Abstract
Rheumatic fever is an immunologically mediated inflammatory disease, that occurs as a delayed sequel to group A streptococcal throat infection, in genetically susceptible individuals. Chronic rheumatic heart disease remains an important public health problem in developing countries. Aetiopathogenesis and guidelines for the diagnosis, prevention and treatment of acute rheumatic fever are reviewed.
MeSH: rheumatic fever, streptococcus, rheumatic heart disease, arthritis, autoimmunity
Introduction
Rheumatic fever (RF) is a multisystem, immunologically mediated inflammatory disease, that occurs as a delayed sequel to group A streptococcal (GAS) infection. Its subsequent complication, chronic rheumatic heart disease (RHD), remains a major public health problem in developing countries.
Epidemiology
The epidemiology of RF in developed countries has changed dramatically over the past decades. In developing areas, the prevalence is still high at up to 24 per 1000 population.1 Rheumatic fever occurs most frequently among children and adolescents between 5 and 18 years,2 coinciding with the age distribution of the highest prevalence of streptococcal infections.
Aetiopathogenesis
The pathogenic mechanisms involved in the development of RF remain unclear. However, it is evident that an abnormal humoral and cellular immune response occurs. Antigenic mimicry between streptococcal antigens, mainly M-protein epitopes and human tissues, such as heart valves, myosin and tropomyosin, brain proteins, synovial tissue and cartilage has been proposed as the triggering factor leading to autoimmunity in individuals with genetic predisposition.3 Several genetic markers of susceptibility have been studied but no consistent association found.4 However, associations with different HLA class II antigens have been observed in several populations.5–13 Molecular mimicry was first demonstrated by humoral immune response. Streptococcal antibodies cross-react with several human tissues including heart, skin, brain, glomerular basement membrane, striated and smooth muscles.14 The presence of CD4+ T cells at lesions sites in the heart has been demonstrated, suggesting a direct role of these cells in the pathogenesis of RHD.15,16 Infiltrating T lymphocytes from heart lesions of severe RHD patients and peripheral T lynphocytes were capable of recognising immunodominant myocardium M5 peptides and valve proteins. These results showed the significance of molecular mimicry between beta hemolytic streptococci and heart tissue assessing the T-cell repertoire leading to local tissue damage in RHD.17,18 Figure 1 illustrates the events that occur during the development of RF/RHD.
Figure 1.
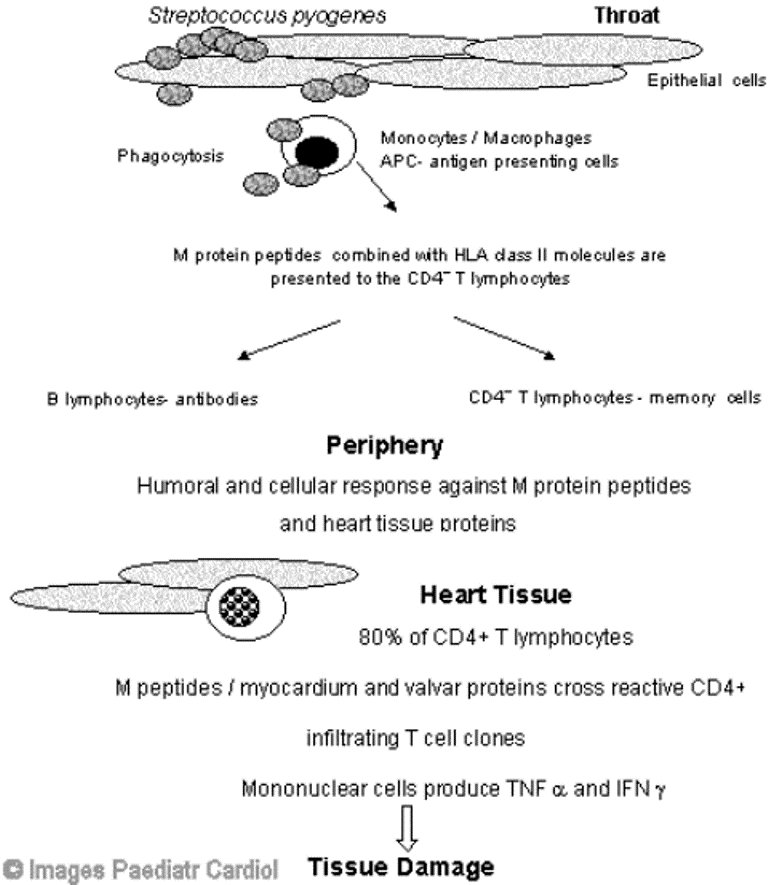
Schematic representation of the aetiopathogenic events occurring during the development of carditis
Major Clinical Manifestations
Arthritis
Arthritis is the most common manifestation, present in 60-80% of patients.19,20 Itu sually affects the peripheral large joints; small joints and axial skeleton are rarely involved. Knees, ankles, elbows and wrists are the most frequently affected. In addition to arthralgia, the joints are red, warm and swollen. Arthritis is characteristically asymmetrical, migratory, and very painful, although some patients may present mild joint complaints. It usually resolves spontaneously at the most in 2 or 3 weeks. Arthritis in ARF has an excellent response to salicylates.21
Approximately one third of patients with joint involvement reported in a series of 786 cases of RF presented with “atypical” articular manifestations, considered by some as a separate entity, i.e., post-streptococcal reactive arthritis. It seems reasonable to include these manifestations as part of the spectrum of RF.19,21 Secondary prophylaxis should be recommended in these situations.
Carditis
Acute carditis was present in 50% of patients in a large recent series.19 It is a pancarditis, but valvular involvement is the rule. The commonest involved valve is the mitral, frequently associated with aortic valve involvement. Right-sided heart valves are rarely affected.
