Abstract
Balloon expandable stents have been used to manage coarctation of the aorta in selected patients with very encouraging results. After the successful use of the Palmaz stents in these lesions newer stents as well as modifications of the Palmaz stents have been introduced to achieve optimal results in these patients. In this review, the older as well as the newer type stents used in coarctation are discussed. Technical considerations to achieve successful stent implantation and choice is outlined.
MeSH: coarctation, stent, catheter intervention, heart disease, congenital
Balloon dilation has gained acceptance in the treatment of coarctation of the aorta both native and recurrent.1–6 A number of patients develop recoarctation after balloon angioplasty7–10 possibly as a result of elastic recoil or long segment stenosis. Additionally in order to achieve successful reduction in the gradient, over sizing of the balloon may be necessary with potential for complications. Stents provide radial strength to avoid recoil of the vessel , and a framework for endothelial growth.10 Over the last few years stents have been used in selected patients to treat coarctation of the aorta both native and recurrent with encouraging results.11–17 Limitations of existing stents were recognized. Desirable characteristics in the implantable stents would include flexibility, relatively small profile to allow introduction in children or small adults, ability to further dilate to adult size as the patient grows, high radial strength, non sharp edges, wide struts to uninhibit flow if covering a vessel and allowing access to the side branches if covered and ability to reposition if necessary. Significant improvement in the existing stent designs as well as introduction of new stents was accomplished in the recent years. The advancement in stent technology aimed at achieving some of these desirable characteristics. The following review discusses the most commonly used stents for coarctation and addresses some of the technical considerations.
Stents
After the initial success in treating iliac artery stenosis,18–20 stents were used to treat stenotic lesions associated with congenital heart disease21–23 both native and post operative and shortly thereafter were used in coarctation of the aorta.11–17 The most commonly used stent is the balloon dilatable type which allows for further expansion of the stent as the patient grows. Self expanding stents have been only infrequently used because of the limitation of further expansion and the limited radial strength.24,25 The following are the stents commonly used for coarctation of the aorta.
I-Palmaz Stents (Johnson & Johnson Interventional Systems Co., Warren, NJ)
The Palmaz stents are balloon expandable stainless steal prostheses. They have a closed cell design which gives them high radial strength but makes them less flexible. Most of these stents are hand mounted and crimped on the delivery balloon. Until recently the large Palmaz stents (P308, 188) were the only ones available in the United States and considered suitable for coarctation of the aorta. The first 2 digits are the length of the stent in mms. The final digit represents the minimum recommended expanded diameter. This in turn reflects on the maximum diameter it can achieve and the radial strength. The wall thickness is .005 inch and the unexpanded diameter is 3.4 mm. All these factors influence the size of the balloon it can be crimped on as well as the minimum sheath size. These stents have a recommended expanded diameter between 8-12 mm but are commonly over dilated to 18-20 mm with some difficulty. A significant number of patients have had P308 stents implanted in the coarctation site with excellent results12–16 (figure 1 and 2, Table I). However over dilation of these stents results in significant foreshortening < 33% at 12 mm and up to 50% at 18 mm15 and is met with significant stent resistance. Smaller Palmaz stents (series ending with the digit 4) have been used sporadically under individual circumstances and are not generally used because of the limited expandability with growth.
Figure 1.
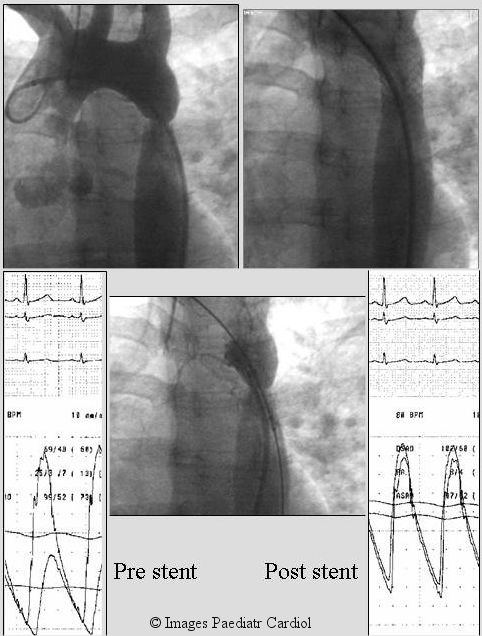
A 30 year old lady with poorly controlled hypertension was diagnosed to have native coarctation (A). A Palmaz P 308 stent was placed as a primary intervention. Post stent, there was no residual angiographic narrowing (B) or significant gradient. On follow-up her lower extremity blood pressure was higher than her arm pressure as is generally seen in normal individuals. She has been taken off anti hypertension treatment and remains normotensive.
Figure 2.
