 - 40 %2,37,38,39 and in the pulsatile pneumatic or electromechanical pumps between 25 - 80 %.40,39,38
Different devices function similarily. Blood is generally drawn at left/right atrial/ventricular level, run into an artificial ventricle and is reintroduced at aortic and/or pulmonary level. With these systems it is possible to maintain a paraphysiological circulation for long periods of time.
Energy sources are pneumatic for devices like Thoratec, Abiomed, Medos, Berlin Heart, Heart Mate and electric for extracorporeal membrane oxygenation, Novacor and Heart Mate. The patient's hemodynamic characteristics determine the use of mono- or biventricular assist devices. Implantation of left ventricular assist devices is contraindicated in the presence of high pulmonary vascular resistance, shunting or if, shortly after implantation of left ventricular assist device, fall in cardiac index, reduced diuresis and central venous pressures > 25 mmHg are recorded: in these cases a biventricular device is needed. In children such problems as low cardiac output syndrome and right heart failure including pulmonary vasoreactive crises after cardiac operations are common.4
Intraaortic balloon pump
Figure 1.
intraaortic balloon pump
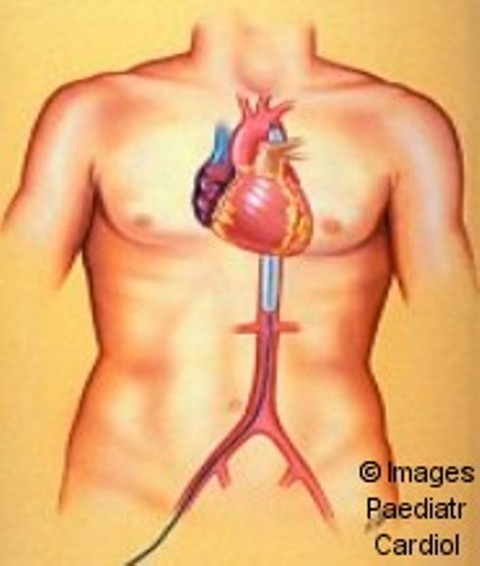
Extracorporeal membrane oxygenation (ECMO)
Figure 2.
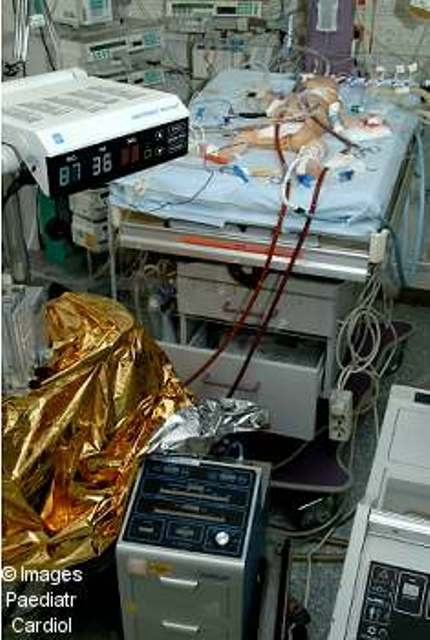
extracorporeal membrane oxygenation in a 7 days old child after Damus-Kaye-Stansel operation
Centrifugal pump ventricular assist device
Figure 3.

Bio-Pump blood pump
Berlin Heart
Figure 4.
Berlin Heart ventricular assist device
Figure 5.

Berlin Heart pediatric pump
Medos
Figure 6.
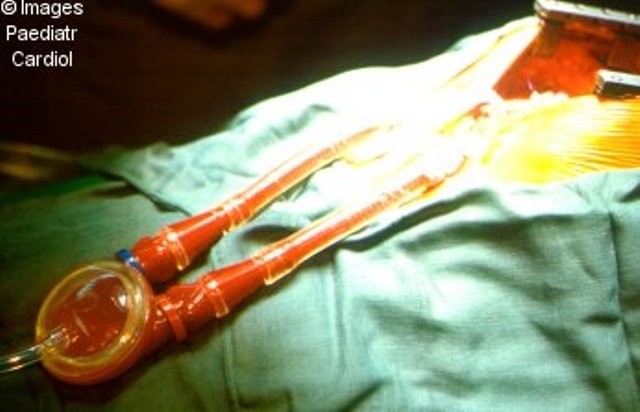
Medos ventricular assist device
Thoratec
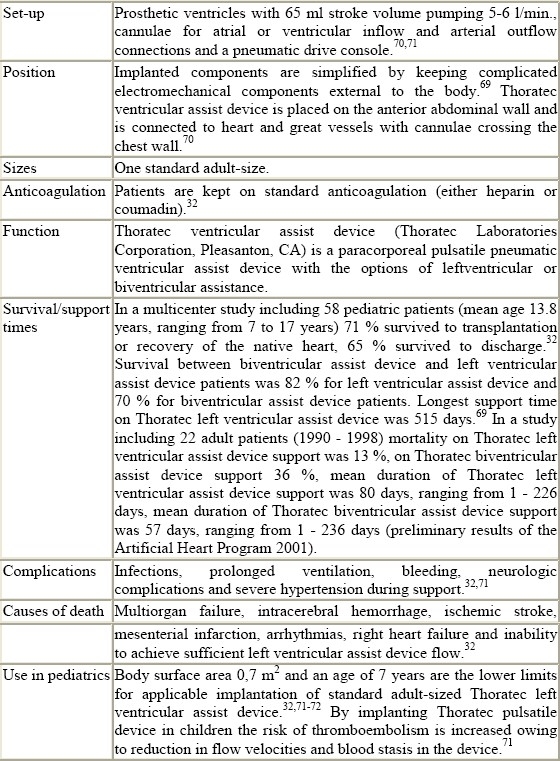
Figure 7.
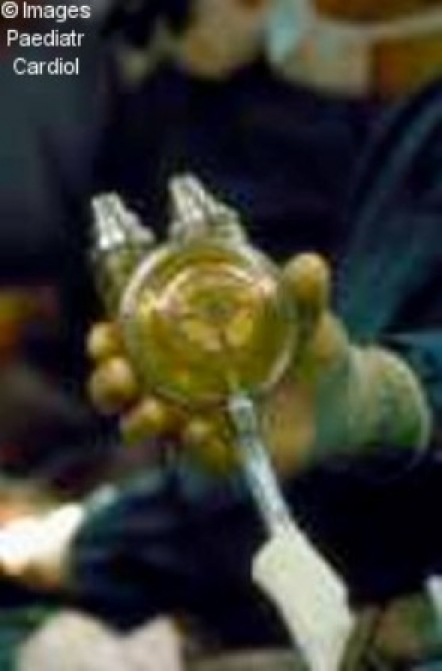
Thoratec pump
Novacor
HeartMate
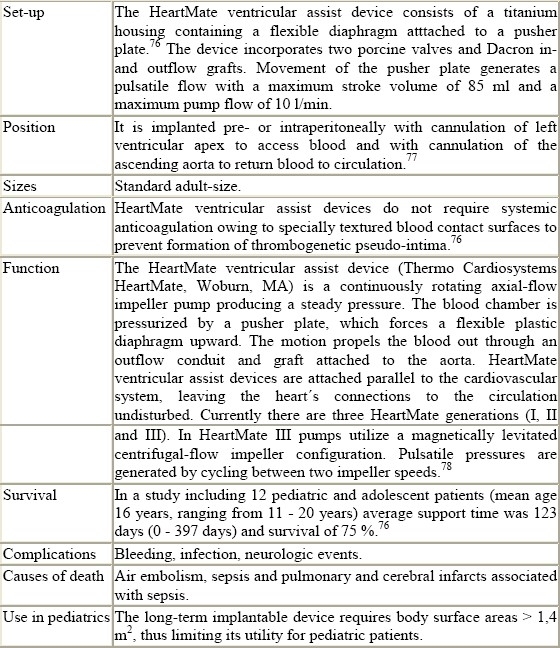
Figure 8.