On pathological examination, the valves are thickened and display rows of small vegetations along their apposing surfaces (Figure 2). Myocarditis is characterised by infiltration of mononuclear cells, vasculitis and degenerative changes of the interstitial connective tissue.22 The pathognomonic lesion is the Aschoff body in the proliferative stage, present in 30 to 40 per cent of biopsies of patients with acute RF.23 It is seen mainly in the interstitial connective tissue of the myocardium, particularly perivascularly (Figure 3).22 Inflammation of the valves consists of oedema and mononuclear cell infiltration of the valvular tissue and the chordae tendineae in the acute phase; fibrosis and calcification occur with maintenance of the inflammatory process.22
Figure 2.
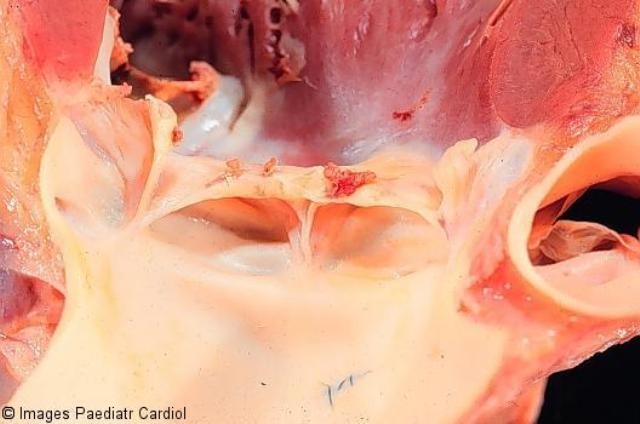
Aortic valve showing active valvulitis. The valve is slightly thickened and displays small vegetations – “verrucae”
Figure 3.
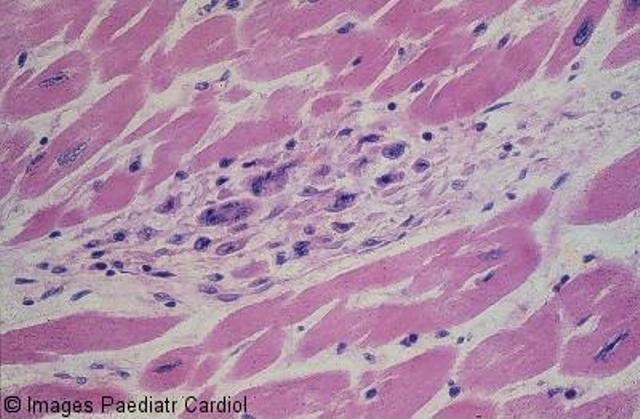
Myocardial Aschoff body – the cells are large, elongated, with large nuclei; some are multinucleate
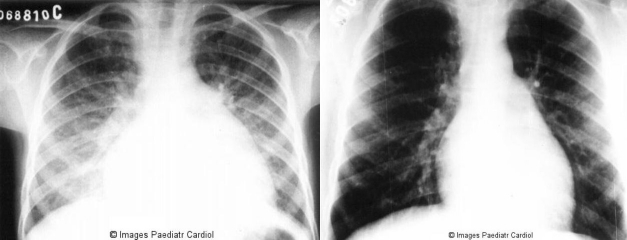
The clinical picture includes high pulse rate, congestive heart failure, arrhytmias and pericardial friction rubs. On the first attack, valvulitis is suspected in the presence of a new apical systolic murmur of mitral regurgitation (associated or not with an apical mid-diastolic murmur) and/or a basal diastolic murmur of aortic regurgitation.24 Cardiomegaly is noted on X-Ray (Figure 4) and on echocardiogram. Myocarditis and/or pericarditis in the absence of valvular involvement is unlikely due to acute RF.24 It is contentious if myocardial disfunction in acute RF is valvular or myocardial in origin.25 In fact, in a subset of patients, the initial presentation may be quite severe, with overt heart failure, fever and toxaemia, making the differential diagnosis with infective endocarditis very difficult, in particular in patients with recurrent rheumatic heart disease.
Figure 4.
Chest radiograph of an 8 year old patient with acute carditis before treatment b: Same patient after 4 weeks
The valvular lesions in RF often result in residual damage. Nevertheless, in milder forms of rheumatic carditis patients may recover from valvulitis without sequelae.1 In the first attack, the lesions are predominantly regurgitant, due to ring dilatation, swollen cusps, chordal rupture or papillary muscle dysfunction. In the chronic phase, obstructive lesions are more frequent. Figures 5–7 depict aspects of the chronic valvar involvement on echocardiography. Figures 8–10 show examples of the pathological presentation of chronic rheumatic valve disease.
Figure 5.
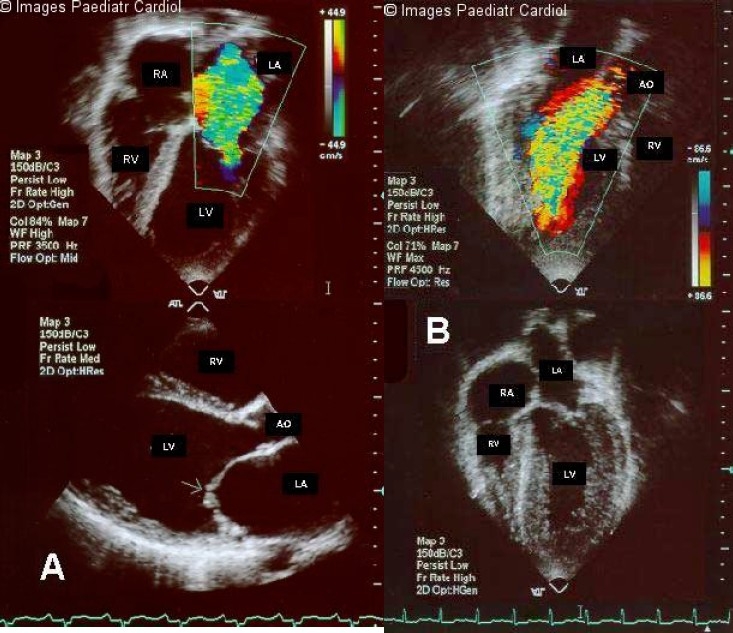
a: Two-dimensional color flow Doppler image of the left ventricular inflow of a patient with mitral regurgitation in the four-chamber view (top panel) and two-dimensional parasternal long-axis view (lower panel), showing lack of apposition of the leaflets of the mitral valve during systole (arrow) b: Color flow Doppler study of a patient with aortic regurgitation, as viewed from the parasternal longaxis view (top panel) and twodimensional fourchamber view, showing hypertrophy and dilatation of the left ventricle (lower panel). LV = left ventricle; LA = left atrium; RV = right ventricle; RA = right atrium; AO = aorta
Figure 7.
