Abstract
Context:
The undercarboxylated form of the osteoblast-secreted protein osteocalcin has favorable effects on fat and glucose metabolism in mice. In human subjects, cross-sectional studies suggest a relevant association.
Objective:
We investigated whether changes in undercarboxylated osteocalcin (ucOC) during osteoporosis treatment are associated with changes in metabolic parameters.
Design, Setting, Participants, and Interventions:
We measured ucOC in sera from a subset of osteoporotic postmenopausal women who were treated with PTH(1-84) or alendronate (n = 64 and n = 33, respectively) during the Parathyroid Hormone and Alendronate study.
Main Outcome Measures:
We measured serum adiponectin, leptin, and insulin and analyzed existing data on body weight, fat mass, and serum glucose concentration. Three-month changes in ucOC levels were evaluated as predictors of 12-month changes in indices of fat and glucose metabolism.
Results:
ucOC levels increased with PTH(1-84) and decreased with alendronate administration (P ≤ 0.01 for both treatment groups). Three-month change in ucOC was inversely associated with 12-month changes in body weight (standardized β = −0.25, P = 0.04) and fat mass (β = −0.23, P = 0.06), after adjustment for the treatment group. Three-month change in ucOC was positively associated with a 12-month change in adiponectin (β = 0.30, P = 0.01), independent of change in fat mass. There were no interactions between treatment and change in ucOC on changes in weight, fat mass, or adiponectin.
Conclusions:
PTH(1-84) increases and alendronate decreases ucOC levels. Changes in ucOC induced by PTH(1-84) and alendronate are associated with changes in metabolic indices. These associations are consistent with observations from animal models and support a role for ucOC in the skeletal regulation of energy metabolism in humans.
Osteocalcin (OC), an osteoblast secretory product, is a component of the bone extracellular matrix and a biochemical marker of bone formation. The undercarboxylated form of the molecule appears to influence fat and glucose homeostasis in animal models and exhibits characteristics of a hormone. Thus, OC is now recognized to have a role in the investigation of the skeleton as an endocrine organ and as a regulator of energy metabolism. Lee et al. (1) demonstrated that mice with a knockout of both OC alleles had increased fat mass, decreased β-cell proliferation and insulin secretion, and insulin resistance. Conversely, mice with high levels of undercarboxylated osteocalcin (ucOC) had small fat pads, increased β-cell proliferation, enhanced insulin sensitivity, and increased expression and serum levels of adiponectin, an insulin-sensitizing adipokine. The mice with high levels of ucOC did not become obese or glucose intolerant under conditions that would usually induce these metabolic abnormalities. In related experiments, ucOC induced adiponectin expression in cultured adipocytes. Administration of recombinant ucOC to wild-type mice decreased fat mass, increased adiponectin expression, improved glucose handling, and attenuated weight gain and glucose intolerance in the setting of a high-fat diet (2).
In human subjects, cross-sectional studies have demonstrated an association between OC, glucose metabolism, and fat mass (3–9). For example, Pittas et al. (4) reported that in older adults, total serum OC was inversely associated with body fat, fasting glucose, and fasting insulin. In a cohort of men and postmenopausal women with type 2 diabetes mellitus, ucOC correlated inversely with percentage trunk fat and with hemoglobin A1c (9).
No interventional trials of the effects of ucOC on human fat and glucose homeostasis have been conducted because ucOC is not available for administration to human subjects. Based on OC's role as a marker of bone formation, we hypothesized that an intervention that increases bone formation would increase ucOC and provide an opportunity to assess resulting changes in energy metabolism. PTH, administered daily for osteoporosis, is such an intervention. In contrast, alendronate, a bisphosphonate, decreases bone formation and has been shown to decrease ucOC (10).