HeartMate pneumatic ventricular assist device
Abiomed
Figure 9.
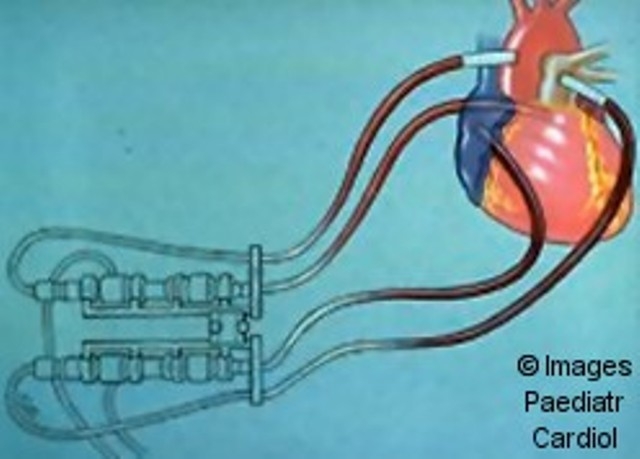
Abiomed ventricular assist device
Jarvik 2000
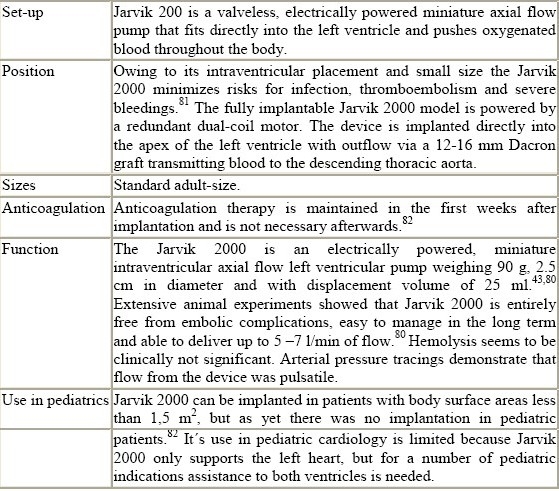
This system has the potential for use as a permanent ventricular assist device or as a bridge to transplantation or recovery in adults and pediatric patients needing left ventricular support exclusively.
Figure 10.
Jarvik 2000
Table 1.
Technical details of ventricular assist devices (summarised)
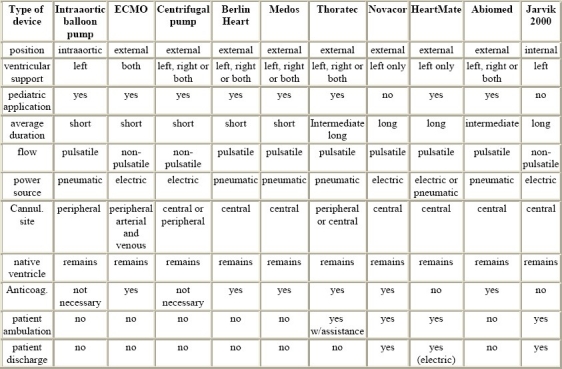
Indications and contraindications for the implantation of ventricular assist devices
No precise guidelines for the implantation of ventricular assist devices in pediatrics exist. The decision for implantation of a ventricular assist device can only be made by assessing the individual clinical situation of each patient with the help of a careful diagnostic approach. The indications for the use of ventricular assist device in pediatric cardiology depend on individual units’ experiences.
The indications for the implantation of ventricular assist devices can be divided in cases of patients without operation like myocarditis, endocarditis, neoplasm or Eisenmenger syndrome, and postoperative indications with deterioration in preoperative ventricular function exacerbated by surgery (for example Bland-White Garland syndrome, hypoplastic left heart syndrome, neoplasm, postoperative change of hemodynamics, arterial switch in transposition of the great arteries after the neonatal period, secondary arterial switch after atrial switch or congenitally corrected transposition of the great arteries, Fontan or Fontan-type procedures with postoperative left ventricular deterioration, pulmonary hypertensive crisis refractory to prostayclin or nitric monoxide treatment, right ventricular failure in tetralogy of Fallot, myocardial failure from prolonged cross-clamp and cardiopulmonary bypass time, Kawasaki disease or procedures with intraoperative complications).5
Surveillance before implantation of a ventricular assist device is directed at registering onset of premonitory manifestations of potentially untreatable low flow rates. Ventricular arrhythmias, hypoxemia and renal dysfunction constitute prodromes of reduced flow and can be taken as indication criteria for ventricular assist devices. Organ dysfunction is also one of the main problems in ventricular assistance owing to the impossibility of determining reversal of organ damage. Contraindications for the implantation of a ventricular assist device include severe renal and hepatic failure, irreversible septic shock, severe neurological damage and hemorrhage.83
Contraindications for implantation of circulatory assist systems are the same as for cardiac transplantation as cardiac recovery is uncertain. Multiorgan failure is a clear contraindication, but early states of multiorgan failure can be reversed by circulatory mechanical assistance. Coagulation disorders, intracranial bleeding and severe neurological complications preclude the implantation of assist devices. Acute or chronic infection impair the implantation of a mechanical assist system,84 but the implantation of an assist system in viral myocarditis is possible.85 Patients after a prolonged run of cardiopulmonary bypass seem to be less favourable for mechanical assist as hemostasis is crucial for eventual outcome. Selection of the most appropriate support system may be decisive for survival of critically ill infants with an otherwise untreatable cardiac condition.
Intraaortic balloon pump
In predominantly right ventricular failure which is often observed in congenital heart disease, intraaortic balloon pump is ineffective. In addition, myocardial ischemia for which intraaortic balloon pumps are useful is an uncommon cause of congestive heart failure in pediatrics. Intraaortic balloon pump appears of therapeutical value for children with a more moderate form of left ventricular dysfunction, but severe cases require the implantation of a left ventricular assist device.
A general indication is low cardiac output despite adequate treatment evinced by poor peripheral perfusion (low mean aortic pressure), metabolic acidosis, mixed venous pO2 < 25 mmHg, urine output < 1 ml/kg/h and left atrial pressure > 20 mmHg.41 Malignant ventricular arrhythmias due to low coronary blood flow and high catecholamine support can profit from intraaortic balloon pump. Contraindications are patent ductus arteriosus, recent coarctation or aortic arch repair and significant aortic valve insufficiency.
