Abstract
Pharmacological closure by indomethacin is customary if symptoms of PDA are not controlled adequately with fluid restriction and diuretics. Its use, however, requires a comprehensive clinical assessment of all the vital perinatal factors and a vigilant monitoring of the sick infant. Prophylactic use of indomethacin is discouraged. The decision to use pharmacological versus surgical treatment or both should be individualized based on evidence-based research and clinician's own experience. Surgical ligation remains the primary mode of therapy in cases of pharmacological treatment failure or recurrence.
MeSH: Ductus Arteriosus, Patent Infant, Premature, Echocardiography, transthoracic, Prostaglandins, Prostaglandin antagonists, Indomethacin
Introduction and background
The ductus arteriosus in the fetus is an important conduit that allows deoxygenated blood to bypass the collapsed lungs and enter the placenta through the descending aorta and umbilical arteries. The placenta acts as an oxygenator and returns oxygen rich blood through the umbilical vein and ductus venosus to the fetal heart. During the first two trimesters, blood flow to the lungs is minimal and it increases substantially during the last trimester. After birth, the ductus arteriosus normally closes within the first several days of life. A persistently patent ductus arteriosus (PDA) can cause significant problems, especially in premature infants.
Physiologic closure of the ductus arteriosus
The outer layer of the ductus is primarily composed of circumferentially oriented muscle fibers.1 During the last trimester of pregnancy, there is rapid growth in medial muscular tissue with subsequent migration into the subintimal space, probably due to increased expression of transforming growth factor 1.1 This growth accompanies a simultaneous increase in the sensitivity of the ductus arteriosus to oxygen tension for closure.1 Oxygen reaches the muscular media either through the lumen or through intramural vasa vasorum coming from the outer muscular layer. Part of the muscular media adjoining the lumen is relatively avascular, devoid of vasa vasorum and therefore poorly oxygenated. During closure of the ductus, the thickness of the avascular zone plays a critical role in causing hypoxia and anatomical remodeling of the ductus. The contraction of circumferential fibers results in narrowing of the ductal lumen whereas contraction of longitudinal fibers leads to shortening of the ductus.
The placenta produces prostaglandins, which maintain prenatal patency of the ductus and, in early gestation, inhibit the ability of the ductus to contract in response to oxygen. The ductus arteriosus itself also produces prostaglandins and nitric oxide-like vasodilators.1 During the postnatal period, final closure of the ductus arteriosus results from increased production of local vasoconstrictors (like endothelin) in response to higher arterial oxygen,1 removal of placental prostaglandin and a decrease in the number of prostaglandin E2 receptors in the ductal wall.
Persistent patency of the ductus arteriosus
During the first 60 hours of life, spontaneous closure of the ductus occurs in 55% of full-term newborn infants. By 2-6 months of age, closure occurs in more than 95% of healthy infants.1 Persistent patency of the ductus arteriosus following birth is inversely related to gestational age. This may be due to the smaller amount of muscular tissue in the media with lower intrinsic tone, and lower responsiveness to oxygen but higher sensitivity to the vasodilating effects of prostaglandin E2 and nitric oxide.1 In clinical practice, most extremely-low-birth-weight infants who are less than 28 weeks gestation have a patent ductus arteriosus (PDA) during the postnatal period. (For an excellent review and images on patent ductus arteriosus in premature infants, please see Karatza et al.).1 Prematurity, however, does not guarantee prolonged patency of the ductus arteriosus. For example, Reller et al. found that the PDA closed spontaneously in 32 of 36 infants with a gestational age of 30-37 weeks and respiratory distress syndrome by the fourth day of life.1 Concurrent infections may increase the release of prostaglandins (6-ketoprostaglandin F1) and tumor necrosis factors, and increase the risk of late ductal opening and closure failures.1
The direction of blood flow across the PDA depends on the balance of pulmonary and systemic vascular resistance. In-utero, the fetal lungs are collapsed and the pulmonary vascular resistance is high. Conversely, the connection of the low resistance placenta to the systemic vascular bed via the umbilical cord allows for low systemic vascular resistance resulting in blood flow from the pulmonary artery to the aorta (i.e. a “right-to-left shunt”) prenatally (for further details, please refer to fetal images1 and figure 1). Despite the increase in smooth muscle in the wall of the pulmonary arterioles during the last trimester, pulmonary vascular resistance falls primarily due to increase in the total cross sectional area of the vascular bed secondary to the growth in the number of respiratory units. This drop in the pulmonary vascular resistance leads to increase in the pulmonary blood flow during the last trimester.
Figure 1.
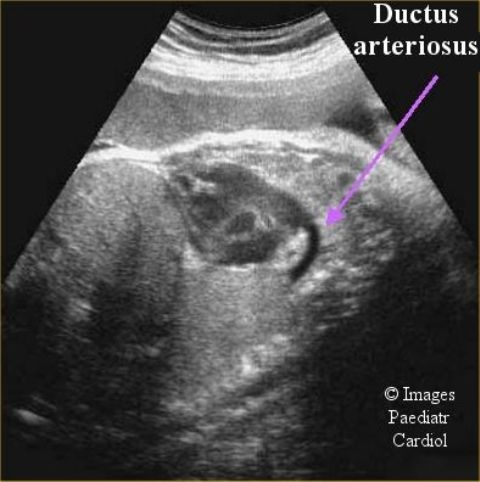
Fetal echocardiogram of the ductus arteriosus (Courtesy of Drs. J. Moodley and Y. Shah)
As the newborn begins to breathe, the lungs expand and the pulmonary vascular resistance drops. The umbilical cord is simultaneously clamped separating the placenta from the systemic bed and the systemic vascular resistance quickly rises above that of the pulmonary bed. As a result, after birth, blood flows from the aorta into the pulmonary artery (i.e. a “left-to-right shunt”). Most of this left-to-right shunt comes from the distal aorta particularly during diastole. A large PDA that offers no resistance of its own (non-restrictive) may result in a considerable increase in pulmonary blood flow eventually leading to high output heart failure despite improved ventricular performance due to decreased afterload.1
Pathology of the PDA in premature infants
In contrast to older children, fetal and neonatal myocardium has a higher non-contractile to contractile mass ratio, and is less compliant.1,2 Therefore, with the increase in preload resulting from ductal left-to-right shunting, higher left ventricular end-diastolic and left atrial pressures ensue early. In addition, rising hydrostatic pressure in pulmonary capillaries from higher pulmonary blood flow exceeds oncotic pressures leading to patchy atelectasis with possible segmental and lobar collapse, further increasing the pulmonary vascular resistance. Premature infants commonly have low oncotic pressure and increased capillary permeability that further worsens the interstitial leak.
