Abstract
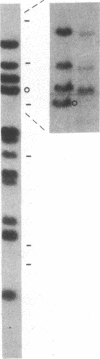
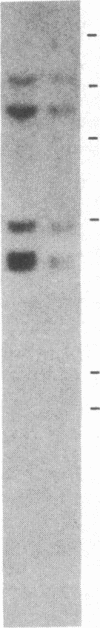
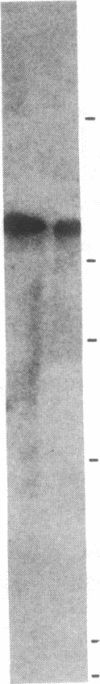
Information relevant to allelic exclusion in T cells has been obtained by a study of cDNA clones corresponding to alpha-chain genes of the T-cell receptor in the rabbit T-cell line RL-5. One clone contains a variable-joining-constant (VJC) sequence encoding a complete alpha chain of the T-cell receptor. A second has an identical constant region and includes a distinct variable-joining (VJ) sequence. However, a single-base deletion in the variable region places the remainder of the second transcript out-of-phase and appears to be the product of a rearrangement involving a variable region of the T-cell receptor alpha-chain pseudogene. Presence of two variable-joining-constant (VJC) transcripts in the same cell line indicates that alpha-chain gene rearrangement is not affected by transcription of a complete alpha-chain mRNA and suggests that steps after mRNA synthesis are involved in the allelic exclusion process for alpha-chain genes. Comparison of rabbit alpha-chain sequences with those of man and mouse revealed interspecies conservation in constant and variable regions. Genomic Southern blot analyses using a rabbit constant region of the T-cell receptor alpha-chain probe revealed the presence of a single constant region gene. Hybridization with variable region probes defined two distinct multigenic subfamilies. Homology between certain rabbit and murine variable regions of the T-cell receptor alpha-chain sequences suggests that the existence of subfamilies predated divergence of these species.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acuto O., Fabbi M., Smart J., Poole C. B., Protentis J., Royer H. D., Schlossman S. F., Reinherz E. L. Purification and NH2-terminal amino acid sequencing of the beta subunit of a human T-cell antigen receptor. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3851–3855. doi: 10.1073/pnas.81.12.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison J. P., Lanier L. L. Identification of antigen receptor-associated structures on murine T cells. Nature. 1985 Mar 7;314(6006):107–109. doi: 10.1038/314107a0. [DOI] [PubMed] [Google Scholar]
- Arden B., Klotz J. L., Siu G., Hood L. E. Diversity and structure of genes of the alpha family of mouse T-cell antigen receptor. 1985 Aug 29-Sep 4Nature. 316(6031):783–787. doi: 10.1038/316783a0. [DOI] [PubMed] [Google Scholar]
- Barth R. K., Kim B. S., Lan N. C., Hunkapiller T., Sobieck N., Winoto A., Gershenfeld H., Okada C., Hansburg D., Weissman I. L. The murine T-cell receptor uses a limited repertoire of expressed V beta gene segments. Nature. 1985 Aug 8;316(6028):517–523. doi: 10.1038/316517a0. [DOI] [PubMed] [Google Scholar]
- Behlke M. A., Spinella D. G., Chou H. S., Sha W., Hartl D. L., Loh D. Y. T-cell receptor beta-chain expression: dependence on relatively few variable region genes. Science. 1985 Aug 9;229(4713):566–570. doi: 10.1126/science.3875151. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Borst J., Prendiville M. A., Terhorst C. Complexity of the human T lymphocyte-specific cell surface antigen T3. J Immunol. 1982 Apr;128(4):1560–1565. [PubMed] [Google Scholar]
