Abstract
Context:
Insulin resistance is associated with inflammation, fibrosis, and hypoxia in adipose tissue.
Objective:
This study was intended to better characterize the extracellular matrix (ECM) and vascularity of insulin-resistant adipose tissue.
Design:
Adipose expression of collagens, elastin, and angiogenic factors was assessed using real-time RT-PCR and immunohistochemistry (IHC) in abdominal sc adipose tissue. Adipocyte-macrophage coculture experiments examined the effects of polarized macrophages on adipose ECM gene expression, and the effects of collagens were measured in an angiogenesis assay.
Participants and Setting:
A total of 74 nondiabetic subjects participated at a University Clinical Research Center.
Interventions:
Interventions included baseline adipose biopsy and measurement of insulin sensitivity.
Main Outcome Measures:
Outcome measures included characterization of vascularity and ECM in adipose tissue.
Results:
CD31 (an endothelial marker) mRNA showed no significant correlation with body mass index or insulin sensitivity. In a subgroup of 17 subjects (nine obese, eight lean), CD31-positive capillary number in obese was decreased by 58%, whereas larger vessels were increased by 70%, accounting for the lack of change in CD31 expression with obesity. Using IHC, obese (compared with lean) subjects had decreased elastin and increased collagen V expression, and adipocytes cocultured with M2 macrophages had reduced elastin and increased collagen V expression. In obese subjects, collagen V was colocalized with large blood vessels, and the addition of collagen V to an angiogenesis assay inhibited endothelial budding.
Conclusions:
The adipose tissue from obese/insulin-resistant subjects has fewer capillaries and more large vessels as compared with lean subjects. The ECM of adipose tissue may play an important role in regulating the expandability as well as angiogenesis of adipose tissue.
The development of obesity is associated with insulin resistance and chronic inflammation (1). There is considerable variability in the relationship between obesity and insulin resistance, with some individuals developing features of metabolic syndrome with only small degrees of weight gain (2).
Much attention has been focused on the adipose tissue changes in inflammation and extracellular matrix (ECM) that occurs with obesity (3, 4). Expansion of fat mass occurs mainly through cellular hypertrophic mechanisms leading to an increase in adipocyte size. With this expansion, considerable tissue remodeling is required, involving stromal cells, preadipocytes, immune cells, and endothelial cells. The adipose tissue from obese, insulin-resistant rodents and humans is characterized by an increase in inflammatory macrophages and fibrosis and increases in components of the ECM, including collagen VI and thrombospondin (5–7).
One prevailing hypothesis surrounding the development of adipose tissue inflammation predicts that adipocyte enlargement results in failed microvasculature expansion, with subsequent hypoxia, adipocyte necrosis, infiltration of macrophages, and a cycle of inflammatory changes, adipokine secretion, and fibrosis (8–10). In support of the hypoxia hypothesis, several studies demonstrated decreased tissue perfusion and decreased CD31-positive labeled capillaries in obese tissue in mice and humans (9, 11, 12). However, some studies suggest that the obese adipose environment has proangiogenic features. One study found little change in adipose blood flow in obese Zucker rats and an increase in blood flow in the GK diabetic rat (13). In another study, mice fed a high-fat diet developed an increased number of newly created blood vessels in sc fat (14).
Another hypothesis associated with the development of adipose hypoxia concerns adipose tissue stiffness. If the ECM surrounding adipocytes does not permit adequate expansion, then adipocytes may be more susceptible to necrosis, and angiogenesis may be impaired (15, 16). Previous studies have demonstrated increased expression of some ECM proteins, including collagen VI and thrombospondin, with obesity and insulin resistance (6, 7, 17). In addition to generating stiffness, however, the ECM can either promote or inhibit angiogenesis. Collagen IV, the major basement membrane protein of blood vessels, inhibits the initial sprouting of vessels during angiogenesis, and many other ECM components play a role in this process (18). For angiogenesis to proceed, constant remodeling of the ECM through proteolytic degradation of ECM components by matrix metalloproteinases (MMP) is important, although the roles of MMP are complex, and trials aimed at targeting MMP to inhibit angiogenesis for tumor growth suppression have been disappointing (19).
In this study, we examined the vascular structures and ECM properties of adipose tissue from insulin-sensitive and insulin-resistant subjects. In insulin-resistant subjects, we observed a decrease in capillary density but an increase in larger blood vessels. A number of ECM components were different in insulin-resistant subjects, including a decrease in elastin and an increase in collagen V. These changes would be predicted to reduce compliance of adipose tissue and inhibit angiogenesis.
