Abstract
Pulmonary arteriovenous malformation is a rare anomaly that presents in several different ways. It can present as an isolated finding, or more often in the context of hereditary haemorrhagic telangiectasia. It can also complicate palliative surgery such as the Glenn operation for complex congenital heart disease with single ventricle physiology. Its management includes transcatheter embolization, which is the preferred mode of therapy, surgery (including resection of the affected lobe, segment, or the fistula itself), or rarely, medical therapy. Complications of the disease itself and of various modes of treatment are relatively common, and patients require close surveillance for possible recurrence, or development of new fistulas. In cases related to the Glenn operation, redirection of hepatic venous flow or heart transplantation may cure the problem.
MeSH: pulmonary arteriovenous malformation, hereditary haemorrhagic telangiectasia, transcatheter embolization, polysplenia syndrome, Glenn operation
Introduction
Pulmonary arteriovenous malformation (PAVM) is a rare cardiovascular anomaly. Most cases are congenital, frequently related to hereditary haemorrhagic telangiectasia (HHT), an autosomal dominant disorder. Isolated cases have also been reported. Rarely, diffuse PAVMs occur in the context of unoperated congenital heart malformations of the type of polysplenia syndrome. Other etiologies include acquired conditions, such as schistosomiasis, tuberculosis, trauma, and juvenile hepatic cirrhosis. Finally, a well recognized acquired cause of PAVM is the Glenn operation, either in its classic form, or its most recent modification of bi-directional cavo-pulmonary anastomosis. Different types of PAVM carry different prognoses and require different types of treatment, specific to each category. In this review article, we describe the most common types of PAVM encountered in paediatric cardiology practice and their management. In order to understand the types of problems encountered in the management of these patients better, we present first 3 illustrative cases, recognizing that these cases do not represent the full spectrum of this multifaceted disease.
Case 1
A 20-year-old man was diagnosed with HHT (Osler-Weber-Rendu disease). He was known to have cyanosis since infancy, as well as multiple cutaneous telangiectasias. His oxygen saturation was 84% by pulse oximetry. His father also had the same cutaneous telangiectasias without cyanosis. The patient had been catheterized in the past and was found to have a large arteriovenous malformation in the left upper lobe, as well as a smaller one in the right upper lobe. He had required 2 partial exchange transfusions because of intense polycythemia with a hematocrit of 73% and hyperviscosity symptoms (headaches and blurred vision). At the age of 24, cardiac catheterization was performed and an attempt was made to place 2 Gianturco-Grifka vascular occlusion devices simultaneously in the main feeding artery. Because of the large size of the proximal feeding artery, it was impossible to stabilize the devices so as to achieve complete occlusion. One device dislodged to a small branch of the fistula without affecting significantly the blood flow to the remaining branches and the other was retrieved back in the sheath. Despite heparin administration, the patient developed deep vein thrombosis of the right leg and required long-term anticoagulation. He remained cyanotic and returned for repeat catheterization at 26 years of age. After demonstration of the fistula with selective injection in the feeding artery (Fig 1a), a 10 Fr long Mullins sheath was placed through the left femoral vein and two Cardio-Seal atrial septal defect occlusion devices (23 and 27 mm respectively), were deployed in the proximal feeding artery of the arteriovenous fistula which came off of the left upper lobe branch (Fig 1b).
Figure 1(a).
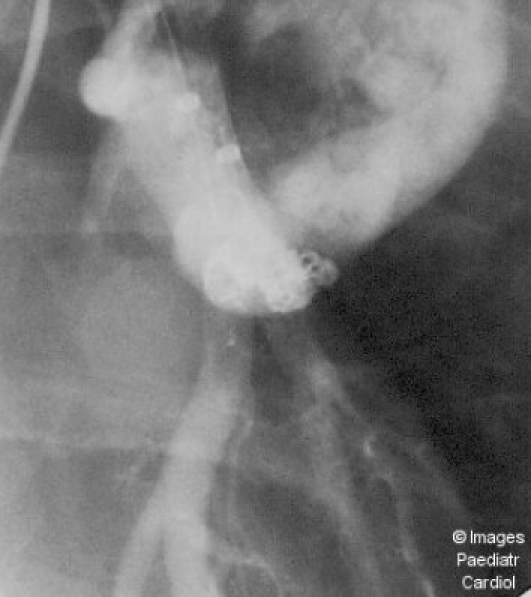
Anteroposterior projection of a left lower lobe artery injection shows a very dilated feeding artery with rapid filling of a tortuous transverse portion of the PAVM
Figure 1(b).
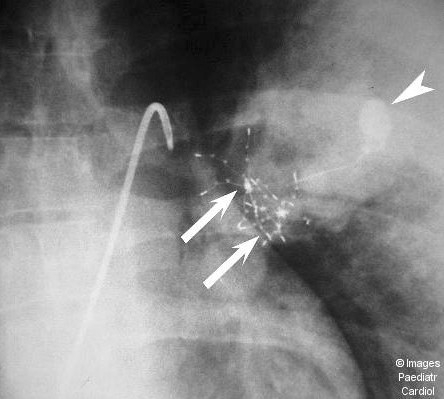
Anteroposterior projection without contrast shows both devices (arrows) deployed in the dilated feeding vessel, as well as the previously placed Gianturco-Grifka device (arrowhead)
These devices achieved near complete occlusion of the fistula (Fig 1c), except for some small branches, which arose very proximally, after the take-off of the feeding artery, near the left-upper lobe branch. The oxygen saturation increased immediately from 85 to 94%, and stabilized later at 90-92%. The patient remained stable and has been followed clinically for 2 years after device placement. Follow-up catheterization has not been performed yet, although it is planned because of the well-known progressive nature of the arteriovenous malformations in Rendu-Osler-Weber disease and the possibility of recanalization after initially successful embolization.
Figure 1(c).