Infants with lower birth weight (<1200 grams) are less able to tolerate a PDA than full term infants. Such infants have underdeveloped alveoli in addition to surfactant deficiency (Figure 2). They also have a less well developed pulmonary lymphatic system and are therefore less capable of eliminating the excess fluid presented to the premature lung by a large PDA. A relatively larger PDA compared to aortic diameter and a relatively lower pulmonary vascular resistance due to less muscle in the pulmonary vascular bed permits relatively larger pulmonary blood flow. This also steals significant blood flow from vessels distal to the duct such as the gut.
Figure 2.
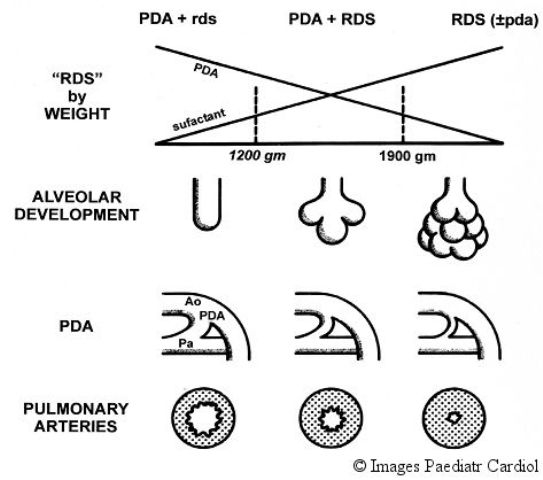
Developmental factors affecting the hemodynamics of the PDA
Respiratory failure is a common consequence of a large PDA in the premature infant. Tachypnea can aid in draining the excess fluid from the interstitial space through the lymphatic system. However, when input exceeds output interstitial edema ensues. This edema may result in decreased CO2 diffusion from capillaries to alveoli and CO2 retention. Consequently air passages are narrowed and respiratory failure and apnea develops more quickly in premature infants as their fragile respiratory muscles tire out. As poor lung compliance is common in most of these infants, this process serves to aggravate any existing respiratory distress syndrome.
Surfactant has become standard therapy for neonatal respiratory distress syndrome associated with prematurity. The introduction of surfactant over the last 10 years has contributed to a decrease in mortality and morbidity in very-low-birth-weight infants.1 Surfactant, however, reduces pulmonary vascular resistance and increases left to right shunting across the ductus. Surfactant does not delay closure of the ductus arteriosus.1,2,3
Perfusion of the systemic vascular bed in the setting of a PDA has been examined. Even with a large PDA, premature infants maintain normal cerebral blood flow, although flow is decreased in post-ductal organs secondary to lower perfusion pressure.1 Auto-regulatory mechanisms in the brain compensate for cerebral blood flow changes associated with ductal shunting that are probably related to diastolic runoff and not to increase in cerebrovascular resistance.1,2
Clinical diagnosis
The classical description of the signs and symptoms of the PDA were described in the 1950s by Dr. Helen Taussig. She described a premature infant with a continuous washing machine-like murmur heard best in the left upper sternal border. She also described bounding pulses due to the rapid runoff, enlarged heart on chest x-ray and evidence of pulmonary congestion and heart failure in the more severe cases. These findings must be properly placed in context of the gestational age of the infant in question. A premature infant being treated in 1950 probably was over 1500 grams in birth weight. Infants being treated in that era were rarely as small as neonates that are routinely cared for in today's neonatal intensive care units.
Therefore, when trying to diagnose a PDA in the modern NICU one must consider the physiologic differences of the extremely low birth weight infant. This is also complicated by the presence of neonatal lung disease such as RDS. Many extremely low birth weight infants have a silent patent ductus arteriosus. They may not have a murmur at all. While bounding pulses are considered the hallmark of the PDA, they may not be detected in the very tiny neonate if that infant does not have enough myocardial reserve to compensate for the rapid run off. Alternatively, bounding pulses may not be found if pulmonary vascular resistance is equal or near equal to systemic vascular resistance. Often the effect of the PDA on the premature infant does not become clear until surfactant deficiency has resolved. When ventilator dependence persists beyond the third day of life in these tiniest patients, surfactant deficiency becomes a less likely cause of cardiopulmonary symptoms. Commonly, after artificial surfactant, oxygen requirements will initially decrease only to increase in a few days. Carbon dioxide retention becomes more of a problem as well. Enlarged heart on chest radiograph and clinical signs of narrow airways will manifest. It is not unusual for the classic murmur of the PDA as well as bounding pulses to appear at this time due to a drop in pulmonary vascular resistance allowing a left to right shunt through the PDA. In general, PDA evaluation by clinical examination alone in the ventilator dependent preterm infants is of limited value.1
The most reliable non-invasive diagnostic tool is echocardiography with Doppler ultrasound. In most infants, a modified parasternal short axis view offers the best window for PDA visualization (Figure 3). This view offers the best opportunity to directly measure the PDA. The secondary effects of the increased flow can estimate the volume load from the left to right ductal shunt. A large shunt leads to dilation of the left atrium and left ventricle, as well as holodiastolic reversal of blood flow distal to the ductus in the descending aorta due to run off into the pulmonary bed. Enlargement of the left atrium reflecting the approximate magnitude of the shunt, can be further supported by left atrial-to-aortic root ratio of >1.3. The presence of a patent fossa ovalis, present in most newborn infants, can confound these measurements.1 In addition, continuous wave Doppler can estimate pulmonary artery pressures by measuring Doppler velocities of PDA flow and tricuspid regurgitation.1
Figure 3.
