Abstract
Context:
Sclerostin, a protein encoded by the SOST gene in osteocytes and an antagonist of the Wnt signaling pathway, is down-regulated by PTH administration. Disorders of parathyroid function are useful clinical settings to study this relationship.
Objective:
The objective of the study was to evaluate sclerostin in two different disorders of parathyroid function, primary hyperparathyroidism and hypoparathyroidism, and to analyze the relationship between sclerostin and PTH, bone markers, and bone mineral density.
Design:
This is a cross-sectional study.
Setting:
The study was conducted at a clinical research center.
Patients:
Twenty hypoparathyroid and 20 hyperparathyroid patients were studied and compared to a reference control group.
Results:
Serum sclerostin was significantly higher in hypoparathyroid subjects than in hyperparathyroid subjects (P < 0.0001) and controls (P < 0.0001). PTH was negatively associated with sclerostin, achieving statistical significance in hypoparathyroidism (r = −0.545; P = 0.02). The bone turnover markers, cross-linked C-telopeptide of type I collagen (CTX) and amino-terminal propeptide of type I collagen (P1NP), were differently associated with sclerostin according to the parathyroid disorder. In primary hyperparathyroidism, bone turnover markers were associated negatively with sclerostin (for P1NP, r = −0.490; P = 0.03). In hypoparathyroidism, bone turnover markers were associated positively with sclerostin (for CTX, r = +0.571; P = 0.01). Although there was no significant correlation between bone mineral density and sclerostin in either parathyroid disorder, there was a significant positive relationship between sclerostin and bone mineral content in hypoparathyroidism.
Conclusions:
The results are consistent with the hypothesis that PTH is a regulator of sclerostin in human disorders of parathyroid function. In addition, the results suggest that bone mineral content may be another factor that influences sclerostin.
Sclerostin is a secretory product of the osteocyte, the most abundant cell in the skeleton (1, 2). Long considered to be an outdated osteoblast, inactive and “entombed” in the mineralized matrix of bone, the osteocyte is now acknowledged to be a master skeletal signaling cell. Responding to mechanical stress and to other environmental influences, the osteocyte signals the two other key bone cells, the osteoblast and the osteoclast, by producing molecules such as sclerostin (3–5).
Sclerostin, a glycoprotein encoded by the osteocyte SOST gene, regulates the activities of the mature osteoblast as well as the osteoblast lineage pathway. Inactivating mutations of the SOST gene lead to exuberant bone growth, as demonstrated in the human disorders sclerosteosis and van Buchem's disease (6–9). Affected individuals have exceedingly dense bones as assessed by radiographic and dual-energy x-ray absorptiometry (DXA) measurements, but they are of normal quality, and fractures have not been reported. A phenotype similar to sclerosteosis is seen in a mouse Sost knockout model (10).
Sclerostin's control of osteoblast action and development is mediated by the anabolic Wnt and BMP signaling pathways. By binding to the LRP5/LRP6 receptor complex, sclerostin inhibits the activity of molecules such as the Wnt pathway's β-catenin, a major activator of anabolic genes in the nucleus of the osteoblast (2, 11–14). Sclerostin also has been shown to inhibit BMP7 secretion by osteocytes in mice (15).
PTH has increasingly been implicated as one of the factors involved in the regulation of sclerostin (16). Evidence that PTH regulates sclerostin expression comes from human and animal studies at both the cellular and molecular level (17–20). Diseases characterized by excessive or deficient PTH provide a useful model to further explore the relationship between PTH and sclerostin. In this study, we measured circulating sclerostin levels in patients with overproduction or deficient PTH, namely primary hyperparathyroidism (PHPT) or hypoparathyroidism (HypoPT).