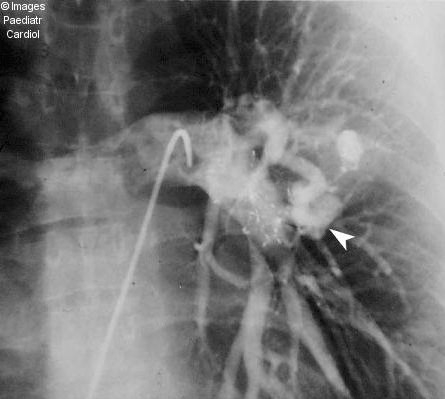
Anteroposterior projection of a left pulmonary artery injection after placement of both devices demonstrates absence of flow within the large PAVM (compare with Fig 1a). The contrast enhanced smaller superimposed lesion (arrowhead) becomes apparent due to absence of contrast in the large PAVM
Case 2
A 15-year-old female presented with cyanosis and clubbing. She also had exophthalmos, scoliosis and mild mental retardation. The chest x-ray showed widening of the mediastinum and marked scoliosis. A 2-D echocardiogram showed significant dilatation of the ascending aorta above the sinuses of Valsalva. Although she did not have excessively long extremities and arachnodactyly, a variant of Marfan's syndrome versus homocystinuria was considered. Because of the severe cyanosis and the aortic aneurysm, she underwent cardiac catheterization. The pulmonary veins and left atrium were entered through a patent foramen ovale. The systemic arterial saturation, as well as that of the left lower pulmonary vein was decreased (80%) and was not changed after administration of oxygen. Pulmonary arterial pressure was mildly elevated at 35/10, with a mean 20 mmHg. The Qp:Qs ratio was markedly decreased at 0,66. Aortography revealed a very large aneurysm of the ascending aorta sparing the sinuses of Valsalva, with a transverse diameter of 7,7 cm and mild aortic insufficiency. Pulmonary angiography showed a large saccular aneurysm arising from the left lower pulmonary artery and connecting directly to the left lower pulmonary vein. There was no significant atrial level shunt. The right pulmonary artery was normal. Because of the large size of the arteriovenous fistula and the coexisting aortic aneurysm, transcatheter embolization was not attempted. The patient was taken to the operating room where a left lower lobectomy was performed. The ascending aorta was replaced by a Hemashield graft above the sinuses of Valsalva. Postoperative recovery was uneventful and her oxygen saturation normalized to 99%. She has been followed for 6 years and remains in relatively good condition although she requires orthopaedic therapy for her scoliosis. The ascending aortic graft has calcified, but there is no progression of the mild aortic regurgitation.
Case 3
A 4-year-old female was diagnosed soon after birth with complex cyanotic congenital heart disease. Chest x-ray showed situs inversus of the abdominal viscera, dextrocardia and decreased pulmonary vascular markings. Two-dimensional echocardiography and cardiac catheterization revealed the following anatomy: situs inversus of the abdominal viscera; hepatic venous drainage directly to the left-sided atrium; left-sided inferior vena cava, continuing through a left-sided azygos vein to a left superior vena cava that connected to the left-sided atrium; right superior vena cava connecting to the coronary sinus, which drained to the left-sided atrium; drainage of the pulmonary veins to the right-sided atrium; common atrioventricular valve; unbalanced AV canal with hypoplastic left ventricle; supero-inferior ventricles; double outlet right ventricle with anterior and rightward aorta and posterior and leftward pulmonary artery; severe subpulmonary and valvar pulmonary stenosis.
She had moderate cyanosis, but was relatively well and thriving until 4 years of age. Subsequently, she developed progressively severe cyanosis and polycythemia, with a hematocrit of 60% and oxygen saturation of 65-70%. She had moderate clubbing with a harsh IV/VI systolic ejection murmur at the right upper sternal border. Cardiac catheterization at 4 years of age showed the above-described anatomy, with low pulmonary artery pressures (mean of 10 mmHg). It was decided to proceed with bilateral anastomoses of the superior venae cavae to the pulmonary arteries which also connected the inferior vena caval flow to the pulmonary circulation through the left azygos vein (Kawashima operation), thus leaving only the hepatic veins draining to the systemic circulation. The main pulmonary artery was disconnected from the right ventricle. The postoperative oxygen saturation was 94%. Over the next 2 years, there was a progressive drop in the arterial oxygen saturation, to 79%. A repeat cardiac catheterization was performed which showed well functioning bilateral cavopulmonary anastomoses, normal central pulmonary arteries, with a diffuse opacification of the lung parenchyma and rapid appearance of contrast in the pulmonary venous circulation, with opacification of large pulmonary veins at the same time with central pulmonary arteries, suggesting diffuse pulmonary arteriovenous communications (Fig 2). The pulmonary artery pressure was normal. She was reoperated, and the hepatic veins were anastomosed through a 22 mm Gore-tex tube graft to the right pulmonary artery. Immediately postoperatively the saturation increased to 85-90%, and over the next six months, it increased further to 97%, suggesting resolution of the pulmonary arteriovenous communications.
Figure 2.
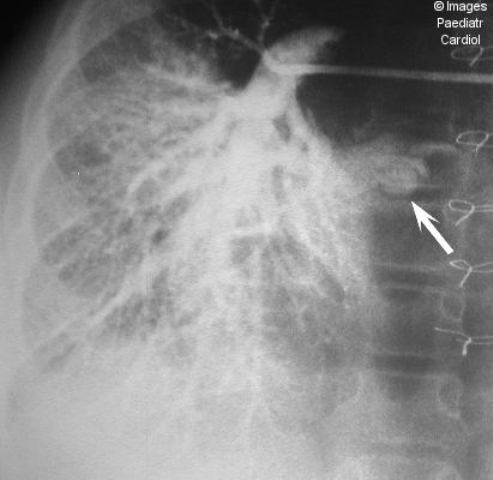
Pulmonary angiography in the Glenn reconstruction (superior vena cava to right pulmonary artery anastomosis) demonstrates simultaneous filling of the pulmonary artery, pulmonary capillary bed and pulmonary veins (arrow) of the right lung
Pathology and pathogenesis
Most cases of PAVM (approximately 60%) are related to HHT, which is an autosomal dominant disorder with variable clinical expression.1 The prevalence of HHT varies from 1:40.000 to as high as 1 in 200 in some parts of the Dutch Antilles. Most patients have mucocutaneous telangiectasias, and various vascular malformations in different organs including PAVM. There are at least 2 genes related to HHT, which have been mapped to chromosomes 9q33-34 and 9q3. The first one encodes a transforming growth factor binding protein, called endoglin.3–5 A much higher incidence of PAVMs has been described in families having mutations of the endoglin gene compared to the ones without the mutations.6