Extracorporeal membrane oxygenation
Biventricular support with extracorporeal membrane oxygenation23 is the predominantly used ventricular assist device in children with heart disease who have failed conventional medical treatment. The use of extracorporeal membrane oxygenation has been proven to be life-saving in children with myocardial failure, because of the advantages of rapid priming of both right and the left heart and support of pulmonary function. Hypoxia and pulmonary hypertension are indications for circulatory support with extracorporeal membrane oxygenation.86
Postoperative support is established for failure to wean from cardiopulmonary bypass, cardiogenic shock or cardiac arrest after cardiac surgery.52,55,87,88 Treatment with high-frequency ventilation, nitric oxide and liquid ventilation can fail when severe hypoxia occurs in the setting of congenital heart disease. Extracorporeal membrane oxygenation is the method of choice in the persistence of intracardiac shunts or in cases of profound respiratory insufficiency accompanying heart failure. Examples of congenital heart diseases often requiring postoperative circulatory support like extracorporeal membrane oxygenation are congenital heart diseases with shunts, total anomalous pulmonary venous connection because of preoperative respiratory problems and postoperative pulmonary hypertensive crisis.64
When myocardial recovery can be expected and immediate weaning of the extracorporeal circuit is not possible, extracorporeal membrane oxygenation is the method of choice for a few days after open heart surgery, for example after switch operation, total anomalous pulmonary vein drainage, Bland White Garland syndrome or after heart transplantation.89
Ishino recommends extracorporeal membrane oxygenation as a first choice for biventricular support after arterial switch operation, particularly in unusual and complex coronary patterns.64,90 When right or biventricular failure develops in infants with imbalanced ventricles, extracorporeal membrane oxygenation is the optimal method of circulatory support. In cases of lung failure and pulmonary hypertension beside underlying cardiac diseases, implantation of extracorporeal membrane oxygenation should be favoured.91 Pulmonary hypertension is a contraindication for the implantation of a left ventricular assist device because of the high risk for right ventricular failure after left ventricular assist device insertion.83
In a study involving cardiac and non-cardiac patients, indications for extracorporeal membrane oxygenation were hypoxia (36 %), cardiac arrest (24 %) and failure to wean from cardiopulmonary bypass (14 %).23 Several studies emphasize failure to wean from cardiopulmonary bypass as a negative prognostic indicator,23,52,62,88 whereas other studies could not find a negative impact.55,57,92
Several studies stress the importance of early institution of extracorporeal membrane oxygenation, before prolonged periods of low cardiac output result in severe organ damage. Low cardiac output in congenital heart disease with shunted single ventricle physiology, including patients with hypoplastic left heart syndrome after neonatal palliation, is an indication for implantation of extracorporeal membrane oxygenation.48,57,88 Blood flow through shunts should be limited to ensure systemic perfusion and to avoid excessive pulmonary “flooding”. Contraindications include malignancy, multisystem organ failure, prematurity and severe central nervous system damage.48,51,56,88
Centrifugal pump ventricular assist device
The clinical application of centrifugal ventricular assist devices has generally been limited to adults and large pediatric patients, but neonates and small pediatric patients requiring ventricular support post-cardiopulmonary bypass are also well supported by a centrifugal ventricular assist device.
In a major study,2,37 the majority of patients receiving Biomedicus supporting systems had undergone palliative or reparative open heart operations (for cyanotic heart vitia with increased or decreased pulmonary flow, right or left sided obstructive lesions, Bland White Garland syndrome) or transplantation, and could not be weaned from cardiopulmonary bypass, a subset therefore presenting with low cardiac output following satisfactory weaning. Only rarely was low cardiac output due to myocarditis or cardiomyopathy a primary indication.93 According to Konertz, indications for implantation of centrifugal pumps are acute (nonsurgical) cardiogenic shock, rescue postcardiotomy support, low cardiac output after long pump runs in complex cardiac reconstructions and bridge to transplantation.84 Centrifugal ventricular assist device can be applied in univentricular circulation, for example after Norwood operation for hypoplastic left heart syndrome or after bidirectional cavopulmonary shunts.2 Contraindications are the same as mentioned for extracorporeal membrane oxygenation.
Novacor
The main indications for implantation of Novacor systems are ischemic or idiopathic cardiomyopathy, acute myocardial infarction and myocarditis in adults.19,74,75
Berlin Heart
Indications for implantation of a Berlin Heart assist device include bridge to transplant in chronic myocardial diseases or end stages of congenital heart disease, rescue therapy after operation for congenital heart disease, acute viral myocarditis,21 cardiomyopathies, myocardial infarction, repeated cardiopulmonary resuscitation and postcardiotomy cardiogenic shock.63,90 The best candidates for its application are patients with biventricular hearts without intracardiac shunting.91 There is the possibility of switching from biventricular assist device to extracorporeal membrane oxygenation for easier weaning once normal functional patterns of the initially akinetic ventricles are observed.63 Contraindications are the same as mentioned for extracorporeal membrane oxygenation.
Thoratec
Patients receiving a Thoratec assist device suffer from various forms of end-stage cardiomyopathies, myocarditis, underlying congenital defects (transposition of the great arteries, Ebstein's anomaly, tetralogy of Fallot) and post-cardiotomy cardiac failure.32
Medos
Indications for implantation of Medos ventricular assist device include heart failure after surgery for congenital heart diseases, cardiomyopathy or acute myocarditis, low cardiac output or cardiac arrest at the intensive care unit following successful weaning from cardiopulmonary bypass.36 In a study at the Charité, Germany, indications were cardiogenic shock and postoperative failure (57 % recovery). Patients in cardiogenic shock had high mortality, only a minority could be successfully weaned or transplanted. In contrast patients with postcardiotomy heart failure had a success rate of 50 % either by weaning or by transplantation.36,68 Contraindications are multiorgan failure, severe coagulopathy, intracranial hemorrhage, neurological impairment and sepsis.36
HeartMate
Severe cardiac failure in chronic dilated cardiomyopathy, resuscitation, acute myocarditis, and Ebstein's anomaly are reasons for implantation of HeartMate ventricular assist device in adolescent and pediatric patients.76
Abiomed BVS 5000
The typical indications are failure to wean from cardiopulmonary bypass, precardiotomy shock, viral myocarditis, myocardial infarction and intractable arrhythmia.22,94 Abiomed BVS 5000 is predominantly used for short-term mechanical support in postcardiotomy patients in cardiogenic shock expecting myocardial.79,56
Predictive factors
The timing of the installation of a ventricular assist device is crucial for outcome of patients needing circulatory support. Several studies have shown that survival is better when extracorporeal life support is instituted after successful weaning from cardiopulmonary bypass.52,53,56,57,88 Therefore the distinction between postcardiotomy patients on ventricular assist device because of failure to wean from cardiopulmonary bypass or because of myocardial dysfunction after successful weaning is important. Several studies group all sorts of postcardiotomy patients together and make comparison with other series difficult.87,96,97 Best survival in patients (47 %) who could not be weaned from cardiopulmonary bypass is reported by Ziomek.57 Overall necessity of ventricular assist device in this report is 6.8 %, a higher usage is reported by Raithel3,98 who found that 8.4 % of pediatric patients required ventricular assist device after cardiac surgery. The key to survival lies in the instution of ventricular assist device before severe organ damage can manifest.58
Patients with incomplete repair of congenital heart disease do badly. Black reports 100 % mortality in this group.49 Therefore complete surgical repair should be a prerequisite for the implantation of a ventricular assist device. Patients who suffer otherwise untreatable cardiac arrest after cardiac surgery may benefit of the installation of a ventricular assist device more than other groups. Del Nido reported survival rates of 64 %.96,99
The risk of embolic events remains a continuing concern. Formation of pseudo-intimas in the inflow conduit of ventricular assist devices was identified as a main source of embolism.100 The development of this friable, easily detachable neo-intima could be reduced by changes in physical characteristics of new inflow conduits: reduced compliance, elimination of graft crimp and shorter length. The dosage of anticoagulant medication must be adjusted to specific requirements of prolonged activation of biological coagulation cascades by using more biocompatible blood contacting surfaces.
