Abstract
Patent ductus arteriosus (PDA), one of the most common congenital heart defects, is an abnormal persistence of a patent lumen in the arterial duct due to an arrest of the natural process of closure after it has served its function as a vital channel in fetal circulation. The histological feature of the arterial duct is entirely different from its adjoining arteries and many intrinsic substances mediate in the process of its normal closure. When existing in isolation, catheter or surgical intervention is usually used for its treatment. Ductal aneurysm is a rare type of PDA. The PDA associated with other congenital heart disease has variable morphology and closing it naturally or by intervention may produce critical symptoms. The PDA and its ligament which represents a closed arterial duct can be part of a vascular ring with abnormal aortic arch formation. It is important to understand the morphological features of PDA so as to choose the optimal strategy for treatment.
MeSH: ductus arteriosus, patent, ligamentum arteriosus (arterial ligament), vascular ring
Introduction
Patent ductus arteriosus (PDA) is one of the most common congenital cardiovascular malformation which accounts for 5 to 10 % in all congenital heart disease.1 It can occur as an isolated anomaly or in association with other cardiac anomalies, like pulmonary atresia, coarctation of the aorta or hypoplastic left heart syndrome.
In the normal heart with a left aortic arch, the arterial duct arises from the left pulmonary artery very close to the bifurcation of the pulmonary trunk and connects to the transition area between aortic arch and descending aorta just distal to the origin of the left subclavian artery. In normal fetal development, the arterial duct is a crucial channel in the circulation conveying deoxygenated blood to the descending aorta.
Normally, the arterial duct closes after the baby is born. Physiologic closure usually occurs soon after birth and the duct is occluded anatomically within 3 months. Very occasionally it narrows and occludes prematurely during fetal life. In some patients, however, the duct fails to close after birth and its lumen remains widely patent.2 This condition is commonly diagnosed as PDA. It is very important for the treatment of PDA to understand the morphology and the closing process of the duct.
Embryology of the arterial duct
In the normal early embryonic stage, the arterial duct exists bilaterally on both right and left sides but the right duct becomes atrophied at around Carnegie stage sixteen (37 to 40 days post embryonic gestation) (Fig 1). The arterial duct is formed from the left sixth embryonic aortic arch from which the pulmonary artery also originates. Their histological compositions of the arterial walls are entirely different.3
Figure 1.
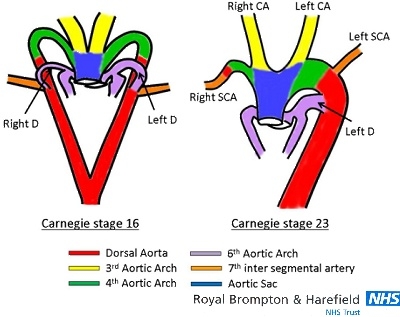
Embryonic development of the normal aortic arch. The arterial duct exists bilaterally on both right and left sides at Carnegie stage 16 (37-40 days of post embryonic gestation). After stage 16, the right-sided arterial duct is atrophied and the right dorsal aorta is obliterated at stage 18 (44-48 days). At the end of arch development (stage 23; 56-60 days) the arterial duct connects to the aortic arch just distal to the left subclavian artery. CA= carotid artery; D= ductus arteriosus; SCA= subclavian artery.
In normal embryogenesis, the duct connects the ipsilateral arch, usually the left-sided aortic arch that is derived from the embryonic fourth arch. The ductal insertion is at the inner curvature of the arch just distal to the ipsilateral left subclavian artery. The latter is derived from cephalad migration of the seventh intersegmental artery on the left side. When the arterial duct is contralateral to the aortic arch, it arises from the brachiocephalic artery or ipsilateral subclavian artery. Rarely, this abnormal origin of the duct can cause isolation of the subclavian artery or right pulmonary stenosis during normal closure of the duct if ductal tissue has extended into the adjoining vessels.4 The isolated right subclavian artery is then supplied retrogradely via the vertebral artery.