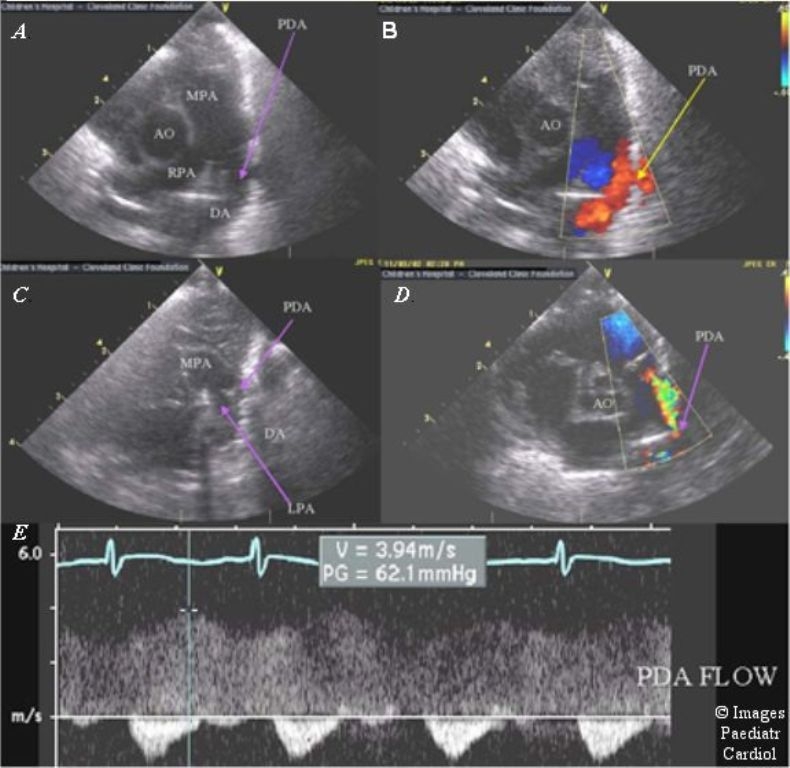
Echocardiography of the PDA. The ductus can be well visualized from the left parasternal area (A) with low velocity flow back into the pulmonary artery from the aorta (B). After therapy with indomethacin the PDA significantly decreases in size (C) with aliasing color Doppler flow in a smaller jet (D), and a high velocity, restrictive spectral Doppler pattern (E). (MPA = main pulmonary artery, RPA = right pulmonary artery, Ao = aorta, PDA = patent ductus arteriosus, DA = descending aorta, LPA = left pulmonary artery)
In the setting of prenatal ductal constriction the Tei index, an echocardiographicaly derived measure of cardiac function, appears to be useful, between the gestational ages of 20-39 weeks, to evaluate depressed right ventricular function.1
The diagnosis of ductus arteriosus aneurysm should be considered when there is a localized saccular or tubular dilatation of the ductus arteriosus. It conceivably results from abnormal elastin formation or abnormal intimal cushion formation. The reported incidence of ductus arteriosus aneurysm, seen after the third trimester, varies between 1.5 - 8.8% and may represent a normal variant.1,2 It may be observed in patients with Marfan, Ehlers-Danlos and Larsen syndrome and has the potential for spontaneous rupture, thromboembolism, erosion, and infection. If large, it can compress the adjoining tissues and structures. Its presence should prompt clinicians to look for the associated syndromes particularly connective tissue diseases.
Pharmacological
Conservative medical management such as diuretics and fluid restriction suffice in many patients with early symptoms. After the second day of life, limiting fluid intake to meet basic requirements of excretion, insensible loss, and growth may lower the risk for development of symptoms related to a PDA in premature infants.1 The ability of a premature infant to tolerate a PDA may be inversely proportional to gestational age. Closure is indicated in the symptomatic infant with a significant volume overload documented by echocardiography.
In the premature infant an important aspect of PDA management is fluid intake. Early fluid restriction to allow for little more than insensible and sensible losses will significantly reduce the risks of PDA, necrotizing enterocolitis, and death at the expense of postnatal weight loss.1 If the signs and symptoms persist, one is left with balancing the various needs of the patient against the problems of treating the symptoms of the PDA.
Simple fluid restriction along with diuretic use is often recommended to control the symptoms of a PDA. Furosemide is commonly used. Although furosemide is a prostaglandin agonist, it does not interfere with PDA closure. Furosemide merely helps the lungs clear fluid and thereby improves the patient's ability to tolerate the PDA. Short-term use of furosemide and fluid restriction requires a close vigilance to prevent dehydration. Long-term use of this drug can have serious and subtle side effects. It causes calcium loss by the kidney and may lead to rickets. It can also cause hypokalemia, which leads to a metabolic alkalosis and carbon dioxide retention that can be misinterpreted as worsening of cardiopulmonary disease leading to an apparent increase in ventilator support. Hypokalemia can be controlled with potassium supplementation but it is difficult to overcome the problem of calcium loss caused by furosemide. For this reason, many clinicians prefer milder thiazide diuretics for long-term use.
One of the core challenges with fluid restriction is nutritional. In a growing infant, long-term fluid restriction limits the amount of calories and minerals that can be given. Providing adequate calories and minerals with fluid restriction requires highly concentrated formulas or parenteral alimentation, which may increase the risk for feeding intolerance and sepsis. For many clinicians, it is a poor trade to control the symptoms of PDA at the expense of good nutrition. Authors recommend the use of Lasix and fluid restriction only for a few days at most. Beyond that, thiazides may be preferred and fluids be restricted only to the level at which adequate calories can be provided for good growth - in a chronically ill premature infant, it is usually no less than 120 ml/Kg/day.
Indomethacin is a potent stimulator of ductal closure. It blocks the enzyme cyclooxygenase inhibiting prostaglandin synthesis thereby facilitating ductal closure (Figure 4). Indomethacin also increases the thickness of the avascular zone by causing constriction of circumferential and longitudinal muscle, decreasing blood flow in the vasa vasorum, and causing vessel wall hypoxia with release of vascular endothelial cell growth factor (VEGF). VEGF induces formation of neointimal mounds and stimulates in-growth of the vasa vasorum during permanent ductal closure.