Usually the PAVM consists of a variable number of pulmonary artery branches, which connect to pulmonary veins without interposition of pulmonary capillaries. Quite often, the connection of the pulmonary arteries and veins has a sacciform appearance, and the feeding arteries are dilated and tortuous. The wall of the arteries is usually thinner and that of the veins thicker than normal. The aneurysmal part is often partially occluded by thrombus. Because of the thin wall of the arteries, which often shows degenerative changes and calcification, intrapulmonary hemorrhage is often noted, which may result in pulmonary hemosiderosis. Rarely, massive hemothorax may occur, if a large branch ruptures into the pleural space.1,2
There are isolated cases of PAVM, not related to HHT, where the fistula is an isolated finding. Sometimes, the PAVM is very large, involving an entire lobe or lung. There are also rare cases of direct communication between the right pulmonary artery and the left atrium.7
A special form of diffuse PAVMs has been recognized increasingly in the context of complex cyanotic congenital heart disease, in which the endocardial anatomy precludes a two-ventricle repair. In the process of a palliative approach to these complex cases, a bi-directional Glenn operation is often performed, in which the superior vena cava is anastomosed to the pulmonary artery, leaving the inferior vena cava draining to the systemic circulation. In some cases, the inferior vena cava drains through an azygos vein to the superior vena cava, leaving only the hepatic veins draining to the systemic circulation after the bi-directional Glenn operation. Several case reports and series of patients have been reported in which a significant number of patients developed a diffuse network of PAVMs at variable intervals after the bi-directional Glenn operation, usually months or years,8–14 but sometimes within days postoperatively.15 Bernstein et al found a 60% prevalence of PAVMs in patients after a cavopulmonary shunt.8 Their incidence was higher in infants who have undergone a bidirectional Glenn operation at ages <6 months (reaching 100%) as well as patients with heterotaxy. Because the common feature of all these cases is the absence of hepatic venous blood from the pulmonary circulation, a hypothesis has been formed that a hepatic factor exists, which when present in the pulmonary circulation, prevents the opening of these arteriovenous communications. In its absence, arteriovenous communications may develop, much like in the case of juvenile hepatic cirrhosis.16 In support of this hypothesis, is the observation that connection of hepatic venous return to the pulmonary circulation, results in resolution of the PAVMs.10,12–14,17–19 The same is true for liver transplantation in cases of hepatic cirrhosis.16
There are also a few case reports of diffuse PAVMs in patients with polysplenia syndrome, who have never been operated on.20–22 An interesting observation in one of these cases20 was the presence of a portal-to-systemic shunt. This may suggest that one or more mesenteric substances entering the systemic venous circulation without undergoing metabolic changes in the liver may also be responsible for development of diffuse PAVMs. Alternatively, the diffuse PAVMs in these patients may represent an arrest in a primitive developmental stage.
Clinical presentation
Cyanosis is the most common feature, which is present in most cases with large enough PAVMs, often from the newborn period.23–25 A progressive increase in cyanosis is often noted because of the opening of new or enlargement of the existing fistulas, and the development of polycythemia. Progression of PAVMs is often noted during pregnancy.26,27 Haemoptysis and haemothorax may occur, especially in patients with HHT and in pregnant women.28 Neurologic sequela are quite common and may occur in up to 33% of patients with HHT.29,30 Both cerebral infarction and cerebral abscess may occur.29 Clubbing is present in patients with long-standing cyanosis. Precordial activity is normal and heart murmurs are either absent or faint. Sometimes a soft bruit is detectable over the involved area, especially with deep inspiration. Multiple cutaneous telangiectasias are common in patients in HHT. Haemorrhage may occur from other areas such as the gastrointestinal and urogenital tract. Bacterial endarteritis has been reported.1
Laboratory findings
Chest x-ray may show one or more rounded or multilobular opacities when the fistulas are large enough (Fig 3). Cardiac size is usually normal. Cardiomegaly may rarely be present when the fistulas are very large and cause a hyperdynamic state. However, the chest film is not a very sensitive diagnostic modality. Pulse oximetry is usually abnormal, with various degrees of desaturation but it may not detect small fistulas. The most sensitive non-invasive screening test for PAVMs, either in the context of complex congenital heart disease or in patients with HHT, is contrast echocardiography.8,31–33 A positive test is defined as the appearance of bubbles in the systemic circulation (Fig 4), at least 3 cardiac cycles after appearance in the right atrium, to exclude intracardiac communications as a cause of right to left shunt.32 In a study comparing different screening tests for PAVMs in patients with HHT (including pulse oximetry in supine and upright position, supine PaO2 in room air and breathing 100% oxygen, chest x-ray and density of contrast in the left atrium during contrast echocardiography), it was concluded that initial screening with contrast echocardiography, followed by measurement of PaO2 while breathing 100% oxygen, was the best screening procedure for identification of patients with PAVMs.33
Figure 3.
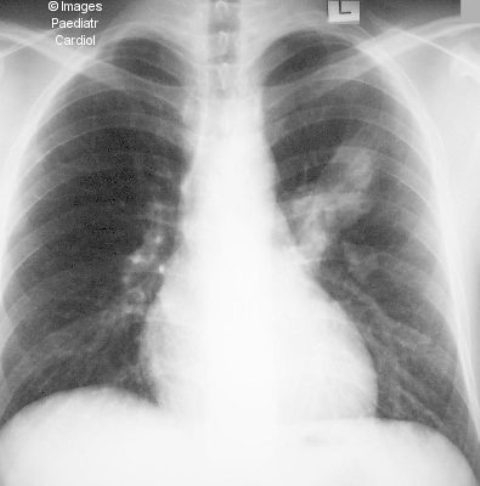
Chest radiograph of patient with huge left-sided PAVM demonstrated as a large opacity in the left lung parenchyma
Figure 4.