Subjects and Methods
Human subjects
Subcutaneous abdominal adipose tissue from 74 subjects were analyzed for this study. The participants signed consent forms approved by the Institutional Review Board from either the University of Arkansas for Medical Sciences or the University of Kentucky, and some of these subjects' samples were analyzed as part of previous studies. No participants had any significant medical history, none were diabetic, and none were taking medications likely to change adipocyte metabolism. All participants underwent an abdominal adipose biopsy through a 3-cm incision, without suction (depth 1–2 cm), and a measurement of insulin sensitivity using the frequently sampled iv glucose tolerance test, as described previously (6, 20). Of the 74 participants, five had either impaired glucose tolerance or impaired fasting glucose, and 64 were women. The mean body mass index (BMI) was 30.1 (range 19–40) kg/m2 and the mean insulin sensitivity index (SI) was 3.79 (range, 0.97–15.2) × 10−4 min−1/μU/ml). For histochemistry and immunohistochemical analysis, a subgroup of the above 74 subjects was studied. This subgroup was representative of the larger group in age and gender and included eight lean subjects [mean BMI = 24 (range 20–28) kg/m2; mean SI = 6.2 (range 2.67–13.6) × 10−4 min−1/μU/ml] and nine obese subjects [mean BMI = 36 (range 32–40) kg/m2; mean SI = 1.8 (range 0.97–2.93) × 10−4 min−1/μU/ml]. Both groups included two males, and there was no significant difference in age. In addition to the differences between groups in BMI and SI, the obese group had higher fasting triglycerides (obese 1.81 mmol/liter, lean 0.80 mmol/liter, P < 0.05) and a nonsignificant trend toward a higher fasting glucose (obese 5.0 mmol/liter, lean 4.7 mmol/liter, P value not significant) and lower high-density lipoprotein (obese 1.3 mmol/liter, lean 1.6 mmol/liter, P value not significant). Four of the obese subjects had impaired glucose tolerance.
RNA isolation and real-time RT-PCR
RNA was isolated from tissue and cells using RNAeasy Lipid Tissue Mini (QIAGEN, Valencia, CA), and RNAqueous phenol-free total RNA kit (Ambion, Austin, TX), respectively. Real-time RT-PCR was performed as described previously (21), using 18S RNA as a standard. Pooled cDNA from the samples being assayed were used to construct standard curves. Hence the data accurately compare samples within each assay and are expressed as arbitrary units. The primer sequences are listed in Supplemental Table 1 (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Histochemistry
Adipose tissue samples were placed in Bouin's fixative, paraffin embedded, and subsequently cut into 5-μm-thick sections and processed as described previously (6).
To detect elastin, slides were placed in a solution containing hematoxylin, ferric chloride, and Weigert's iodine. For collagen, slides were stained pink with Van Gieson solution. For quantification, sections were photographed using a Nikon Eclipse 55i microscope fitted with a DSRiL digitial camera. Each section was photographed using the ×4 objective, and the field of view was moved to the right, retaining 20% of the first field until the entire section was photographed. The NIS-Elements software stitching algorithm was used to produce a single large composite picture. Images were quantified using NIH ImageJ. The background was subtracted from each image, the image threshold was set to identify the stained areas, and the area of stain to total area was calculated and expressed as percentage of total area.
Immunohistochemistry
Paraffin sections were prepared as described previously (6). After blocking with 2% horse serum, primary collagen antibodies were incubated at a dilution of 1:50 for 1 h and then rinsed and incubated with peroxidase-conjugated secondary antibody contained in Vector Laboratories (Burlingame, CA) Immpress kits. Collagen V (Santa Cruz Biotechnology, Santa Cruz, CA; sc20648) was detected with a rabbit polyclonal antibody to COL5A1 (collagen α 1 type V) protein. Additional collagens detected were collagen I (Santa Cruz Biotechnology; sc59772), collagen II (Santa Cruz Biotechnology; 59958), collagen III (Santa Cruz Biotechnology; sc28888), and collagen IV (Santa Cruz Biotechnology; sc59814). For fluorescent images, Alexa Fluor-conjugated secondary antibodies were used. NIH ImageJ was used to quantify the area of collagen for each section.
An antibody to the endothelial cell marker CD31 (22) (Santa Cruz Biotechnology; SC1506) was used to label blood vessels. Similar staining was obtained using antibodies to von Willebrand factor and to lectin-tetramethylrhodamine isothiocyanate (data not shown) (9). To differentiate between capillaries and small vessels, double staining was performed with CD31 and anti-α smooth muscle actin (ASMA) (Santa Cruz Biotechnology; SC130616). Alexa Flour-conjugated secondary antibodies were used for visualization. Cellular structures positive for CD31 only were counted as capillaries. CD31-stained structures exhibiting ASMA staining were counted as a blood vessel. The number of capillaries or vessels were normalized both to the number of adipocytes and to the area being measured and were counted in fibrotic and nonfibrotic areas. A nonfibrotic location contained adipocytes and was at least three adipocyte cell diameters from fibrosis visualized by collagen staining, and a fibrotic area was defined as vessels within fibrotic material or within three adipocyte cell diameters of fibrosis.