The effects of treatment-induced changes in ucOC on fat and glucose homeostasis have not been studied to date. An opportunity to test the hypothesis relating changes in ucOC to energy metabolism in human subjects is the PTH and Alendronate (PaTH) study (11). The PaTH study compared PTH(1-84) and alendronate, providing us the opportunity to relate expected increases (PTH) and decreases (alendronate) in ucOC with potential perturbations in indices of energy metabolism. We tested the hypothesis that increases in ucOC are associated with improvements in metabolic parameters, including decreases in body weight and fat mass, and increases in serum adiponectin.
Participants and Methods
The PaTH study was a randomized, double-blinded clinical trial that compared the effects of PTH(1-84), alendronate, or their combination on bone mineral density (BMD) in postmenopausal women with low baseline BMD. Detailed descriptions of the study design, recruitment, and interventions have been published previously (11) but are summarized below.
Study design
Participants completed a 2-wk run-in period and then were randomized to one of three treatments for the first year of the trial: PTH(1-84) plus placebo alendronate (n = 119), alendronate plus placebo PTH(1-84) (n = 60), or PTH(1-84) plus alendronate (n = 60). The efficacy outcome variables in the original trial were areal BMD by dual-energy x-ray absorptiometry, volumetric BMD by quantitative computed tomography, and biochemical markers of bone turnover.
This ancillary study used data from the first year of the trial because the second year did not include a PTH(1-84) arm. We studied a subset of participants from the PTH(1-84) (plus placebo alendronate) and alendronate [plus placebo PTH(1-84)] groups; sampling was restricted to the pool with complete outcome data, including fat mass measurements, which were not available for all participants. The sampling design was designed to maximize variability in change in ucOC. We expected that the average effects of PTH(1-84) and alendronate on ucOC would differ in direction and so sampled women from both arms. However, the greater variability in the effects of PTH(1-84) on bone turnover and BMD, compared with alendronate (12), led us to expect greater variability in changes of ucOC as well; thus, we oversampled women in the PTH(1-84) group. Specifically we included PTH(1-84) and alendronate-treated participants in a ratio of approximately 2:1 [66 participants from the PTH(1-84) group and 34 participants from the alendronate group], randomly sampling those numbers within each treatment stratum. We excluded three participants [two from the PTH(1-84) group and one from the alendronate group] who lost more than 8.5 kg of weight during the 1-yr period because weight loss of this magnitude suggests either successful intentional caloric restriction or illness, which were not consistent with our research questions. The final sample consisted of 64 women from the PTH(1-84) group and 33 from the alendronate group and provided 80% power to detect correlations of 0.29 or greater, with a two-sided α of 0.05.
The PaTH study protocol was approved by the institutional review board at each clinical center, and all participants provided written informed consent before enrollment. Except for one clinician at the coordinating center responsible for reports to the Data and Safety Monitoring Board, investigators and participants remained blinded to treatment group assignment.
Participants
Postmenopausal women aged 55–85 yr were recruited from four U.S. clinical centers: Bangor, ME; Minneapolis, MN; New York, NY; and Pittsburgh, PA. Each participant had a femoral neck, total hip, and/or spine BMD T-score of −2.5 or less or −2.0 or less with at least one of the following risk factors: age 65 yr or older, a history of postmenopausal vertebral or nonvertebral fracture, or a maternal history of hip fracture. Women were excluded if they had medical conditions or took medications known to affect bone or mineral metabolism or if they had used a bisphosphonate for more than 12 months or for more than 4 wk in the previous 12 months.
Treatment
Participants in the PTH(1-84) treatment arm received PTH(1-84) 100 μg daily, injected sc in the morning (NPS Pharmaceuticals, Parsippany, NJ). Consistent with the double-blind trial design, they took a placebo alendronate pill daily. Participants in the alendronate arm took alendronate 10 mg daily, ingested orally in the morning after an overnight fast (Merck, Whitehouse Station, NJ). They also injected placebo PTH(1-84) daily. All participants received daily supplements of calcium carbonate (elemental calcium 500 mg; GlaxoSmithKline, Philadelphia, PA) and vitamin D3 (400 IU; Rugby Laboratories, Norcross, GA).