Histology and anatomical closure of the arterial duct
Histologically, the wall of the arterial duct is easily distinguishable from the fibro-elastic walls of the aorta and pulmonary trunk/artery (Fig. 2).5,6 While the great arteries have walls composed of elastic layers, the ductal wall is mainly muscular. The wall of the normal arterial duct is lined on its luminal aspect by an intimal layer of endothelial cells that overlies an internal elastic lamina. The elastic lamina is fragmented and sometimes split up into several layers. It is interrupted by intimal cushions that lie underneath it. The media of the ductal wall mainly consists of circularly arranged smooth muscle cells with minimal elastic fibres in between. The inner layer of the media has been described as having a longitudinal arrangement of the muscle bundles whereas the muscle bundles in the outer layer are circularly arranged (Fig 2). However, the methodical studies of von Hayek found the media to be composed of two spiral arrangements of smooth muscles that are in opposite directions.7 The outer spiral is more acute, giving the impression of circularly arranged smooth muscle fibres whereas the inner spiral is more gradual so the fibres appear longitudinal.
Figure 2.
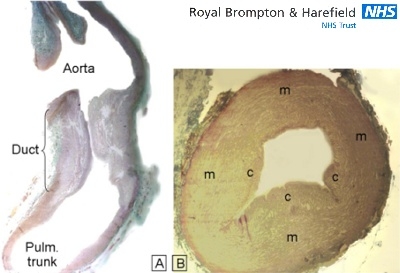
Histologic sections through two normal ducts from neonates. (A)This longitudinal section shows the distinctive appearance of the ductal wall composed mainly of smooth muscle compared to the walls of the aorta and pulmonary trunk which are composed primarily of elastic tissue. (B) This transverse section shows the intimal cushions (c) that protrude toward the ductal lumen. m=media. [trichrome stain]
The process of the closure of the arterial duct occurs in two steps.8 The first stage is due to constriction of the smooth muscle in the media of the ductal wall, which produces shortening and an increase in wall thickness. This change of the wall of the arterial duct develops significant ischemic hypoxia which leads to produce several protein factors, such as vascular endothelial growth factor, transforming growth factor and other inflammatory mediators. At the same time, the intimal cushions (or mounds) enhance the thickening of the ductal wall by proliferation of connective tissue and, later, increase in elastic tissue while smooth muscle fibres become less conspicuous. The cushions disrupt the internal elastic membrane and form swollen protrusions into the ductal lumen. Cushions along opposite walls gradually unite and finally obliterate the lumen. Macroscopically, the intimal cushions give the luminal surface of the ductal wall an irregular appearance whereas the walls of the adjoining great arteries are usually smooth. The first step is more evident at the pulmonary end than the aortic end which may remain open as an aortic ampulla after the remaining part of the duct has become occluded and ligamentous.
The second stage of the closure is due to proliferation of connective tissue in the intima and media. Atrophy of smooth muscle cells ultimately transforms the muscular vessel into a non-contractile ligament represented by a mass of dense elastic and fibrous tissue that occasionally contains a slit-like lumen. This process continues several weeks. The arterial duct is completely closed by 8 weeks of age in 88% of infants with a normal cardiovascular system.9
Mechanism of functional closure of the arterial duct
It is well known that oxygen tension and prostaglandins have significant effects on ductal closure. They act directly on the smooth muscle of the duct but in opposite directions with increasing oxygen tension constricting the duct and prostaglandins relaxing it. Reduction of prostaglandin synthesis by cyclooxgenase (COX) inhibitor causes ductal constriction in the fetus.10
Furthermore, there are various vasoactive substances (including; nitric oxide, bradykinin, endogenous catecholamines) which mediate ductal closure. Nitric oxide is a vasodilater which contributes to patency of the duct. In both animal and human studies, nitric oxide inhibitor has been shown to cause strong constriction of the duct with non-steroid anti-inflammatory drug (indomethacin).11
Morphology of PDA
Isolated PDA
The most common malformation is PDA occurring in isolation. Histologically, the internal elastic lamina in the PDA is generally intact and the intimal cushions are absent or are less well formed than normal (Fig. 3).6
Figure 3.