Angiogenesis assay
An endothelial tube formation assay (CBA-200; Cell Biolabs, San Diego, CA) was used to determine whether collagens affected angiogenesis. ECM gel prepared from Engelbreth-Holm-Swarm (EHS) tumor cells was plated into 96-well plates and incubated at 37 C to allow a three-dimensional gel matrix to form. Human umbilical vein endothelial cells (HUVEC((Invitrogen, Carlsbad, CA; C00325PA) were plated at a density of 50,000 per well. Collagen I (Sigma Chemical Co., St. Louis, MO; C7774-5MG, human placenta) and collagen V (Sigma; C3657-5MG, human placenta) were suspended in 1% acetic acid and added in soluble form 30 min after HUVEC were plated. The number of branch points in each well was scored and normalized to total area.
Tissue culture with adipocytes and polarized macrophages
Polarized THP-1 cells, induced to differentiate into M1, M2a, and M2c macrophages, were cocultured with human adipocytes derived from stem cells (23), as described previously (6). After 24 h coculture, the wells (macrophages) and the inserts (adipocytes) were separated, and RNA was extracted.
Statistical analysis
All data from samples were expressed as mean ± sem. Student's two-sample t tests were used to compare groups with respect to continuous variables. Pearson's correlation coefficients were used to describe the linear association between variables. SI was not normally distributed and therefore was analyzed on a log scale.
Results
Adipose tissue vascularity
Previous papers have suggested that the adipose tissue of obese rodents and humans is hypoxic, demonstrating decreased capillary density (9, 11, 24). We examined gene expression of CD31 in adipose tissue from nondiabetic subjects covering a range of BMI and SI, expecting to see a decrease in expression in obese, insulin-resistant subjects due to a decrease in capillaries. However, we found that CD31 was not associated with BMI or SI, and VEGF mRNA was only weakly associated with BMI (Supplemental Table 2).
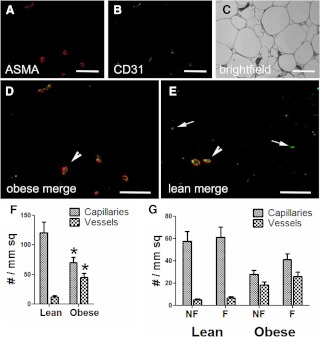
To better examine adipose tissue vascularity, we used immunofluorescence to assess the number, location, and characteristics of blood vessels in adipose tissue from eight lean, insulin-sensitive subjects and eight obese insulin-resistant subjects, and representative images are shown in Fig. 1. Larger blood vessels can be distinguished from capillaries based on the presence or absence of the ring of ASMA surrounding the endothelial staining (stained with CD31). The number of capillaries and larger vessels were counted and normalized to either adipocyte number or tissue area (Fig. 1F). When compared with lean subjects, obese subjects demonstrated decreased numbers of capillaries and increased numbers of larger vessels. This pattern was similar regardless of whether the data were expressed per adipocyte or total area. Adipose fibrosis plays an important role in insulin resistance, and we analyzed capillaries and large vessels relative to fibrosis, as described in Subjects and Methods. In obese subjects, both fibrotic and nonfibrotic areas contained approximately equal numbers of large vessels and capillaries (Fig. 1G). Lean subjects have much less fibrosis, but even the fibrotic areas contained mostly capillaries.
Fig. 1.
Identification of blood vessels in lean and obese adipose tissue. Obese (A–D) and lean (E) adipose tissue samples were immunoreacted with antibodies to CD31 (B, green) to identify endothelial cells and ASMA (A, red) to delineate the vessel wall, overlaid in D. C, The bright-field overlay of the same image. In the merged images from a representative obese (D) and lean (E) subject, larger blood vessels (arrowheads) are recognizable because they accumulate both ASMA and CD31. Capillaries are small and display only endothelial cell staining (arrows). Magnification, ×200; bar, 125 μm. F, Images from obese and lean subjects were scored for the number of capillaries and larger vessels, as described in Subjects and Methods, and normalized to cross-sectional area. Vessels are defined as structures that stained with CD31 and ASMA, and capillaries stained for only CD31. G, The areas containing the capillaries and vessels were categorized as fibrotic and nonfibrotic. *, P < 0.05 vs. lean.
ECM in adipose tissue
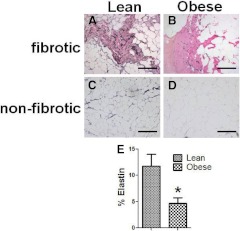
The difference in the relative abundance of large vessels and capillaries between lean and obese individuals could be due to changes in the ECM of the adipose tissue, which can affect endothelial cell sprouting and vessel extension. One ECM component that was differentially expressed between obese and lean subjects was elastin. As shown in Fig. 2, elastin was found to be abundant in lean subjects' adipose tissue, especially in the fibrotic areas, although some elastin was also found associated with adipocytes in the nonfibrotic areas. In obese subjects, however, the amount of elastin protein was lower, and the elastin fibrils were not as long and filamentous as in lean subjects. Quantification of elastin staining showed that adipose tissue from obese individuals had less than 6% total elastin content (normalized to total area) compared with approximately 12% in lean individuals (Fig. 2E). In addition, the elastin protein level was significantly associated with insulin sensitivity (SI: r = 0.49; n = 17; P < 0.05). Despite this significant relationship between elastin protein, however, there was no significant relationship between elastin mRNA levels and either BMI or SI (Supplemental Table 2).