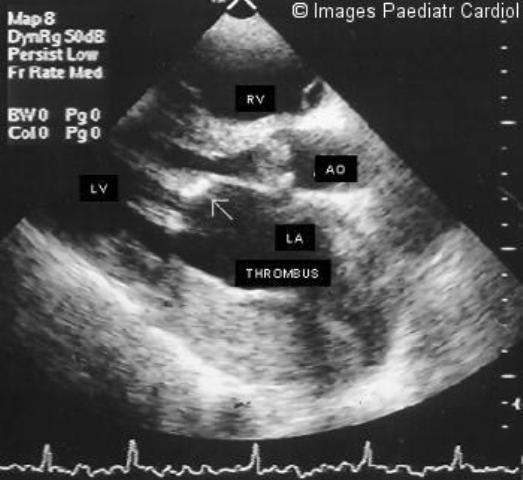
Two-dimensional parasternal long-axis view of a patient with mitral stenosis, showing thickened valve cusps (arrow), with poor leaflet separation in diastole. Left atrium is enlarged, with a thrombus in the posterior aspect of it. Aortic valve is also stenotic
Figure 8.
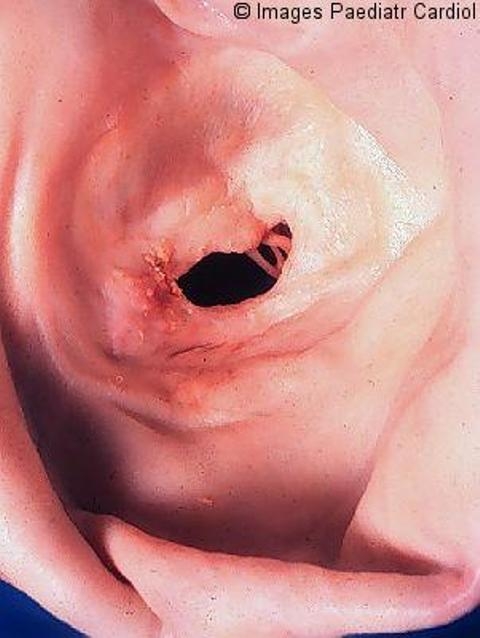
Stenotic mitral valve seen from left atrium. Both commissures are fused; the cusps are severely thickened. The left atrium is huge. The valve is both incompetent and stenotic
Figure 10.
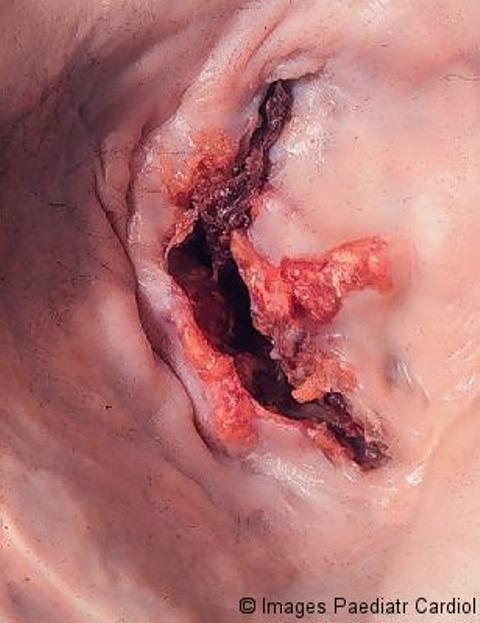
Stenotic mitral valve seen from left atrium, showing fusion of commissures, thickening and calcification of the cusps
Figure 6.
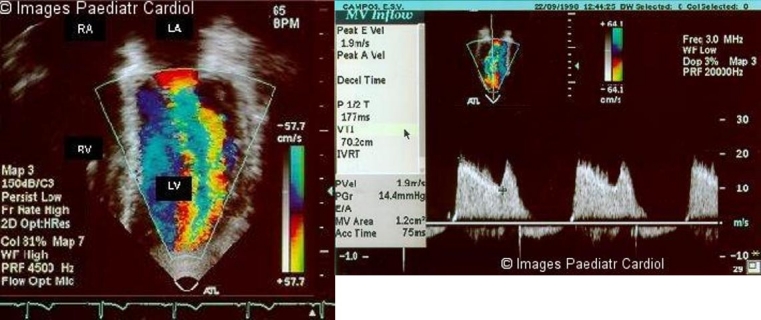
a: Two-dimensional color flow Doppler study of the left ventricular inflow of a patient with mitral stenosis b: Pressure gradient half-time and mitral area is calculated from the diastolic mitral orifice Doppler velocities signals (lower panel). LV = left ventricle; LA = left atrium; RV = right ventricle; RA = right atrium
Figure 9.

Opened stenotic mitral valve showing thickening distorted cusps, adherent commissures with calcification and thrombus deposition, and thickening, fusion and shortening of chordae tendinae
Sydenham's chorea
Sydenham's chorea is characterized by involuntary movements, specially of the face and limbs, muscle weakness, disturbances of speech and gait. Children usually exhibit concomitant psycologic dysfunction, especially obsessive-compulsive disorder, increased emotional lability, hyperactivity, irritablility and age-regressed behavior.26–28 It is usually a delayed manifestation, and is often the sole manifestation of ARF. However, chorea may occur in association with other major manifestations of RF, particularly in the first attack.29 Evidence of a recent GAS infection is often difficult to document. Most of the patients experience resolution of the symptomatology after a few months.3,24 However, a recurrence rate up to 32% has been described, despite the regular use of secondary benzathine penicillin prophylaxis. Some believe that these episodes represent exacerbations rather than distinct attacks of acute RF.29,30
Subcutaneous nodules
Subcutaneous nodules are rarely seen and when present, they are usually associated with severe carditis. They are painless, firm, movable, measuring around 0.5 to 2 cm. They are usually located over extensor surfaces of the joints, particularly knees, wrists and elbows (Figure 11).24
Figure 11.