Left ventricular venting to ensure complete decompression of the left heart is an important factor contributing to the patient's outcome. Ventricular overdistension can be minimized by maintenance of low central venous pressures and high flow rates.57 Otherwise left ventricular overdistension may result in elevation of left ventricular end-diastolic pressures when ventricular function is decreased and forward pulsatile flow is low. This may result in decreased coronary perfusion particularly of left ventricular subendocardial layers. In biventricular failure venting of the left ventricle is therefore advisable.62
A possible key to success in circulatory support (with the exception of the bridge to transplant concept) with ventricular assist devices lies in the reversibility of heart dysfunction. Once myocardial architecture is destroyed, no support can regenerate myocardial tissue. Emplacement of a ventricular assist device reduces pre- and afterload, this reduction in left ventricular volume decreases wall stress during ventricular assist device support period and helps avoid cell damage and cell loss in cardiomyopathies.101 Unloading of the heart by using mechanical circulatory support appears to reverse pathological ventricular remodeling occuring in cardiomyopathies.102 It is crucial to install circulatory support via ventricular assist device in time before myocardial architecture deteriorates.
Different assist devices can be combined, for example Biomedicus pump with intraaortic balloon pump to ensure pulsatile flow.59 Loss of pulsatility has been blamed for development of capillary leakage during prolonged pump runs.103
In order to support children with severe cardiac dysfunction following open heart surgery,it is possible to use extracorporeal membrane oxygenation without oxygenator as so-called “no membrane oxygenator-ventricular assist device”.104 In this configuration, the circuit becomes a roller pump ventricular assist device. This system, as opposed to the centrifugal pump system, retains the desirable effects of extracorporeal membrane oxygenation including pump servo-regulation, pressure monitoring, access ports for fluid/drug administration, air bubble detection, in-line blood gas monitoring and heat exchanger. In patients with a single ventricle, for example after Norwood stage I procedure, oxygenation is provided by the children's own lungs by leaving aortopulmonary shunts open.105
In a minority of patients, unloading of the left ventricle during left ventricular assist device allows a recovery of left ventricular function such that these patients can be weaned from circulatory assistance. In these selected patients ventricular assist device should not be a bridge to cardiac transplantation but rather to recovery.
Future development
The duration of circulatory support is extending continuously with the ultimate goal of total replacement of the heart. There is a growth in the number of patients with decompensated heart insufficiency in the adult and pediatric population, which cannot be sufficiently dealt with by heart transplants owing to the shortage of donor hearts. An alternative surgical solution could be extended use of ventricular assist devices as temporary or permanent implantation.
Children in advanced cardiac failure and in profound cardiogenic shock who would otherwise die immediately may be kept alive using ventricular assist devices and may either completely recover or qualify for successful heart transplantation. Widely used devices such as extracorporeal membrane oxygenation or other ventricular assist devices may sustain the circulation for several days or weeks. These systems require continuous intensive care, and chances for extubation and mobilization are very small. Pediatric ventricular assist devices should allow ambulation and rehabilitation of the patient. Rehabilitation is of vital importance in the adult and pediatric population. An implantable device of appropriate size would meet the needs of rehabilitation and ambulation. The aim is to design a device that could be used for long periods of time as a bridge to heart transplantation and with the possibility of its use as a permanent heart replacement device.
The Jarvik 2000 is a potential device that could fulfill these criteria.72 The number of heart transplantations is limited due to shortage of donor hearts. In order to salvage patients suffering from severe end-stage cardiac diseases, artificial hearts as permanent implants may alleviate this problem. At the same time an increased number of transplant-contraindicated patients are fitted with circulation assist devices of a permanent kind.
The possibility of having a new generation of systems so constructed to reduce the rate of complications, to minimize overall dimensions and to be easily managed (home care) should radically improve the risk-benefit ratio and economic concerns. However there are various problems to be solved: in addition to smaller, cheaper, simpler and more easily controllable pulsatile ventricular assist devices, there is a need for compact, durable and reliable nonpulsatile pumps, which can be completely implanted as a bridge to transplantation and/or for permanent use.
References
- 1.Waldenberger FR. Pathophysiological considerations concerning uni- and biventricular mechanical cardiac assist. Int J Artif Org. 1997;20:684–91. [PubMed] [Google Scholar]
- 2.Karl TR, Horton SB. Centrifugal pump ventricular assist device in pediatric cardiac surgery. In: Duncan BW, editor. Mechanical support for cardiac and respiratory failure in pediatric patients. New York: Marcel Dekker; 2001. pp. 21–47. [Google Scholar]
- 3.Raithel SC, Pennington DG, Boegner E, Fiore A, Weber TR. Extracorporeal membrane oxygenation in children after cardiac surgery. Circulation. 1992;86:305–10. [PubMed] [Google Scholar]
- 4.Herwig V, Severin M, Waldenberger FR, Konertz W. Medos HIA assist system: first experiences with mechanical circulatory assist in infants and children. Int J Artif Org. 1997;20:692–4. [PubMed] [Google Scholar]