Figure 4.
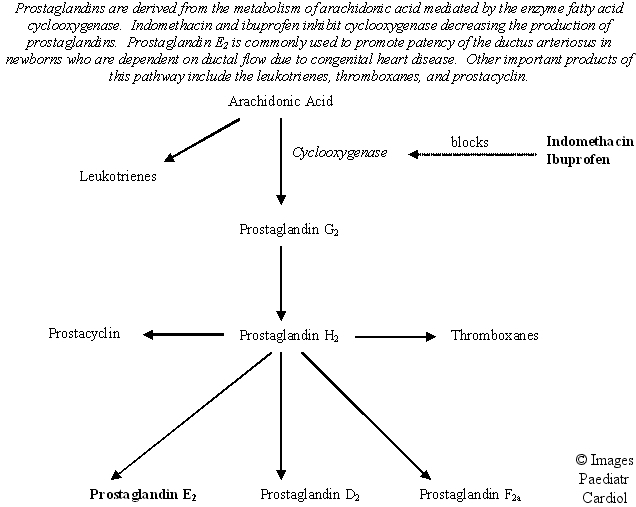
Biosynthesis of arachidonic acid
Indomethacin is the most commonly used agent for medical closure of a ductus. Most studies have shown that the use of indomethacin in closing the PDA has reduced the need for subsequent surgical closure. The appropriate time to administer indomethacin is debatable. The three primary strategies are as follows:
Use of indomethacin within 24 hours of life as a prophylactic treatment
Before or at early onset of clinical symptoms
After the development of clinical symptoms
Given as prophylactic treatment within 24 hours of birth, indomethacin reduces the incidence of a symptomatic PDA, the need for surgical closure, and the incidence of grade 3 and 4 intraventricular hemorrhage (pooled relative risk = 0.66 [95% CI 0.53 to 0.82]). Closure of the PDA by itself, however, does not improve the outcome in terms of mortality, necrotizing enterocolitis (NEC), bronchopulmonary dysplasia, or retinopathy of prematurity. Additionally, there are serious concerns among many clinicians regarding side effects of indomethacin including transient oliguria and altered renal function; decrease in cerebral, mesenteric, and renal blood flow; altered platelet function; and necrotizing enterocolitis or gastrointestinal perforation. Consequently, use of indomethacin in contraindicated in infants with a bleeding diathesis, necrotizing enterocolitis, or renal failure.
Indomethacin is commonly used in institutions caring for premature infants; however, the administration of this medication is quite variable. This makes it difficult to compare the side effects, or their lack of, reported in various studies. Confounding variables include post-natal age, gestational age, dose, delivery, and the rate of delivery, prenatal use of medications, surfactant use, and infections. A lack of documentation regarding the exact mode of delivery adds to the confusion. For example, the effectiveness and safety of IV infusion over 36 hours versus the conventional mode of infusion may be different and needs further evaluation. In addition, the risk for necrotizing enterocolitis and a higher rate of ductus reopening remains a concern in extremely premature infants (<27 weeks of gestational age).
Notwithstanding several contraindications, should clinicians elect to use indomethacin, based on our own experience and review of the literature, we recommend the following strategy to minimize the side effects of indomethacin. To avoid compromise in cerebral and gastrointestinal blood flow, infants should receive 0.1- 0.2 mg/kg IV of indomethacin slowly over a minimum of 30 minutes followed immediately by 1 mg/kg of furosemide. A total of 3 doses can be given depending on the clinical response & indomethacin levels. The usefulness and the factors that may prompt prolonged indomethacin therapy are still under investigation. Although successful closure of the PDA by indomethacin can be achieved even after 10 days of postnatal age, close monitoring of indomethacin levels and clinical response is required for optimal management of all infants to minimize its side effects.
Furosemide given immediately after indomethacin may prevent the renal side effects of indomethacin therapy without interfering with efficacy of indomethacin for the closure of PDA. In addition, it may help improve pulmonary compliance. Concomitant dopamine therapy in an attempt to increase the renal blood flow, however, is of no benefit and it does not reduce the magnitude of the indomethacin-induced oliguria.
Obstetrical use of indomethacin to treat premature labor can affect the postnatal response of the ductus to medical therapy. In-utero exposure to indomethacin results in production of endothelial nitric oxide synthase, increased nitric oxide production, loss of smooth muscle cells, and loss of contractile capacity. In such cases, prostaglandins play a minimal role in the closure of the ductus arteriosus and premature infants become unresponsive to indomethacin therapy. These children have an increased incidence of surgical closure of the PDA. It remains to be seen whether cyclooxygenase-1-selective inhibitors would provide effective delay of premature labor without adverse effects to fetus.
Ibuprofen, another non-selective cyclooxygenase inhibitor, given on the third day of postnatal life appears to be as effective as indomethacin for PDA closure but less likely to induce oliguria. It may be too early to judge its full safety profile as evidenced by a recent report of 3 very premature infants who developed pulmonary hypertension after ibuprofen prophylaxis.
Hydrocortisone decreases responsiveness of the ductus arteriosus smooth muscle to PGE2. Prenatal administration of betamethasone decreased the incidence of clinically significant PDA from 34% to 18% by 24 hours. Many clinicians, who could not use indomethacin, either due to contraindications for drug use or personal preference, found postnatal use of low dose corticosteroids useful in the management of hemodynamically significant PDA. Recent concerns about neurodevelopmental delay in very-low-birth-weight infants associated with the use of dexamethasone strongly argue against steroid use in these infants.
Non-Pharmacological Therapies
Surgical ligation
In small infants that are not a candidate for, or who have failed, medical therapy, surgical ligation remains an effective alternative. In a randomized, controlled trial of prophylactic surgical ligation of PDA, the incidence of NEC decreased from 30% to 8%, however, it had no significant effect on other outcome measures like death, bronchopulmonary dysplasia, retinopathy of prematurity, and intraventricular hemorrhage. The classical approach via a left lateral sternotomy with ligation can be performed in an operating room or at the bedside with low mortality. The use of anterior extra-pleural approach reported in 5 patients is a relatively new technique and more studies are needed prior to its recommendation for general use. Alternatively, to minimize trauma to the chest wall, a video-assisted thoracoscopic approach has been used successfully in some institutions.