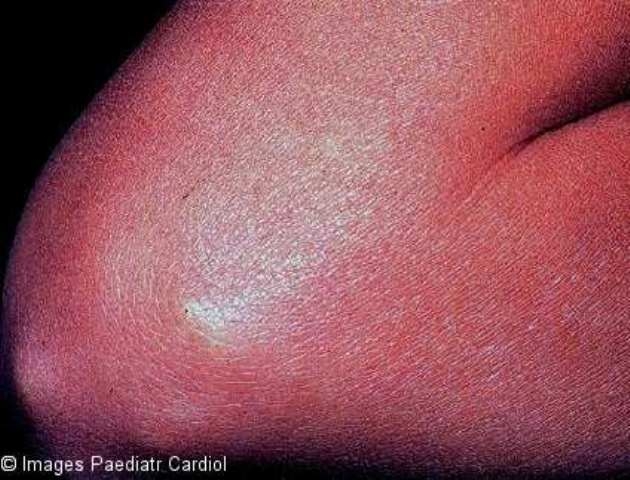
Subcutaneous nodule on the extensor surface of elbow of a patient with acute RF
Erythema marginatum
This is an evanescent, erythematous, non-pruritic rash with pale centers and rounded or serpiginous margins. Lesions occur mainly on the trunk and proximal extremities and may be induced by application of heat (Figure 12).24,31
Figure 12.
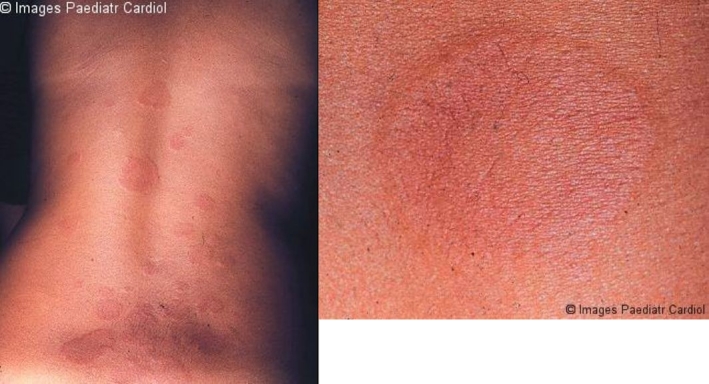
a: Erythema marginatum on the trunk, showing erythematous lesions with pale centers and rounded or serpiginous margins b: Closer view of erythema marginatum in the same patient
Diagnosis
With the exception of Sydenham's chorea, which has a latency period of several months, the clinical manifestations of acute RF present after about 3 weeks following the streptococcal throat infection. It usually begins with nonspecific symptoms, such as fever, malaise and persistent pallor.3
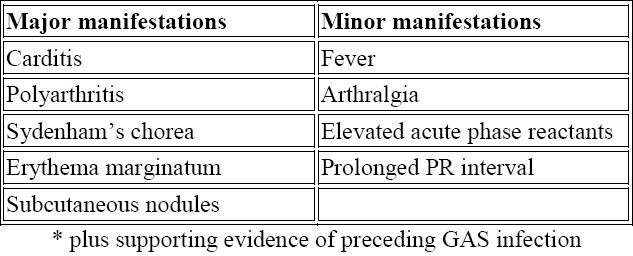
There is no specific clinical or laboratory test that establishes the diagnosis of RF. Diagnosis is based on the revised Jones Criteria24 (Table 1). Arthritis, carditis, chorea, and less frequently, subcutaneous nodules and erythema marginatum are major manifestations of RF. If supported by evidence of preceding streptococcal infection, the presence of two major manifestations or one major and two minor manifestations indicates a high probability of acute RF. The two exceptions to this requirement are Sydenham's chorea and indolent carditis. Other manifestations include arthralgia, serositis and involvement of the kidneys and lungs.3
Table 1.
Guidelines for the diagnosis of initial attack of rheumatic fever (Jones Criteria, 1992 Update).24
Differential diagnosis
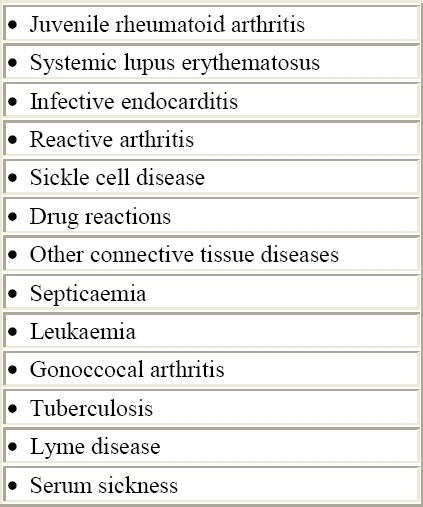
The differential diagnosis is extensive because of the lack of specificity of many of the clinical and laboratory findings in RF and also the unavailability of a laboratory test to confirm the diagnosis. Several diseases need to be considered. Table 2 includes those more often presenting clinical difficulties to the clinician. Juvenile rheumatoid arthritis (JRA) and other connective tissue diseases often should be considered. The articular involvement in JRA usually lasts longer than in RF. It is generally polyarticular and symmetrical, typically affecting the small joints of the hands. Patients complain of increased pain in the morning or after long periods of immobility. Arthritis of the cervical spine occurs in about half of the cases but is also quite frequent in acute RF.3
Table 2.