- 5.Däbritz S, Messmer BJ. Individual center experiences in pediatric machanical circulatory support for bridge-to-transplant and myocardial recovery. In: Hetzer R, Hennig E, Loebe, editors. Mechanical circulatory support. Darmstadt: Springer; 1994. pp. 21–31. [Google Scholar]
- 6.Legallois JJC. Expérience sur le principe de la vie, notamment sur celui des mouvements du coeur, et sur le siège de ce principe; suivies du rapport fit à la première classe de l’Institut sur celles relatives aux mouvements du coeur. Paris: D’Hautel; 1812. [Google Scholar]
- 7.Fye WB. Julien Jean Cesar Legallois. Clin Cardiol. 1995;18:599–600. doi: 10.1002/clc.4960181015. [DOI] [PubMed] [Google Scholar]
- 8.Bernard C. Discours prononcé à sa réception à l’Académie française le 27 mai 1869. Paris: Didier; 1869. [Google Scholar]
- 9.Liotta D, Hall CW, Cooley DA, De Bakey ME. Prolonged ventricular bypass with intrathoracic pumps. Trans Am Soc Artif Intern Organs. 1964;10:154–6. [PubMed] [Google Scholar]
- 10.Gibbon JH., Jr Application of a mechanical heart and lung apparatus to cardiac surgery. Minnesota Medicine. 1954;37:171–80. [PubMed] [Google Scholar]
- 11.Fou AA, John H, Gibbon The first years of the heart-lung machine. Tex Heart Inst J. 1997;24:1–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Brown L, Goldenberg AL, Dennis C. Myocardial assist: comparison of left heart bypass with counterpulsation. Surg Forum. 1966;17:152–4. [PubMed] [Google Scholar]
- 13.Moulopoulos SD, Crosby MJ, Wildevuur C, Kolff J. Mechanical assistance to the circulation: principle and evaluation of results. Med Res Eng. 1968;7:8–9. [PubMed] [Google Scholar]
- 14.Kantrowitz A, Tjonneland S, Freed PS, Phillips SJ, Butner AN, Sherman JL., Jr Initial clinical experience with intraaortic balloon pumping in cardiogenic shock. JAMA. 1968;203:113–8. [PubMed] [Google Scholar]
- 15.Spencer FC, Eisman B, Trinkle JK, Rossi NP. Assisted circulation for cardiac failure following intracardiac surgery with cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1965;49:56–73. [PubMed] [Google Scholar]
- 16.DeVries WC, Anderson JL, Joyce LD, Anderson FL, Hammond EH, Jarvik RK, Kolff WJ. Clinical use of the total artificial heart. New Engl J Med. 1984;310:273–8. doi: 10.1056/NEJM198402023100501. [DOI] [PubMed] [Google Scholar]
- 17.Vigano M, Martinelli L. Modified method for Novacor left ventricular assist device implantation. Ann Thorac Surg. 1996;61:247–9. doi: 10.1016/0003-4975(95)00943-4. [DOI] [PubMed] [Google Scholar]
- 18.Robbins RC, Kown MH, Portner PM, Oyer PE. The totally implantable Novacor left ventricular assist system. Ann Thorac Surg. 2001;71:S162–5. doi: 10.1016/s0003-4975(00)02643-6. [DOI] [PubMed] [Google Scholar]
- 19.Loisance D, Cooper GJ, Deleuze PH. Bridge to transplantation with the wearable Novacor left ventricular assist system: operative technique. Eur J Cardiothorac Surg. 1995;9:95–8. doi: 10.1016/s1010-7940(05)80026-0. [DOI] [PubMed] [Google Scholar]
- 20.Vetter HO, Kaulbach HG, Schmitz C, Forst H, Überfuhr P, Kreuzer E, Pfeiffer M, Brenner P, Dewald O, Reichart B. Experience with the Novacor left ventricular assist system as a bridge to cardiac transplantation, including the new wearable system. J Thorac Cardiovasc Surg. 1995;109:74–80. doi: 10.1016/S0022-5223(95)70422-1. [DOI] [PubMed] [Google Scholar]
- 21.Alexi-Meskishvili V, Hetzer R, Weng Y, Stiller B, Loebe M, Potapov Y. The use of the Berlin Heart in children. In: Duncan BW, editor. Mechanical support for cardiac and respiratory failure in pediatric patients. New York: Marcel Dekker; 2001. pp. 287–314. [Google Scholar]
- 22.Samuels LE, Holmes EC, Matthew PT, Thomas P, Entwistle JC, III, Rohinton JM, Narula J, Wechsler AS. Management of acute cardiac failure with mechanical assist: experience with the Abiomed BVS 5000. Ann Thorac Surg. 2001;71:S67–72. doi: 10.1016/s0003-4975(00)02644-8. [DOI] [PubMed] [Google Scholar]
- 23.Duncan BW. Extracorporeal membrane oxygenation for children with cardiac disease. In: Duncan BW, editor. Mechanical support for cardiac and respiratory failure in pediatric patients. New York: Marcel Dekker; 2001. pp. 1–20. [Google Scholar]
- 24.Soeter JR, Mamiya RT, Sprague AY, McNamara JJ. Prolonged extracorporeal oxygenation for cardiorespiratory failure after tetralogy correction. J Thorac Cardiovasc Surg. 1973;66:214–8. [PubMed] [Google Scholar]
- 25.Bartlett R, Gazzaniga A, Fong S, Burns N. Prolonged extracorporeal cardiopulmonary support in man. J Thorac Cardiovasc Surg. 1974;68:918–32. [PubMed] [Google Scholar]
- 26.Bartlett RH, Gazzaniga AB, Fong SW, Jefferies MR, Roohk HV, Haiduc N. Extracorporeal membrane oxygenator support for cardiopulmonary failure. J Thorac Cardiovasc Surg. 1977;73:375–86. [PubMed] [Google Scholar]
- 27.Hill JD, DeLaval MR, Fallat RJ, Bramson ML, Eberhart RC, Schulte HD, Osborn JJ, Barber J, Gerbode F. Acute respiratory insufficiency: treatment with prolonged extracorporeal oxygenation. J Thorac Cardiovasc Surg. 1972;64:551–62. [PubMed] [Google Scholar]
- 28.Pyle RB, Helton WC, Johnson FW, Hornung JR, Hunt CE, Trumball HR, Lindsay WG, Nicoloff DM. Clinical use of the membrane oxygenator. Arch Surg. 1975;110:966–70. doi: 10.1001/archsurg.1975.01360140110022. [DOI] [PubMed] [Google Scholar]
- 29.ECMO Registry Report. Ann Arbor: Extracorporeal life support organization. 1998 Jul [Google Scholar]
- 30.Pollock JC, Charlton MC, Williams WG, Edmonds JF, Trusler GA. Intraaortic balloon pumping in children. Ann Thorac Surg. 1980;29:522–8. doi: 10.1016/s0003-4975(10)61697-9. [DOI] [PubMed] [Google Scholar]
- 31.Veasy JG, Blalock RC, Orth JL, Boucek MM. Intraaortic balloon pumping in infants and children. Circulation. 1983;5:330–3. doi: 10.1161/01.cir.68.5.1095. [DOI] [PubMed] [Google Scholar]