Subjects and Methods
Subjects
Serum samples were obtained from ongoing HypoPT and PHPT studies at the Metabolic Bone Diseases Unit of Columbia University Medical Center. A convenience sample consisting of 20 subjects (10 postmenopausal women and 10 men) with HypoPT and 20 subjects (10 postmenopausal women and 10 men) with PHPT was chosen. HypoPT was defined as chronic hypocalcemia in association with levels of PTH below the PTH assay reference range (<10 pg/ml). PHPT was defined by hypercalcemia in association with levels of PTH in the upper range or above the PTH assay reference range (>65 pg/ml). Exclusion criteria included: liver or kidney disease, Paget's disease, rheumatoid arthritis, diabetes mellitus, Cushing's syndrome, multiple myeloma or any other malignancy, current treatment with any bisphosphonate, glucocorticoids, calcitonin, raloxifene, estrogens, fluoride, lithium, or methotrexate. Serum was obtained, and DXA measurements were made before any patient underwent pharmacological (PTH for HypoPT) or surgical (parathyroidectomy for PHPT) treatment for their disease. Sclerostin serum of 31 healthy subjects (15 men and 16 postmenopausal women; age range, 37 to 79 yr) were used as the control group (provided by TECOmedical AG, Sissach, Switzerland). The study was approved by the Institutional Review Board of Columbia University Medical Center, and all subjects gave written informed consent.
Serum measurements
Blood samples were prepared, and sera were immediately frozen at −70 C. Serum sclerostin levels were measured using an ELISA developed by TECOmedical Group. This ELISA uses a biotinylated polyclonal antibody as well as a horseradish peroxidase-labeled secondary monoclonal antibody that specifically recognizes human sclerostin. The detection limit of the assay is 0.15 ng/ml. No study subjects demonstrated sclerostin levels below this detection limit. Intra- and interassay precision are 1.6 and 2.7%, respectively. Results for the sclerostin measurements are reported throughout in nanograms per milliliter (multiply by 44 to convert to picomoles per liter).
PTH was measured in duplicate using the immunoradiometric assay for the quantitative determination of human total intact PTH as developed by Scantibodies Laboratories, Inc. (Santee, CA). Normal range is 14–66 pg/ml. Interassay precision is 8.4%, and the intraassay precision is 5.7%. Serum amino-terminal propeptide of type I collagen (P1NP), cross-linked C-telopeptide of type I collagen (CTX), and 25-hydroxyvitamin D (25-OHD) were measured simultaneously by immunochemiluminescence assays on the IDS-iSYS Multi-Discipline automated analyzer (Immunodiagnostics Systems, Scottsdale, AZ). Normal range for P1NP is 27.7–127.6 ng/ml. Sensitivity is below 1 ng/ml, and intra- and interassay precision are below 4% and below 6%, respectively. Dynamic range for CTX is 0.033–6.000 ng/ml, and intra- and interassay precision is 4.9 and 8.8%, respectively. Normal reference values for 25-OHD are set at above 30 ng/ml, with sensitivity of 5.5 ng/ml and intra- and interassay precision of below 8% and below 10%, respectively.
Bone mineral density (BMD)
BMD was measured at the lumbar spine (LS; L1–L4), total hip (TH), femoral neck (FN), and distal one-third radius (1/3 radius) by DXA (Hologic 4500W; Hologic Inc., Bedford, MA). The short-term in vivo precision error (root-mean-square sd) was 0.026 g/cm2 for L1–L4, 0.032 g/cm2 for the TH, 0.041 g/cm2 for the FN, and 0.033 g/cm2 for the forearm.
Statistical analysis
Results are expressed as mean ± sem. Serum chemistry measures were log-transformed before analysis, and tests were adjusted for age and unequal variances when appropriate. HypoPT and PHPT groups were evaluated by two-sided independent t-tests. Each clinical group was compared with controls by independent t-test also. Pearson correlations were used to assess the association between sclerostin and bone mineral indices. Linear regression was used to fit a least squares prediction line between sclerostin (log10) and CTX (log10) and P1NP (log10) separately for each group. A value of P < 0.05 was considered significant. Statistical analysis was performed using SAS, version 9.2 (SAS Institute, Inc., Cary, NC).