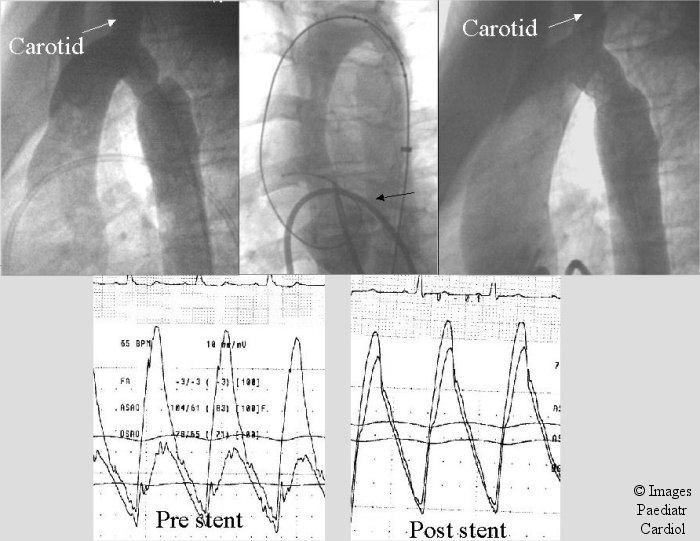
A 14 year old patient with recurrent coarctation after subclavian flap angioplasty. A transseptal approach (black arrow) was utilized to obtain accurate assessment of stent position prior to deployment to avoid the carotid artery. Notice accurate stent position in relation to the carotid. The systolic gradient decreased from 35 to 4 mm.Hg.
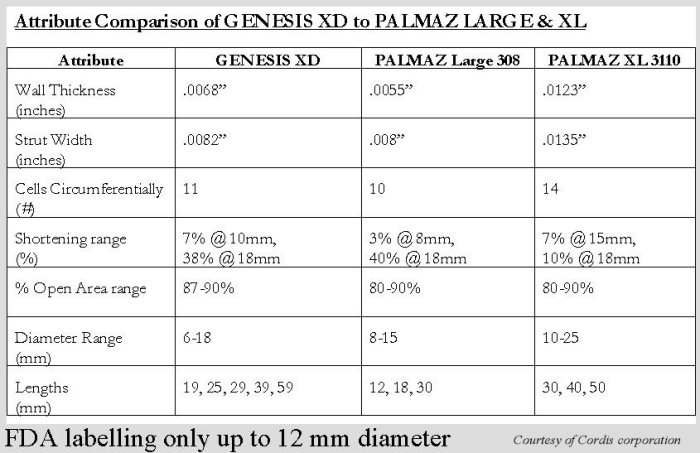
Table 1.
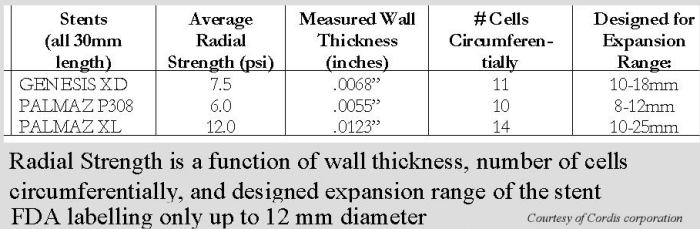
Comparison of Genesis and Palmaz stents
Extra large diameter Palmaz stents
These have became available in the United States and were designated P3110, 3010, 4010, 5010, (4014, 5014 outside the US). The numbering implications are similar to the 8-series outlined earlier. The wall thickness is .013 in. These stents are expandable to 25 mm while maintaining their radial strength. Their foreshortening ranges between 2.5% at 10 mm and 23% at 25 mm (off label). The 3010 is available balloon mounted on 12 mm delivery balloon. The others are hand mounted on the selected balloons. These stents require relatively larger balloons for adequate hand crimping and larger sheaths than the series ending with 8. (figure 3–5, tables 1 & 2). They offer a significant advantage because of their expandability, radial strength and less fore shortening.
Figure 3.
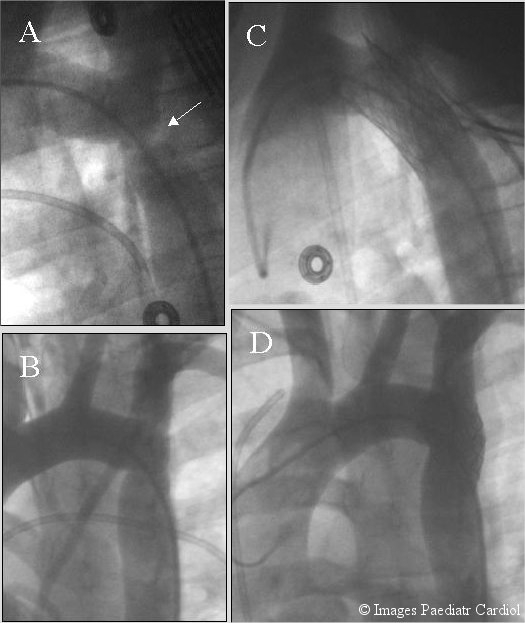
A 15 year old referred with diagnosis of coarctation (A & B) and poorly controlled hypertension. Angiogram shows severe coarctation seen better on lateral view (A). A Palmaz P-3110 stent (C & D) was placed eliminating the gradient which was 53 mm Hg.
Figure 5.
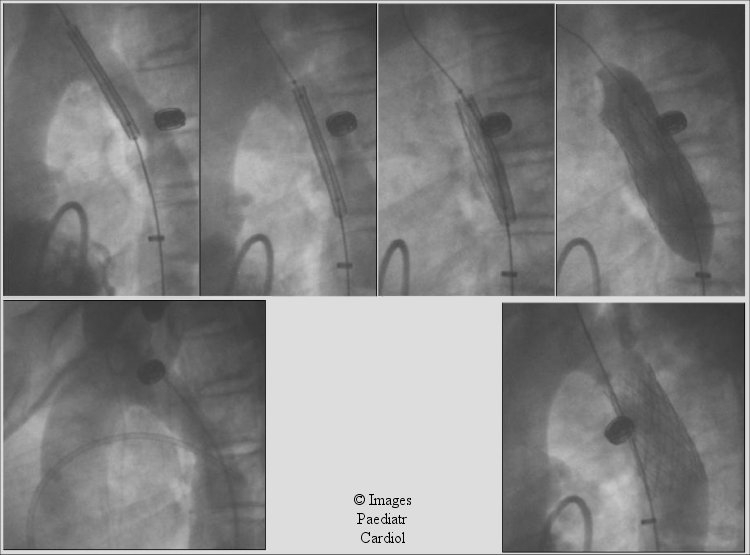
Lateral projection of patient described in Figure 4. Notice the multiple angiograms obtained using the transseptally placed left ventricular catheter allowing for fine adjustment of stent position prior to deployment.