Differential diagnosis of rheumatic fever
Cardiac involvement presents primarily as pericarditis therefore infective endocarditis should be considered in patients with recurrent RF presenting with persistent fever of unknown origin. Sometimes the differential diagnosis can be difficult, especially in those presenting with a more severe clinical presentation. Splenomegaly, vascular and immmunologic phenomena, demonstration of vegetations on echocardiogram and positive blood cultures are indicative of infective endocarditis. Gallium-67 cardiac scintigraphy can be helpful in this setting.
Systemic lupus erythematosus (SLE) shares clinical characteristics with acute RF. Arthralgia and transient arthritis are common. SLE affects multiple organs, including kidney, central nervous system, skin, and blood. Diagnosis is made on clinical grounds and confirmed by serologic studies.32
Laboratory studies
Acute phase reactants are useful in helping to recognize acute RF and also to exclude other diseases. C-reactive protein and erythrocyte sedimentation rates are helpful in monitoring inflammatory activity.24
Laboratory evidence of a preceding GAS infection should be sought, either by demonstration of GAS in the throat by culture or rapid streptoccocal antigen test, or using streptococcal antibody tests. Elevated or rising titers of antistreptolysin O (ASO) occur in more than 80% of patients with acute GAS pharyngitis.24 There is a remarkable response during the acute phase of RF. The test specificity has been shown to be 93% with ASO titers above 960 IU/ml.33
Prolonged P-R interval relative to heart rate is a nonspecific finding, present in more than one third of the patients. Low-voltage QRS complexes and ST segment changes may be found in the presence of pericarditis and pericardial effusion.3
Endomyocardial biopsy is invasive and does not appear to provide additional diagnostic information where there is a clinical consensus about the diagnosis of RF, with a diagnostic sensitivity in one relatively large study of 27%. It should be limited to clinical investigation.23,34
Cardiac scanning scintigraphy has been shown to be a reliable method distinguishing acute from chronic, inactive RHD and also in the follow-up of active carditis (Figure 13).35–37
Figure 13.
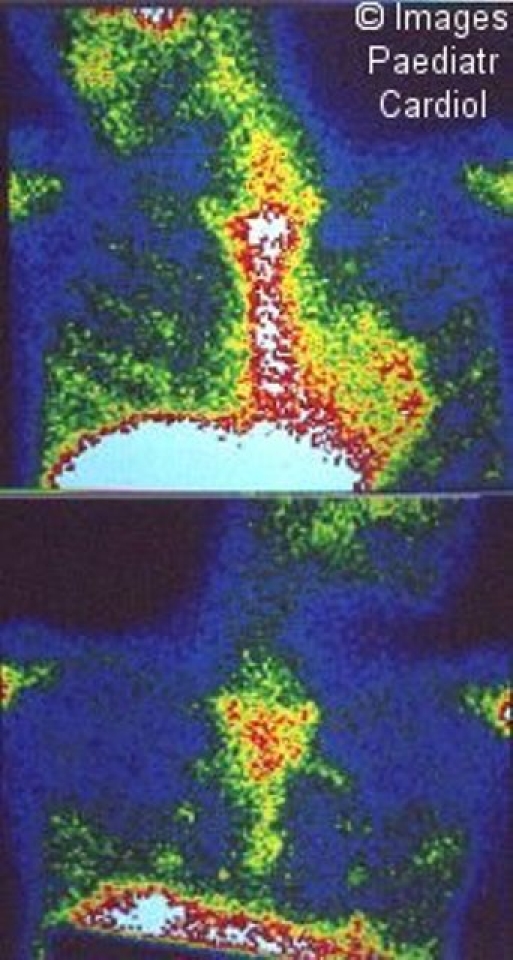
Gallium-67 scintigraphy of the myocardium, showing on left, a positive study, with hypercaptation on the heart projection and, on the right, a negative study
The role of echocadiography to diagnose valvulitis without auscultatory findings has been debated.34,38–41 At present, subauscultatory valve regurgitation is not considered a diagnostic criterion for RF.24
Treatment
Prevention
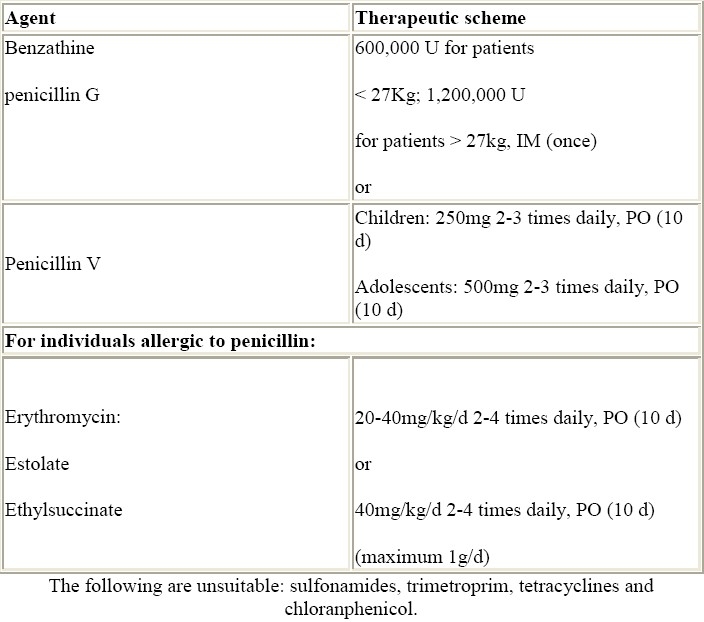
Prevention of initial attacks of RF (primary prevention) requires the eradication of GAS from the pharynx. Table 3 shows the currently recommended treatment schedules.42 Emphasis should be given to the need of eradication of GAS as part of the treatment of acute RF.
Table 3.