Results
Baseline biochemical and DXA data
Demographic data are shown in Table 1. HypoPT and PHPT subjects were of similar age and body mass index. As expected, PTH levels were much lower in the HypoPT group (7.3 ± 0.9 pg/ml) than in the PHPT group (91.9 ± 13.1 pg/ml). Also as expected, since vitamin D supplementation is a key part of treatment for HypoPT and 25-OHD metabolism in the kidney by 25-hydroxyvitamin d-1α-hydroxylase is influenced by PTH and therefore in PHPT is typically metabolized at a faster rate, 25-OHD concentrations were higher in HypoPT than in PHPT (P = 0.002). In HypoPT, CTX and P1NP concentrations were significantly lower than in PHPT (P < 0.001) reflecting lower and higher bone turnover rates respectively. BMD at all sites was greater in HypoPT than in PHPT (Table 1).
Table 1.
Baseline characteristics, biochemical indices, and BMD measurements
| HypoPT | PHPT | P value | |
|---|---|---|---|
| Age (yr) | 58.3 ± 1.4 | 59.2 ± 1.4 | NS |
| BMI (kg/m2) | 28.6 ± 1.2 | 25.8 ± 1.2 | NS |
| Intact PTH (pg/ml) | 7.3 ± 0.9 | 91.9 ± 13.1 | <0.0001 |
| 25-OHD (ng/ml) | 52 ± 5 | 32.8 ± 3 | 0.002 |
| Calcium (mg/dl) | 8.5 ± 0.2 | 10.6 ± 0.2 | <0.0001 |
| CTX (ng/ml) | 0.110 ± 0.01 | 0.780 ± 0.13 | <0.0001 |
| P1NP (ng/ml) | 22.7 ± 2 | 55.9 ± 8 | <0.0001 |
| LS BMD (g/cm2) | 1.201 ± 0.05 | 0.847 ± 0.03 | <0.0001 |
| TH BMD (g/cm2) | 1.027 ± 0.05 | 0.826 ± 0.03 | 0.0013 |
| FN BMD (g/cm2) | 0.863 ± 0.04 | 0.654 ± 0.02 | <0.0001 |
| 1/3 radius BMD (g/cm2) | 0.738 ± 0.02 | 0.673 ± 0.02 | <0.05 |
Data are expressed as mean ± sem (n = 20 per group). Normal ranges are given in the text. BMI, Body mass index; NS, not significant.
Sclerostin measurements
Serum sclerostin levels were much higher in HypoPT than in PHPT and normal controls (Fig. 1). Both genders demonstrated significantly higher levels of sclerostin in HypoPT but the comparisons in men (P < 0.0001) were greater than in women (P < 0.05). This observation reflects higher levels of sclerostin in men vs. HypoPT women. In PHPT subjects, sclerostin levels were lower than controls but the difference was small. There was no gender difference in PHPT or in controls (Table 2).
Fig. 1.
Serum sclerostin levels (mean ± sem) in HypoPT vs. PHPT and normal controls. ***, P < 0.0001. P values were calculated on log-transformed data (see Statistical analysis).
Table 2.
Serum sclerostin
| HypoPT | PHPT | Controls | |
|---|---|---|---|
| All | 1.118 ± 0.09a,b | 0.656 ± 0.04a | 0.715 ± 0.04b |
| Female | 0.859 ± 0.05c,d | 0.670 ± 0.05c | 0.648 ± 0.05d |
| Males | 1.376 ± 0.14a,b | 0.643 ± 0.07a | 0.802 ± 0.08c |
Data are expressed as mean (ng/ml) ± sem, and P values were calculated on log-transformed data (see Statistical analysis). Male data higher than females are shown in bold.
P < 0.0001 HypoPT vs. PHPT.
P < 0.0001 HypoPT vs. control.
P < 0.05 HypoPT vs. PHPT.
P < 0.05 HypoPT vs. control.
PTH and serum sclerostin showed negative associations in HypoPT and in PHPT with the r-value in the HypoPT group achieving significance (r = −0.545, P = 0.02). The slopes of the relationships did not significantly differ between the 2 diseases (Fig. 2). In PHPT, there was a significant negative correlation between serum calcium concentration and sclerostin (r = −0.546; P = 0.02).
Fig. 2.
Relationship between PTH and sclerostin in HypoPT and PHPT.