Table 2.
Further comparison of Genesis and Palmaz stents
The Palmaz Genesis stent
The Palmaz-Genesis stent (Cordis / Johnson and Johnson) was introduced to overcome some of the short falls of the Plamaz stents. It was designed to have lower profile, minimize shortening, add radial strength, and increase flexibility. It has a closed cell design based on the FlexSegemnt technology (Figure 6) which improves flexibility, reduces shortening and improves scaffolding in a bend. The original Genesis® stent was successful in negotiating tight curves and had good radial strength. However the maximum diameter which can be achieved is less than optimal for coarctation. Subsequently the Palmaz Genesis, large and extra large diameters (XD), were introduced to allow larger diameter expansion while retaining the favorable characteristics of the genesis stent. The Genesis XD is expandable off label to 18 mm in bench work while maintaining flexibility and excellent radial force. (Tables 1 and 2). It has equivalent or higher strength to the Palmaz P-308 stent with less shortening. Its application and expandability for coarctation remains to be tested in a large group of patients.
Figure 6.
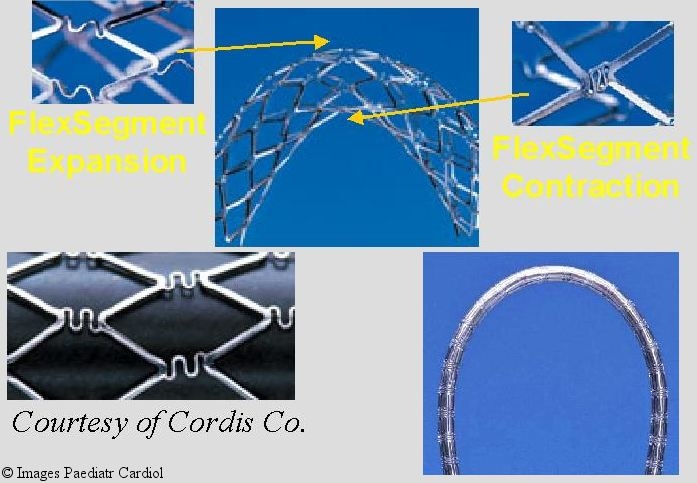
Flex segment technology used in manufacturing the Palmaz Genesis stent:allows for better negotiation of curves while maintaining radial strength
II-The Intrastent (IT) double strut® (Intratherapeutics, St. Paul, MN, recently acquired by ev3, Plymouth, MN)
The IT LD stent was introduced in an attempt to improve on the only stent existing at that time considered suitable for coarctation (namely the Palmaz). This new stent has an open cell design composed of parallel struts of laser cut 316L stainless steel.26,27 The unexpanded diameter is 3.8 mm length with no foreshortening at 12 mm diameter. The design allows for better flexibility and maneuverability. The maneuverability may be a significant advantage when placing the stent in tortuous areas through tight curves as the pulmonary arteries, though this is not necessarily the case in coarctation. The flexibility does, however, offer some possible advantage when implanted along the curve of the aortic arch. The initially introduced Intrastent (double strut LD) was tested in bench research up to 12 mms. against the Palmaz stent, and was felt to have comparable radial strength. Expanding the stent beyond 12 or 15 mms. has not been as encouraging as what initially was hoped. It was noted to have less radial strength,26,27 (and personal experience). Elastic recoil of the stent was noticed on deflation of the balloon in certain lesions requiring high radial strength. Additionally, distortion of the stent with either shortening or elongation has been recognized when larger diameters were attempted. This stent has not gained popularity in coarctation which may require diameter beyond 12-15 mms. The same company introduced two stents which are expandable to larger diameters while maintaining the integrity of the stent as well as the radial strength. The Mega LD® (unexpanded diameter 3.8 mm) and Max LD® (unexpanded diameter 4.5 mm) stents were introduced (series S17 and S18 respectively) in varying lengths (16, 26, 36 mm). These stents can be expanded up to 18 and 25 mms., respectively, with < 25 % shortening at the largest diameters. They also maintain good radial strength. These stents have the possible advantage of conforming to the curve of the aortic arch, (figures 7–9) while maintaining the radial strength as well as the advantage of open cell design especially if crossing a side branch. These however require slightly larger balloons and sheaths for adequate crimping and deployment because of their larger unexpanded diameter compared to the Palmaz stents (Series-8).
Figure 7.