Trans-catheter closure
Although coil occlusion has been performed in infants, a large short PDA, which is the typical anatomy in symptomatic newborns and premature infants, is difficult to close. In addition, there is a significant risk of obstructing the descending aorta or left pulmonary artery, which are small caliber vessels in neonates. In children with a smaller ductus and less significant left to right shunting closure can be deferred until they are older. Out of the neonatal period, cardiac catheterization with coil occlusion of the PDA has become the primary mode of closure (Figure 5). Newer occlusion devices similar to ones used for ASD closure are being developed for closure of the large PDA in older children and adults.
Figure 5.
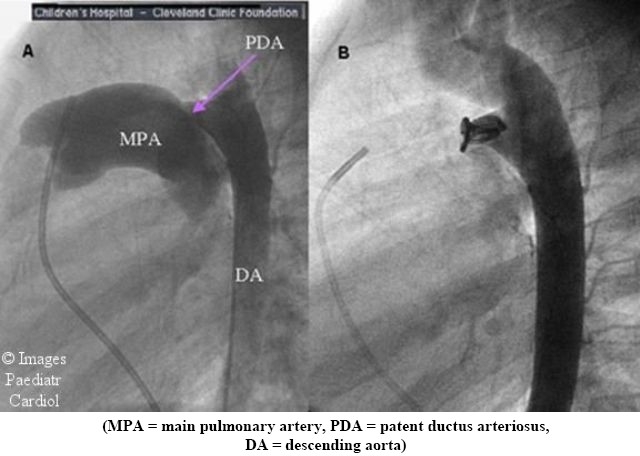
Closure of a PDA by coil catheterization. (A) Injection into the aorta reveals a large PDA at baseline. (B) Following placement of a coil the angiographic dye no longer crosses into the pulmonary artery confirming ductal closure
Pharmacological management versus surgical closure
Pharmacological management (usually indomethacin) and/or mechanical closure (usually surgical ligation) are considered if symptoms of PDA are not controlled adequately with fluid restriction and/or diuretics. Choosing between these approaches is still difficult and controversial. While studies have shown that closure of the PDA is beneficial, it is by no means clear as to the best method of closure, timing or patient selection. Indomethacin does close the PDA in many cases but not all. In general, indomethacin is most effective at closing the PDA in infants who need it the least i.e. 32-36 weeks gestation larger infants who are better able to tolerate the hemodynamic effects of a PDA. The earlier the infant's gestation, the less likely it is that the PDA is going to respond to indomethacin. Indomethacin failure rate in neonates weighing less than 800 g can be high (43%). Also, neonates who are beyond 10 days postnatal age may be less responsive to indomethacin.
Side effects of indomethacin are potentially serious. As mentioned above, these include altered renal function; decreased cerebral, mesenteric, and renal blood flow; altered platelet function; necrotizing enterocolitis (NEC); and bowel perforation. Consequently, use of indomethacin is contraindicated in patients with bleeding diathesis, necrotizing enterocolitis or oliguria. When given to a patient with high pulmonary vascular resistance, it can cause severe hypoxia. Necrotizing enterocolitis is a particular problem in the patients who tend to have the most difficulty with the PDA i.e. the very tiny infant. With increasing prematurity, the risk for the development of NEC as well as its severity increases.
Consequently, many clinicians are opting for closure of the PDA by mechanical means in small premature infants (<800 grams). Risks of PDA closure in the hands of an experienced surgeon tend to be small and limited to the usual proximate consequences of surgery like surgical mishaps, infection and chylothorax. In view of our excellent surgical outcome, we rarely use indomethacin in the extreme premature infants. We also have a zero incidence of surgical NEC over the last several years treating over 70 premature infants weighing <1500 grams. With the availability of adequate facilities and trained staff, many neonatal intensive care units prefer to perform the PDA ligation in the unit, thus avoiding the need to move the already critically ill patient.
Summary
Although effective, we do not recommend prophylactic indomethacin treatment or prophylactic surgical ligation as it will lead to unnecessary indomethacin and surgical exposure to a large number of preterm infants who may never develop PDA related symptoms requiring pharmacological and/or surgical closure. The decision to use pharmacological versus surgical treatment or both should be based on giving careful consideration to the above factors, individual infant, evidence based research, experience of surgical and nursing team, and clinician's own experience. The data on the success of therapy and its impact on the overall mortality vary depending on various prenatal, natal, and postnatal factors including the experience of the surgical and medical staff, time and mode of medical treatment, and the type of medical therapy. Until further information is available, in cases of pharmacological treatment failure or recurrence, the optimal management remains surgical closure. More studies are needed regarding the safety and effectiveness of ibuprofen in extremely premature infants.