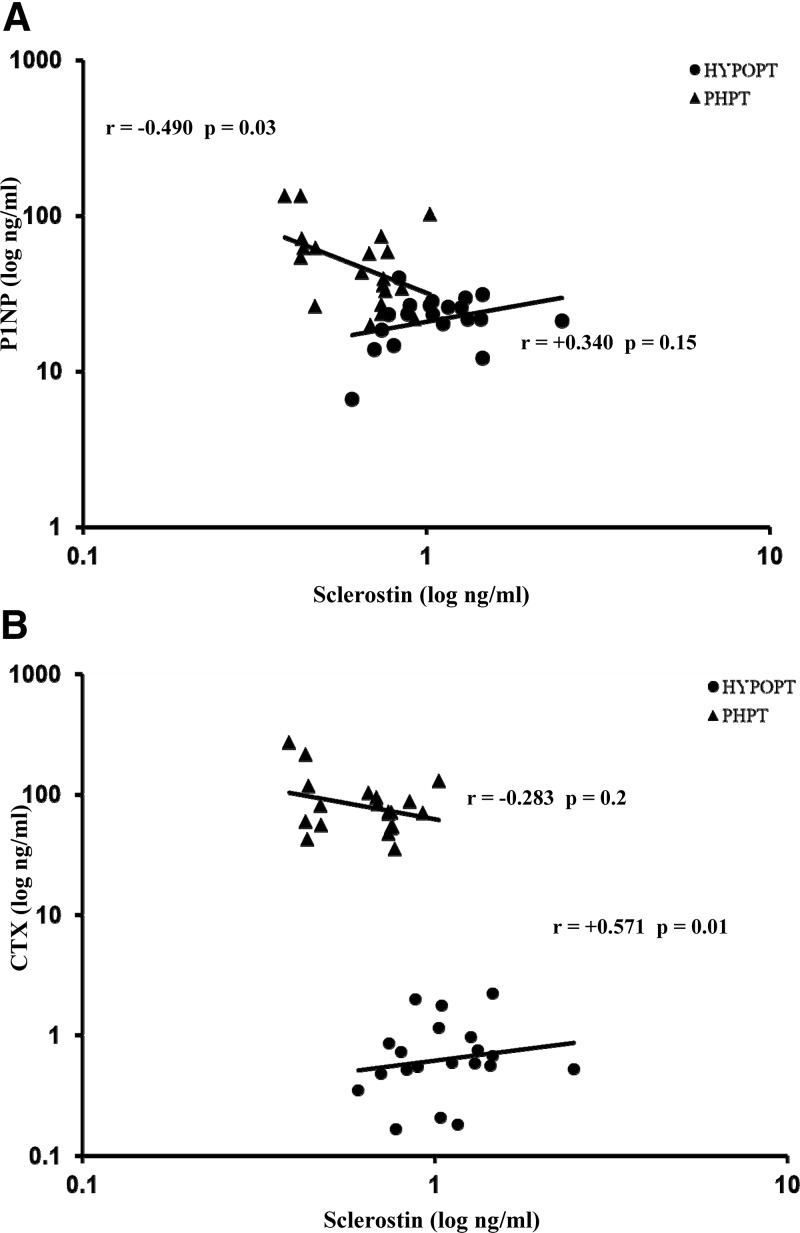
The association between serum sclerostin and bone turnover markers, CTX and P1NP, differed according to the parathyroid disorder. In PHPT, as expected, there was an inverse association between the markers and sclerostin, with the r-value between sclerostin and P1NP achieving statistical significance (r = −0.490, P = 0.03; Fig. 3). CTX and P1NP values were significantly associated with each other (r = +0.846, P < 0.001). In HypoPT subjects, while bone markers were also significantly associated with each other (r = +0.627, P = 0.004), serum sclerostin levels, unexpectedly, were correlated positively with CTX and P1NP. The positive relationship between sclerostin and CTX achieved statistical significance (r = +0.571, P = 0.01; Fig. 3). No significant correlation was seen between sclerostin and daily supplemental intake of calcium, calcitriol and vitamin D in the HypoPT.