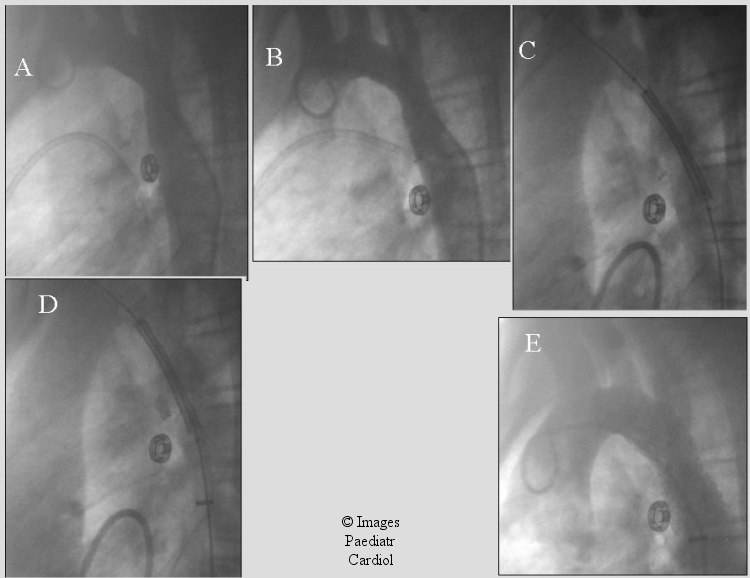
13 year old with recurrent coarctation after end to end repair. A) shows initial coarctation appearance. B) post balloon angioplasty there was mild improvement. Intra-therapeutics (Max) stent was subsequently placed (C & D). Stent position was adjusted based on the LV angiogram. E) The gradient decreased from 35 mm Hg to 1 mmHg after stent placement
Figure 9.
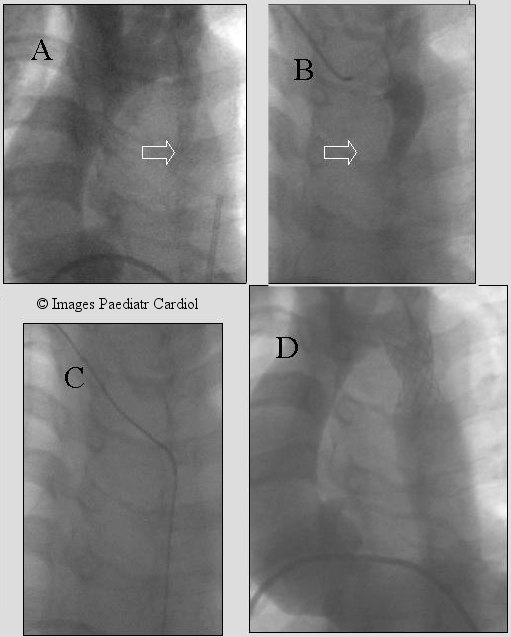
Severe native coarctation in a 12 year old. Retrograde crossing was difficult because of the presence of dilated collaterals. A wire was passed from the brachial artery (B & C), snared from the groin thus establishing the accurate track prior to successful stent placement (D). The gradient decreased at implantation from 80 mmHg to 25. Redilation was performed 9 months later.
III -Cheatham-Platinum (CP) Stent (NuMed, Hopkinton, NY)
The CP stent was developed by NuMed (Hopkinton, New York), in collaboration with John Cheatham, MD, to provide improvement on the stents available at that time which was the Palmaz. This stent was made from heat-tempered 90% platinum and 10% Iridium, .01 inch wire arranged in a zig pattern.16 The number of the zigs is variable between 6-8 per row and will affect the final diameter, profile and the strength of the stent as well as the percent shortening. If the target lesion expected diameter is < 15 mm then a 6 zig would be sufficient especially if it is in small child thus having a lower profile stent. The latter, however, would have limited application for coarctation because of the usually larger diameter required. The 8 zig / row configuration can be dilated up to 24 mm but has larger profile (28) and would offer a better choice for treating coarctation. A custom made 10 zig stent can be made and dilatable up to 30 mm with < 20 % shortening but would have a larger profile requiring larger sheath. Initial published results of this stent are encouraging.16 There was rare incidence of stent fatigue fracture.
Technical tips for implantation
This section is not intended to be a full discussion of the implantation technique, but rather to present a few tips reflecting personal preference and may or may not be applicable to every patient or operator. We, however, feel that these are helpful to achieve successful stent implantation.
1. Wire choice
The wire is chosen to provide extra support (for example: extra stiff or super stiff wires) to avoid stent / balloon migration during implantation. We position the wire to ensure that the stiff part is well in the position of implantation and extends proximally as much as possible keeping the floppy end distant from the implantation site. This is accomplished by placing the tip of the wire in the right subclavian artery (or in the left subclavian, depending on the location of the coarctation) or occasionally by making a loop in the ascending aorta. (figures 2, 4, 5, 7 and 8)
Figure 4.
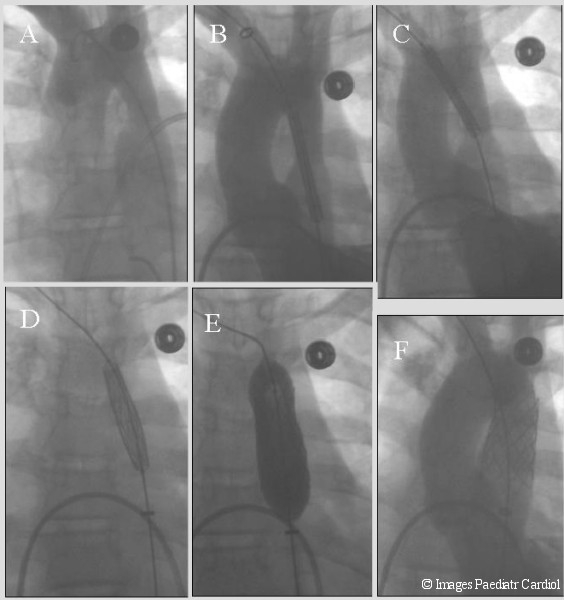
Recurrent coarctation involving a relatively long segment (A). A Palmaz P- 4010 stent was used (B). The wire was placed in the right subclavian (B-D). Notice the use of BIB balloon. Inflation of the inner balloon (D) allows for initial expansion of the stent center followed by the outer balloon (E). After implantation there was excellent angiographic and hemodynamic result (F).