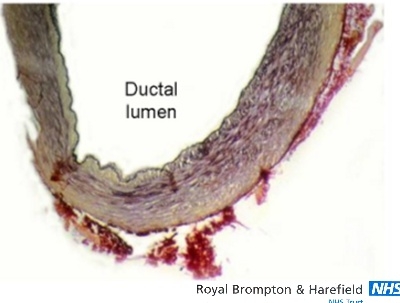
Histology image showing part of a persistently patent duct cut in cross-section. The internal elastic lamina is intact and appears as a black line lining the ductal lumen. Intimal cushions are barely formed, leaving a widely patent lumen. [elastic van Geison stain]
The PDA connects the upper descending aorta to the bifurcation of the main pulmonary artery (pulmonary trunk) close to the origin of the left pulmonary artery. Ductal anatomy varies in terms of its size (length and diameter), its shape, and its topography in relation to adjacent structures. In the fetus and the newborn the duct is wide and appears as a direct continuation of the pulmonary trunk. From the pulmonary bifurcation, it runs nearly parallel to the aortic arch and unites with the beginning of the descending aorta approximately 1cm distal to the origin of the left subclavian artery. It enters the descending aorta at an acute angle of 30° to 35° and overlaps its aortic entrance when viewed in antero-posterior projection. When it is large it bulges beyond the lateral wall of the aorta. In the fetus the pulmonary end of the duct is related to the left border of the sternum and the second left costal cartilage but it shifts lower into the second interspace in the first few months after birth. This corresponds posteriorly to the level of the sixth rib and the sixth intercostal space. From anterior to posterior the inferior surface of the duct is related to the left pulmonary artery, the recurrent laryngeal nerve, the left bronchus, and the left vagus nerve.
The usual size of the duct in the newborn varies from 7 to 11mm long and 4 to 5mm in diameter. However, extreme lengths or diameters can occur. The shape of the duct varies considerably. Morphologically, the duct may be tubular, funnel shaped, long and meandering, short or have no length at all (window duct), or is aneurysmal (Fig. 4, 5, 6). The classification of Krichenko and colleagues12 based on the appearance of the ductal lumen on angiography and the location of the ductal insertions relative to the air shadow of the trachea is a useful aid when considering interventional device closure.
Figure 4.
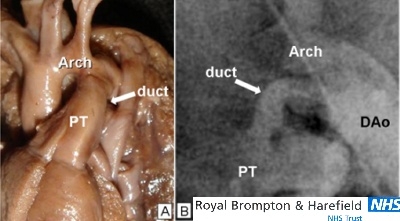
The tubular shaped duct. (A)This neonatal duct is widely patent. The pulmonary trunk, duct and descending aorta forms a continuous channel. (B) This angiogram of a tubular duct that is without a restrictive lesion to flow. This shape could be unsuitable for catheter occlusion by coils owing to the risk of coil embolization. (Arch= aortic arch; PT= pulmonary trunk; DAo= descending aorta)
Figure 5.
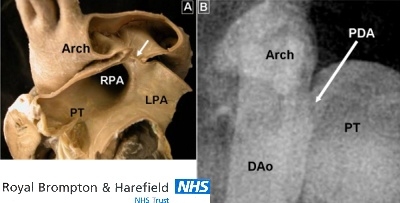
The short duct. (A)This PDA (arrow) is extremely short and resembles a window between the pulmonary bifurcation and the underside of the aortic arch. (B)This angiocardiogram shows a duct with a similar morphology. Arch= aortic arch; DAo= descending aorta; LPA= left pulmonary artery; PDA= patent arterial duct; PT= pulmonary trunk; RPA= right pulmonary artery
Figure 6.
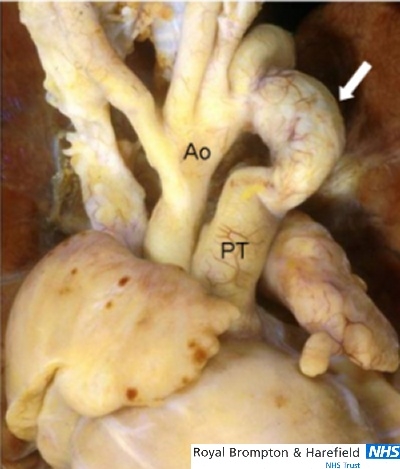
The aneurysmal duct. This specimen viewed from the front shows a long duct with an aneurysmal bulge (arrow).