Fig. 2.
Elastin expression in adipose tissue. Adipose elastin was visualized with a histochemical stain, as described in Subjects and Methods. Representative fibrotic (A) and nonfibrotic (C) areas of a lean subject are compared with fibrotic (B) and nonfibrotic (D) areas from an obese subject. Elastin was present in fibrotic areas and to a lesser extent in nonfibrotic areas of lean subjects. Obese subjects exhibited much less elastin staining. Magnification, ×200; bar, 125 μm. E, The amount of elastin was quantified as described in Subjects and Methods, and total elastin in lean and obese subjects is shown, expressed as a percentage of total area. *, P < 0.05.
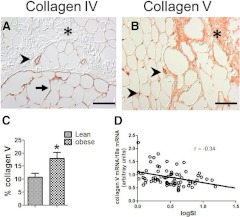
Adipose collagen VI is increased in obese subjects (6, 7), and the subjects in this study also demonstrated significant correlations between collagen VI and obesity (Supplemental Table 2). Collagens I, III, IV, VII, VIII, and X were all expressed by adipose tissue and by adipocytes in culture, whereas collagens II and IX were expressed at very low levels. Although there were no significant differences between lean and obese subjects in the expression of these collagens (data not shown), collagen V was differentially expressed at both the RNA and protein levels. Figure 3B shows a representative image of collagen V expression in adipose tissue from an obese individual. Collagen V was abundant in fibrotic areas as well as in large blood vessels; collagen V preferentially accumulated in obese subjects (Fig. 3C), and there was a significant inverse relationship between collagen V protein and SI (r = −0.52; n = 17; P < 0.05). Figure 3A shows collagen IV staining for comparison. Collagen IV is a component of basement membranes and in adipose tissue was noted to encircle both adipocytes and the endothelial cell layer of vessels. Collagen IV was not present in fibrotic areas and was not altered with obesity. Using RNA isolated from adipose tissue of 74 subjects, covering a wide range of BMI and insulin sensitivity, we found a significant inverse relationship between collagen V expression and SI, suggesting insulin-resistant subjects have higher levels of collagen V (Fig. 3D and Supplemental Table 2).
Fig. 3.
Collagen V localizes to fibrotic areas and large vessels. The immunohistochemical staining pattern of collagens IV (A) and V (B) are shown. Collagen IV, a basement membrane protein, was most abundant in blood vessels (arrowheads) and surrounding adipocytes (arrow) and was seldom found in fibrotic areas (asterisk) within the adipose tissue section. In contrast, collagen V was very abundant in fibrotic areas (asterisk) and surrounding blood vessels (arrowheads). Magnification, ×200; bar, 125 μm. C, Quantification of collagen V staining in lean and obese (BMI > 27 kg/m2) subjects. D, Relationship between collagen V mRNA abundance and SI. *, P < 0.05.
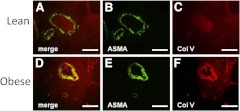
To better define the relationship between collagen V, blood vessels, and insulin resistance, we used immunofluorescence to label both ASMA and collagen V, and the typical staining patterns for collagen V in lean and obese subjects are shown in Fig. 4. The large blood vessels in lean subjects expressed low levels of collagen V, with only a very thin band of collagen V staining just below the endothelial basement membrane and also within the ECM surrounding the vessel. However, a thick band of collagen V was localized to the large vessels of obese subjects and extended from the endothelial basement membrane to the outer vessel wall. This thick band of collagen V staining was present in 80% of large blood vessels in obese adipose tissue.
Fig. 4.
Collagen V localization in large vessels in obese subjects. Adipose tissue sections from lean (A–C) and obese (D–F) subjects were immunoreacted with antibodies against ASMA (B and E, green) and collagen V (C and F, red) to localize collagen V to blood vessels in merged images (A and D). Vessels from lean subjects exhibited very little collagen V accumulation. Vessels from obese subjects contained significantly more collagen V. In most vessels in obese subjects, collagen V could be seen to extend from the endothelial basement membrane to the outer edges of the vessel. Magnification, ×200; bar, 125 μm.