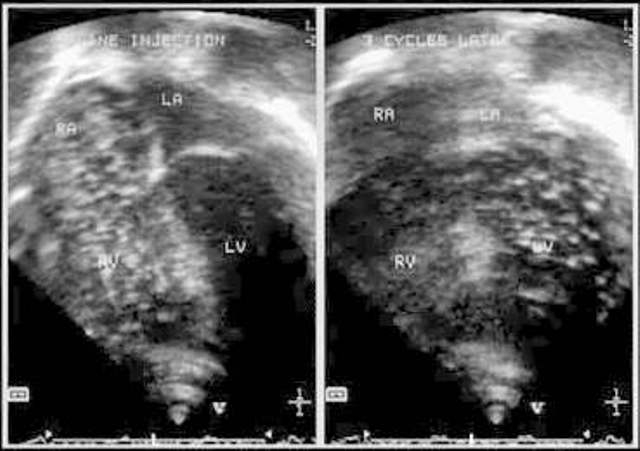
Two-dimensional echocardiogram with injection of agitated saline through a peripheral vein, demonstrates opacification of right-sided chambers, and 3 cycles later, contrast appears in left heart chambers, suggesting intrapulmonary shunting
Radionuclide testing using Tc99m labeled albumin microspheres is also a useful test, which can provide a quantitative measurement of the right-to-left shunt by measuring the fraction of the injected dose of microspheres reaching the kidneys.34 Chest computed tomography may demonstrate PAVMs quite accurately (Fig 5a and b).35 Recently, contrast enhanced magnetic resonance angiography was compared to helical CT. It was found quite sensitive (75%) and very specific (100%). All significant PAVMs with a feeding vessel diameter >3 mm were detected. Thus, contrast enhanced magnetic resonance angiography seems to be a very useful nonionizing and noninvasive procedure for the diagnosis and exact anatomic localization of PAVMs.36
Figure 5(a).
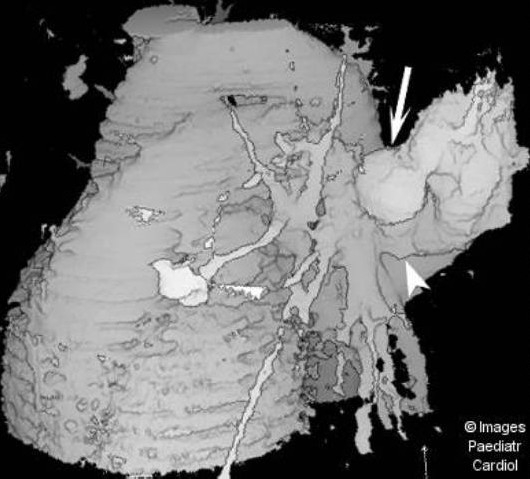
Surface shaded display reconstructed image from a set of contrast enhanced spiral CT slices demonstrates the huge PAVM (arrow) and its large feeding artery (arrowhead), as well as the two smaller lesions
Figure 5(b).
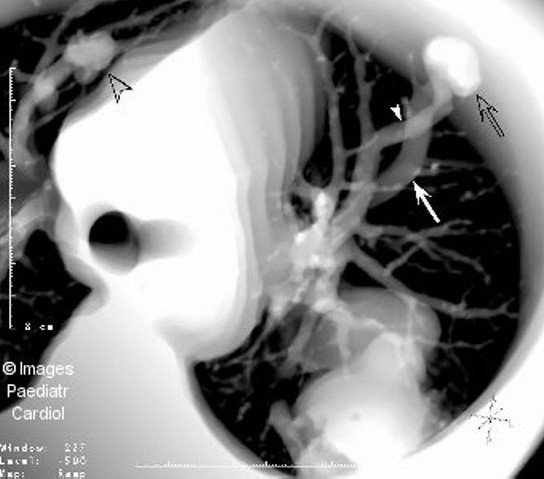
Thick section multiplanar reformatted image from a set of contrast enhanced spiral CT slices shows part of the huge PAVM, as well as a small PAVM in the right upper lobe (open arrowhead). A smaller PAVM (open arrow) with its feeding artery (arrowhead) and dilated draining vein (arrow) is demonstrated in the left lung
Cardiac catheterization and pulmonary angiocardiography are the most useful and definitive tests, which show the exact location and anatomy of the PAVM and may guide therapeutic embolization. Pulmonary artery pressures are either normal or low because of the decreased pulmonary vascular resistance, especially when the fistulas are large or diffuse. The feeding vessel(s) will be demonstrated during injection in the appropriate pulmonary artery. Rapid opacification of the pulmonary veins and left atrium will be demonstrated. When the fistulas are small, they may not be opacified unless selective injection is performed in the branch of the pulmonary artery providing blood supply to the PAVM.37 Manipulation of catheters in the fistula should be careful, since rupture has been reported. However, selective injections are necessary if therapeutic embolization is planned (see below).
Management and long-term outcome
Three forms of management are available for the various types of PAVMs: a) transcatheter embolization therapy (using different devices such as coils, detachable balloons, and various devices designed for occlusion of atrial septal defects and patent arterial ducts), b) surgical, including lobectomy, segmentectomy or fistulectomy, redirection of hepatic venous flow to the pulmonary circulation, and heart or heart/lung transplantation and c) medical (including pharmacologic management) in a few cases. Each form of therapy is discussed below.
A. Transcatheter embolization
This is the most widely used form of therapy for large isolated PAVMs. Treatment is indicated in patients with PAVMs with feeding arteries larger than 3 mm in diameter, even when they are asymptomatic, since the risk of stroke and other systemic emboli is at least 30%29,38,39,41 and may be as high as 70% in patients with diffuse PAVMs.40 The two most widely used types of devices are coils and detachable balloons.
Dutton et al reported their experience in 53 patients who underwent 102 procedures for coil embolization of PAVMs.41 Forty-two (79%) had HHT and 19 (36%) had suffered neurologic complications. All PAVMs with feeding arteries greater than 3 mm were occluded with steel coils. Oxygen saturation increased from a mean of 89% pre-embolization to 94%. Mean shunt fraction (calculated with radionuclide scanning with 99mTc albumin macroaggregates) decreased from 23 ± 2% to 9 ± 1%. All procedures were successful, but 18 complications occurred, four of which were potentially serious (2 coil embolizations in the systemic circulation, one myocardial puncture and one cerebrovascular accident). However no lasting sequelae were noted.
Haitjema et al reported their results and long-term follow-up of coil embolization in 32 patients.42 Overall, 92 PAVMs were embolized (all fistulas with a feeding artery greater than 3 mm) with significant improvement in systemic oxygenation. Treatment was incomplete in 2 patients, one of whom was referred for surgical treatment. Mean follow-up time was 25 (1-72) months, with 14 patients being followed for more than 2 years. Recanalization occurred in 2 patients after 22 and 28 months respectively, emphasizing the need for long-term follow-up. Complications occurred in 18% of patients, including pleural effusion in 4, arrhythmia in 1 and dislodgement of coils in the left ventricle in 2. To avoid this complication, the authors have started to use balloon occlusion of the proximal feeding artery to stop blood flow before releasing the coils.
