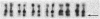Abstract
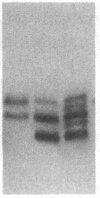
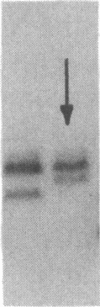
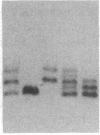
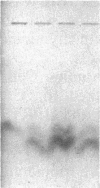
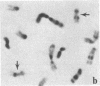
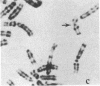
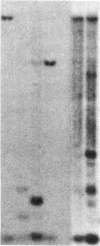
Mammalian sex-dosage compensation is mediated by maintaining activity of only one X chromosome. The asynchronous DNA synthesis characterizing the silent human X chromosome is thought to be reversible only during ontogeny of oocytes. We have previously shown that the glucose-6-phosphate dehydrogenase (G6PD) locus (G6PD) on the allocyclic X chromosome in chorionic villi is partially expressed. We now show that in hybrids derived from a clone of chorionic villi cells (heterozygous for G6PD A) and mouse A9 cells, the loci for G6PD, hypoxanthine phosphoribosyltransferase (HPRT) and phosphoglycerate kinase are expressed on both human X chromosomes; the human X chromosomes carrying either G6PD A or B replicate synchronously with each other and with murine chromosomes. The X chromosome with G6PD A was identified as the original late-replicating X, because methylation in the body of the HPRT gene on this chromosome remained characteristic of the inactive X chromosome. These results indicate that X-chromosome inactivation is completely reversible in cells of trophoblast origin; induction of full transcriptional activity is accompanied by acquisition of isocyclic replication, showing an intimate relationship between these processes. The molecular events responsible for this reversal may be similar to those occurring during maturation of oocytes. Chorionic villi and derivative hybrids provide in vitro models for exploring early events that program the single active X chromosome.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIDSON R. G., NITOWSKY H. M., CHILDS B. DEMONSTRATION OF TWO POPULATIONS OF CELLS IN THE HUMAN FEMALE HETEROZYGOUS FOR GLUCOSE-6-PHOSPHATE DEHYDROGENASE VARIANTS. Proc Natl Acad Sci U S A. 1963 Sep;50:481–485. doi: 10.1073/pnas.50.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber R. A., Davidson R. L. Maintenance of replication patterns in human-mouse hybrids retaining only one human chromosome. Somatic Cell Genet. 1979 Jul;5(4):519–528. doi: 10.1007/BF01538885. [DOI] [PubMed] [Google Scholar]
- Goldman M. A., Holmquist G. P., Gray M. C., Caston L. A., Nag A. Replication timing of genes and middle repetitive sequences. Science. 1984 May 18;224(4650):686–692. doi: 10.1126/science.6719109. [DOI] [PubMed] [Google Scholar]
- Graves J. A., Young G. J. X-chromosome activity in heterokaryons and hybrids between mouse fibroblasts and teratocarcinoma stem cells. Exp Cell Res. 1982 Sep;141(1):87–97. doi: 10.1016/0014-4827(82)90071-4. [DOI] [PubMed] [Google Scholar]
- Hors-Cayla M. C., Heuertz S., Frezal J. Coreactivation of four inactive X genes in a hamster x human hybrid and persistence of late replication of reactivated X chromosome. Somatic Cell Genet. 1983 Nov;9(6):645–657. doi: 10.1007/BF01539470. [DOI] [PubMed] [Google Scholar]
- Jolly D. J., Esty A. C., Bernard H. U., Friedmann T. Isolation of a genomic clone partially encoding human hypoxanthine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5038–5041. doi: 10.1073/pnas.79.16.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M., Mohandas T., Shapiro L. J. Cell cycle-specific reactivation of an inactive X-chromosome locus by 5-azadeoxycytidine. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1215–1219. doi: 10.1073/pnas.79.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan B., DeMars R. Localized Derepression on the Human Inactive X Chromosone in Mouse-Human Cell Hybrids. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1510–1514. doi: 10.1073/pnas.72.4.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratzer P. G., Chapman V. M. X chromosome reactivation in oocytes of Mus caroli. Proc Natl Acad Sci U S A. 1981 May;78(5):3093–3097. doi: 10.1073/pnas.78.5.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester S. C., Korn N. J., DeMars R. Derepression of genes on the human inactive X chromosome: evidence for differences in locus-specific rates of derepression and rates of transfer of active and inactive genes after DNA-mediated transformation. Somatic Cell Genet. 1982 Mar;8(2):265–284. doi: 10.1007/BF01538681. [DOI] [PubMed] [Google Scholar]
- Littlefield J. W. The use of drug-resistant markers to study the hybridization of mouse fibroblasts. Exp Cell Res. 1966 Jan;41(1):190–196. doi: 10.1016/0014-4827(66)90558-1. [DOI] [PubMed] [Google Scholar]
- Lyon M. F. X-chromosome inactivation and developmental patterns in mammals. Biol Rev Camb Philos Soc. 1972 Jan;47(1):1–35. doi: 10.1111/j.1469-185x.1972.tb00969.x. [DOI] [PubMed] [Google Scholar]