- 32.Reinhartz O, Keith FM, El-Banayosy A, McBride LR, Robbins RC, Copeland JG, Farrar DJ. Multicenter Experience with the Thoratec ventricular assist device in children and adolescents. J Heart Lung Trans. 2001;20:439–48. doi: 10.1016/s1053-2498(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 33.Farrell DM, LaPierre RA, Howe RJ, Matte GS. Management of the ventricular assist device circuit for pediatric cardiac patients. In: Duncan BW, editor. Mechanical support for cardiac and respiratory failure in pediatric patients. New York: Marcel Dekker; 2001. pp. 159–68. [Google Scholar]
- 34.Karl TR, Sano S, Horton S, Mee RB. Centrifugal pump left heart assist in pediatric cardiac operations. J Thorac Cardiovasc Surg. 1991;102:624–30. [PubMed] [Google Scholar]
- 35.Del Nido PJ, Ducan BW, Mayer JE, Jr, Wessel DL, LaPierre RA, Jonas RA. Left ventricular assist device improves survival in children with left ventricular dysfunction after repair of anomalous origin of the left pulmonary artery. Ann Thorac Surg. 1999;67:169–72. doi: 10.1016/s0003-4975(98)01309-5. [DOI] [PubMed] [Google Scholar]
- 36.Konertz WF. Clinical applications in children of the Medos ventricular assist device. In: Duncan BW, editor. Mechanical support for cardiac and respiratory failure in pediatric patients. New York: Marcel Dekker; 2001. pp. 269–85. [Google Scholar]
- 37.Ibrahim AE, Ducan BW, Blume ED, Jonas RA. Long-term follow-up of pediatric patients requiring mechanical circulatory support. Ann Thorac Surg. 2000;69:186–92. doi: 10.1016/s0003-4975(99)01194-7. [DOI] [PubMed] [Google Scholar]
- 38.Minami K, Körner MM, Posival H, El-Banayosy A, Körfer R. Mechanical ventricular support in postcardiotomy cardiac failure. Transplantforum. 1995;1:16–20. [Google Scholar]
- 39.Reichenbacher EW, Myers JL, Waldhausen JA. Current status of cardiac surgery: a 40 year review. JACC. 1989;3:535–44. doi: 10.1016/0735-1097(89)90089-2. [DOI] [PubMed] [Google Scholar]
- 40.Loisance DY, Jansen PG, Wheeldon DR, Portner PM. Long-term mechanical circulatory support with the wearable Novacor left ventricular assist system. Eur J Cardiothorac Surg. 2000;18:220–4. doi: 10.1016/s1010-7940(00)00488-7. [DOI] [PubMed] [Google Scholar]
- 41.Hawkins JA, Minich LL. Intra-aortic balloon counterpulsation for children with cardiac disease. In: Duncan BW, editor. Mechanical support for cardiac and respiratory failure in pediatric patients. New York: Marcel Dekker; 2001. pp. 49–60. [Google Scholar]
- 42.Cascade PN, Rubenfire M, Kantrowitz A. Radiographic aspects of the phase-shift balloon pump. Radiology. 1972;103:299–302. doi: 10.1148/103.2.299. [DOI] [PubMed] [Google Scholar]
- 43.Frazier OH, Cooley DA. Use of cardiac assistance devices as bridges to cardiac transplantation: Review of current status and report of the Texas Heart Institute's Experience. In: Unger F, editor. Assisted Circulation. Heidelberg: Springer Verlag; 1989. pp. 247–59. [Google Scholar]
- 44.Pennington DG, Swartz MT. Circulatory support in infants and children. Ann Thorac Surg. 1993;55:233–7. doi: 10.1016/0003-4975(93)90529-q. [DOI] [PubMed] [Google Scholar]
- 45.Park JK, Hsu KT, Gersony WM. Intraaortic balloon pump management of refractory congestive heart failure in children. Pediatr Cardiol. 1993;14:19–22. doi: 10.1007/BF00794839. [DOI] [PubMed] [Google Scholar]
- 46.Del Nido PJ, Swan PR, Benson LN, Bohn D, Charlton MC, Coles JG, Trusler GA, Williams WG. Successful use of intraaortic balloon pumping in a 2-kilogram infant. Ann Thorac Surg. 1988;46:574–6. doi: 10.1016/s0003-4975(10)64704-2. [DOI] [PubMed] [Google Scholar]
- 47.Veasy JG, Webster LG. Intra-aortic balloon pumping in children. Heart Lung. 1985;14:548–55. [PubMed] [Google Scholar]
- 48.Duncan BW, Hraska V, Jonas RA, Wessel DL, del Nido PJ, Laussen PC, Mayer JE, Lapierre RA, Wison JM. Mechanical circulatory support in children with cardiac disease. J Thorac Cardiovasc Surg. 1999;117:529–542. doi: 10.1016/s0022-5223(99)70333-8. [DOI] [PubMed] [Google Scholar]
- 49.Black MD, Coles JG, Williams WG, Rebeyka IM, Trusler AG, Bohn D, Gruenwald C, Freedom R. Determinants of success in pediatric cardiac surgery undergoing extracorporeal membrane oxygenation. Ann Thorac Surg. 1995;60:133–8. [PubMed] [Google Scholar]
- 50.Karl TR. Mechanical circulatory support at the Royal Children's Hospital. In: Hetzer R, Hennig E, Loebe M, editors. Mechanical Circulatory support. Berlin: Springer; 1997. pp. 7–20. [Google Scholar]
- 51.Dalton HJ, Siewers RD, Fuhrman BP, del Nido PJ, Thompson AE, Shaver MG, Dowhy M. Extracorporeal membrane oxygenation for cardiac rescue in children with severe myocardial dysfunction. Crit Care Med. 1993;21:1020–1028. doi: 10.1097/00003246-199307000-00016. [DOI] [PubMed] [Google Scholar]
- 52.Klein MD, Shaen KW, Whittlesey GC, Pinksy WW, Arciniegas E. Extracorporeal membrane oxygenation for the circulatory support of children after repair of congenital heart disease. J Thorac Cardiovasc Surg. 1990;100:498–505. [PubMed] [Google Scholar]
- 53.Tracy TF, Delosh T, Bartlett RH. Extracorporeal life support organization 1994. ASAIO Trans. 1994:1017–9. [PubMed] [Google Scholar]
- 54.Rogers AJ, Trento A, Siewers R, Griffith BP, Hardesty RL, Pahl E, Beerman LB, Fricker FJ, Fischer DR. Extracorporeal membrane oxygenation for postcardiotomy shock in children. Ann Thorac Surg. 1989;47:903–6. doi: 10.1016/0003-4975(89)90032-5. [DOI] [PubMed] [Google Scholar]
- 55.Delius RE, Bove EL, Meliones JN, Custer JR, Moler FW, Crowley D, Amerikia A, Behrendt DM, Bartlett RH. Use of extracorporeal life support in patients with congenital heart disease. Crit Care Med. 1992;20:1216–22. doi: 10.1097/00003246-199209000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Weinhaus L, Canter C, Noetzel M, McAlister W, Spray TL. Extracorporeal membrane oxygenation for circulatory support after repair of congenital heart defects. Ann Thorac Surg. 1989;48:206–12. doi: 10.1016/0003-4975(89)90071-4. [DOI] [PubMed] [Google Scholar]
- 57.Ziomek S, Harrell JE, Fasules JW, Faulkner SC, Chipman SW, Moss M, Frazier E, Van Devanter SH. Extracorporeal membrane oxygenation for cardiac failure after congenital heart operation. Ann Thorac Surg. 1992;54:861–8. doi: 10.1016/0003-4975(92)90638-k. [DOI] [PubMed] [Google Scholar]