The largest experience with embolization has been reported from Yale University. White et al reported 221 patients with PAVMs treated with embolotherapy.38,39 A subset of these patients had very large PAVMs with a feeding artery greater than 8 mm.39 These authors used a combination of coils and balloons according to the size of the feeding artery, with coils being used for smaller vessels, balloons for medium-size and balloons in a nest of coils in very large vessels. Most patients were cured in the first procedure, but approximately 20% required a second or a third attempt. Acute periprocedural complications included pleural effusion (possibly due to limited pulmonary infarction) in approximately 30%, angina (possibly due to coronary air embolism) in 2% and embolization of a device in 4%. However, no long-term sequelae were noted. The authors reported that although balloon deflation was a common finding, this occurred at least 3 weeks after embolization and no late dislodgement of balloons was observed.
From the above series, it can be concluded that both coils and detachable balloons are effective in occluding even large PAVMs. Although complications are relatively common, most are treatable and leave no sequelae. However, serious complications can occur. Haitjema et al, reported a 47-year-old patient with HHT, who had a hepatic arteriovenous fistula in addition to a PAVM, in whom several unusual complications happened.43 A detachable balloon deflated and migrated into a hepatic artery. A coil migrated into the left ventricle and was retrieved later with a forceps. Pericardial tamponade occurred, possibly due to ventricular perforation at the time of coil retrieval from the left ventricle. Finally, although pulmonary artery pressures were normal pre-embolization, significant pulmonary hypertension was noted post-embolization, with acute right heart failure. This was attributed to the combination of the high-flow state due to the large intra-hepatic shunt and the acute afterload faced by the pulmonary arteries after occlusion of the PAVM, which was a low resistance pathway.
An important question is: Is there a patient too young to undergo transcatheter embolization of a PAVM? Several authors44,45 have described occlusion of large PAVMs with multiple feeding vessels that caused severe cyanosis in newborns. Gianturco steel coils were used in all cases. All procedures were successful, with no serious complications, although a second procedure was required in one of the cases several months later, due to recurrence of fistulae. A relatively large number of coils was required in each session (8-10) to achieve complete occlusion of all feeding arteries.
There are other devices that have been found useful in embolization of PAVMs. The Gianturco-Grifka Vascular occlusion device consists of a sac filled with a wire which is delivered through a catheter, and can reach a final size of 9 mm. Ebeid et al reported a successful occlusion of a large PAVM in a 12-year-old girl using 3 Gianturco-Grifka Vascular occlusion devices.46 We have used this device in a 24-year-old patient with HHT and a large PAVM unsuccessfully due to the large size of the feeding artery. Occlusion of the feeding artery was achieved in a second procedure, using two Cardioseal double-umbrella devices.47 An earlier version of this device, the Bard Clamshell Septal Occluder Device, has been used to close a direct communication between the right pulmonary artery and the left atrium.37 Other authors have described use of the Amplatzer duct-occluder device for successful embolization of large PAVMs that were not suitable for coil embolization.48,49
A special type of diffuse PAVMs presents a more difficult problem. Faughan et al reported on 16 patients with this type of PAVMs, out of a large population of 351 patients from 3 referral centers.40 These patients have a very high incidence of stroke or brain abscess (up to 70%). In a subset of this population, a form of embolization called pulmonary flow redistribution was used. The procedure consisted first of temporary occlusion of the lobar arteries of the most affected lobes with a balloon catheter. If oxygen saturation improved, permanent occlusion followed. This allowed redistribution of blood flow to the unaffected or less severely affected lobes. Although this resulted in significant improvement of cyanosis immediately, one patient had massive hemoptysis 7 years after the procedure. This was found to be due to a very dilated bronchial artery to the lobe that had been embolized and was treated with transcatheter embolization of the bronchial artery.
B. Surgical treatment
Although the success rate of transcatheter embolization with coils, balloons and other devices described above is quite high, there is also a significant rate of recanalization after initial successful embolization which varies between 5-15%.39,50 In addition, growth of small fistulas or development of new ones may occur. Despite the generally acceptable complication rate, most of which are transient, there are rarely serious complications as described in the previous section. For the above reasons, some authors believe that surgical treatment should be the preferred therapeutic option.51 This is especially true, if the fistula is very large, like our case 2 above, as well as other cases in the literature. Segmental resection is possible if all feeding arteries are identified and selectively ligated.52 Otherwise, lobectomy is preferred.52,53 Selective resection of the fistula (so called “fistulectomy”) has been reported in a case of a large isolated PAVM, as an alternative to segmental or lobar resection54 so as to avoid removal of healthy lung parenchyma. Resection of a PAVM with use of video-assisted thoracic surgery has also been reported.55
Patients with PAVMs that have developed after a bidirectional Glenn operation, or a Kawashima operation, can clearly benefit from inclusion of the hepatic venous return in the pulmonary circulation. Several series of patients and case reports have described resolution of PAVMs after completion of a modified Fontan in patients with a bidirectional Glenn, or redirection of hepatic venous flow to the pulmonary circulation using an intra- or extra-cardiac baffle.12,17,56 A patient with anomalous hepatic venous drainage to the left atrium, intact atrial septum, and PAVMs, was found to have reversal of the pulmonary vascular anomalies after diversion of the anomalous hepatic drainage to the right atrium.18
Patients with complex congenital heart disease who have developed PAVMs may have particularly difficult postoperative course, sometimes requiring additional forms of support such as extracorporeal membrane oxygenation (ECMO) and use of nitric oxide.57 Resolution of PAVMs has also been reported after orthotopic heart transplantation, in a patient who had undergone a Kawashima operation and had profound cyanosis and ventricular dysfunction.58
Medical therapy
There is a potential role –although limited- for medical therapy in patients with PAVMs. Sands et al reported two unusual cases of diffuse PAVMs, one in the context of periportal fibrosis and the other with dyskeratosis congenita with ataxia, both of which derived significant improvement in systemic oxygenation with oral nifedipine.59 We have also observed resolution of cyanosis and return of oxygen saturation to normal values after night-time oxygen administration in the patient with polysplenia syndrome and PAVMs who has been previously reported.20 The improvement in oxygenation has persisted several years after discontinuation of oxygen therapy [unpublished observation]. It is possible that in these cases, vasodilation of pulmonary arterioles may have lead to diversion of blood flow through normal capillaries and away from the abnormal arteriovenous communications, resulting in improved oxygenation. This may also be the mechanism by which nitric oxide improves oxygenation postoperatively.57
Conclusion
Pulmonary arteriovenous malformation represents a multifaceted disease, with many different causes. Hereditary haemorrhagic telangiectasia is a commonly associated disorder. Treatment in most cases consists of transcatheter embolization using different devices. Although recanalization and complications do occur, the outcome in general is satisfactory. Surgical treatment is reserved for very large or very diffuse fistulas. A special form of PAVMs, related to bidirectional Glenn procedures in patients with complex congenital heart disease, may be cured by redirection of hepatic venous flow to the lungs. Medical therapy has a very limited -sometimes adjunctive- role in the management of diffuse PAVMs.