In vitro analysis of ECM expression
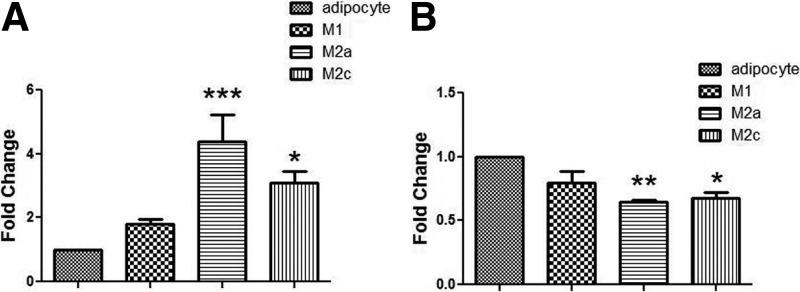
To understand the interactions between adipocytes and macrophages in the expression of both elastin and collagen V, we used an in vitro coculture system involving human adipocytes and polarized M1, M2a, and M2c macrophages, as described in Subjects and Methods. Macrophages expressed very low levels of elastin and collagen V (data not shown), and the gene expression profiles in adipocytes are shown in Fig. 5. Collagen V was expressed in human adipocytes, and coculture with M2a and M2c macrophages resulted in an increase in collagen V expression (Fig. 5A). Coculture with M2a and M2c macrophages resulted in decreased elastin expression by adipocytes (Fig. 5B). Because most of the macrophages in obese human adipose tissue are M2 macrophages (6), these experiments suggest that the chronic low-grade inflammatory environment comprised of M2 macrophages affects the expression of ECM components by adipocytes.
Fig. 5.
Macrophages alter elastin and collagen V gene expression in adipocytes. As described in Subjects and Methods, a coculture system was used to determine the effects of macrophage coculture on adipocyte ECM expression. THP-1 monocytes were differentiated into M1, M2a, or M2c polarized macrophages and cocultured with mature adipocytes for 24 h. Gene expression of collagen V (A) and elastin (B) was quantified by real-time RT-PCR in adipocytes alone or after culture with the polarized macrophages, as indicated. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. adipocytes alone.
ECM effects on angiogenesis
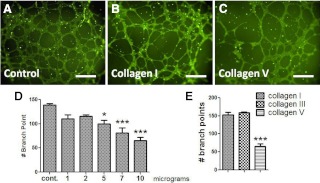
Previous studies have suggested that collagen V may disrupt endothelial adherence to vascular structures (25). We hypothesized that elevated collagen V surrounding adipose vessels may contribute to decreased angiogenesis and examined the effects of collagen V on endothelial proliferation in an in vitro angiogenesis assay. HUVEC were layered onto a proangiogenic ECM in the presence or absence of collagens I, III, and V in the medium, and the number of endothelial branch points were counted as an indicator of angiogenesis. As shown in Fig. 6, the addition of collagen I or III had no effect on angiogenesis. However, the addition of increasing concentrations of collagen V led to a dose-dependent decrease in angiogenesis. These data suggest that the adipocyte production of collagen V may contribute to the decrease in vascularity of adipose tissue.
Fig. 6.
Collagen V inhibits angiogenesis. HUVEC were layered onto an ECM, as described in Subjects and Methods, and medium was then added in the presence of increasing concentrations of the respective collagens. A–C, Micrographs show the extent of endothelial cell branching after 10 h incubation alone (A) or with the addition of collagen I (B) and collagen V (C) at 10 μg/ml. Magnification, ×200; bar, 125 μm. D, Quantification of the branch points from the addition of increasing concentrations of collagen V. E, Collagen I, III, and V were added to the ECM at a concentration of 10 μg/ml, followed by incubation for 10 h and counting of branch points. *, P < 0.05 vs. control; ***, P < 0.01 vs. control.
Discussion
Many studies have highlighted the changes in adipose tissue that occur with obesity and insulin resistance, which include macrophage infiltration, adipokine expression, altered expression of ECM proteins, and evidence for hypoxia (10, 16, 26). However, the precise sequence of events and the relative predominance of any one process are unclear. The use of rodent models of insulin resistance or obesity has identified many of these changes in adipose tissue. For example, adipose tissue inflammation and macrophage infiltration can be reduced through genomic deletion of the TNFα receptor or MCP1 (monocyte chemotactic protein-1) (or its receptor) (27–29). Alteration of the ECM through the deletion of collagen VI results in adipose tissue that can expand without the development of inflammation (15). Studies in humans have confirmed many of these findings but have also highlighted important differences. The adipose tissue of insulin-resistant humans accumulates macrophages and expresses inflammatory cytokines, and increased collagen VI and other ECM components have been described (6, 7, 30). However, mice fed a high-fat diet develop a large number of crown-like structures containing mostly M1 macrophages, and there is an M2 to M1 phenotypic change that occurs with obesity and reversed with exercise or peroxisome proliferator-activated receptor-γ agonist treatment (31–33). However, human adipose tissue contains relatively few crown-like structures, and the macrophages are more M2 in their characteristics (6, 34) and are strongly associated with fibrosis (6).
