Abstract
We present a patient with tetralogy of Fallot who developed bilateral branch pulmonary artery stenosis following surgical repair that was refractory to balloon dilatation. Simultaneous stenting of both pulmonary arteries produced a good result.
MeSH: Stents, Pulmonary Artery
Introduction
Congenital pulmonary artery (PA) branch stenosis can occur in isolation, as part of a syndrome or in conjunction with other cardiac defects; quite often, PA branch stenosis occurs after surgical repair of congenital heart disease. Significant narrowing of the pulmonary artery origins can lead an overall reduction in pulmonary blood flow or to disproportionate distribution to the two lungs. In addition, an increase in right ventricular systolic pressure will result in right ventricular hypertrophy and possible failure. Moreover, coexistence of pulmonary regurgitation is made worse by PA branch stenosis. A right ventricular peak systolic pressure equal to or greater than 50% of the aortic systolic pressure or quantitative pulmonary perfusion scans showing ipsilateral lung perfusion <35% than predicted in unilateral stenoses are indications for intervention.
Percutaneous transluminal balloon angioplasty for PA branch stenosis is frequently of limited effectiveness in decreasing the pressure gradient, may have significant complications such as vessel rupture, dissection, aneurysm formation or even death and is often followed by recurrence. The latter has become less problematic with the advent of balloon expandable stents.1–4 The radial force of the stent holds the vessel open after deployment, counteracting the natural elastic recoil of the arterial wall. This elastic recoil of the vessel also helps to anchor the stent in place until epithelisation occurs. The collapsed stent is introduced over a deflated balloon catheter into the femoral vein through a long and wide Mullins sheath. Once the stent has been correctly positioned the balloon is inflated to deliver the stent over the stenosis in the vessel. The balloon is then deflated and withdrawn. Balloon expandable stents have added advantage that they can be further dilated when necessary as may be the case with growing children or in case of subsequent restenosis.
We present a patient with tetralogy of Fallot who developed bilateral branch pulmonary artery stenosis following surgical repair that was refractory to balloon dilatation. We describe simultaneous stenting of both PAs with a pleasing result.
Patient
Our patient, a 16 year old female had a repair of tetralogy of Fallot at the age of 14 months following a severe cyanotic spell. At operation the ventricular septal defect was closed with a Gortex patch and infundibular resection was carried out together with a trans-annular patch. She was left with a small residual ventricular septal defect which closed spontaneously, pulmonary regurgitation and mild residual infundibular stenosis. Postoperatively echocardiography demonstrated a dilated main pulmonary artery and narrowing of the origin of the RPA with marked turbulence on colour flow mapping and peak Peak Doppler gradients were up to 60 mm Hg.
Fifteen years after surgery, balloon angioplasty was carried out fro bilateral branch PA stenosis. The right had two levels of stenosis: main pulmonary artery (MPA) to distal right pulmonary artery (RPA) gradient 22 = mm Hg) and the left was tightly stenosed at its origin: MPA to left pulmonary artery (LPA) gradient = 30 mm Hg. The procedure did not produce any significant amelioration in gradients or angiographic appearance.
A further intervention was carried out at a later date with the intention to place stents to the origins of both PAs. Access to the LPA was from the right femoral vein and the RPA from the left femoral vein and a superstiff wire was used on each side.
Figure 1.
RV angiogram – note RPA and LPA stenoses (arrows)
Figure 2.
MPA angiogram further delineating LPA stenosis
As the LPA stenosis was complex and severe, it was decided to predilate with a high pressure balloon prior to stenting (figure 3).
Figure 3.
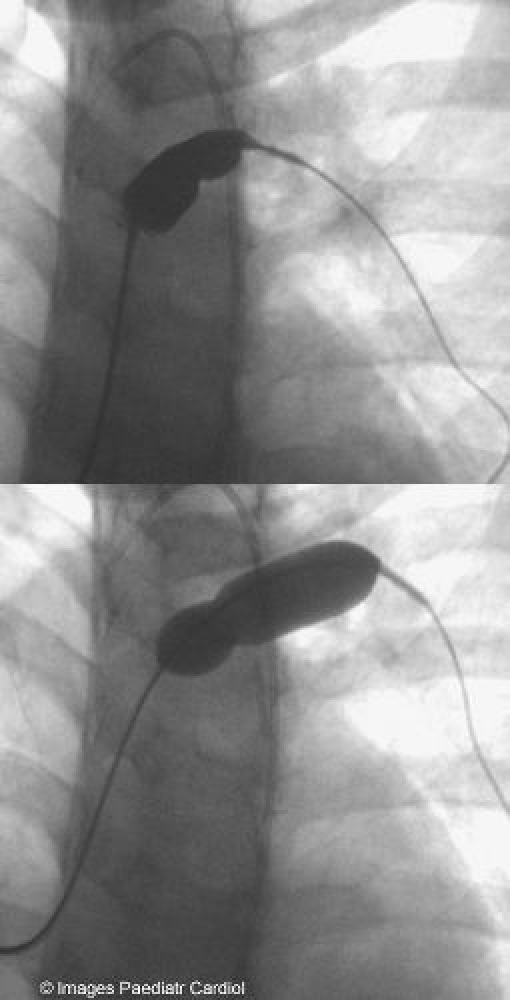
Predilatation of LPA
Two separate Mullins sheaths sizes (11F on the left side and 12F on the right side) were introduced in the PAs distal to the stenosis (figure 4).