- Brenner M. B., Trowbridge I. S., Strominger J. L. Cross-linking of human T cell receptor proteins: association between the T cell idiotype beta subunit and the T3 glycoprotein heavy subunit. Cell. 1985 Jan;40(1):183–190. doi: 10.1016/0092-8674(85)90321-6. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Ritchie K. A., Hammer R. E., O'Brien R. L., Arp B., Storb U. Expression of a microinjected immunoglobulin gene in the spleen of transgenic mice. Nature. 1983 Nov 24;306(5941):332–336. doi: 10.1038/306332a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien Y. H., Gascoigne N. R., Kavaler J., Lee N. E., Davis M. M. Somatic recombination in a murine T-cell receptor gene. Nature. 1984 May 24;309(5966):322–326. doi: 10.1038/309322a0. [DOI] [PubMed] [Google Scholar]
- Chien Y., Becker D. M., Lindsten T., Okamura M., Cohen D. I., Davis M. M. A third type of murine T-cell receptor gene. Nature. 1984 Nov 1;312(5989):31–35. doi: 10.1038/312031a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gascoigne N. R., Chien Y., Becker D. M., Kavaler J., Davis M. M. Genomic organization and sequence of T-cell receptor beta-chain constant- and joining-region genes. Nature. 1984 Aug 2;310(5976):387–391. doi: 10.1038/310387a0. [DOI] [PubMed] [Google Scholar]
- Hayday A. C., Diamond D. J., Tanigawa G., Heilig J. S., Folsom V., Saito H., Tonegawa S. Unusual organization and diversity of T-cell receptor alpha-chain genes. 1985 Aug 29-Sep 4Nature. 316(6031):828–832. doi: 10.1038/316828a0. [DOI] [PubMed] [Google Scholar]
- Kronenberg M., Goverman J., Haars R., Malissen M., Kraig E., Phillips L., Delovitch T., Suciu-Foca N., Hood L. Rearrangement and transcription of the beta-chain genes of the T-cell antigen receptor in different types of murine lymphocytes. Nature. 1985 Feb 21;313(6004):647–653. doi: 10.1038/313647a0. [DOI] [PubMed] [Google Scholar]
- LIND P. E., BURNET F. M. Recombination between virulent and non-virulent strains of influenza virus. II. The behaviour of virulence markers on recombination. Aust J Exp Biol Med Sci. 1957 Feb;35(1):67–78. doi: 10.1038/icb.1957.8. [DOI] [PubMed] [Google Scholar]
- Malissen M., Minard K., Mjolsness S., Kronenberg M., Goverman J., Hunkapiller T., Prystowsky M. B., Yoshikai Y., Fitch F., Mak T. W. Mouse T cell antigen receptor: structure and organization of constant and joining gene segments encoding the beta polypeptide. Cell. 1984 Jul;37(3):1101–1110. doi: 10.1016/0092-8674(84)90444-6. [DOI] [PubMed] [Google Scholar]
- McIntyre B. W., Allison J. P. Biosynthesis and processing of murine T-cell antigen receptor. Cell. 1984 Oct;38(3):659–665. doi: 10.1016/0092-8674(84)90260-5. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten P., Yokota T., Rothbard J., Chien Y., Arai K., Davis M. M. Structure, expression and divergence of T-cell receptor beta-chain variable regions. Nature. 1984 Nov 1;312(5989):40–46. doi: 10.1038/312040a0. [DOI] [PubMed] [Google Scholar]
- Pernis B., Chiappino G., Kelus A. S., Gell P. G. Cellular localization of immunoglobulins with different allotypic specificities in rabbit lymphoid tissues. J Exp Med. 1965 Nov 1;122(5):853–876. doi: 10.1084/jem.122.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitts T. H., Lefranc M. P., Stinson M. A., Sims J. E., Schroder J., Steinmetz M., Spurr N. L., Solomon E., Goodfellow P. N. The chromosomal location of T-cell receptor genes and a T cell rearranging gene: possible correlation with specific translocations in human T cell leukaemia. EMBO J. 1985 Jun;4(6):1461–1465. doi: 10.1002/j.1460-2075.1985.tb03803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitts T. H., Stinson A., Forster A., Foroni L., Luzzatto L., Catovsky D., Hammarström L., Smith C. I., Jones D., Karpas A. Heterogeneity of T-cell beta-chain gene rearrangements in human leukaemias and lymphomas. EMBO J. 1985 Sep;4(9):2217–2224. doi: 10.1002/j.1460-2075.1985.tb03917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Meuer S. C., Fitzgerald K. A., Hussey R. E., Hodgdon J. C., Acuto O., Schlossman S. F. Comparison of T3-associated 49- and 43-kilodalton cell surface molecules on individual human T-cell clones: evidence for peptide variability in T-cell receptor structures. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4104–4108. doi: 10.1073/pnas.80.13.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie K. A., Brinster R. L., Storb U. Allelic exclusion and control of endogenous immunoglobulin gene rearrangement in kappa transgenic mice. Nature. 1984 Dec 6;312(5994):517–520. doi: 10.1038/312517a0. [DOI] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. A third rearranged and expressed gene in a clone of cytotoxic T lymphocytes. Nature. 1984 Nov 1;312(5989):36–40. doi: 10.1038/312036a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim G. K., Yagüe J., Nelson J., Marrack P., Palmer E., Augustin A., Kappler J. Primary structure of human T-cell receptor alpha-chain. Nature. 1984 Dec 20;312(5996):771–775. doi: 10.1038/312771a0. [DOI] [PubMed] [Google Scholar]
- Siu G., Kronenberg M., Strauss E., Haars R., Mak T. W., Hood L. The structure, rearrangement and expression of D beta gene segments of the murine T-cell antigen receptor. 1984 Sep 27-Oct 3Nature. 311(5984):344–350. doi: 10.1038/311344a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Toyonaga B., Yanagi Y., Suciu-Foca N., Minden M., Mak T. W. Rearrangements of T-cell receptor gene YT35 in human DNA from thymic leukaemia T-cell lines and functional T-cell clones. 1984 Sep 27-Oct 3Nature. 311(5984):385–387. doi: 10.1038/311385a0. [DOI] [PubMed] [Google Scholar]
- Trowsdale J., Kelly A., Lee J., Carson S., Austin P., Travers P. Linkage map of two HLA-SB beta and two HLA-SB alpha-related genes: an intron in one of the SB beta genes contains a processed pseudogene. Cell. 1984 Aug;38(1):241–249. doi: 10.1016/0092-8674(84)90546-4. [DOI] [PubMed] [Google Scholar]
- Tunnacliffe A., Kefford R., Milstein C., Forster A., Rabbitts T. H. Sequence and evolution of the human T-cell antigen receptor beta-chain genes. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5068–5072. doi: 10.1073/pnas.82.15.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tykocinski M. L., Marche P. N., Max E. E., Kindt T. J. Rabbit class I MHC genes: cDNA clones define full-length transcripts of an expressed gene and a putative pseudogene. J Immunol. 1984 Oct;133(4):2261–2269. [PubMed] [Google Scholar]
- Weaver D., Costantini F., Imanishi-Kari T., Baltimore D. A transgenic immunoglobulin mu gene prevents rearrangement of endogenous genes. Cell. 1985 Aug;42(1):117–127. doi: 10.1016/s0092-8674(85)80107-0. [DOI] [PubMed] [Google Scholar]
- Weiler E. Differential activity of allelic gamma-globulin genes in antibody-producing cells. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1765–1772. doi: 10.1073/pnas.54.6.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winoto A., Mjolsness S., Hood L. Genomic organization of the genes encoding mouse T-cell receptor alpha-chain. 1985 Aug 29-Sep 4Nature. 316(6031):832–836. doi: 10.1038/316832a0. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Clark S. P., Taylor S., Sohn U., Wilson B. I., Minden M. D., Mak T. W. Organization and sequences of the variable, joining and constant region genes of the human T-cell receptor alpha-chain. 1985 Aug 29-Sep 4Nature. 316(6031):837–840. doi: 10.1038/316837a0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]