Primary prevention of rheumatic fever.42
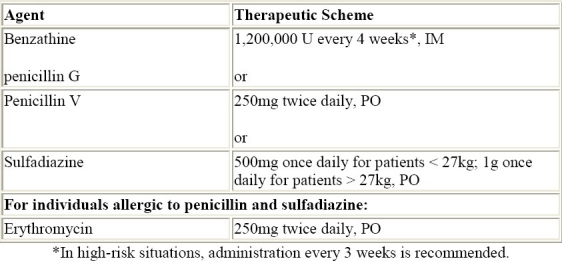
Antibiotic prophylaxis is the safest way to prevent recurrent attacks of acute RF and is recommended for patients with well-documented RF. The recommendations from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young of the American Heart Association are shown on Table 4.42
Table 4.
Secondary prevention of rheumatic fever.42
Arthritis
Salicylates remain the first-line drugs in the treatment of arthritis. The response is usually excellent. Treatment should be started at 80 to 100 mg/kg/day (maximum, 4g daily) for 3-4 weeks. Naproxen (10-15mg/kg/day, bid) is an alternative drug, with very good response. Other nonsteroidal antiinflammatory drugs also can be used.
Carditis
Moderate to severe carditis is usually an indication for cortiscoteroids although efficacy in reducing sequelae has not been proven so far. Albert at al. performed a meta-analysis of the literature on the treatment of rheumatic carditis, comparing corticosteroids and salicylates in preventing valvular damage. It seems clear that corticosteroids are superior to salicylates in rapidly resolving acute manifestations, but the advantage of the former in preventing a pathologic murmur at 1 year posttreatment was not statistically significant.43 Prednisone, 2mg/kg/day (maximum, 60mg/day) is used for two weeks and after that, the dose is gradually tapered, reducing 20 to 25% of the previous dose every week. Some advocate the concomitant use of salicylates to avoid rebound. In severe carditis, therapy may be initiated with intravenous methylprednisolone.44,45 Intravenous immunoglobulin seems not to alter the extent and severity of carditis or decrease chronic morbitidy.46
Heart failure usually responds to steroids. Bed rest is always recommended and should be planned on an individual basis. Diuretics and vasodilators may be used in patients with more severe haemodynamic decompensation. Digoxin should be used with caution because of the risk of toxicity in the presence of active myocarditis.47 Surgical treatment in the acute stage should be considered when clinical therapy is ineffective to control cardic failure. Valve repair, although technically more difficult, is the first choice for younger patients.48
Chorea
Treatment with haloperidol (initial dose of 0.5 to 1mg/kg/day, maximum, 5mg/day)49 or valproic acid (15-20 mg/kg/day)50,51 are helpful in decreasing the severity of involuntary movements but may not improve the behavioral symptoms. Carbamazepine has also been suggested as a first-line treatment for Sydenham's chorea.52 Alternatively, phenobarbital also may be used, 5-7mg/kg/day, tid. Treatment is usually maintained for 8-12 weeks. Intravenous immunoglobulin therapy has been suggested.26
Acknowledgments
The authors would like to acknowledge the assistance of Dr. Clovis Artur Almeida da Silva, from the Department of Paediatrics, Rheumatology Unity (director), University of Sao Paulo Medical School, who provided invaluable help with the illustrations and suggestions on the text. We would also like to thank Prof. Maria de Lourdes Higuchi, director of the Laboratory of Pathology, Heart Institute (InCor), University of Sao Paulo Medical School, for the pictures on the pathological specimens.
References
- 1.Stollerman GH. Rheumatic fever. Lancet. 1997;349:935–942. doi: 10.1016/S0140-6736(96)06364-7. [DOI] [PubMed] [Google Scholar]
- 2.Community control of rheumatic heart disease in developing countries: 2. Strategies for prevention and control. WHO Chron. 1980;34:389–395. [PubMed] [Google Scholar]
- 3.da Silva NA, Pereira BA. Acute rheumatic fever. Still a challenge. Rheum Dis Clin North Am. 1997;23:545–568. doi: 10.1016/s0889-857x(05)70347-1. [DOI] [PubMed] [Google Scholar]
- 4.Gibofsky A, Khanna A, Suh E, Zabriskie JB. The genetics of rheumatic fever: relationship to streptococcal infection and autoimmune disease. J Rheumatol Suppl. 1991;30:1–5. [PubMed] [Google Scholar]
- 5.Ayoub EM, Barrett DJ, Maclaren NK, Krischer JP. Association of class II human histocompatibility leukocyte antigens with rheumatic fever. J Clin Invest. 1986;77:2019–2026. doi: 10.1172/JCI112531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anastasiou-Nana MI, Anderson JL, Carlquist JF, Nanas JN. HLA-DR typing and lymphocyte subset evaluation in rheumatic heart disease: a search for immune response factors. Am Heart J. 1986;112:992–997. doi: 10.1016/0002-8703(86)90311-x. [DOI] [PubMed] [Google Scholar]
- 7.Rajapakse CN, Halim K, Al Orainey I, Al Nozha M, Al Aska AK. A genetic marker for rheumatic heart disease. Br Heart J. 1987;58:659–662. doi: 10.1136/hrt.58.6.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jhinghan B, Mehra NK, Reddy KS, Taneja V, Vaidya MC, Bhatia ML. HLA, blood groups and secretor status in patients with established rheumatic fever and rheumatic heart disease. Tissue Antigens. 1986;27:172–178. doi: 10.1111/j.1399-0039.1986.tb01517.x. [DOI] [PubMed] [Google Scholar]