Fig. 3.
Relationship between sclerostin and the bone formation marker P1NP (A) and the bone resorption marker CTX (B) in HypoPT and PHPT.
There was no association between serum sclerostin and BMD at any site in HypoPT or PHPT, but a strong and significant correlation was seen between sclerostin with bone mineral content (BMC) in HypoPT subjects (LS BMC, r = +0.648, P = 0.003; TH BMC, r = +0.663, P = 0.002; FN BMC, r = +0.778, P < 0.0001; and 1/3 radius BMC, r = +0.643, P = 0.003) (Fig. 4).
Fig. 4.
Relationship between sclerostin and BMD (A) and BMC (B) in HypoPT and PHPT.
Discussion
These results confirm and extend recent studies of serum sclerostin in the circulation of patients with several different metabolic bone diseases (21–24). These studies have been enabled by the availability of validated assays for sclerostin (25), an advance over earlier approaches (17–19). Drake et al. (20) recently showed a significant correlation between bone marrow plasma and serum concentrations of sclerostin. Thus, it is likely that although sclerostin is a locally active signaling molecule, circulating levels are of clinical relevance.
Human and animal studies have shown that PTH regulates sclerostin expression (17–20, 24). The relationship is primarily a negative one with levels of sclerostin reduced in the presence of PTH (17–20, 22). In this report, we took advantage of two disorders of parathyroid function, HypoPT and PHPT, to explore this relationship to a greater extent than has previously been reported. In a direct comparison between HypoPT and PHPT, we have shown that sclerostin levels are much higher in HypoPT than in PHPT. HypoPT subjects also demonstrate much higher levels of sclerostin than control subjects. The correlation between sclerostin and PTH in PHPT was not significant, although the slopes of the sclerostin-PTH relationships were identical, suggesting that the lack of a significant relationship may relate to the limited number of subjects with PHPT we studied. A relationship between sclerostin and PTH in PHPT was recently shown by Kaji et al. (26). Moreover, Drake et al. (20) have demonstrated that sclerostin levels fell 14 d after PTH exposure. The observations are consistent with our current understanding that PTH is a negative regulator of sclerostin. Consistent with this point, in PHPT, sclerostin levels are lower than controls but the difference did not achieve statistical significance. Our data, nevertheless, are in agreement with the report of Lierop et al. (24), who demonstrated significantly lower sclerostin levels in PHPT than in controls. Similar to our study, there was substantial overlap in values between groups.
The more dramatic difference in sclerostin levels in HypoPT vs. PHPT subjects may reflect the fact that the catabolic actions of PTH predominate in PHPT. In this setting, therefore, PTH is likely to be preferentially using its catabolic receptor activator of nuclear factor κB ligand-mediated biochemical pathway and not the sclerostin-mediated Wnt-signaling pathway associated with its anabolic actions (27, 28). Hence, in PHPT, PTH may have a smaller effect on sclerostin than it would have in another situation such as when PTH is used as an anabolic therapy for osteoporosis. Consistent with this hypothesis, the negative relationship between sclerostin and PTH was stronger in the HypoPT subjects (r = −0.545; P = 0.02) than in the PHPT subjects (r = −0.316; P = 0.2).
Another noteworthy feature of this report is the relationship between sclerostin and bone turnover markers. Previous studies, which have analyzed the relationship between sclerostin levels and circulating markers of bone turnover, have not been consistent (20, 22–24, 29). Mödder et al. (29) noted a negative correlation between bone formation [bone-specific alkaline phosphatase (BAP), P1NP] and bone resorption (CTX) markers only in postmenopausal women at least 60 yr old. In another study by Gaudio et al. (23), sclerostin was negatively associated with BAP but positively associated with CTX. However, several other studies have not shown a significant relationship between bone turnover markers and sclerostin levels (22, 24, 30). Our study provides additional insight into this issue, with results that suggest that these relationships are influenced by the parathyroid disorder. As expected, in PHPT, a negative relationship was seen between sclerostin and CTX and between sclerostin and P1NP. The effect was greater on P1NP than on CTX. The greater effect of sclerostin on an index of bone formation than one of bone resorption is consistent with recent studies of sclerostin antibodies in which bone formation markers are markedly increased (31). In the study of Padhi et al. (31), subjects who received a single sc dose of the sclerostin monoclonal antibody (AMG 785) demonstrated not only an increase in bone formation markers (maximum percentage change with 10 mg/kg dose sc, P1NP, +184%; BAP, +126%; and osteocalcin, +176%) but also a decrease in bone resorption marker CTX (−54%).