References
- 1.Silver MM, Freedom RM, Silver MD, Olley PM. The morphology of the human newborn ductus arteriosus: a reappraisal of its structure and closure with special reference to prostaglandin E1 therapy. 1981;12:1123-1136. Hum Pathol. 1981;12:1123–1136. doi: 10.1016/s0046-8177(81)80333-4. [DOI] [PubMed] [Google Scholar]
- 2.Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330:1431–1438. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 3.Fay FS. Guinea pig ductus arteriosus. I. Cellular and metabolic basis for oxygen sensitivity. Am J Physiol. 1971;221:470–479. doi: 10.1152/ajplegacy.1971.221.2.470. [DOI] [PubMed] [Google Scholar]
- 4.Snider SR, Chen YQ, Oprysko PR, Mauray F, Tse MM, Lin E, Koch C, Clyman RI. Combined prostaglandin and nitric oxide inhibition produces anatomic remodeling and closure of the ductus arteriosus in the premature newborn baboon. Pediatr Res. 2001;50:365–373. doi: 10.1203/00006450-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Coceani F, Kelsey L. Endothelin-1 release from lamb ductus arteriosus: relevance to postnatal closure of the vessel. Can J Physiol Pharmacol. 1991;69:218–221. doi: 10.1139/y91-033. [DOI] [PubMed] [Google Scholar]
- 6.Connuck D, Sun JP, Super DM, Kirchner HL, Fradley LG, Harcar-Sevcik RA, Salavator A, Singer L, Mehta SK. Incidence of patent ductus arteriosus and patent foramen ovale in normal infants. Am J Cardiol. 2002;89:244–247. doi: 10.1016/s0002-9149(01)02214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siassi B, Blanco B, Cabol L, Coran AG. Incidence and clinical features of patent ductus arteriosus in low-birth-weight infants: a prospective study of 150 consecutively born infants. Pediatrics. 1976;57:347–351. [PubMed] [Google Scholar]
- 8.Karatza AA, Azzopardi DV, Gardiner HM. The persistently patent arterial duct in the premature infant. Images Paediatr Cardiol. 2001;6:4–17. [PMC free article] [PubMed] [Google Scholar]
- 9.Reller MD, Colasurdo MA, Rice MJ, McDonald RW. The timing of spontaneous closure of the ductus arteriosus in infants with respiratory distress syndrome. Am J Cardiol. 1990;66:75–78. doi: 10.1016/0002-9149(90)90739-n. [DOI] [PubMed] [Google Scholar]
- 10.Gonzales A, Sosenko IRS, Chandar J, Hummler H, Claure N, Bancalari E. Influence of infection of patent ductus arteriosus and chronic lung disease in premature infants weighing 1000 grams or less. J Pediatr. 1996;128:470–478. doi: 10.1016/s0022-3476(96)70356-6. [DOI] [PubMed] [Google Scholar]
- 11.Todros T, Capuzzo E, Gaglioti P. Prenatal diagnosis of congenital anomalies. Images Paediatr Cardiol. 2001;7:3–18. [PMC free article] [PubMed] [Google Scholar]
- 12.Kimball TR, Ralston MA, Khoury P, Crump RG, Cho FS, Reuter JH. Effect of ligation of patent ductus arteriosus on left ventricular performance and its determinants in premature infants. J Am Coll Cardiol. 1996;27:193–197. doi: 10.1016/0735-1097(95)00452-1. [DOI] [PubMed] [Google Scholar]
- 13.Friedman WF. The intrinsic physiologic properties of the developing heart. Prog Cardiovasc Dis. 1972;16:87–111. doi: 10.1016/0033-0620(72)90006-0. [DOI] [PubMed] [Google Scholar]
- 14.Romero T, Covell J, Friedman WF. A comparison of pressure-volume relations of the fetal, newborn, and adult heart. Am J Physiol. 1972;222:1285–1290. doi: 10.1152/ajplegacy.1972.222.5.1285. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz RM, Luby AM, Scanlon W, Kellogg RJ. Effect of surfactant on morbidity, mortality, and resource use in newborn infants weighing 500 to 1500 g. 1994;330:1476-8140. N Engl J Med. 1994;330:1476–8140. doi: 10.1056/NEJM199405263302102. [DOI] [PubMed] [Google Scholar]
- 16.Clyman RI, Jobe A, Heymann M, Ikegami M, Roman C, Payne B, Mauray F. Increased shunt through the patent ductus arteriosus after surfactant replacement therapy. J Pediatr. 1982;100:101–107. doi: 10.1016/s0022-3476(82)80247-3. [DOI] [PubMed] [Google Scholar]
- 17.Kaapa P, Seppanen M, Kero P, Saraste M. Pulmonary hemodynamics after synthetic surfactant replacement in neonatal respiratory distress syndrome. J Pediatr. 1993;123:115–119. doi: 10.1016/s0022-3476(05)81553-7. [DOI] [PubMed] [Google Scholar]
- 18.Reller MD, Rice MJ, McDonald RW. Review of studies evaluating ductal patency in the premature infant. J Pediatr. 1993;122:S59–62. doi: 10.1016/s0022-3476(09)90044-0. [DOI] [PubMed] [Google Scholar]
- 19.Shimada S, Kasai T, Konishi M, Fujiwara T. Effects of patent ductus arteriosus on left ventricular output and organ blood flows in preterm infants with respiratory distress syndrome treated with surfactant. J Pediatr. 1994;125:270–277. doi: 10.1016/s0022-3476(94)70210-1. [DOI] [PubMed] [Google Scholar]
- 20.Wright LL, Baker KR, Hollander DI, Wright JN, Nagey DA. Cerebral blood flow velocity in term newborn infants: changes associated with ductal flow. J Pediatr. 1988;112:768–773. doi: 10.1016/s0022-3476(88)80700-5. [DOI] [PubMed] [Google Scholar]
- 21.Martin CG, Snider R, Katz SM, Peabody JL, Brady JP. Abnormal cerebral blood flow patterns in preterm infants with a large patent ductus arteriosus. J Pediatr. 1982;101:587–593. doi: 10.1016/s0022-3476(82)80715-4. [DOI] [PubMed] [Google Scholar]