Biochemical assessment
Blood was drawn in the morning at baseline and 1, 3, and 12 months after randomization. Blood was drawn approximately 24 h after the last injection because participants were instructed not to inject PTH(1-84) on the morning the blood was drawn. Samples did not have to be obtained in the fasting state. Analytes including glucose levels were measured as the samples were collected (SmithKline Beecham Clinical Laboratories, Van Nuys, CA), and then specimens were stored at −70 C. For this substudy, specimens drawn at baseline and after 12 months of treatment were batch analyzed in a central laboratory (Maine Medical Center Research Institute, Scarborough, ME) for levels of total OC, ucOC, adiponectin, leptin, and insulin. In addition, total OC and ucOC were measured on specimens drawn after 3 months of treatment. Total OC was measured with the N-MID Osteocalcin ELISA (IDS Ltd., Fountain Hills, AZ), with intra- and interassay coefficients of variation (CV) of 4.0 and 1.8%, respectively. ucOC was measured with the Glu-OC EIA (Takara Bio Inc., Otsu, Shiga, Japan), with intra- and interassay CV of 8.3 and 5.2%, respectively. This assay has low cross-reactivity with carboxylated OC (13, 14) and has shown utility in other published studies (15, 16), despite the fact that it detects and may overestimate certain large ucOC fragments (14). Adiponectin was measured with the human total adiponectin/Acrp30 quantikine ELISA (R&D Systems, Minneapolis, MN) with intra- and interassay CV of 6.5 and 3.6%, respectively. Leptin was measured with the human leptin quantikine ELISA (R&D Systems), with intra- and interassay CV of 4.4 and 3.2%, respectively. Insulin was measured by ELISA (ALPCO, Salem, NH), with intra- and interassay CV of 6.8 and 4.3%, respectively. One participant with one specimen CV for insulin greater than 80%, even when repeated, was excluded from analyses with insulin or insulin to glucose ratio as outcome variable. Four participants with specimens with CV for insulin of greater than 20%, even when repeated, were included in the main analysis but excluded in a subsequent sensitivity analysis. These CV were determined by the laboratory used for this substudy.
Assessment of body weight, fat mass, and other covariates
At baseline and month 12, weight was measured on a standard balance beam scale. Height was measured using a calibrated, wall-mounted Harpenden stadiometer, and body mass index was calculated as weight (kilograms) per height2 (square meters). Whole-body fat (grams) and areal BMD (grams per square centimeter) were measured at baseline and month 12 by dual-energy x-ray absorptiometry (Hologic QDR 4500A or Delphi densitometer; Hologic, Bedford, MA).
At baseline, participants self-reported prior medical diagnoses including diabetes; medications; and smoking, categorized as never, former, or current.
Statistical analysis
Differences in baseline characteristics by treatment group were assessed using χ2, Mann-Whitney, and Student's t tests. For each treatment group, we used Wilcoxon signed-rank or paired t tests as appropriate to determine whether study outcomes changed between baseline and 3 or 12 months. Similarly, we used Mann-Whitney and t tests to assess between-group differences in these changes.
Next, we used Spearman's rank correlation to characterize the unadjusted associations of a 3-month change in ucOC with 12-month changes in other study outcomes. We focused on 3-month rather than 12-month changes in ucOC because most of the observed change occurred within the first 3 months (11). In addition, this 3-month change mostly precedes the 12-month changes in weight, fat mass, adiponectin, leptin, insulin, glucose and insulin to glucose ratio that we considered as outcomes, making causal interpretation of the associations we saw more plausible.
Finally, we used linear models to estimate adjusted associations between 3-month change in ucOC and 12-month changes in each metabolic measure, controlling for age and treatment group. Because 12-month changes in leptin and insulin levels and insulin to glucose ratio were not normally distributed, we analyzed changes in log-transformed levels. We used residuals to check the assumptions of normality and linearity, and we checked for influential points. We report standardized regression coefficients, which are scale-free effect measures similar in interpretation to correlation coefficients.