Surprisingly, in HypoPT the correlation between sclerostin and bone turnover markers is a positive one. At this point, a clear understanding of this relationship is not apparent, but several possibilities come to mind. The higher levels of sclerostin in the setting of low levels of bone turnover markers could reflect a secondary or compensatory effect of sclerostin in response to independent non-PTH factors such as bone mass. Another explanation is that PTH may act as a necessary facilitator for the inhibitory action of sclerostin on bone formation. In the absence of PTH, sclerostin might be permissive and unable to serve as a regulatory molecule. Recent data by Rhee et al. (32) are consistent with this concept. Mice overexpressing sclerostin with a constitutively active PTH 1 receptor in osteocytes have lower BMD compared with mice overexpressing sclerostin only. Consistent with this idea, fibroblast growth factor-23, another osteocyte-driven cell product, requires a threshold PTH level in order for it to be functional (33, 34).
Mödder et al. (29) have reported that men have higher sclerostin levels than women. We were able to show a higher level of sclerostin only in male HypoPT subjects. Although consistent with the reports of Mödder et al. (29) and Schett and Hennies (25), our data did not universally confirm these findings in PHPT and in controls. Perhaps this could be accounted for by our relatively small sample size.
Published data on a correlation between BMD and circulating sclerostin are also inconsistent. Mirza et al. (22) found a negative correlation between femoral neck BMD and sclerostin in all subjects (pre- and postmenopausal women), but after adjustment for age, the relationship was no longer significant. Mödder et al. (29) performed a total body scan in a population-based sample of 362 patients and found a positive association between total body bone mass and total body BMC with circulating sclerostin in middle-aged and elderly subjects, but not in the younger population. Polyzos et al. (30) have also noted a positive correlation with LS BMD and T-score. A positive association between sclerostin and BMD is rather paradoxical because sclerostin plays a regulatory role in bone mineralization by inhibiting osteoblastic activity.
Although we did not find a correlation between sclerostin and BMD in either group, we found a strong positive correlation between sclerostin and BMC at all four sites (LS, FN, TH, and 1/3 radius) in HypoPT but not in PHPT. The correlation between BMC and sclerostin was sustained after adjustment for PTH levels. In addition to PTH, then, the results suggest that BMC may be another one of several factors that influence sclerostin. It is likely that higher BMC is associated with more numerous osteocytes and that osteocyte number drives this relationship. With further studies, the nature of these relationships between sclerostin, PTH, and bone mass will become more apparent.
Acknowledgments
We thank Marieluise Wippermann (TECOmedical Group) for providing us with the sclerostin assay materials.
This work was supported by Grants DK32333, DK06950, DK066329, and K24DK074457 from the National Institutes of Health and by Grant FD002525 from the Food and Drug Administration.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BAP
- Bone-specific alkaline phosphatase
- BMC
- bone mineral content
- BMD
- bone mineral density
- CTX
- cross-linked C-telopeptide of type I collagen
- DXA
- dual-energy x-ray absorptiometry
- FN
- femoral neck
- HypoPT
- hypoparathyroidism
- LS
- lumbar spine
- 25-OHD
- 25-hydroxyvitamin D
- PHPT
- primary hyperparathyroidism
- P1NP
- serum amino-terminal propeptide of type I collagen
- 1/3 radius
- one third distal radius
- TH
- total hip.