References
- 1.Hodgson CH, Burchell HB, Good CA, Claggett OT. Hereditary hemorrhagic telangiectasis and pulmonary arteriovenous fistula: survey of a large family. N Engl J Med. 1959;261:625. doi: 10.1056/NEJM195909242611301. [DOI] [PubMed] [Google Scholar]
- 2.Wagenvoort CA, Heath D, Edwards JE. In: The Pathology of Pulmonary Vasculature. Thomas Charles C., editor. Springfield IL; 1964. p. 441. [Google Scholar]
- 3.McDonald MT, Papenberg KA, Ghosh S, Glatfelter AA, Biesecker BB, Helmbold EA, Markel DS, Zolotor A, McKinnon WC, Vanderstoep JL. A disease locus for hereditary hemorrhagic telangiectasia maps to chromosome 9q33-34. Nat Genet. 1994;6:197–204. doi: 10.1038/ng0294-197. [DOI] [PubMed] [Google Scholar]
- 4.Shovlin CL, Hughes JMB, Tuddenham EGD, Temperley I, Perembelon YF, Scott J, Seidman CE, Seidman JG. A gene for hereditary hemorrhagic telangiectasia maps to chromosome 9q3. Nat Genet. 1994;6:205–9. doi: 10.1038/ng0294-205. [DOI] [PubMed] [Google Scholar]
- 5.McAllister KA, Grogg KMM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J. Endoglin, a TGFbinding protein of endothelial cells, is the gene for hereditary hemorrhagic telangiectasia type 1. Nat Genet. 1994;8:345–51. doi: 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- 6.Berg JN, Guttmacher AE, Marchuk DA, Porteous ME. Clinical heterogeneity in hereditary hemorrhagic telangiectasia: are pulmonary arteriovenous malformations more common in families linked to endoglin? J Med Genet. 1996;33:256–7. doi: 10.1136/jmg.33.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucas EV, Jr, Lund CV, Edwards JE. Direct communication of a pulmonary artery with the left atrium: an unusual variant of the pulmonary arteriovenous fistula. Circulation. 1961;24:1409. doi: 10.1161/01.cir.24.6.1409. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein HS, Brook MM, Silverman NH, Bristow J. Development of Pulmonary arteriovenous fistulae in children after cavopulmonary shunt. Circulation. 1995;92(suppl II):II-309–II-314. doi: 10.1161/01.cir.92.9.309. [DOI] [PubMed] [Google Scholar]
- 9.Srivastava D, Preminger T, Lock JE, Mandell V, Keane JF, Mayer JE, Kozakewich H, Spevak PJ. Hepatic venous blood and the development of pulmonary arteriovenous malformations in congenital heart disease. Circulation. 1995;92:1217–1222. doi: 10.1161/01.cir.92.5.1217. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs ML, Pourmoghadam KK, Geary EM, Wright KL, Zales VR. Pulmonary arteriovenous malformations after cavopulmonary connection. Ann Thorac Surg. 2000;69:634–5. doi: 10.1016/s0003-4975(99)01084-x. [DOI] [PubMed] [Google Scholar]
- 11.Kim SJ, Bae EJ, Cho DJ, Park IS, Kim YM, Kim WH, Kim SH. Development of pulmonary arteriovenous fistulas after bidirectional cavopulmonary shunt. Ann Thorac Surg. 2000;70:1918–22. doi: 10.1016/s0003-4975(00)02164-0. [DOI] [PubMed] [Google Scholar]
- 12.Mahle WT, Rychik J, Rome JJ. Clinical significance of pulmonary arteriovenous malformations after staging bi-directional cavopulmonary anastomosis. Am J Cardiol. 2000;86:239–41. doi: 10.1016/s0002-9149(00)00868-7. [DOI] [PubMed] [Google Scholar]
- 13.Vettukattil JJ, Slavik Z, Lamb RK, Monro IL, Keeton BR, Tsang VT, Aldous AJ, Zivanovic A, Johns S, Lewington V, Salmon AP. Intrapulmonary arteriovenous shunting may be a universal phenomenon on patients with the superior cavopulmonary anastomosis: a radionuclide study. Heart. 2000;83:425–8. doi: 10.1136/heart.83.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinohara T, Yokoyama T. Pulmonary arteriovenous malformation in patients with total cavopulmonary shunt. What role does lack of hepatic venous flow to the lungs play? Pediatr Cardiol. 2001;22:343–6. doi: 10.1007/s002460010243. [DOI] [PubMed] [Google Scholar]
- 15.Pandurangi UM, Shah MJ, Murali R, and Cherian KM. Rapid onset of pulmonary arteriovenous malformations after cavopulmonary anastomosis. doi: 10.1016/s0003-4975(99)00407-5. [DOI] [PubMed] [Google Scholar]
- 16.Barbe T, Losay J, Grimon G. Pulmonary arteriovenous shunting in children with liver disease. J Pediatr. 1995;126:571–9. doi: 10.1016/s0022-3476(95)70351-9. [DOI] [PubMed] [Google Scholar]
- 17.Knight WB, Mee RBB. A cure for pulmonary arteriovenous fistulas? Ann Thorac Surg. 1995;59:999–1001. doi: 10.1016/0003-4975(94)00735-p. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Menkis AH, Rosenberg HC. Reversal of pulmonary arteriovenous malformation after diversion of anomalous hepatic drainage. Ann Thorac Surg. 1998;65:848–9. doi: 10.1016/s0003-4975(98)00011-3. [DOI] [PubMed] [Google Scholar]
- 19.Bacha EA, Jonas RA, Mayer JE., Jr Management of pulmonary arteriovenous malformations after surgery for complex congenital heart disease. J Thorac Cardiovasc Surg. 2000;119:175–6. doi: 10.1016/s0022-5223(00)70237-6. [DOI] [PubMed] [Google Scholar]
- 20.Papagiannis J, Kanter RJ, Effman EL, Pratt PC, Marcille R, Browning IB, 3d, Armstrong BE. Polysplenia with pulmonary arteriovenous malformations. Pediatr Cardiol. 1993;14:127–9. doi: 10.1007/BF00796995. [DOI] [PubMed] [Google Scholar]