Figure 8.
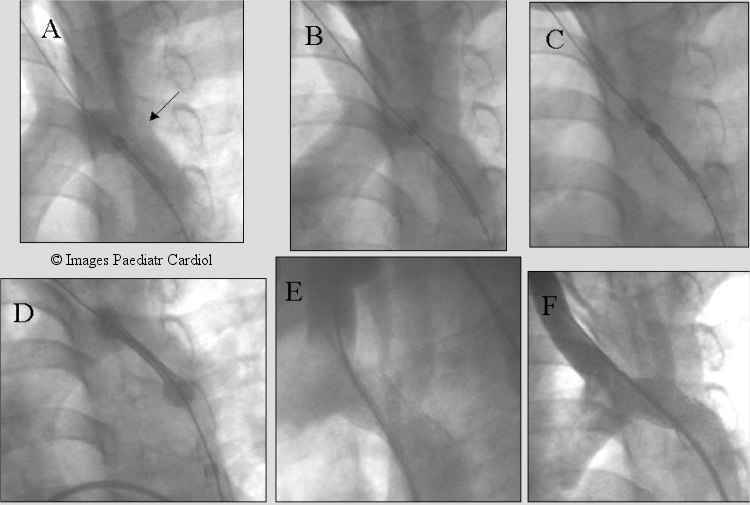
Recurrent coarctation after subclavian flap aortoplasty (arrow). Note the proximity to the left carotid artery. Multiple LV angiograms obtained to accurately place the stent before and after uncovering the stent. The two ends of the balloon were allowed to expand slightly (C & D) with further imaging prior to fully deploying the stent (Max, IT Stent).
2. Complex arch anatomy
In these patients, the position of the stent has to be very accurate to avoid unnecessary covering of the proximal branches or extension to distal aneurysms. We usually enter the left ventricle from prograde fashion, either using a transseptal puncture (figure 2, 4, 5, 7) or through a PFO (figure 8) and place an angiographic catheter in the left ventricle to obtain angiograms and pressure measurements, before, during and after deployment. Multiple angiograms are obtained; before and after uncovering the stent with adjustment of stent position as necessary.
3. Stent choice
The ideal stent is probably not available, but certain characteristics make some stents more attractive than others in different positions of the coarctation. The stent should be chosen that is expandable to an adult size aorta. A more flexible stent may be better suited if the implantation is across the arch (figures 7, 8). If the stent may cover side branches or neck vessels, then an open cell design may be more attractive.
4. Balloon choice and inflation
The perfect balloon would be of low profile, high pressure, have enough material to allow easy crimping of the stent and resistant to rupture. These facts are difficult to achieve because a thin balloon would be more prone to rupture, and more difficult to accurately mount and crimp the stent. A larger material balloon will require a larger sheath. However the industry has achieved significant improvement in the balloon designs and material. Another consideration is the length of the balloon in relation to the stent i.e. shorter versus longer. Both offer some potential advantages and is interventionalist dependent. The shorter balloons offer the advantage of inflating the inner part of the stent first thus avoiding the potential complications of flaring of the ends of the stents resulting in balloon rupture or vessel wall perforation. However it can result in stent sliding or embolization making it difficult to accurately place the stent. The longer balloons allow more precise placement and repositioning if necessary before fully inflating the balloon. We generally use balloons slightly longer than the stent or the BIB balloon (NuMed, Hopkinton, NY), which offers two balloons, one shorter (inner) and one longer (outer) than the stent. When we use the longer balloon method and if precise positioning of the stent is required, we find it helpful to initially uncover only the distal part of the balloon (the end further from the operator). The balloon is slightly inflated, thus allowing the distal part to slightly expand (Figure 8C). We obtain further pictures and reposition, if necessary. When we are comfortable with the position, the balloon and stent are fully uncovered and further mild inflation is done to allow the proximal part of the balloon (the end close to the operator) to inflate (figure 8D). This is done before fully inflating the balloon, thus the stent is held in accurate position by the two partially inflated ends of the balloon. An angiogram is obtained to confirm the position of the stent and readjustment is done as necessary with repeat angiograms. When the position is accurate, the stent is fully inflated. During the inflation, steady pressure is maintained by the operator on the balloon to ensure the balloon/stent will not travel. Maintaining the extra support wire in that position, as well as the steady hand of the operator with gentle push, should maintain the stent in its position. If during the inflation, there is any movement of the balloon, this can also be readjusted by minimal hand manipulation or alternatively to stop the inflation and reposition before the full inflation. Alternatively, the BIB balloon allows inflating the center of the stent and the inner balloon first (Figure 4). This also may allow partial repositioning of the stent before the full inflation. Once the stent has been deployed, repeat angiograms are obtained from the catheter in the LV to confirm there is no dissection and the accurate position of the stent. The pressure from the LV is compared with the pressure obtained from the arterial end thus estimating the residual gradient. Based on the result of the angiogram and the residual gradient, if any, mild further dilatation can be done. Achieving a perfect result on the first time implanting the stent especially if this is a primary stenting is not necessary. Further dilatation after six months to a year can be done.