Usually the PDA has a tubular shape or it may be funnel-shaped with a wide aortic orifice and a considerably narrower pulmonary end which is restrictive to flow (Fig. 7). The aortic ampulla is prominent. The narrower orifice is thought to represent the site of initial impetus for closure.
Figure 7.
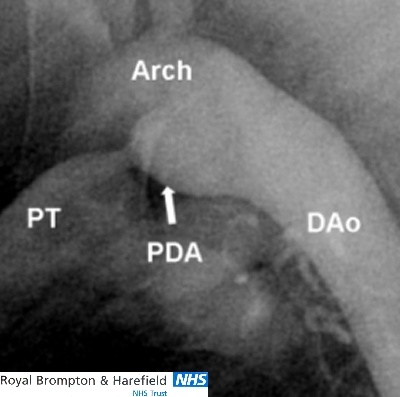
Angiographic image of a funnel-shaped duct. The PDA arises from the inner curvature of the upper descending aorta. The pulmonary end is very narrow and restrictive to flow. Arch= aortic arch; PDA= patent arterial duct; PT= pulmonary trunk, DAo= descending aorta
The short duct may be narrowed at its aortic end and also appear funnel-shaped.13 Occasionally, the duct may have no length and the opening resembles a window between the aorta and pulmonary artery (Fig 5). Coil embolization and reopening of the ductal channel is the most common complication when treating this shape of PDA using the transcatheter route.14 Video-Assisted Transscopic Surgery or conventional surgery, can be considered in this type.15 The window duct will likely require patch closure rather than ligation.
The tubular shaped duct has a uniform calibre or it may have a constriction in the middle giving its lumen an hour-glass appearance. In some cases, there are multiple constrictions within.
Ducts that are long and meandering or form right angles are rarely seen in isolation. They are more often associated with other congenital heart defects.
The aneurysmal arterial duct is rare type. Most cases result from partial closure of the aortic end after the pulmonary end has occluded, trapping blood to from a clot in the ductal lumen. (Fig. 6) Others are aneurysmal ducts with narrow openings at both ends suggesting the closure process was initiated at both ends but not within the duct itself. The probable complications of both types are dissection, rupture or thromboemboli. In case of aneurysmal dilatation, left vocal cord paralysis can occur by compression of the left laryngeal nerve.
PDA in association with other congenital heart disease
There are various morphological features of the arterial duct with other congenital heart anomalies. In hearts with severe right heart obstructive lesion like pulmonary atresia or stenosis patency of the duct is important for blood supply to the lungs. Generally, the duct is wider than normal but is narrower than the aorta. In cases with pulmonary atresia the duct may be long, considerably more tortuous, narrow, and may insert at right angles to the aorta (Fig. 8).16
Figure 8.
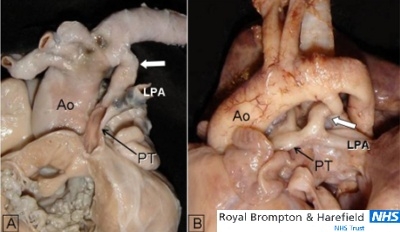
Two specimens of long and meandering (curly) duct indicated with white arrows. (A)This specimen viewed from the front has muscular atresia of the right ventricular outflow tract. (B)This specimen with critical valvar pulmonary stenosis and double outlet right ventricle is viewed from the left side. Ao= aorta; LPA= left pulmonary artery; PT= pulmonary trunk.
In the duct-dependent systemic circulation like hypoplastic left heart syndrome or interrupted aortic arch ductal constriction can cause critical symptoms in early infancy. Usually the duct is straight with a tubular shape and is significantly wider than the aorta (Fig. 9).17 Extensions of ductal tissue into the aortic lumen of hypoplastic left heart can be a substrate for coarctation lesion.
Figure 9.