- Martin G. R., Epstein C. J., Travis B., Tucker G., Yatziv S., Martin D. W., Jr, Clift S., Cohen S. X-chromosome inactivation during differentiation of female teratocarcinoma stem cells in vitro. Nature. 1978 Jan 26;271(5643):329–333. doi: 10.1038/271329a0. [DOI] [PubMed] [Google Scholar]
- McBurney M. W., Strutt B. J. Genetic activity of X chromosomes in pluripotent female teratocarcinoma cells and their differentiated progeny. Cell. 1980 Sep;21(2):357–364. doi: 10.1016/0092-8674(80)90472-9. [DOI] [PubMed] [Google Scholar]
- Migeon B. R., Sprenkle J. A., Do T. T. Stability of the "two active X" phenotype in triploid somatic cells. Cell. 1979 Nov;18(3):637–641. doi: 10.1016/0092-8674(79)90118-1. [DOI] [PubMed] [Google Scholar]
- Migeon B. R. Stability of X chromosomal inactivation in human somatic cells. Nature. 1972 Sep 8;239(5367):87–89. doi: 10.1038/239087a0. [DOI] [PubMed] [Google Scholar]
- Migeon B. R., Wolf S. F., Axelman J., Kaslow D. C., Schmidt M. Incomplete X chromosome dosage compensation in chorionic villi of human placenta. Proc Natl Acad Sci U S A. 1985 May;82(10):3390–3394. doi: 10.1073/pnas.82.10.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migeon B. R., Wolf S. F., Mareni C., Axelman J. Derepression with decreased expression of the G6PD locus on the inactive X chromosome in normal human cells. Cell. 1982 Jun;29(2):595–600. doi: 10.1016/0092-8674(82)90175-1. [DOI] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Bishop D. F., Desnick R. J., Shapiro L. J. Frequency of reactivation and variability in expression of X-linked enzyme loci. Am J Hum Genet. 1984 Jul;36(4):916–925. [PMC free article] [PubMed] [Google Scholar]
- Monk M., Harper M. I. Sequential X chromosome inactivation coupled with cellular differentiation in early mouse embryos. Nature. 1979 Sep 27;281(5729):311–313. doi: 10.1038/281311a0. [DOI] [PubMed] [Google Scholar]
- Pai G. S., Sprenkle J. A., Do T. T., Mareni C. E., Migeon B. R. Localization of loci for hypoxanthine phosphoribosyltransferase and glucose-6-phosphate dehydrogenase and biochemical evidence of nonrandom X chromosome expression from studies of a human X-autosome translocation. Proc Natl Acad Sci U S A. 1980 May;77(5):2810–2813. doi: 10.1073/pnas.77.5.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M., Stolzmann W. M., Baranovskaya L. I. Replication variants of the human inactive X chromosome. I. Variability within lymphocytes of single individuals. Chromosoma. 1982;85(3):405–412. doi: 10.1007/BF00330363. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Wolf S. F., Migeon B. R. Evidence for a relationship between DNA methylation and DNA replication from studies of the 5-azacytidine-reactivated allocyclic X chromosome. Exp Cell Res. 1985 Jun;158(2):301–310. doi: 10.1016/0014-4827(85)90455-0. [DOI] [PubMed] [Google Scholar]
- Takagi N., Yoshida M. A., Sugawara O., Sasaki M. Reversal of X-inactivation in female mouse somatic cells hybridized with murine teratocarcinoma stem cells in vitro. Cell. 1983 Oct;34(3):1053–1062. doi: 10.1016/0092-8674(83)90563-9. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Feinberg A. P. Use of restriction fragment length polymorphisms to determine the clonal origin of human tumors. Science. 1985 Feb 8;227(4687):642–645. doi: 10.1126/science.2982210. [DOI] [PubMed] [Google Scholar]
- Von Kap-Herr C., Mukherjee B. B. Stability of inactive X-chromosome in mouse embryoid body-mule cell and transformed mouse cell-mule cell heterokaryons. Exp Cell Res. 1977 Feb;104(2):369–376. doi: 10.1016/0014-4827(77)90102-1. [DOI] [PubMed] [Google Scholar]
- Wolf S. F., Dintzis S., Toniolo D., Persico G., Lunnen K. D., Axelman J., Migeon B. R. Complete concordance between glucose-6-phosphate dehydrogenase activity and hypomethylation of 3' CpG clusters: implications for X chromosome dosage compensation. Nucleic Acids Res. 1984 Dec 21;12(24):9333–9348. doi: 10.1093/nar/12.24.9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S. F., Jolly D. J., Lunnen K. D., Friedmann T., Migeon B. R. Methylation of the hypoxanthine phosphoribosyltransferase locus on the human X chromosome: implications for X-chromosome inactivation. Proc Natl Acad Sci U S A. 1984 May;81(9):2806–2810. doi: 10.1073/pnas.81.9.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S. F., Migeon B. R. Clusters of CpG dinucleotides implicated by nuclease hypersensitivity as control elements of housekeeping genes. Nature. 1985 Apr 4;314(6010):467–469. doi: 10.1038/314467a0. [DOI] [PubMed] [Google Scholar]
- Yen P. H., Patel P., Chinault A. C., Mohandas T., Shapiro L. J. Differential methylation of hypoxanthine phosphoribosyltransferase genes on active and inactive human X chromosomes. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1759–1763. doi: 10.1073/pnas.81.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]