5. Extent of dilatation
When implanting the stent, the goal is to achieve the largest possible diameter and eliminate any gradient to decrease the need for redilation. However the concerns are always the possibility of tear or aneurysm formation which may be encountered with over dilation We have adopted certain criteria to help achieve safe and effective stent implantation. We measure carefully the descending aorta at the level of the diaphragm and the proximal transverse arch. The balloon is genrerally chosen to be equal to the lesser of the 2 measurements not to exceed 3 times the size of the stenotic segment. The balloon is inflated to expand and deploy the stent which may not be the full diameter of the balloon or the full pressure. When the stent is expanded well we repeat angiography and pressure measurement. If there is significant residual gradient and no dissection, repeat balloon dilation using the same balloon at the maximum pressure is done. Repeat assessment is performed. If there is still significant gradient and no dissection, and we have not exceeded 3 times the size of the stenotic segment, a slightly larger balloon may be used. Subsequently flaring of the ends using a lower pressure inflation may be performed if necessary though it is not essential. Repeat assessment is done. If there is significant residual gradient we would be reluctant to perform further dilation at this point. The patient would be brought back to the catheterization laboratory for repeat dilation at a later date. We like to wait at least 6 months. Achieving a perfect result is not necessary at the time of stent implantation.
6. Anticoagulation
Patients are maintained on aspirin for at least one year after the procedure to avoid platelet aggregation. Since this is a high pressure, high flow area it was not felt necessary to use warfarin to anti-coagulate the patients.14
Discussion
Coarctation, if left untreated, poses a significant health risk and up to 90% of the patients with isolated coarctation may die before age fifty years of complications of coarctation including aortic rupture, intracranial hemorrhages, hypertension, endocarditis or heart failure.29,30 With treatment of coarctation and aggressive management of this disease, significant improvement in the long-term survival of these patients has been seen and is hopefully going to improve with effective and successful management. The major burden on the interventional cardiologist is to choose the right approach for the specific patient, whether surgery, balloon or stent. Each of these modalities have a role for the specific patient. The patient's age weight history of surgery influences in great deal the management strategy. Though the use of stents to treat coarctation of the aorta has been relatively recent, its use to treat peripheral vascular stenosis dates to the mid 1980s18–20 and later in the management of other stenotic lesions in congenital heart disease both native and post operative.21–23 Early animal work in experimental coarctation model21,32 showed promising results. However, in another animal model few instances of rupture occurred with redilation.33 It appeared that in this specific animal model the rupture occurred when the dilation was attempted at significantly higher balloon diameter to stenotic lesion ratio. Slowly but steadily the use of stents was introduced in the management of coarctation. The initial and follow up results have been encouraging with successful gradient reduction.12–17,34 Similar to redilation in other lesions,32 redilation of the stented coarctation has been possible with further gradient reduction. Few complications however have been observed.11,15,16,36. Some appear to be related to using a significantly larger balloon to stenotic site ratio or related to balloon choice. Recoarctation following balloon angioplasty alone has been seen in 18-31 % of patients with native coarctation and in 9-80% of postoperative coarctation,8,9,11 depending on the age of the patient, the nature of the lesion and size of balloon used. Late follow up of angioplasty patients showed that 32% of the patients with recurrent coarctation had not had adequate gradient relief (<20 mm. Hg) at the initial angioplasty despite adequate balloon / isthmus ratio.8,11 Intravascular stents can maintain the patency of a stenotic lesion by eliminating the elastic recoil of the tissue and can extend over a relatively long segment of stenosis, thus decreasing the need for over expansion or causing intimal tear in these vessels.10,37 Short and intermediate term follow up have shown that stented coarctation have good ( if not better) gradient relief12–15 and usually the recurrence is attributed to somatic growth14,17 or conservative initial dilation (personal experience) to avoid using too high balloon to stenosed segment ratio exceeding (3-4 times) in the initial implantation. In few patients17 neointimal growth causing mild restenosis was seen in growing children who were stented < 6 years old.
Extension of a stent into the orifices of the branches of the aortic arch is a concern as the struts can cause obstruction to the flow in major vessels. In adult size patients, however, the struts of a dilated stent are very small relative to the diameter of the subclavian and carotid arteries. Published reports for coarctation as well as in other sites14,36 have indicated continued patency and unimpeded flow in these vessels. This however must be taken into account during stent implantation. Every effort should be made to minimize the extension of the stent across the side branches if possible. In those instances where a stent will have to cross the side branches there may be a potential advantage for the open cell design though the experience with these stents in that position is limited at this time.
In conclusion, in selected patients with coarctation of the aorta stent implantation may be a feasible and improved option to relieve the stenosis. Short and mid-term follow-up of these patients have shown encouraging results. Further studies, especially addressing long term follow up, are needed.