Figure 4.
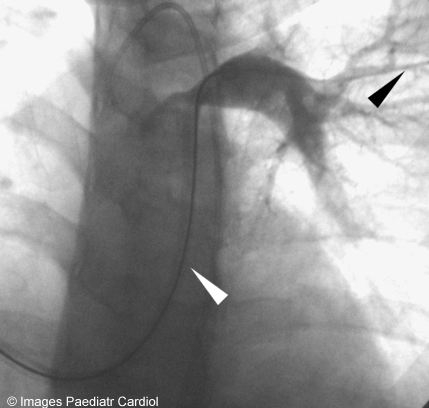
Angiogram through a Mullins sheath (white arrow) being placed over a superstiff wire (black arrow)
Figure 5.
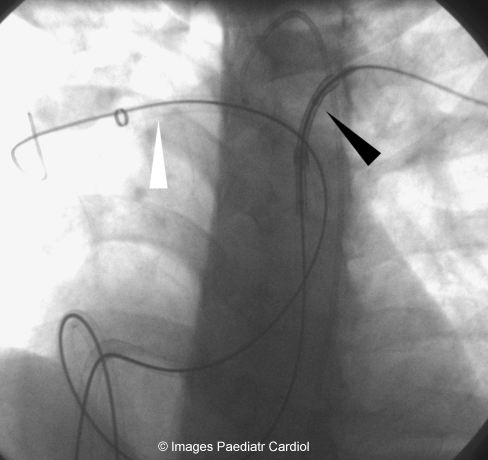
Mullins sheaths in both branch PAs – stent mounted over balloon being delivered to the LPA
Figure 6.
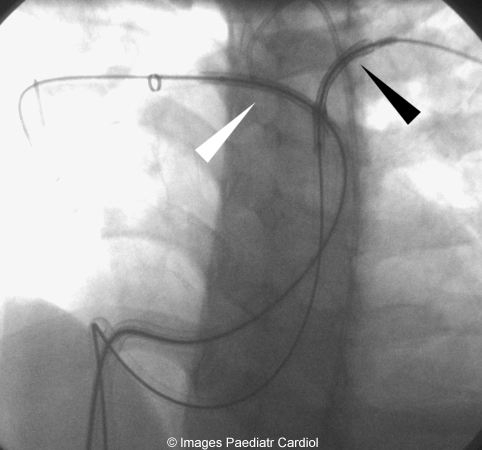
Both stents being placed prior to deployment
Figure 7.
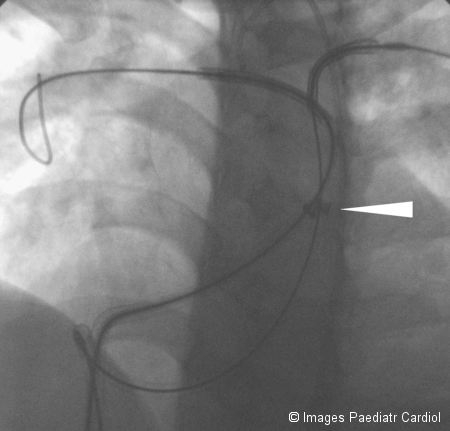
Both Mullins sheaths withdrawn (tip of sheaths indicated by arrow) exposing both stents
Two 59 mm Genesis Cordis stents were used. The RPA stent was deployed on an 18mm Crystal balloon while a 16 mm MaxiLD balloon was used for the LPA stent. Precise positioning of the stents was evaluated by angiography through the Mullins sheath. Simultaneous inflation of the balloons by 2 operators working synchronously led to the deployment of the 2 stents at the same time; this is a crucial part of the technique in order to avoid displacement of one of the stents or occlusion of a PA branch. The anaesthetist provided a short period of apnoea during balloon inflation in order to minimise balloon movement or displacement.
Figure 8.
Simultaneous inflation of both stents
The proximal and distal ends of the stents were flared open by inflating the balloon distally and proximally.
Figure 9.
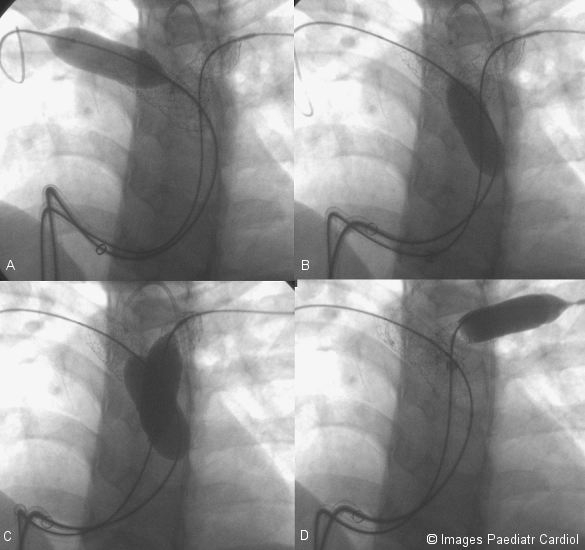
Proximal and distal ends of both stents being flared open. A. Distal end of RPA stent B. Proximal end of RPA stent C. Proximal ends of both stents D. Distal end of LPA stent
Figure 10.