This study examined the expression of a number of different genes in sc adipose tissue related to hypoxia, angiogenesis, and ECM in subjects covering a range of BMI and SI. This study did not examine visceral adipose tissue, which is more highly correlated with insulin resistance. Although there was a positive correlation between BMI and VEGF gene expression, there was no significant relationship between CD31 or HIF1α (hypoxia-inducible factor) and either BMI or SI. Previous studies have described evidence for hypoxia in rodent models of obesity, and a human study found a lower mean pO2 in obese (compared with lean) subjects (9). Although the changes in human adipose tissue are likely not as extreme as found in rodents, it is possible that more evidence of hypoxia would have been observed if extremely obese or diabetic subjects were studied.
Although the expression of markers of vascularity were not correlated well with obesity, immunohistochemical analysis of vascular organization identified significant differences between lean and obese subjects. When compared with lean, insulin-sensitive subjects, obese subjects demonstrated fewer capillaries but a greater number of larger blood vessels. The increase in larger vessels in the obese subjects would likely explain the lack of a difference in CD31 expression but would be consistent with a trend toward hypoxia, because large vessels have a greater diffusion barrier and would likely not deliver O2 as well as capillaries. Angiogenesis is a normal component of the wound-healing process and is closely associated with inflammation. These data would suggest that the angiogenic process is disordered in obese subjects, such that larger vessels and fewer capillaries are formed. This process occurs not only in fibrotic areas but also in nonfibrotic areas, suggesting that these large vessels not only are associated with scars but also may be related to changes in the ECM throughout the tissue.
It is hard to separate obesity from insulin resistance, and this study was limited by the study of subjects who differed in both BMI and SI. However, a recent study performed arrays on nondiabetic, BMI-matched subjects at the extremes of insulin sensitivity and found many categories of altered gene expression, including genes involved in angiogenesis and ECM (35). Thus, this study is consistent with other studies that have examined adipose tissue changes with the development of insulin resistance (36).
Several previous studies have noted increased fibrosis and increased collagen VI in human adipose tissue (6, 7, 17). To better characterize other ECM proteins, we examined the expression of other collagens and elastin. Although collagens II and IX were expressed only at very low levels, other collagens were present. Because ECM proteins in tissues may have a very slow turnover, regulation at the level of expression may not necessarily be an accurate reflection of ECM protein regulation. Therefore, we used histochemical methods to examine lean and obese adipose tissues for ECM components. As shown in Fig. 2, major differences in elastin accumulation were found. In lean subjects, staining of long strands was observed throughout the adipose tissue, even in nonfibrotic areas, whereas there was little elastin staining in obese subjects, and the staining that was present included mostly short strands. There was no association between elastin mRNA levels and obesity, suggesting that much posttranscriptional processing takes place, or perhaps elastin is targeted by proteases during the process of adipose remodeling (37, 38).
With weight gain, adipocytes expand considerably, and previous studies have suggested that the flexibility of the ECM permits a good expansion of adipose tissue in a manner that would favor angiogenesis and lead to obesity with limited metabolic consequences (10). The knockout of collagen VI resulted in a mouse that could become obese with less adipose inflammation and less insulin resistance (15). To our knowledge, this is the first report of abnormalities in elastin as a result of obesity or insulin resistance. Because of the role of elastin in allowing flexibility and stretch, this deficiency of elastin in the adipose tissue of insulin-resistant subjects may be important in causing the impairment of angiogenesis and increased inflammation.
Another adipose ECM protein that differed between lean and obese subjects was collagen V. A recent microarray study suggested that collagen V was increased in obese humans (39). Among our subjects, there was no significant association between collagen V expression and BMI, but there was a significant inverse association with SI. Although this relationship was not strong, the location of collagen V was very different based on immunohistochemical analyses. Collagen V (but not collagen IV) was present in the fibrotic areas of adipose tissue, even in the fibrotic areas in lean subjects, comparable to collagen VI (6). Obesity specifically altered organization of collagen V associated with the vasculature. A thin rim of collagen V was observed as a component of the vessel wall of larger vessels in lean subjects, but in obese subjects, considerably more collagen V was localized in the vessel wall. Using in vitro coculture of adipocytes and macrophages, we demonstrated expression of collagen V by adipocytes and a significant up-regulation when cocultured with M2a and M2c macrophages. As described by us previously, M2c macrophages express high levels of TGFβ and are highly prevalent in the adipose tissue of obese subjects (6).
Fibrosis and the deposition of ECM can affect the angiogenic properties of tissues. Once endothelial cells begin sprouting, a number of ECM proteins must be degraded to allow vessel extension. Some ECM proteins, such as collagen IV, must be secreted to stabilize the vessel sprout (40). The strong association of collagen V with larger blood vessels and in fibrotic areas is of interest because of the known association of collagen V with vascular function. Previous studies have suggested that collagen V may inhibit endothelial cell adherence to the basement membrane substrate and impair endothelial migration (25, 41), and collagen V is known to have a domain that binds thrombospondin, which is also antiangiogenic (42, 43). Furthermore, there is a known association between collagen V and scleroderma, which is characterized by intense fibrosis and microvascular occlusion (44). Therefore, the increase vascular collagen V in obese adipose tissue may impair angiogenesis. To examine the effects of collagen V on angiogenesis, collagen V was added to an in vitro angiogenesis assay. Compared with collagen I and collagen III, collagen V significantly inhibited endothelial budding into vessels in a dose-dependent fashion.