References
- 1.Anjos R, Quershi SA, Rosenthal E, Murdoch I, Hayes A, Parsons J, Baker EJ, Tynan M. Determinants of hemodynamic results of balloon dilation of aortic recoarctation. Am J Cardiol. 1992;69:665–671. doi: 10.1016/0002-9149(92)90161-q. [DOI] [PubMed] [Google Scholar]
- 2.Kan JS, Whilte RI, Mitchell SE, Farmlett EJ, Donahoo JS, Gardner TJ. Treatment of restenosis of coarctation by percutaneous transluminal angioplasty. Circulation. 1983;68:1087–1094. doi: 10.1161/01.cir.68.5.1087. [DOI] [PubMed] [Google Scholar]
- 3.Lock JE, Keane JF, Fellows KE. The use of catheter intervention procedures for congenital heart disease. J Am Coll Cardiol. 1986;7:1420–1423. doi: 10.1016/s0735-1097(86)80166-8. [DOI] [PubMed] [Google Scholar]
- 4.Rao PS, Wilson AD, Chopra PS. Immediate and follow-up results of balloon angioplasty of postoperative recoarctation in infants and children. Am Heart J. 1990;120:1315–1320. doi: 10.1016/0002-8703(90)90242-p. [DOI] [PubMed] [Google Scholar]
- 5.Rao PS. Balloon angioplasty of aortic coarctation: A review. Clin Cardiol. 1989;12:618–628. doi: 10.1002/clc.4960121103. [DOI] [PubMed] [Google Scholar]
- 6.Rao PS. Balloon Angioplasty for aortic recoarctation following previous surgery. In: Rao, editor. Transcatheter Therapy In Pediatric Cardiology. Wiley-Liss; 1993. pp. 206–208. [Google Scholar]
- 7.Gersony WM. Coarctation of the Aorta. In: Adams FH, Emmanouilides GC, Riemenschneider TA, editors. “Heart Disease in Infants, Children and Adolescents”. 4th ed. Baltimore: William & Wilkins; 1989. pp. 243–255. [Google Scholar]
- 8.Mendelsohn AM, Lloyd TR, Crowley DC, Sandhu SK, Kocis KC, Beekman RH. Late follow-up of balloon angioplasty in children with a native coarctation of the aorta. Am J Cardiol. 1994;74:696–700. doi: 10.1016/0002-9149(94)90312-3. [DOI] [PubMed] [Google Scholar]
- 9.Rao PS, Galal O, Smith PA, Wilson A. Five to nine year follow-up results of balloon angioplasty of native coarctation in infants and children. J Am Coll Cardiol. 1996;27:462–470. doi: 10.1016/0735-1097(95)00479-3. [DOI] [PubMed] [Google Scholar]
- 10.Ovaert C, Benson LN, Nykanene D, Freedom RM. Transcatheter treatment of coarctation of the aorta: a review. Pediatr Cardiol. 1998;19:27–44. doi: 10.1007/s002469900243. [DOI] [PubMed] [Google Scholar]
- 11.Hellenbrand WE, Allen HD, Golinko RJ, Hagler DJ, Lutin W, Kan J. Balloon angioplasty for aortic recoarctation: results of valvuloplasty and angioplasty of congenital anomalies registry. Am J Cardiol. 1990;65:793–797. doi: 10.1016/0002-9149(90)91390-r. [DOI] [PubMed] [Google Scholar]
- 12.de Lezo JS, Pan M, Romero M. Balloon-expandable stent repair of severe coarctation of aorta. Am Heart J. 1995;129:1002–1008. doi: 10.1016/0002-8703(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 13.Bulbul ZR, Bruckheimer E, Love JC, Fahey JT, Hellenbrand WE. Implantation of balloon-expandable stents for coarctation of the aorta: implantation data and short-term results. Cathet Cardiovasc Diagn. 1996;39:36–42. doi: 10.1002/(SICI)1097-0304(199609)39:1<36::AID-CCD7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 14.Ebeid MR, Prieto LP, Latson LA. The use of balloon expandable stents in coarctation of the aorta: short and intermediate term follow up. J Am Coll Cardiol. 1997;30:1847–1852. doi: 10.1016/s0735-1097(97)00408-7. [DOI] [PubMed] [Google Scholar]
- 15.Hamadan MA, Maheshwari S, Fahey JT, Hellenbrand WE. Endovascular stents for coarctation of the aorta: initial results and intermediate-term follow-up. J Am Coll Cardiol. 2001;38:1518–1523. doi: 10.1016/s0735-1097(01)01572-8. [DOI] [PubMed] [Google Scholar]
- 16.Cheatham JP. Stenting of coarctation of the aorta. catheter cardiovascular intervention. 2001;54:112–125. doi: 10.1002/ccd.1249. [DOI] [PubMed] [Google Scholar]
- 17.de Lezo JS, Pan M, Romero M, Medina A, Segura J, Lafuente M, Pavlovic D, Hernandez E, Melian F, Espada J. Immediate and follow-up findings after stent treatment for severe coarctation of the aorta. Am J Cardiol. 1999;83:400–406. doi: 10.1016/s0002-9149(98)00877-7. [DOI] [PubMed] [Google Scholar]
- 18.Palmaz JC, Richter GM, Noeldge G, Schatz RA, Robison PD, Gardiner GA, Jr, Becker GJ, McLean GK, Denny DF, Jr, Lammer J. Intraluminal stents in atherosclerotic iliac artery stenosis: preliminary report of a multicenter study. Radiology. 1988;168:727–731. doi: 10.1148/radiology.168.3.2970098. [DOI] [PubMed] [Google Scholar]