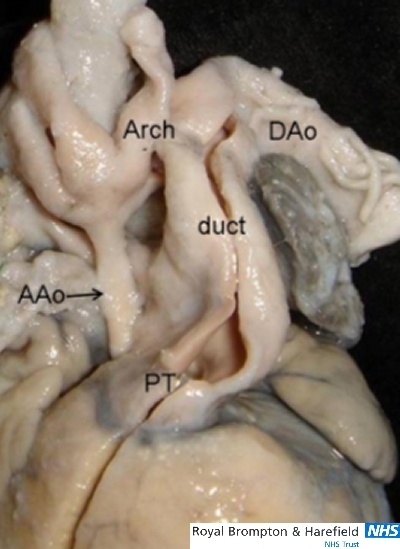
This specimen viewed from the front shows a widely patent arterial duct associated with aortic atresia in the setting of hypoplastic left heart syndrome. The aortic arch and coronary arteries are supplied retrogradely via the arterial duct. AAo= ascending aorta; Arch= aortic arch; DAo= descending aorta; PT= pulmonary trunk.
PDA with aortic arch malformation
PDA is most frequently found on the left side but it is occasionally found on the right side in association with a right aortic arch and mirror-imaged arrangement of the neck and arm arteries. In abnormal development of the embryonic aortic arch, the PDA, or its ligament, can contribute to the formation of a vascular ring that encircles the trachea and esophagus.
There are several patterns of vascular rings involving the arterial duct.18 The right aortic arch with retroesophageal component occasionally forms vascular ring when it is tethered to the left pulmonary artery by a left duct that arises from the upper part of the descending aorta. As part of the right aortic arch and retroesophageal right subclavian artery lie behind the oesophagus, the trachea and esophagus become surrounded by the ascending aorta, the pulmonary trunk and the left PDA (or ligament) (Fig 10).
Figure 10.
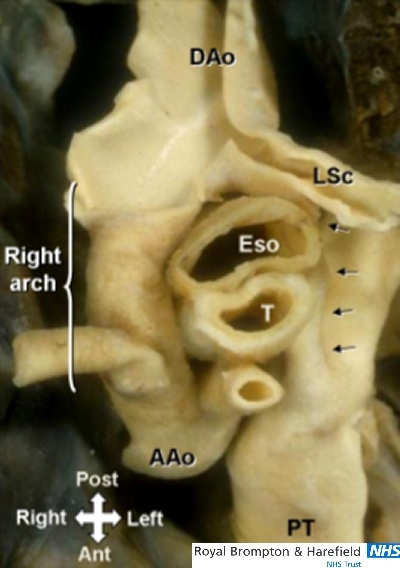
This ‘aerial’ view of a right aortic arch with left ductal ligament(arrows) shows the vascular ring around the trachea(T) and esophagus(Eso) pedicle. AAo= ascending aorta; LSc= left subclavian artery; PT= pulmonary trunk.
The left aortic arch with right descending aorta is a possible combination for vascular ring, but it is uncommon.19 In combination with right PDA or ligament the left aortic arch forms a vascular ring. A rare situation was reported in a patient with dextrocardia in whom the left-sided PDA caused tracheal compression.
Conclusions
PDA can show various shapes with or without associated congenital heart and vascular anomalies. Nowadays, there are several choices for treating PDA – pharmacologic, surgery or transcatheter intervention. Selection of treatment depends on the shape and size of the PDA, age of patient, and hemodynamic condition. Appreciation of the location and morphological features of PDA is necessary for selecting the optimal treatment option for the individual patient.