- 9.Guilherme L, Weidebach W, Kiss MH, Snitcowsky R, Kalil J. Association of human leukocyte class II antigens with rheumatic fever or rheumatic heart disease in a Brazilian population. Circulation. 1991;83:1995–1998. doi: 10.1161/01.cir.83.6.1995. [DOI] [PubMed] [Google Scholar]
- 10.Weidebach W, Goldberg AC, Chiarella JM, Guilherme L, Snitcowsky R, Pileggi F, et al. HLA class II antigens in rheumatic fever. Analysis of the DR locus by restriction fragment-length polymorphism and oligotyping. Hum Immunol. 1994;40:253–258. doi: 10.1016/0198-8859(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 11.Maharaj B, Hammond MG, Appadoo B, Leary WP, Pudifin DJ. HLA-A, B, DR, and DQ antigens in black patients with severe chronic rheumatic heart disease. Circulation. 1987;76:259–261. doi: 10.1161/01.cir.76.2.259. [DOI] [PubMed] [Google Scholar]
- 12.Olmez U, Turgay M, Ozenirler S, Tutkak H, Duzgun N, Duman M, et al. Association of HLA class I and class II antigens with rheumatic fever in a Turkish population. Scand J Rheumatol. 1993;22:49–52. doi: 10.3109/03009749309095114. [DOI] [PubMed] [Google Scholar]
- 13.Guedez Y, Kotby A, El Demellawy M, Galal A, Thomson G, Zaher S, et al. HLA class II associations with rheumatic heart disease are more evident and consistent among clinically homogeneous patients. Circulation. 1999;99:2784–2790. doi: 10.1161/01.cir.99.21.2784. [DOI] [PubMed] [Google Scholar]
- 14.Stollerman GH. Rheumatogenic streptococci and autoimmunity. Clin Immunol Immunopathol. 1991;61:131–142. doi: 10.1016/s0090-1229(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 15.Raizada V, Williams RC, Jr, Chopra P, Gopinath N, Prakash K, Sharma KB, et al. Tissue distribution of lymphocytes in rheumatic heart valves as defined by monoclonal anti-T cell antibodies. Am J Med. 1983;74:90–96. doi: 10.1016/0002-9343(83)91124-5. [DOI] [PubMed] [Google Scholar]
- 16.Kemeny E, Grieve T, Marcus R, Sareli P, Zabriskie JB. Identification of mononuclear cells and T cell subsets in rheumatic valvulitis. Clin Immunol Immunopathol. 1989;52:225–237. doi: 10.1016/0090-1229(89)90174-8. [DOI] [PubMed] [Google Scholar]
- 17.Guilherme L, Cunha-Neto E, Coelho V, Snitcowsky R, Pomerantzeff PM, Assis RV, et al. Human heart-infiltrating T-cell clones from rheumatic heart disease patients recognize both streptococcal and cardiac proteins. Circulation. 1995;92:415–420. doi: 10.1161/01.cir.92.3.415. [DOI] [PubMed] [Google Scholar]
- 18.Guilherme L, Oshiro SE, Fae KC, Cunha-Neto E, Renesto G, Goldberg AC, et al. T-cell reactivity against streptococcal antigens in the periphery mirrors reactivity of heart-infiltrating T lymphocytes in rheumatic heart disease patients. Infect Immun. 2001;69:5345–5351. doi: 10.1128/IAI.69.9.5345-5351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.da Silva CH. Rheumatic fever: a multicenter study in the state of Sao Paulo. Pediatric Committee--Sao Paulo Pediatric Rheumatology Society. Rev Hosp Clin Fac Med Sao Paulo. 1999;54:85–90. doi: 10.1590/s0041-87811999000300004. [DOI] [PubMed] [Google Scholar]
- 20.Kiss MHB. Articular involvement in rheumatic fever. Rev.Soc.Cardiol.Estado de Sao Paulo. 1993;3:26–31. [Google Scholar]
- 21.Ayoub EM, Majeed HA. Poststreptococcal reactive arthritis. Curr Opin Rheumatol. 2000;12:306–310. doi: 10.1097/00002281-200007000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Pomerance A. Cardiac involvement in rheumatic and ‘collagen’ diseases. In: Pomerance A, Davies MJ, editors. The pathology of the heart. Oxford: Blackwell Scientific Publications; 1975. pp. 279–306. [Google Scholar]
- 23.Narula J, Chopra P, Talwar KK, Reddy KS, Vasan RS, Tandon R, et al. Does endomyocardial biopsy aid in the diagnosis of active rheumatic carditis? Circulation. 1993;88:2198–2205. doi: 10.1161/01.cir.88.5.2198. [DOI] [PubMed] [Google Scholar]
- 24.Guidelines for the diagnosis of rheumatic fever. Jones Criteria, 1992 update. Special Writing Group of the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young of the American Heart Association. JAMA. 1992;268:2069–2073. [PubMed] [Google Scholar]
- 25.Essop MR, Wisenbaugh T, Sareli P. Evidence against a myocardial factor as the cause of left ventricular dilation in active rheumatic carditis. J Am Coll Cardiol. 1993;22:826–829. doi: 10.1016/0735-1097(93)90197-9. [DOI] [PubMed] [Google Scholar]
- 26.Dajani AS, Bisno AL, Chung KJ, Durack DT, Gerber MA, Kaplan EL, et al. Prevention of rheumatic fever: a statement for health professionals by the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease of the Council on Cardiovascular Disease in the young, the American Heart Association. Pediatr Infect Dis J. 1989;8:263–266. [PubMed] [Google Scholar]
- 27.Asbahr FR, Negrao AB, Gentil V, Zanetta DM, da Paz JA, Marques-Dias MJ, et al. Obsessive-compulsive and related symptoms in children and adolescents with rheumatic fever with and without chorea: a prospective 6-month study. Am J Psychiatry. 1998;155:1122–1124. doi: 10.1176/ajp.155.8.1122. [DOI] [PubMed] [Google Scholar]
- 28.Mercadante MT, Busatto GF, Lombroso PJ, Prado L, Rosario-Campos MC, do VR, et al. The psychiatric symptoms of rheumatic fever. Am J Psychiatry. 2000;157:2036–2038. doi: 10.1176/appi.ajp.157.12.2036. [DOI] [PubMed] [Google Scholar]