- 19.Palmaz JC, Garcia OJ, Schatz RA, Rees CR, Roeren T, Richter GM, Noeldge G, Gardiner GA, Jr, Becker GJ, Walker C. Placement of balloon-expandable intraluminal stents in iliac arteries: first 171 procedures. Radiology. 1990;174:969–975. doi: 10.1148/radiology.174.3.174-3-969. [DOI] [PubMed] [Google Scholar]
- 20.Palmaz JC, Encarnacion CE, Garcia OJ, Schatz RA, Rivera FJ, Laborde JC, Dougherty SP. Aortic bifurcation stenosis: treatment with intravascular stents. J Vasc Interv Radiol. 1991;2:319–23. doi: 10.1016/s1051-0443(91)72250-1. [DOI] [PubMed] [Google Scholar]
- 21.Mullins CE, O’Laughlin MP, Vick GW, 3d, Mayer DC, Myers TJ, Kearney DL, Schatz RA, Palmaz JC. Implantation of balloon-expandable intravascular grafts by catheterization in pulmonary arteries and systemic veins. Circulation. 1988;77:188–199. doi: 10.1161/01.cir.77.1.188. [DOI] [PubMed] [Google Scholar]
- 22.O’Laughlin MP, Slack MC, Grifka RG, Perry SB, Lock JE, Mullins CE. Implantation and intermediate-term follow-up of stents in congenital heart disease. Circulation. 1993;88:605–14. doi: 10.1161/01.cir.88.2.605. [DOI] [PubMed] [Google Scholar]
- 23.Powell AJ, Lock JE, Keane JF, Perry SB. Prolongation of RV-PA conduit life span by percutaneous stent implantation. Intermediate-term results. Circulation. 1995;92:3282–8. doi: 10.1161/01.cir.92.11.3282. [DOI] [PubMed] [Google Scholar]
- 24.Redington AN, Hayes AM, Yen Ho S. Transcatheter stent implantation to treat aortic coarctation in infancy. Br Heart J. 1993;69:80–82. doi: 10.1136/hrt.69.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Redington AN, Weil J, Somerville J. Self expanding stents in congenital heart disease. Br Heart J. 1994;72:378–383. doi: 10.1136/hrt.72.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreutzer J, Rome JJ. Open-cell design stents in congenital heart disease: a comparison of intrstent vs. palmaz stents. Catheter Cardiovasc Interv. 2002;56:400–409. doi: 10.1002/ccd.10180. [DOI] [PubMed] [Google Scholar]
- 27.Rutledge JM, Mullins CE, Nihill MR, Grifka RG, Vincent JA. Initial experience with intratherapeutics Intrastent Doublestrut LD stents in patients with congenital heart defects. Catheter Cardiovasc Interv. 2002;56:541–548. doi: 10.1002/ccd.10251. [DOI] [PubMed] [Google Scholar]
- 28.Cheatham JP. Improved stents for pediatric applications. Prog Pediatr Cardiol. 2001;14:95–115. [Google Scholar]
- 29.Campbell M. Natural history of coarctation of the aorta. Br Heart J. 1970;32:633–640. doi: 10.1136/hrt.32.5.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirklin JW, Barratt-Boyes BG, editors. Cardiac Surgery. New York: Churchill-Livingston; 1992. Coarctation of the Aorta and Interrupted Aortic Arch; pp. 1263–1325. [Google Scholar]
- 31.Morrow WR, Smith VC, Ehler WJ, VanDellen AF, Mullins CE. Balloon angioplasty with stent implantation in experimental coarctation of the aorta. Circulation. 1994;89:2677–2683. doi: 10.1161/01.cir.89.6.2677. [DOI] [PubMed] [Google Scholar]
- 32.Grifka RG, Vick GW, 3rd, O’Laughlin MP, Myers TJ, Morrow WR, Nihill MR, Kearney DL, Mullins CE. Balloon expandable intravascular stents: aortic implantation and late further dilation in growing minipigs. Am Heart J. 1993;126:979–984. doi: 10.1016/0002-8703(93)90715-l. [DOI] [PubMed] [Google Scholar]
- 33.Mendelsohn AM, Dorostkar PC, Moorehead CP. Stent Redilation in canine models of congenital heart disease: pulmonary artery stenosis and coarctation of the aorta. Cathet Cardiovasc Diagn. 1996;38:430–440. doi: 10.1002/(SICI)1097-0304(199608)38:4<430::AID-CCD24>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 34.Rosenthal E, Qureshi SA, Tynan M. Stent implantation for aortic recoarctation. Am Heart J. 1995;129:1220–1221. doi: 10.1016/0002-8703(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 35.Ing FF, Grifka RG, Nihill MR, Mullins CE. Repeat dilation of intravascular stents in congenital heart defects. Circulation. 1995;92:893–897. doi: 10.1161/01.cir.92.4.893. [DOI] [PubMed] [Google Scholar]
- 36.Magee AG, Brezinska-Rajszys G, Qureshi SA. Stent implantation for aortic coartcation and recoarctation. Heart. 1999;82:600–606. doi: 10.1136/hrt.82.5.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hornung TS, Benson LN, McLaughlin PR. Interventions for aortic coarctation. Cardiol Rev. 2002;10:139–148. doi: 10.1097/00045415-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Schatz RA, Plamaz JC, Tio FO, Garcia F, Garcia O, Reuter SR. Balloon-expandable intra-coronary stents in the adult dog. Circulation. 1987;76:450–457. doi: 10.1161/01.cir.76.2.450. [DOI] [PubMed] [Google Scholar]