Acknowledgments
The Cardiac Morphology Unit receives funding support from the Royal Brompton and Harefield Hospital Charitable Fund
References
- 1.Mitchell SC, Korones SB, Berendes HW. Congenital heart disease in 56,109 births. Incidence and natural history. Circulation. 1971;43:323–332. doi: 10.1161/01.cir.43.3.323. [DOI] [PubMed] [Google Scholar]
- 2.Ettedgui JA, Sharland GK, Chita SK, Cook A, Fagg N, Allan LD. Absent pulmonary valve syndrome with ventricular septal defect: role of the arterial duct. Am J Cardiol. 1990;66:233–234. doi: 10.1016/0002-9149(90)90598-u. [DOI] [PubMed] [Google Scholar]
- 3.O’Rahilly R. Human embryology and teratology. Wiley-Lyss. 2001 [Google Scholar]
- 4.Becker AE, Becker MJ, Edwards JE. Congenital anatomic potentials for subclavian steal. Chest. 1971;60:4–13. doi: 10.1378/chest.60.1.4. [DOI] [PubMed] [Google Scholar]
- 5.Gittenberger-de Groot AC, Moulaert AJ, Harinck E, Becker AE. Histopathology of the ductus arteriosus after prostaglandin E1 administration in ductus dependent cardiac anomalies. Br Heart J. 1978;40:215–220. doi: 10.1136/hrt.40.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho SY, Anderson RH. Anatomical closure of the ductus arteriosus: a study in 35 specimens. J Anat. 1979;128:829–836. [PMC free article] [PubMed] [Google Scholar]
- 7.Hayek HV. der funktionelle Bau der Naberlarterien und des ductus Botalli. Z Anat Entwicklungsgesch. 1935;105:15. [Google Scholar]
- 8.Cassels D. The ductus arteriosus. Charles C Thomas. 1973:75–95. [Google Scholar]
- 9.Christie A. Normal closing time of the foramen ovale and the ductus arteriosus. Am J Dis Child. 1930;40:323. [Google Scholar]
- 10.Clyman RI. Ibuprofen and patent ductus arteriosus. N Engl J Med. 2000;343:728–730. doi: 10.1056/NEJM200009073431009. [DOI] [PubMed] [Google Scholar]
- 11.Hammerman C, Kaplan M. Comparative tolerability of pharmacological treatments for patent ductus arteriosus. Drug Saf. 2001;24:537–551. doi: 10.2165/00002018-200124070-00005. [DOI] [PubMed] [Google Scholar]
- 12.Krichenko A, Benson LN, Burrows P, Moes CA, McLaughlin P, Freedom RM. Angiographic classification of the isolated, persistently patent ductus arteriosus and implications for percutaneous catheter occlusion. Am J Cardiol. 1989;63:877–880. doi: 10.1016/0002-9149(89)90064-7. [DOI] [PubMed] [Google Scholar]
- 13.Grunenfelder J, Bartram U, Van Praagh R, et al. The large window ductus: a surgical trap. Ann Thorac Surg. 1998;65:1790–1791. doi: 10.1016/s0003-4975(98)00280-x. [DOI] [PubMed] [Google Scholar]
- 14.Daniels CJ, Cassidy SC, Teske DW, Wheller JJ, Allen HD. Reopening after successful coil occlusion for patent ductus arteriosus. J Am Coll Cardiol. 1998;31:444–450. doi: 10.1016/s0735-1097(97)00491-9. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs JP, Giroud JM, Quintessenza JA, et al. The modern approach to patent ductus arteriosus treatment: complementary roles of video-assisted thoracoscopic surgery and interventional cardiology coil occlusion. Ann Thorac Surg. 2003;76:1421–1427. doi: 10.1016/s0003-4975(03)01035-x. discussion 1427-1428. [DOI] [PubMed] [Google Scholar]
- 16.Elzenga NJ, Gittenberger-de Groot AC. The ductus arteriosus and stenoses of the pulmonary arteries in pulmonary atresia. Int J Cardiol. 1986;11:195–208. doi: 10.1016/0167-5273(86)90179-8. [DOI] [PubMed] [Google Scholar]
- 17.Boucek MM, Mashburn C, Kunz E, Chan KC. Ductal anatomy: a determinant of successful stenting in hypoplastic left heart syndrome. Pediatr Cardiol. 2005;26:200–205. doi: 10.1007/s00246-004-0965-1. [DOI] [PubMed] [Google Scholar]
- 18.Edwards J. Anomalies of the derivatives of the aortic arch system. Med Clin North Am. 1948;32:925–949. doi: 10.1016/s0025-7125(16)35662-0. [DOI] [PubMed] [Google Scholar]
- 19.Berman W, Jr, Yabek SM, Dillon T, Neal JF, Akl B, Burstein J. Vascular ring due to left aortic arch and right descending aorta. Circulation. 1981;63:458–460. doi: 10.1161/01.cir.63.2.458. [DOI] [PubMed] [Google Scholar]
- 20.Scherer D, Westcott JL. Dextrocardia, left aortic arch, and tracheal compression.An unusual type of vascular ring. Radiology. 1972;103:383–384. doi: 10.1148/103.2.383. [DOI] [PubMed] [Google Scholar]