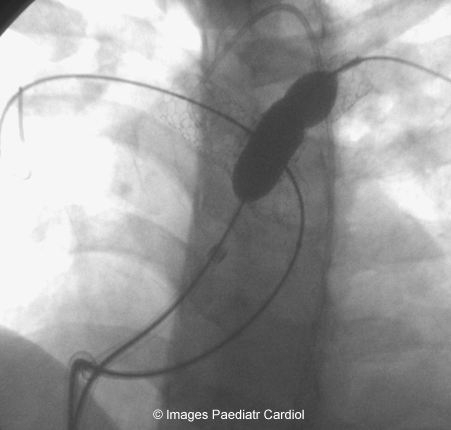
LPA stent being reinflated
Figure 11.
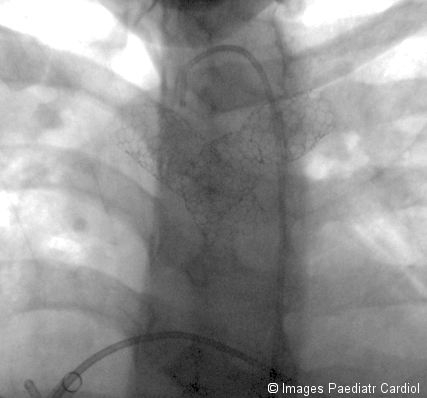
Final result
Figure 12.
Final angiographic result
The gradients across the PAs on this occasion fell from 42 to 10 mm Hg on the right and from 50 to 8 mm Hg on the left. RV pressure fell from 70/- to 32/- following the procedure. There were no complications.
Figure 13.
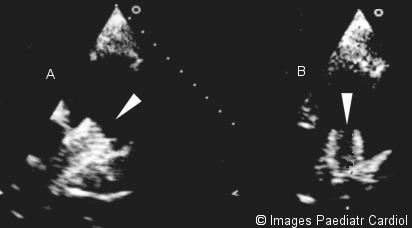
Echocardiographic parasternal short axis view showing A. RPA stent B. LPA stent. Note stent mesh structure clearly visualised on 2D echocardiography
Figure 14.
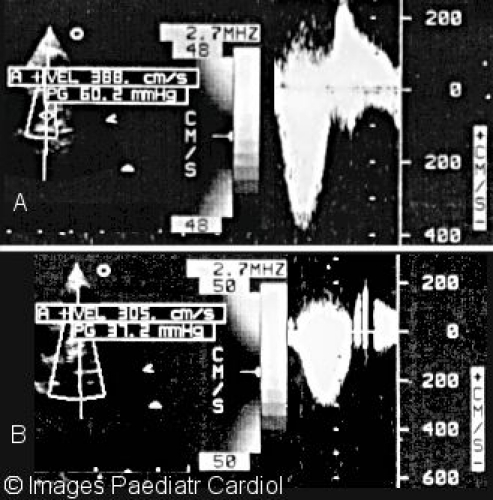
Continuous wave Doppler of right ventricular outflow tract and LPA showing A. Peak gradient before (60 mmHg) and B. Peak gradient after (37 mmHg) stenting
Conclusion
Bilateral PA origin stenosis may require stenting and this should be done simultaneously. Pre-dilatation may be indicated in some cases and stability of the stents prior to deployment is essential using superstiff wires, apnoea and, if the degree of pulmonary regurgitation is severe, consider a large dose of adenosine, esmolol infusion or fast pacing.
References
- 1.Chandar JS, Wolfe SB, Rao PS. Role of stents in the management of congenital heart defects. J Invasive Cardiol. 1996;8:314–325. [PubMed] [Google Scholar]
- 2.Shaffer KM, Mullins CE, Grifka RG. Intravascular Stents in congenital heart disease: short and long-term results from a large single-center experience. J Am Coll Cardiol. 1998;31:661–667. doi: 10.1016/s0735-1097(97)00535-4. [DOI] [PubMed] [Google Scholar]
- 3.Ing FF, Grifka RG, Nihill MR, Mullins CE. Repeat dilation of intravascular stents in congenital heart defects. Circulation. 1995;92:893–897. doi: 10.1161/01.cir.92.4.893. [DOI] [PubMed] [Google Scholar]
- 4.Rao PS. Stents in the management of congenital heart disease in pediatric and adult patients. Indian Heart J. 2001;53:714–730. [PubMed] [Google Scholar]