In summary, these studies identified a number of novel findings in the adipose tissue of obese, insulin-resistant subjects, including a simultaneous increase in large blood vessels and decrease in capillaries, a decrease in elastin, and an increase in collagen V localization around large vessels. Based on the properties of these structural proteins, these changes in ECM components would be expected to make adipose tissue more stiff and less accommodating for adipocyte expansion and capillary proliferation, all of which would promote insulin resistance.
Supplementary Material
Acknowledgments
We thank Regina Dennis at the University of Arkansas for Medical Sciences and Stacy BeBout at the University of Kentucky for their assistance with subject recruitment.
This work was supported by Grants DK80327 (to P.A.K.), DK71349 (to C.A.P. and P.A.K.), and AG20941 (to C.A.P.); M01RR14288 of the General Clinical Research Center; CTSA Grants 1UL1RR033173 and 1UL1RR029884; a Merit Review Grant from the Veterans Administration (to N.R.); and P20RR021954.
Disclosure Summary: The authors have no conflicts to disclose.
Footnotes
- ASMA
- Anti-α smooth muscle actin
- BMI
- body mass index
- ECM
- extracellular matrix
- HUVEC
- human umbilical vein endothelial cells
- MMP
- matrix metalloproteinase
- SI
- insulin sensitivity index.
References
- 1. de Luca C, Olefsky JM. 2008. Inflammation and insulin resistance. FEBS Lett 582:97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP. 1993. Quantification of the relationship between insulin sensitivity and β-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 42:1663–1672 [DOI] [PubMed] [Google Scholar]
- 3. Lee MJ, Wu Y, Fried SK. 2010. Adipose tissue remodeling in pathophysiology of obesity. Curr Opin Clin Nutr Metab Care 13:371–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Suganami T, Ogawa Y. 2010. Adipose tissue macrophages: their role in adipose tissue remodeling. J Leukoc Biol 88:33–39 [DOI] [PubMed] [Google Scholar]
- 5. Varma V, Yao-Borengasser A, Bodles AM, Rasouli N, Phanavanh B, Nolen GT, Kern EM, Nagarajan R, Spencer HJ, 3rd, Lee MJ, Fried SK, McGehee RE, Jr, Peterson CA, Kern PA. 2008. Thrombospondin-1 is an adipokine associated with obesity, adipose inflammation, and insulin resistance. Diabetes 57:432–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spencer M, Yao-Borengasser A, Unal R, Rasouli N, Gurley CM, Zhu B, Peterson CA, Kern PA. 2010. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am J Physiol Endocrinol Metab 299:E1016–E1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pasarica M, Gowronska-Kozak B, Burk D, Remedios I, Hymel D, Gimble J, Ravussin E, Bray GA, Smith SR. 2009. Adipose tissue collagen VI in obesity. J Clin Endocrinol Metab 94:5155–5162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trayhurn P, Wood IS. 2004. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 92:347–355 [DOI] [PubMed] [Google Scholar]
- 9. Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. 2009. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 58:718–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rutkowski JM, Davis KE, Scherer PE. 2009. Mechanisms of obesity and related pathologies: the macro- and microcirculation of adipose tissue. FEBS J 276:5738–5746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. 2007. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56:901–911 [DOI] [PubMed] [Google Scholar]
- 12. Pang C, Gao Z, Yin J, Zhang J, Jia W, Ye J. 2008. Macrophage infiltration into adipose tissue may promote angiogenesis for adipose tissue remodeling in obesity. Am J Physiol Endocrinol Metab 295:E313–E322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kampf C, Bodin B, Källskog O, Carlsson C, Jansson L. 2005. Marked increase in white adipose tissue blood perfusion in the type 2 diabetic GK rat. Diabetes 54:2620–2627 [DOI] [PubMed] [Google Scholar]
- 14. Balwierz A, Polus A, Razny U, Wator L, Dyduch G, Tomaszewska R, Scherneck S, Joost H, Dembinska-Kiec A. 2009. Angiogenesis in the New Zealand obese mouse model fed with high fat diet. Lipids Health Dis 8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. 2009. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol 29:1575–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Virtue S, Vidal-Puig A. 2010. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome–an allostatic perspective. Biochim Biophys Acta 1801:338–349 [DOI] [PubMed] [Google Scholar]
- 17. Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, Basdevant A, Guerre-Millo M, Poitou C, Zucker JD, Bedossa P, Clément K. 2010. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 59:2817–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ucuzian AA, Gassman AA, East AT, Greisler HP. 2010. Molecular mediators of angiogenesis. J Burn Care Res 31:158–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Egeblad M, Werb Z. 2002. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2:161–174 [DOI] [PubMed] [Google Scholar]