References
- 1. Parfitt AM. 1977. The cellular basis of bone turnover and bone loss: a rebuttal of the osteocytic resorption–bone flow theory. Clin Orthop Relat Res 127:236–247 [PubMed] [Google Scholar]
- 2. van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE, ten Dijke P, Löwik CW. 2004. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med 199:805–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonewald LF, Johnson ML. 2008. Osteocytes, mechanosensing and Wnt signaling. Bone 42:606–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tanaka K, Yamaguchi Y, Hakeda Y. 1995. Isolated chick osteocytes stimulate formation and bone-resorbing activity of osteoclast-like cells. J Bone Miner Metab 13:61–70 [Google Scholar]
- 5. Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Löwik CW, Reeve J. 2005. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J 19:1842–1844 [DOI] [PubMed] [Google Scholar]
- 6. Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den Ende J, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Van Hul W. 2001. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 10:537–543 [DOI] [PubMed] [Google Scholar]
- 7. Wergedal JE, Veskovic K, Hellan M, Nyght C, Balemans W, Libanati C, Vanhoenacker FM, Tan J, Baylink DJ, Van Hul W. 2003. Patients with van Buchem disease, an osteosclerotic genetic disease, have elevated bone formation markers, higher bone density, and greater derived polar moment of inertia than normal. J Clin Endocrinol Metab 88:5778–5783 [DOI] [PubMed] [Google Scholar]
- 8. Hamersma H, Gardner J, Beighton P. 2003. The natural history of sclerosteosis. Clin Genet 63:192–197 [DOI] [PubMed] [Google Scholar]
- 9. van Bezooijen RL, ten Dijke P, Papapoulos SE, Löwik CW. 2005. SOST/sclerostin, an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev 16:319–327 [DOI] [PubMed] [Google Scholar]
- 10. Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D'Agostin D, Kurahara C, Gao Y, Cao J, Gong J, Asuncion F, Barrero M, Warmington K, Dwyer D, Stolina M, Morony S, Sarosi I, Kostenuik PJ, Lacey DL, Simonet WS, Ke HZ, Paszty C. 2008. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23:860–869 [DOI] [PubMed] [Google Scholar]
- 11. Semënov M, Tamai K, He X. 2005. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem 280:26770–26775 [DOI] [PubMed] [Google Scholar]
- 12. van Bezooijen RL, Svensson JP, Eefting D, Visser A, van der Horst G, Karperien M, Quax PH, Vrieling H, Papapoulos SE, ten Dijke P, Löwik CW. 2007. Wnt but not BMP signaling is involved in the inhibitory action of sclerostin on BMP-stimulated bone formation. J Bone Miner Res 22:19–28 [DOI] [PubMed] [Google Scholar]
- 13. Johnson ML, Kamel MA. 2007. The Wnt signaling pathway and bone metabolism. Curr Opin Rheumatol 19:376–382 [DOI] [PubMed] [Google Scholar]
- 14. Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D. 2005. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem 280:19883–19887 [DOI] [PubMed] [Google Scholar]
- 15. Krause C, Korchynskyi O, de Rooij K, Weidauer SE, de Gorter DJ, van Bezooijen RL, Hatsell S, Economides AN, Mueller TD, Löwik CW, ten Dijke P. 2010. Distinct modes of inhibition by sclerostin on bone morphogenetic protein and Wnt signaling pathways. J Biol Chem 285:41614–41626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kramer I, Keller H, Leupin O, Kneissel M. 2010. Does osteocytic SOST suppression mediate PTH bone anabolism? Trends Endocrinol Metab 21:237–244 [DOI] [PubMed] [Google Scholar]
- 17. Keller H, Kneissel M. 2005. SOST is a target gene for PTH in bone. Bone 37:148–158 [DOI] [PubMed] [Google Scholar]
- 18. Silvestrini G, Ballanti P, Leopizzi M, Sebastiani M, Berni S, Di Vito M, Bonucci E. 2007. Effects of intermittent parathyroid hormone (PTH) administration on SOST mRNA and protein in rat bone. J Mol Histol 38:261–269 [DOI] [PubMed] [Google Scholar]