- 21.Kapur S, Rome J, Chandra RS. Diffuse pulmonary arteriovenous malformation in a child with polysplenia syndrome. Pediatr Pathol Lab Med. 1995;15:463–8. doi: 10.3109/15513819509026982. [DOI] [PubMed] [Google Scholar]
- 22.Kawata H, Kishimoto H, Ikawa S, Ueno T, Nakajima T, Kayatani F, Inamura N, Nakada T. Pulmonary and systemic arteriovenous fistulas in patients with left isomerism. Cardiol Young. 1998;8:290–4. doi: 10.1017/s1047951100006788. [DOI] [PubMed] [Google Scholar]
- 23.Allen SW, Whitfield JM, Clarke DR, Sujansky E, Wiggins JW. Pulmonary arteriovenous malformations in the newborn: a familial case. Pediatr Cardiol. 1993;14:58–61. doi: 10.1007/BF00794850. [DOI] [PubMed] [Google Scholar]
- 24.Grady RM, Sharkey AM, Bridges ND. Transcatheter coil embolization of a pulmonary arteriovenous malformation in a neonate. Br Heart J. 1994;71:370–71. doi: 10.1136/hrt.71.4.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fletcher SE, Cheatham JP, Bolam DL. Primary transcatheter treatment of congenital pulmonary arteriovenous malformation causing cyanosis of the newborn. Cathet Cardiovasc Interv. 2000;50:52–3. doi: 10.1002/(sici)1522-726x(200005)50:1<48::aid-ccd9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 26.Esplin MS, Varner MW. Progression of pulmonary arteriovenous malformation during pregnancy: case report and review of the literature. Obstet Gynecol Surv. 1997;52:248–53. doi: 10.1097/00006254-199704000-00022. [DOI] [PubMed] [Google Scholar]
- 27.Gershon AS, Faughan ME, Chon KS, Pugash RA, Clark JA, Bohan MJ, Henderson KJ, Hyland RH, White RI., Jr Transcatheter embolotherapy of maternal pulmonary arteriovenous malformations during pregnancy. Chest. 2001;119:470–7. doi: 10.1378/chest.119.2.470. [DOI] [PubMed] [Google Scholar]
- 28.Ference BA, Shannon TM, White RI, Jr, Zawin M, Burdge CM. Life-threatening pulmonary hemorrhage with pulmonary arteriovenous malformations and hereditary hemorrhagic telangiectasia. Chest. 1994;106:1387–90. doi: 10.1378/chest.106.5.1387. [DOI] [PubMed] [Google Scholar]
- 29.Moussouttas M, Fayad P, Rosenblatt M, Hashimoto M, Pollak J, Henderson K, Ma TY, White RI., Jr Pulmonary arteriovenous malformations: cerebral ischemia and neurologic manifestations. Neurology. 2000;55:959–64. doi: 10.1212/wnl.55.7.959. [DOI] [PubMed] [Google Scholar]
- 30.Kjeldsen AD, Oxhoj H, Andersen PE, Green A, Vase P. Prevalence of pulmonary arteriovenous malformations (PAVMs) and occurrence of neurological symptoms in patients with hereditary hemorrhagic telangiectasia (HHT) J Intern Med. 2000;248:255–62. doi: 10.1046/j.1365-2796.2000.00725.x. [DOI] [PubMed] [Google Scholar]
- 31.Chang RK, Alejos JC, Atkinson D, Jensen R, Drant S, Galindo A, Laks H. Bubble contrast echocardiography in detecting pulmonary arteriovenous shunting in children with univentricular heart after cavopulmonary anastomosis. J Am Coll Cardiol. 1999;33:2052–8. doi: 10.1016/s0735-1097(99)00096-0. [DOI] [PubMed] [Google Scholar]
- 32.Nanthakumar K, Graham AT, Robinson TI, Grande P, Pugash RA, Clarke JA, Hutchinson SJ, Mandzia JL, Hyland RH, Faughan ME. Contrast echocardiography for detection of hereditary hemorrhagic telangiectasia. Am Heart J. 2001;141:243–6. doi: 10.1067/mhj.2001.112682. [DOI] [PubMed] [Google Scholar]
- 33.Kjeldsen AD, Oxhoj H, Andersen PE, Elle B, Jacobsen JP, Vase P. Pulmonary arteriovenous malformations: screening procedures and pulmonary angiography in patients with hereditary hemorrhagic telangiectasia. Chest. 1999;116:432–9. doi: 10.1378/chest.116.2.432. [DOI] [PubMed] [Google Scholar]
- 34.Chilvers ER, Peters AM, George P. Quantification of right-to-left shunt through pulmonary arteriovenous malformations using Tc albumin microspheres. Clin Radiol. 1988;39:611–614. doi: 10.1016/s0009-9260(88)80065-5. [DOI] [PubMed] [Google Scholar]
- 35.Remy J, Remy-Jardin M, Wattine L, Deffontaines C. Pulmonary arteriovenous malformations: evaluation with CT of the chest before and after treatment. Radiology. 1992;182:809–16. doi: 10.1148/radiology.182.3.1535899. [DOI] [PubMed] [Google Scholar]
- 36.Khalil A, Farres MT, Mangiapan G, Tassart M, Bigot JM, Carette MF. Pulmonary arteriovenous malformations. Chest. 2000;117:1399–1403. doi: 10.1378/chest.117.5.1399. [DOI] [PubMed] [Google Scholar]
- 37.Preminger TJ, Perry SB, Burrows PE. Vascular anomalies. In: Emmanouilides GC, Riemenschneider TA, Allen HD, editors. Heart Disease in Infants, Children and Adolescents. Baltimore: Williams and Wilkins; 1995. pp. 791–810. [Google Scholar]
- 38.White RI, Lynch-Nyhan A, Terry P, Buescher PC, Farmlett EJ, Charnas L, Shuman K, Kim W, Kinnison M, Mitchell SE. Pulmonary arteriovenous malformations: techniques and long-term outcome of embolotherapy. Radiology. 1988;169:663–9. doi: 10.1148/radiology.169.3.3186989. [DOI] [PubMed] [Google Scholar]
- 39.Lee DW, White RI, Egglin TK, et al. Embolotherapy of large pulmonary arteriovenous malformations: long-term results. Ann Thorac Surg. 1997;64:930–40. doi: 10.1016/s0003-4975(97)00815-1. [DOI] [PubMed] [Google Scholar]