- 20. Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. 2003. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther 5:1003–1015 [DOI] [PubMed] [Google Scholar]
- 21. Di Gregorio GB, Yao-Borengasser A, Rasouli N, Varma V, Lu T, Miles LM, Ranganathan G, Peterson CA, McGehee RE, Kern PA. 2005. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes 54:2305–2313 [DOI] [PubMed] [Google Scholar]
- 22. Almendro N, Bellón T, Rius C, Lastres P, Langa C, Corbí A, Bernabéu C. 1996. Cloning of the human platelet endothelial cell adhesion molecule-1 promoter and its tissue-specific expression. Structural and functional characterization. J Immunol 157:5411–5421 [PubMed] [Google Scholar]
- 23. Rasouli N, Yao-Borengasser A, Varma V, Spencer HJ, McGehee RE, Jr, Peterson CA, Mehta JL, Kern PA. 2009. Association of scavenger receptors in adipose tissue with insulin resistance in nondiabetic humans. Arterioscler Thromb Vasc Biol 29:1328–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Trayhurn P, Wang B, Wood IS. 2008. Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br J Nutr 100:227–235 [DOI] [PubMed] [Google Scholar]
- 25. Fichard A, Kleman JP, Ruggiero F. 1995. Another look at collagen V and XI molecules. Matrix Biol 14:515–531 [DOI] [PubMed] [Google Scholar]
- 26. Rasouli N, Kern PA. 2008. Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab 93:S64–S73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. 1997. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature 389:610–614 [DOI] [PubMed] [Google Scholar]
- 28. Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. 2006. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116:1494–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr 2005. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 116:115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr 2003. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lumeng CN, Bodzin JL, Saltiel AR. 2007. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prieur X, Mok CY, Velagapudi VR, Núñez V, Fuentes L, Montaner D, Ishikawa K, Camacho A, Barbarroja N, O'Rahilly S, Sethi JK, Dopazo J, Orešič M, Ricote M, Vidal-Puig A. 2011. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and m2/m1 polarization in obese mice. Diabetes 60:797–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kawanishi N, Yano H, Yokogawa Y, Suzuki K. 2010. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc Immunol Rev 16:105–118 [PubMed] [Google Scholar]
- 34. Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Györi G, Zlabinger GJ, Stulnig TM. 2007. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 31:1420–1428 [DOI] [PubMed] [Google Scholar]
- 35. Elbein SC, Kern PA, Rasouli N, Yao-Borengasser A, Sharma NK, Das SK. 2011. Global gene expression profiles of subcutaneous adipose and muscle from glucose-tolerant, insulin-sensitive, and insulin-resistant individuals matched for BMI. Diabetes 60:1019–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sun K, Kusminski CM, Scherer PE. 2011. Adipose tissue remodeling and obesity. J Clin Invest 121:2094–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chun TH, Hotary KB, Sabeh F, Saltiel AR, Allen ED, Weiss SJ. 2006. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell 125:577–591 [DOI] [PubMed] [Google Scholar]
- 38. Unal R, Yao-Borengasser A, Varma V, Rasouli N, Labbate C, Kern PA, Ranganathan G. 2010. Matrix metalloproteinase-9 is increased in obese subjects and decreases in response to pioglitazone. J Clin Endocrinol Metab 95:2993–3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Henegar C, Tordjman J, Achard V, Lacasa D, Cremer I, Guerre-Millo M, Poitou C, Basdevant A, Stich V, Viguerie N, Langin D, Bedossa P, Zucker JD, Clement K. 2008. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol 9:R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Davis GE, Senger DR. 2005. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res 97:1093–1107 [DOI] [PubMed] [Google Scholar]
- 41. Yamamoto K, Yamamoto M, Noumura T. 1992. Disassembly of F-actin filaments in human endothelial cells cultured on type V collagen. Exp Cell Res 201:55–63 [DOI] [PubMed] [Google Scholar]
- 42. Mumby SM, Raugi GJ, Bornstein P. 1984. Interactions of thrombospondin with extracellular matrix proteins: selective binding to type V collagen. J Cell Biol 98:646–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Voskerician G, Anderson JM, Ziats NP. 1997. Inhibition of migration of endothelial cells by type V collagen and high molecular weight kininogens. Biomed Sci Instrum 33:172–177 [PubMed] [Google Scholar]
- 44. Bezerra MC, Teodoro WR, de Oliveira CC, Velosa AP, Ogido LT, Gauditano G, Parra ER, Capelozzi VL, Yoshinari NH. 2006. Scleroderma-like remodeling induced by type V collagen. Arch Dermatol Res 298:51–57 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.