- 19. Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O'Brien CA, Manolagas SC, Jilka RL. 2005. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology 146:4577–4583 [DOI] [PubMed] [Google Scholar]
- 20. Drake MT, Srinivasan B, Mödder UI, Peterson JM, McCready LK, Riggs BL, Dwyer D, Stolina M, Kostenuik P, Khosla S. 2010. Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab 95:5056–5062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mödder UI, Clowes JA, Hoey K, Peterson JM, McCready L, Oursler MJ, Riggs BL, Khosla S. 2011. Regulation of circulating sclerostin levels by sex steroids in women and in men. J Bone Miner Res 26:27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mirza FS, Padhi ID, Raisz LG, Lorenzo JA. 2010. Serum sclerostin levels negatively correlate with parathyroid hormone levels and free estrogen index in postmenopausal women. J Clin Endocrinol Metab 95:1991–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gaudio A, Pennisi P, Bratengeier C, Torrisi V, Lindner B, Mangiafico RA, Pulvirenti I, Hawa G, Tringali G, Fiore CE. 2010. Increased sclerostin serum levels associated with bone formation and resorption markers in patients with immobilization-induced bone loss. J Clin Endocrinol Metab 95:2248–2253 [DOI] [PubMed] [Google Scholar]
- 24. van Lierop AH, Witteveen JE, Hamdy NA, Papapoulos SE. 2010. Patients with primary hyperparathyroidism have lower circulating sclerostin levels than euparathyroid controls. Eur J Endocrinol 163:833–837 [DOI] [PubMed] [Google Scholar]
- 25. Schett G, Hennies M. 2009. Development of a sclerostin ELISA for clinical use. Bone 44:S278 [Google Scholar]
- 26. Kaji H, Imanishi Y, Sugimoto T, Seino S. 2011. Comparisons of serum sclerostin levels among patients with postmenopausal osteoporosis, primary hyperparathyroidism and osteomalacia. Exp Clin Endocrinol Diabetes 119:440–444 [DOI] [PubMed] [Google Scholar]
- 27. Poole KE, Reeve J. 2005. Parathyroid hormone—a bone anabolic and catabolic agent. Curr Opin Pharmacol 5:612–617 [DOI] [PubMed] [Google Scholar]
- 28. Lee SK, Lorenzo JA. 1999. Parathyroid hormone stimulates TRANCE and inhibits osteoprotegerin messenger ribonucleic acid expression in murine bone marrow cultures: correlation with osteoclast-like cell formation. Endocrinology 140:3552–3561 [DOI] [PubMed] [Google Scholar]
- 29. Mödder UI, Hoey KA, Amin S, McCready LK, Achenbach SJ, Riggs BL, Melton LJ, 3rd, Khosla S. 2011. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res 26:373–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Polyzos SA, Anastasilakis AD, Bratengeier C, Woloszczuk W, Papatheodorou A, Terpos E. 11 January 2011. Serum sclerostin levels positively correlate with lumbar spinal bone mineral density in postmenopausal women—the six-month effect of risedronate and teriparatide. Osteoporos Int doi: 10.1007/s00198-010-1525-6 [DOI] [PubMed] [Google Scholar]
- 31. Padhi D, Jang G, Stouch B, Fang L, Posvar E. 2011. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res 26:19–26 [DOI] [PubMed] [Google Scholar]
- 32. Rhee Y, Allen MR, Condon K, Lezcano V, Ronda AC, Galli C, Olivos N, Passeri G, O'Brien CA, Bivi N, Plotkin LI, Bellido T. 2011. PTH receptor signaling in osteocytes governs periosteal bone formation and intracortical remodeling. J Bone Miner Res 26:1035–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gupta A, Winer K, Econs MJ, Marx SJ, Collins MT. 2004. FGF-23 is elevated by chronic hyperphosphatemia. J Clin Endocrinol Metab 89:4489–4492 [DOI] [PubMed] [Google Scholar]
- 34. Geller JL, Khosravi A, Kelly MH, Riminucci M, Adams JS, Collins MT. 2007. Cinacalcet in the management of tumor-induced osteomalacia. J Bone Miner Res 22:931–937 [DOI] [PubMed] [Google Scholar]