- 29.Terreri MT, Roja SC, Len CA, Faustino PC, Roberto AM, Hilario MO. Sydenham's chorea--clinical and evolutive characteristics. Sao Paulo Med J. 2002;120:16–19. doi: 10.1590/S1516-31802002000100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berrios X, Quesney F, Morales A, Blazquez J, Bisno AL. Are all recurrences of “pure” Sydenham chorea true recurrences of acute rheumatic fever? J Pediatr. 1985;107:867–872. doi: 10.1016/s0022-3476(85)80177-3. [DOI] [PubMed] [Google Scholar]
- 31.Secord E, Emre U, Shah BR, Tunnessen WW., Jr Picture of the month: Erythema marginatum in acute rheumatic fever. Am.J.Dis.Child. 1992;146:637. [PubMed] [Google Scholar]
- 32.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 33.Machado CSM, Ortiz K, Martins ALB, Martins RS, Machado NC. Antistreptolysin O titer profile in acute rheumatic fever diagnosis. J Pediatr (Rio J) 2001;77(2):105–111. doi: 10.2223/jped.185. [DOI] [PubMed] [Google Scholar]
- 34.Stollerman GH. Rheumatic fever in the 21st century. Clin Infect Dis. 2001;33:806–814. doi: 10.1086/322665. [DOI] [PubMed] [Google Scholar]
- 35.Soares J, Jr, Snitcowsky R, Meneghetti JC, Hironaka FH, Assis RVC, Giorgi MCP, et al. Gallium-67 imaging in the diagnosis and follow-up of rheumatic carditis. Eur.J.Nucl.Med. 1990;16:S5. [Google Scholar]
- 36.Soares J, Jr, Snitcowsky R, Assis RV, Izaki M, Giorgi MCP, Meneghetti JC, et al. Carditis in rheumatic fever - importance of scintigraphic diagnosis. J.Nucl.Med. 1992;33:995. [Google Scholar]
- 37.Kao CH, Hsieh KS, Wang YL, Chen CW, Wang SJ, Yeh SH. 99Tcm-HMPAO-labelled white blood cell scanning for the detection of carditis in the differentiation of rheumatic fever and inactive rheumatic heart disease in children. Nucl Med Commun. 1992;13:478–481. doi: 10.1097/00006231-199206000-00051. [DOI] [PubMed] [Google Scholar]
- 38.Davies C, Sahn D. Detection and significance of subclinical mitral regurgitation by colour Doppler techniques. Heart. 2001;85:369–370. doi: 10.1136/heart.85.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Figueroa F, Berrios X, Gutierrez M, Carrion F, Goycolea JP, Riedel I, et al. Anticardiolipin antibodies in acute rheumatic fever. J Rheumatol. 1992;19:1175–1180. [PubMed] [Google Scholar]
- 40.Narula J, Chandrasekhar Y, Rahimtoola S. Diagnosis of active rheumatic carditis. The echoes of change. Circulation. 1999;100:1576–1581. doi: 10.1161/01.cir.100.14.1576. [DOI] [PubMed] [Google Scholar]
- 41.Wilson NJ, Neutze JM. Echocardiographic diagnosis of subclinical carditis in acute rheumatic fever. Int J Cardiol. 1995;50:1–6. doi: 10.1016/0167-5273(95)02325-q. [DOI] [PubMed] [Google Scholar]
- 42.Dajani A, Taubert K, Ferrieri P, Peter G, Shulman S. Treatment of acute streptococcal pharyngitis and prevention of rheumatic fever: a statement for health professionals. Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, the American Heart Association. Pediatrics. 1995;96:758–764. [PubMed] [Google Scholar]
- 43.Albert DA, Harel L, Karrison T. The treatment of rheumatic carditis: a review and meta-analysis. Medicine (Baltimore) 1995;74:1–12. doi: 10.1097/00005792-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 44.DiSciascio G, Taranta A. Rheumatic fever in children. Am Heart J. 1980;99:635–658. doi: 10.1016/0002-8703(80)90739-5. [DOI] [PubMed] [Google Scholar]
- 45.Herdy GV, Pinto CA, Olivaes MC, Carvalho EA, Tchou H, Cosendey R, et al. Rheumatic carditis treated with high doses of pulsetherapy methylprednisolone. Results in children over years. Arq Bras Cardiol. 1999;72:601–606. doi: 10.1590/s0066-782x1999000500007. [DOI] [PubMed] [Google Scholar]
- 46.Voss LM, Wilson NJ, Neutze JM, Whitlock RM, Ameratunga RV, Cairns LM, et al. Intravenous immunoglobulin in acute rheumatic fever: a randomized controlled trial. Circulation. 2001;103:401–406. doi: 10.1161/01.cir.103.3.401. [DOI] [PubMed] [Google Scholar]
- 47.Thatai D, Turi ZG. Current guidelines for the treatment of patients with rheumatic fever. Drugs. 1999;57:545–555. doi: 10.2165/00003495-199957040-00007. [DOI] [PubMed] [Google Scholar]
- 48.Pomerantzeff PM, Brandao CM, Faber CM, Grinberg M, Cardoso LF, Tarasoutchi F, et al. Mitral valve repair in rheumatic patients. Heart Surg Forum. 2000;3:273–276. [PubMed] [Google Scholar]
- 49.Marques-Dias MJ, Mercadante MT, Tucker D, Lombroso P. Sydenham's chorea. Psychiatr Clin North Am. 1997;20:809–820. doi: 10.1016/s0193-953x(05)70346-4. [DOI] [PubMed] [Google Scholar]
- 50.Daoud AS, Zaki M, Shakir R, al Saleh Q. Effectiveness of sodium valproate in the treatment of Sydenham's chorea. Neurology. 1990;40:1140–1141. doi: 10.1212/wnl.40.7.1140. [DOI] [PubMed] [Google Scholar]
- 51.Genel F, Arslanoglu S, Uran N, Saylan B. Sydenham's chorea: clinical findings and comparison of the efficacies of sodium valproate and carbamazepine regimens. Brain Dev. 2002;24:73–76. doi: 10.1016/s0387-7604(01)00404-1. [DOI] [PubMed] [Google Scholar]
- 52.Harel L, Zecharia A, Straussberg R, Volovitz B, Amir J. Successful treatment of rheumatic chorea with carbamazepine. Pediatr Neurol. 2000;23:147–151. doi: 10.1016/s0887-8994(00)00177-6. [DOI] [PubMed] [Google Scholar]