- 22.Urquhart DS, Nicholl RM. How good is clinical examination at detecting a significant patent ductus arteriosus in the preterm neonate? Arch Dis Child. 2003;88:85–86. doi: 10.1136/adc.88.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans N, Iyer P. Assessment of ductus arteriosus shunt in preterm infants supported by mechanical ventilation: effect of interatrial shunting. J Pediatr. 1994;125:778–785. doi: 10.1016/s0022-3476(94)70078-8. [DOI] [PubMed] [Google Scholar]
- 24.Musewe NN, Poppe D, Smallhorn JF, Hellman J, Whyte H, Smith B, Freedom RM. Doppler echocardiographic measurement of pulmonary artery pressure from ductal Doppler velocities in the newborn. J Am Coll Cardiol. 1990;15:446–456. doi: 10.1016/s0735-1097(10)80076-2. [DOI] [PubMed] [Google Scholar]
- 25.Mori Y, Rice MJ, McDonald RW, Reller MD, Wanitkun S, Harada K, Sahn DJ. Evaluation of systolic and diastolic ventricular performance of the right ventricle in fetuses with ductal constriction using the Doppler Tei Index. Am J Cardiol. 2001;88:1173–1178. doi: 10.1016/s0002-9149(01)02056-2. [DOI] [PubMed] [Google Scholar]
- 26.Dyamenahalli U, Smallhorn JF, Geva T, Fouron JC, Cairns P, Jutras L, Hughes V, Rabinovitch M, Mason CAE, Hornberger LK. Isolated ductus arteriosus aneurysm in the fetus and infant: a multi-institutional experience. J Am Coll Cardiol. 2000;36:262–269. doi: 10.1016/s0735-1097(00)00707-5. [DOI] [PubMed] [Google Scholar]
- 27.Jan SL, Hwang B, Fu YC, Chai JW, Chi CS. Isolated neonatal ductus arteriosus aneurysm. J Am Coll Cardiol. 2002;39:342–347. doi: 10.1016/s0735-1097(01)01736-3. [DOI] [PubMed] [Google Scholar]
- 28.Bell EF, Warburton D, Atonestreet BS, Oh W. Effect of fluid administration on the development of symptomatic patent ductus arteriosus and congestive heart failure in premature infants. N Engl J Med. 1980;302:598–604. doi: 10.1056/NEJM198003133021103. [DOI] [PubMed] [Google Scholar]
- 29.Bell EF, Acarregui MJ. Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2001;(3):CD000503. doi: 10.1002/14651858.CD000503. [DOI] [PubMed] [Google Scholar]
- 30.Clyman RI, Seidner SR, Kajino H, Roamn C, Koch CJ, Ferrara N, Waleh N, Mauray F, Chen YQ, Perkett EA, Quinn T. VEGF regulates remodeling during permanent anatomic closure of the ductus arteriosus. Am J Physiol Regul Integr Comp Physiol. 2002;282:R199–206. doi: 10.1152/ajpregu.00298.2001. [DOI] [PubMed] [Google Scholar]
- 31.Fowlie PW, Davis PG. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev. 2002;(3):CD000174. doi: 10.1002/14651858.CD000174. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts RS, Saigal S, Solimano A, Vincer M, Wright LL. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med. 2001;344:1966–1972. doi: 10.1056/NEJM200106283442602. [DOI] [PubMed] [Google Scholar]
- 33.Hammerman C, Glaser J, Schimmel MS, Ferber B, Kaplan M, Eidelman AI. Continuous versus multiple rapid infusions of indomethacin: effects of cerebral blood flow velocity. Pediatrics. 1995;95:244–248. [PubMed] [Google Scholar]
- 34.Fujii AM, Brown E, Mirochnick M, O’Brien S, Kaufman G. Neonatal necrotizing enterocolitis with intestinal perforation in extremely premature infants receiving early indomethacin treatment for patent ductus arteriosus. J Perinatol. 2002;22:535–540. doi: 10.1038/sj.jp.7210795. [DOI] [PubMed] [Google Scholar]
- 35.Narayanan M, Cooper B, Weiss H, Clyman RI. Prophylactic indomethacin:factors determining permanent ductus arteriosus closure. J Pediatr. 2000;136:330–337. doi: 10.1067/mpd.2000.103414. [DOI] [PubMed] [Google Scholar]
- 36.Quinn D, Cooper B, Clyman RI. Factors associated with permanent closure of the ductus arteriosus: a role for prolonged indomethacin therapy. Pediatrics. 2002;110:e10. doi: 10.1542/peds.110.1.e10. [DOI] [PubMed] [Google Scholar]
- 37.Shaffer CL, Gal P, Ransom JL, Carlos RQ, Smith MS, Davey AM, Dimaguila MAVT, Brown YL, Schall SA. Effect of age and birth weight on indomethacin pharmacodynamics in neonates treated for patent ductus arteriosus. Crit Care Med. 2002;30:343–348. doi: 10.1097/00003246-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 38.Yeh TF, Wilks A, Singh J, Betkerur M, Lilien L, Pildes RS. Furosemide prevents the renal side effects of indomethacin therapy in premature infants with patent ductus arteriosus. J Pediatr. 1982;101:433–437. doi: 10.1016/s0022-3476(82)80079-6. [DOI] [PubMed] [Google Scholar]
- 39.Najak ZD, Harris EM, Lazzara A, Pruitt AW. Pulmonary effects of furosemide in preterm infants with lung disease. J Pediatr. 1983;102:758–763. doi: 10.1016/s0022-3476(83)80253-4. [DOI] [PubMed] [Google Scholar]
- 40.Fajardo CA, Whyte RK, Steele BT. Effect of dopamine on failure of indomethacin to close the patent ductus arteriosus. J Pediatr. 1992;121:771–775. doi: 10.1016/s0022-3476(05)81914-6. [DOI] [PubMed] [Google Scholar]
- 41.Barrington K, Brion LP. Dopamine versus no treatment to prevent renal dysfunction in indomethacin-treated preterm newborn infants. Cochrane Database Syst Rev. 2002;(3):CD003213. doi: 10.1002/14651858.CD003213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clyman RI, Chen YQ, Chemtob S, Mauray F, Kohl T, Varma DR, Roman C. In utero remodeling of the fetal lamb ductus arteriosus. Circulation. 2001;103:1806–1812. doi: 10.1161/01.cir.103.13.1806. [DOI] [PubMed] [Google Scholar]