- 58.Ferrazzi P, Glauber M, Di Domencio A, Fiocchi R, Mamprin F, Gamba A, Crupi G, Cossolini M, Parenzan L. Assisted circulation for myocardial recovery after repair of congenital heart disease. Eur J Cardiothorac Surg. 1991;5:419–24. doi: 10.1016/1010-7940(91)90187-o. [DOI] [PubMed] [Google Scholar]
- 59.Khan A, Gazzaniga AB. Mechanical circulatory assistance in paediatric patients with cardiac failure. Cardiovasc Surg. 1996;4:43–9. doi: 10.1016/0967-2109(96)83782-3. [DOI] [PubMed] [Google Scholar]
- 60.Thuys CA, Mullaly RJ, Horton SB, O’Connor EB, Cochrane AD, Brizard CPR, Karl TR. Centrifugal ventricular assist in children under 6 kg. Eur J Cardiothorac Surg. 1998;13:130–4. doi: 10.1016/s1010-7940(97)00310-2. [DOI] [PubMed] [Google Scholar]
- 61.Ratcliffe MB, Bavaria JE, Wenger RK, Bogen DK, Edmunds LH., Jr Left ventricular mechanics of ejecting postischemic hearts during left ventricular circulatory assistance. J Thorac Cardiovasc Surg. 1991;101:245–55. [PubMed] [Google Scholar]
- 62.Langley SM, Sheppard SV, Tsang VT, Monro JL, Lamb RK. When is extracorporeal life support worthwile following repair of congenital heart disease in children? Eur J Cardiothorac Surg. 1998;13:520–5. doi: 10.1016/s1010-7940(98)00055-4. [DOI] [PubMed] [Google Scholar]
- 63.Stiller B, Daehnert I, Weng G, Hennig E, Hetzer R, Lange PE. Children may survive severe myocarditis with prolonged use of biventricular assist devices. Heart. 1999;82:237–40. doi: 10.1136/hrt.82.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ishino K, Alexi-Meskishvili V, Weng Y, Loebe M, Uhlemann F, Lange PE, Hetzer R. Mechanical circulatory support for post cardiotomy cardiogenic shock in infants. ASAIO J. 1996;42:M735–M738. doi: 10.1097/00002480-199609000-00086. [DOI] [PubMed] [Google Scholar]
- 65.Reul H. Design, development and testing of blood pumps. In: Hwang NHC, editor. In: Advances in Cardiovascular Engineering. New York: Plenum Press; 1993. pp. 237–58. [Google Scholar]
- 66.Shum-Tim D, Duncan BW, Hraska V, Friehs I, Shin’oka T, Jonas RA. Evaluation of a pulsatile pediatric ventricular assist device in an acute right heart failure model. Ann Thorac Surg. 1997;64:1374–80. doi: 10.1016/S0003-4975(97)00901-6. [DOI] [PubMed] [Google Scholar]
- 67.Weyand M, Kececioglu D, Schmid C, Kehl HG, Tandler R, Loick HM, Scheld HH. Successful bridging to cardiac transplantation in a dystrophic infant with the use of a new paracorporeal pneumatic pump. J Thorac Surg. 1997;114:505–7. doi: 10.1016/S0022-5223(97)70206-X. [DOI] [PubMed] [Google Scholar]
- 68.Konertz W, Hotz H, Schneider M, Redlin M, Reul H. Clinical experience with the Medos HIA-VAD system in infants and children: a preliminary report. Ann Thorac Surg. 1997;63:1138–44. doi: 10.1016/s0003-4975(97)00063-5. [DOI] [PubMed] [Google Scholar]
- 69.Reichenbach SH, Farrar DJ, Hill JD. A versatile intracorporeal ventricular assist device based on the Thoratec VAD system. Ann Thorac Surg. 2001;71:S171–5. doi: 10.1016/s0003-4975(00)02616-3. [DOI] [PubMed] [Google Scholar]
- 70.Farrar DJ, Hill JD. Thoratec ventricular assist device principal investigators. Recovery of major organ function in patients awaiting heart transplantation with Thoratec ventricular assist device. J Heart Lung Transplant. 1994:1125–32. [PubMed] [Google Scholar]
- 71.Copeland JG, Arabia FA, Smith RG. Bridge to transplantation with a Thoratec left ventricular assist device in a 17-kg child. Ann Thorac Surg. 2001;71:1003–4. doi: 10.1016/s0003-4975(00)02268-2. [DOI] [PubMed] [Google Scholar]
- 72.Ashton RC, OZ MC, Michler RE, Champsaur G, Catanese KA, Hsu DT, Addonizio LJ, Quaegebeur JM. Left ventricular assist device options in pediatric patients. ASAIO J. 1995;41:M227–80. doi: 10.1097/00002480-199507000-00011. [DOI] [PubMed] [Google Scholar]
- 73.Müller J, Wallukat G, Weng YG, Dandel M, Spiegelsberger S, Semrau S, Brandes K, Theodoris V, Loebe M, Meyer R, Hetzer R. Weaning from mechanical cardiac support in patients with idiopathic dilated cardiomyopathy. Circulation. 1997;96:542–9. doi: 10.1161/01.cir.96.2.542. [DOI] [PubMed] [Google Scholar]
- 74.El-Banayosy A, Deng M, Loisance DY, Vetter H, Gronda E, Loebe M, Vigano M. The European experience of Novacor left ventricular assist (LVAS) therapy as a bridge to transplant: a retrospective multi-centre study. Eur J of Cardiothorac Surg. 1999;15:835–41. doi: 10.1016/s1010-7940(99)00107-4. [DOI] [PubMed] [Google Scholar]
- 75.Deng CD, Loebe M, El-Banayosy A, Gronda E, Jansen PGM, Vigano M, Wieselthaler GM, Reichart B, Vitali E, Pavie A, Mesana T, Loisance DY, Wheeldon DR, Portner PM. Mechanical circulation support of advanced heart failure. Circulation. 2001;103:231–237. doi: 10.1161/01.cir.103.2.231. [DOI] [PubMed] [Google Scholar]
- 76.Helman DN, Oz MC, Galantowicz ME. The use of the Heartmate left ventricular assist device in children. In: Duncan BW, editor. Mechanical support for cardiac and respiratory failure in pediatric patients. New York: Marcel Dekker; 2001. pp. 259–67. [Google Scholar]
- 77.Emery RW, Joyce LD. Directions in care and assistance. J Cardiac Surg. 1991;6:400–14. doi: 10.1111/j.1540-8191.1991.tb00337.x. [DOI] [PubMed] [Google Scholar]
- 78.Loree HM, II, Bourque K, Gernes DB, Richardson JS, Poirier VL, Barletta N, Fleischli A, Foiera G, Gempp TM, Schoeb R, Litwak KN, Akimoto T, Kameneva M, Watach MJ, Litwa P. The Heartmate III: design and in vivo studies of a Maglev centrifugal left ventricular assist device. Artif Org. 2001;25:386–91. doi: 10.1046/j.1525-1594.2001.025005386.x. [DOI] [PubMed] [Google Scholar]
- 79.Marelli D, Laks H, Fazio D, Hamilton MA, Fonarow GC, Meehan DA, Moriguchi JD. Mechanical assist strategy using the BVS 5000i for patients with heart failure. Ann Thorac Surg. 2000;70:59–66. doi: 10.1016/s0003-4975(00)01252-2. [DOI] [PubMed] [Google Scholar]
- 80.Kaplon RJ, Oz MC, Kwiatkowski PA, Levin HR, Shah AS, Jarvik RK, Rose EA. Miniature axial flow pump for ventricular assistance in children and small adults. J Thorac Cardiovasc Surg. 1996;111:13–8. doi: 10.1016/S0022-5223(96)70396-3. [DOI] [PubMed] [Google Scholar]