- 40.Faughan ME, Lui YW, Wirth JA, Pollak JS, Fayad PB, Wirth JA, Rosenblatt MM, Dickey KW, Burdge CM. Diffuse pulmonary arteriovenous malformations: Characteristics and prognosis. Chest. 2000;117:31–38. doi: 10.1378/chest.117.1.31. [DOI] [PubMed] [Google Scholar]
- 41.Dutton JA, Jackson JE, Hughes JM, Whyte MK, Peters AM, Ussov W, Allison DJ. Pulmonary arteriovenous malformations: results of treatment with coil embolization in 53. AJR. 1995;165:1119–25. doi: 10.2214/ajr.165.5.7572487. [DOI] [PubMed] [Google Scholar]
- 42.Haitjema TJ, Overtoom TThC, Westerman CJJ, Lammers JW. Embolization of pulmonary arteriovenous malformations: results and follow-up in 32 patients. Thorax. 1995;50:719–23. doi: 10.1136/thx.50.7.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haitjema T, ten Berg MJ, Overtoom TThC. Unusual complications after embolization of a pulmonary arteriovenous malformation. Chest. 1996;109:1401–4. doi: 10.1378/chest.109.5.1401. [DOI] [PubMed] [Google Scholar]
- 44.Grady RM, Sharkey AM, Bridges ND. Transcatheter coil embolization of a pulmonary arteriovenous malformation in a neonate. Br Heart J. 1994;71:370–71. doi: 10.1136/hrt.71.4.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fletcher SE, Cheatham JP, Bolam DL. Primary transcatheter treatment of congenital pulmonary arteriovenous malformation causing cyanosis of the newborn. Cathet Cardiovasc Intervent. 2000;50:48–51. doi: 10.1002/(sici)1522-726x(200005)50:1<48::aid-ccd9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 46.Ebeid MR, Braden DS, Gaymes CH, Joransen JA. Closure of a large pulmonary arteriovenous malformation using multiple Gianturco-Grifka vascular occlusion devices. Cathet Cardiovasc Intervent. 2000;49:426–9. doi: 10.1002/(sici)1522-726x(200004)49:4<426::aid-ccd17>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 47.Apostolopoulou SC, Kelekis NL, Papagiannis J, Hausdorf G, Rammos S. Transcatheter occlusion of a large pulmonary arteriovenous malformation with use of a Cardioseal device. J Vasc Interv Radiol. 2001;12:767–9. doi: 10.1016/s1051-0443(07)61452-3. [DOI] [PubMed] [Google Scholar]
- 48.Waight DJ, Hijazi ZM. Pulmonary arteriovenous malformations: transcatheter embolization options. Cathet Cardiovasc Intervent. 2000;50:52–53. doi: 10.1002/(sici)1522-726x(200005)50:1<52::aid-ccd10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 49.Naanaa-Baccar H, Godart F, Francart C, Vaksmann G, Breviere GM, Rey C. Left monoplegia revealing pulmonary arteriovenous fistula in an adolescent : occlusion with an Amplatzer occluder. Arch Mal Coeur Vaiss. 2001;94:504–8. [PubMed] [Google Scholar]
- 50.Remy-Jardin M, Wattine L, Remy J. Transcatheter occlusion of pulmonary arterial circulation and collateral supply: failures, incidents and complications. Radiology. 1991;180:669–705. doi: 10.1148/radiology.180.3.1871280. [DOI] [PubMed] [Google Scholar]
- 51.Puskas JD, Allen MS, Moncure AC, Wain JC, Hilgenberg AAD, Wright C, Grillo HC, Mathisen DJ. Pulmonary arteriovenous malformations : therapeutic options. Ann Thorac Surg. 1993;56:253–8. doi: 10.1016/0003-4975(93)91156-h. [DOI] [PubMed] [Google Scholar]
- 52.Storck M, Mickley V, Abendroth D, Sunder-Plassmann L. Pulmonary arteriovenous malformations: aspects of surgical therapy. Vasa. 1996;25:54–9. [PubMed] [Google Scholar]
- 53.Wechsler J, Jedlicka V, Kerwitzer J, Novotny J, Pavlik M, Pestal A, Capov I. A case of pulmonary arteriovenous malformation treated by lobectomy. Acta Chir Hung. 1999;38:53–5. [PubMed] [Google Scholar]
- 54.Schroder C, Frohlich G, Harms C, Kleckow M, Macchiarini P. Fistulectomy as an alternative to segmentectomy for pulmonary arteriovenous fistula. J Thorac Cardiovasc Surg. 2001;122:386–8. doi: 10.1067/mtc.2001.113742. [DOI] [PubMed] [Google Scholar]
- 55.Temes RT, Paramsothy P, Endara SA, Wernly JA. Resection of a solitary pulmonary arteriovenous malformation by video-assisted thoracic surgery. J Thorac Cardiovasc Surg. 1998;116:878–9. doi: 10.1016/S0022-5223(98)00441-3. [DOI] [PubMed] [Google Scholar]
- 56.Uemura H, Yagihara T, Hattori R, Kawahira Y, Tsukano S, Watanabe K. Redirection of hepatic venous drainage after total cavopulmonary shunt in left isomerism. Ann Thorac Surg. 1999;68:1731–5. doi: 10.1016/s0003-4975(99)00665-7. [DOI] [PubMed] [Google Scholar]
- 57.Bacha E, Jonas RA, Mayer JE. Management of pulmonary arteriovenous malformations after surgery for complex congenital heart disease. J Thorac Cardiovasc Surg. 2000;119:175–6. doi: 10.1016/s0022-5223(00)70237-6. [DOI] [PubMed] [Google Scholar]
- 58.Graham K, Sondheimer H, Schaffer M. Resolution of cavopulmonary-shunt associated pulmonary arteriovenous malformation after heart transplantation. J Heart Lung Transplant. 1997;16:1271–4. [PubMed] [Google Scholar]
- 59.Sands A, Dalzell E, Craig B, Shields M. Multiple pulmonary arteriovenous fistulas in childhood. Pediatr Cardiol. 2000;21:493–6. doi: 10.1007/s002460010120. [DOI] [PubMed] [Google Scholar]