- 43.Loftin CD, Trivedi DB, Langenbach R. Cyclooxygenase-1-selective inhibition prolongs gestation in micewithout adverse effects on the ductus arteriosus. J Clin Invest. 2002;110:549–557. doi: 10.1172/JCI14924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Overmeire BV, Smets K, Lecoutere D, DeBroek HV, Weyler J, Groote KD, Langhendries JP. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. N Engl J Med. 2000;343:674–681. doi: 10.1056/NEJM200009073431001. [DOI] [PubMed] [Google Scholar]
- 45.Varvarigou A, Bardin CL, Beharry K, Chemtob S, Papageorgiou A, Aranda JV. Early ibuprofen administration to prevent patent ductus arteriosus in premature newborn infants. JAMA. 1996;275:539–544. [PubMed] [Google Scholar]
- 46.Gournay V, Savagner C, Thiriez G, Kuster A, Roze JC. Pulmonary hypertension after ibuprofen prophylaxis in very preterm infants. Lancet. 2002;359:1486–1488. doi: 10.1016/S0140-6736(02)08424-6. [DOI] [PubMed] [Google Scholar]
- 47.Clyman RI, Mauray F, Roman C, Rudolph AM, Heymann MA. Glucocorticoids alter the sensitivity of the lamb ductus arteriosus to prostaglandin E2. J Pediatr. 1981;98:126–128. doi: 10.1016/s0022-3476(81)80558-6. [DOI] [PubMed] [Google Scholar]
- 48.Clyman RI, Ballard PL, Sniderman S, Ballard RA, Roth R, Heymann MA, Granberg JP. Prenatal administration of betamethasone for prevention of patent ductus arteriosus. J Pediatr. 1981;98:123–125. doi: 10.1016/s0022-3476(81)80557-4. [DOI] [PubMed] [Google Scholar]
- 49.Blackmon LR, Bell EF, Engle WA, Kanto WP, Jr, Martin GI, Miller CA, Rosenfeld W, Speer ME, Stark AR. (Committee on fetus and newborn). Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics. 2002;109:330–338. doi: 10.1542/peds.109.2.330. [DOI] [PubMed] [Google Scholar]
- 50.Cassady G, Crouse DT, Kirklin JW, Strange MJ, Joiner CH, Godoy G, Odrezin GT, Cutter GR, Kirklin JK, Pacifico AD, et al. A randomized, controlled trial of very early prophylactic ligation of the ductus arteriosus in babies who weighed 1000 g or less at birth. N Engl J Med. 1989;320:1511–1516. doi: 10.1056/NEJM198906083202302. [DOI] [PubMed] [Google Scholar]
- 51.Mortier E, Ongenae M, Vermassen F, Van Aken J, De Roose J, Van Haesebrouck P, Vandeveire B, Rolly G. Operative closure of patent ductus arteriosus in the neonatal intensive care unit. Acta Chir Belg. 1996;6:266–268. [PubMed] [Google Scholar]
- 52.Niinikoski H, Alanen M, Parvinen T, Aantaa R, Ekblad H, Kero P. Surgical closure of patent ductus arteriosus in very-low-birth-weight infants. Pediatr Surg Int. 2001;17:338–341. doi: 10.1007/s003830000515. [DOI] [PubMed] [Google Scholar]
- 53.Russell JL, Leblanc JG, Potts JE, Sett SS. Is surgical closure of patent ductus arteriosus a safe procedure in premature infants? Int Surg. 1998;83:358–360. [PubMed] [Google Scholar]
- 54.Mazzera E, Brancaccio G, Feltri C, Michielon G, DiDonato R. Minimally invasive surgical closure of patent ductus arteriosus in premature infants: a novel approach. J Card Surg. 2002;17:292–294. doi: 10.1111/j.1540-8191.2001.tb01143.x. [DOI] [PubMed] [Google Scholar]
- 55.Burke RP. Reducing the trauma of congenital heart surgery. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2001;4:216–228. [PubMed] [Google Scholar]
- 56.Burke RP. Video-assisted thoracoscopic surgery for patent ductus arteriosus. Pediatrics. 1994;93:823–825. [PubMed] [Google Scholar]
- 57.Schaarschmidt K, Kerremanns I, Schleef J, Forster R, Pattyn P, Stratmann U, Willital GH, Scheld HH. Laparoscopic and thoracoscopic surgery in infancy and childhood, the Munster/Gent experience. Technol Health Care. 1996;3:263–271. [PubMed] [Google Scholar]
- 58.Lloyd RJ, Zinman R, Sharratt GP, Hanna BD. Transvenous closure of patent ductus arteriosus in a sick 2780g infant. Can J Cardiol. 1996;12:300–302. [PubMed] [Google Scholar]
- 59.Cotton RB, Stahlman MT, Bender HW, Graham TP, Catterton WZ, Kovar I. Randomized trial of early closure of symptomatic patent ductus arteriosus in small preterm infants. J Pediatr. 1978;93:647–651. doi: 10.1016/s0022-3476(78)80910-x. [DOI] [PubMed] [Google Scholar]
- 60.Merritt TA, Harris JP, Roghmann K, Wood B, Campanella V, Alexson C, Manning J, Shapiro DL. Early closure of the patent ductus arteriosus in very low-birth-weight infants: a controlled trial. J Pediatr. 1981;99:281–286. doi: 10.1016/s0022-3476(81)80479-9. [DOI] [PubMed] [Google Scholar]
- 61.Trus T, Winthrop AL, Pipe S, Shah J, Langer JC, Lau GY. Optimal management of patent ductus arteriosus in the neonate weighing less than 800 g. J Pediatr Surg. 1993;28:1137–1139. doi: 10.1016/0022-3468(93)90148-e. [DOI] [PubMed] [Google Scholar]
- 62.Palder SB, Schwartz MZ, Tyson KR, Marr CC. Management of patent ductus arteriosus: a comparison of operative v pharmacologic treatment. J Pediatr Surg. 1987;22:1171–1174. doi: 10.1016/s0022-3468(87)80730-3. [DOI] [PubMed] [Google Scholar]
- 63.Palder SB, Schwartz MZ, Tyson KR, Marr CC. Association of closure of patent ductus arteriosus and development of necrotizing enterocolitis. J Pediatr Surg. 1988;23:422–423. doi: 10.1016/s0022-3468(88)80439-1. [DOI] [PubMed] [Google Scholar]
- 64.Gavilanes AW, Heineman E, Herpers MJ, Blanco CE. Use of neonatal intensive care unit as a safe place for neonatal surgery. Arch Dis Child Fetal Neonatal Ed. 1997;76:F51–53. doi: 10.1136/fn.76.1.f51. [DOI] [PMC free article] [PubMed] [Google Scholar]