- 81.Myers TJ, Khan T, Frazier OH. Infectious complications associated with ventricular assist systems. ASAIO J. 2000;46:S28–36. doi: 10.1097/00002480-200011000-00034. [DOI] [PubMed] [Google Scholar]
- 82.Frazier OH, Myers TJ, Jarvik RK, Westaby S, Pigott DW, Gregoric ID, Khan T, Tamez DW, Conger JL, Macris MP. Research and development of an implantable, axial-flow left ventricular assist device: the Jarvik 2000 heart. Ann Thorac Surg. 2001;71:S125–32. doi: 10.1016/s0003-4975(00)02614-x. [DOI] [PubMed] [Google Scholar]
- 83.Williams MR, Quaegebeur JM, Hsu DT, Addonizio LJ, Kichuk MR, Oz MC. Biventricular assist device as a bridge to transplantation in a pediatric patient. Ann Thorac Surg. 1996;62:578–80. [PubMed] [Google Scholar]
- 84.Konertz W, Reul H. Mechanical circulatory support in children. Int J Artif Organs. 1997;20:657–8. [PubMed] [Google Scholar]
- 85.Acker MA. Mechanical circulatory support for patients with acute-fulminant myocarditis. Ann Thorac Surg. 2001;71:S73–6. doi: 10.1016/s0003-4975(00)02628-x. [DOI] [PubMed] [Google Scholar]
- 86.Hunkeler NM, Canter CE, Donze A, Spray TL. Extracorporeal life support in cyanotic congenital heart disease before cardiovascular operation. Am J Cardiol. 1992;69:790–3. doi: 10.1016/0002-9149(92)90507-u. [DOI] [PubMed] [Google Scholar]
- 87.Anderson HL, Attorri RJ, Custer JR, Chapman AR, Bartlett RH. Extracorporeal membrane oxygenation for pediatric cardiopulmonary failure. J Thorac Cardiovasc Surg. 1990;99:1011–21. [PubMed] [Google Scholar]
- 88.Walters HL, Hakimi M, Rice MD, Lyons JM, Whittlesea GC, Klein MD. Pediatric cardiac surgical ECMO: multivariate analysis of risk for hospital death. Ann Thorac Surg. 1995;60:329–337. doi: 10.1016/0003-4975(95)00410-m. [DOI] [PubMed] [Google Scholar]
- 89.Hetzer R, Loebe M, Potapov EV, Weng Y, Stiller B, Hennig E, Alexi-Meskishvili, Lange PE. Circulatory support with pneumatic paracorporeal ventricular assist device in infants and children. Ann Thorac Surg. 1998;66:1498–506. doi: 10.1016/s0003-4975(98)00914-x. [DOI] [PubMed] [Google Scholar]
- 90.Ishino K, Loebe M, Uhlemann F, Weng Y, Hennig E, Hetzer R. Circulatory support with paracorporeal ventricular assist device (VAD) in infants and children. Eur J Cardiothorac Surg. 1997;11:965–72. doi: 10.1016/s1010-7940(97)01149-4. [DOI] [PubMed] [Google Scholar]
- 91.Konertz W. Mechanical circulatory assist in pediatric patients. Int J Artif Organs. 1997;20:681–3. [PubMed] [Google Scholar]
- 92.Delius RE, Zwischenberger JB, Cilley R, Behrendt DM, Bove EL, Deeb GM, Crowley D, Heidelberger KP, Bartlett RH. Prolonged extracorporeal life support of pediatric and adolescent cardiac transplant patients. Ann Thorac Surg. 1990;50:791–5. doi: 10.1016/0003-4975(90)90688-3. [DOI] [PubMed] [Google Scholar]
- 93.Loebe M, Hennig E, Müller J, Spiegelsberger S, Weng Y, Hetzer R. Long-term mechanical circulatory support as a bridge to transplantation, for recovery from cardiomyopathy, and for permanent replacement. Eur J Cardio Thorac Surg. 1997;11:S18–24. [PubMed] [Google Scholar]
- 94.Chen JM, Spanier TB, Gonzalez JJ, Marelli D, Flannery MA, Tector KA, Cullinane S, Mehmet CO. Improved survival in pateints with acute myocarditis using external pulsatile mechanical ventricular assistance. J Heart Lung Transplant. 1999;18:351–7. doi: 10.1016/s1053-2498(98)00054-0. [DOI] [PubMed] [Google Scholar]
- 95.Jett GK. Abiomed BVS 5000: experience and potential advantages. Ann Thorac Surg. 1996;61:301–4. doi: 10.1016/0003-4975(95)01009-2. [DOI] [PubMed] [Google Scholar]
- 96.Del Nido PJ, Dalton HJ, Thompson AE, Siewers RD. Extracorporeal membrane oxygenator rescue in children during cardiac arrest after cardiac surgery. Circulation. 1992;86:300–4. [PubMed] [Google Scholar]
- 97.Journois DJ, Pouard P, Mauriat P, Malhere T, Vouhe P, Safron D. Inhaled nitric oxide as a therapy for pulonary hypertension after operations for congenital defects. J Thorac Cardiovasc Surg. 1994;107:1129–35. [PubMed] [Google Scholar]
- 98.Raithel SC, Boegner E, Fiore A, Pennington DG. Extracorporeal membrane oxygenation in children following cardiac surgery. Circulation. 1991;84:240. [PubMed] [Google Scholar]
- 99.Del Nido PJ, Armitage JM, Fricker FJ, Cipriani L, Dayal G, Park SC, Siewers RD. Extracorporeal membrane oxygenation support as a bidge to pediatric heart transplantation. Circulation. 1994;90:66–9. [PubMed] [Google Scholar]
- 100.Houel A, Moczar M, Clerin V, Loisance DY. Pseudo intima in inflow conduit of left ventricular assist devices. Ann Thorac Surg. 1999;68:717–24. doi: 10.1016/s0003-4975(99)00527-5. [DOI] [PubMed] [Google Scholar]
- 101.Nakatani S, McCarthy PM, Kottke-Marchant K, Harasaki H, James KB, Savage RM, Thomas JD. Left ventricular echocardiographic and histologic changes: impact of chronic unloading by an implantable ventricular assist device. JACC. 1996;27:894–901. doi: 10.1016/0735-1097(95)00555-2. [DOI] [PubMed] [Google Scholar]
- 102.Dipla K, Mattiello JA, Jeevanandam V, Houser SR, Margulies KB. Myocyte recovery after mechanical circulatory support in humans with end-sstage heart failure. Circulation. 1998;97:2316–2322. doi: 10.1161/01.cir.97.23.2316. [DOI] [PubMed] [Google Scholar]
- 103.Taylor KM. Pulsatile and non-pulsatile perfusion. In: Minami K, Körfer R, Wada J, editors. Cardiac thoracic surgery. Amsterdam: Elsevier; 1992. pp. 57–65. [Google Scholar]
- 104.Darling EM, Kaemmer D, Lawson DS, Jaggers JJ, Ungerleider RM. Use of ECMO without the oxygenator to provide ventricular support after Norwood Stage I procedures. Ann Thorac Surg. 2001;71:735–6. doi: 10.1016/s0003-4975(00)02063-4. [DOI] [PubMed] [Google Scholar]
- 105.Jaggers JJ, Forbess J, Shah A, Meliones JN, Kirshbom PM, Miller CE, Ungerleider RM. Extracorporeal membrane oxygenation (ECMO) for post-cardiotomy failure in children: significance of shunt management in the single ventricle. Ann Thorac Surg. 2000;69:1476–83. doi: 10.1016/s0003-4975(00)01330-8. [DOI] [PubMed] [Google Scholar]