We also examined the relationships between the 12-month change in ucOC and 12-month changes in metabolic measures, but we report only those results in which they are meaningfully different from those for 3-month change in ucOC.
In addition, motivated by the very different effects of PTH(1-84) and alendronate on ucOC, we tested for modification of the effects of changes in the ucOC by treatment group. Finally, we checked for mediation of the ucOC effects on adiponectin by change in fat mass by comparing the estimated effects on adiponectin before and after adjustment for change in fat mass.
Data were analyzed using Stata 10 software (StataCorp, College Station, TX).
Results
Baseline participant characteristics and adherence to treatment
Participants included in this ancillary study were 70.1 ± 6.9 yr old, and 96% were Caucasian (Table 1). The median baseline ucOC level was 4.5 ng/ml, which was 23% of the total OC level. One participant self-reported a history of diabetes. However, she had never taken medications for diabetes, and her glucose levels at baseline and month 12 were less than 126 mg/dl.
Table 1.
Baseline characteristics of study participants
| All (n = 97) | PTH (1-84) (n = 64) | Alendronate (n = 33) | P value (between groups) | |
|---|---|---|---|---|
| Age (yr) | 70.1 ± 6.9 | 70.0 ± 7.2 | 70.1 ± 6.4 | 0.93 |
| Race, n (%) | 0.17 | |||
| Native American | 1 (1%) | 0 (0%) | 1 (3%) | |
| Asian | 3 (3%) | 3 (5%) | 0 (0%) | |
| White | 93 (96%) | 61 (95%) | 32 (97%) | |
| Weight (kg) | 65.0 ± 13.1 | 66.4 ± 13.9 | 62.3 ± 11.0 | 0.14 |
| Body mass index (kg/m2) | 25.3 ± 4.6 | 26.0 ± 4.7 | 24.0 ± 4.0 | 0.04 |
| Total body fat (kg) | 23.6 ± 8.3 | 24.5 ± 8.6 | 21.7 ± 7.4 | 0.11 |
| Percentage body fat (%) | 36.0 ± 6.4 | 36.7 ± 6.1 | 34.6 ± 6.8 | 0.14 |
| Smoking status, n (%) | 0.39 | |||
| Never | 44 (45%) | 31 (48%) | 13 (39%) | |
| Former | 44 (45%) | 26 (41%) | 18 (55%) | |
| Current | 9 (9%) | 7 (11%) | 2 (6%) | |
| BMD (g/cm2) | ||||
| Femoral neck | 0.604 ± 0.086 | 0.603 ± 0.090 | 0.605 ± 0.081 | 0.94 |
| Total hip | 0.712 ± 0.102 | 0.706 ± 0.105 | 0.725 ± 0.097 | 0.39 |
| Total spine | 0.765 ± 0.113 | 0.764 ± 0.113 | 0.767 ± 0.113 | 0.91 |
| Serum biochemical parameters | ||||
| Total OC (ng/ml) | 18.6 (14.3–23.2) | 18.6 (16.1–24.0) | 18.9 (13.8–22.9) | 0.36 |
| ucOC (ng/ml) | 4.5 (2.9–6.8) | 4.4 (2.9–6.9) | 4.6 (2.8–6.5) | 0.87 |
| ucOC to total OC ratio | 0.23 (0.18–0.30) | 0.22 (0.18–0.30) | 0.26 (0.18–0.33) | 0.40 |
| Adiponectin (μg/ml) | 12.9 (10.0–17.0) | 13.0 (9.1–18.5) | 11.9 (10.3–15.7) | 0.89 |
| Leptin (ng/ml) | 11.6 (6.8–20.7) | 13.0 (7.1–23.3) | 10.2 (4.4–18.9) | 0.25 |
| Insulin (μIU/ml) | 11.9 (7.2–19.9) | 12.0 (7.4–23.4) | 10.2 (7.2–17.1) | 0.44 |
| Glucose (mg/dl) | 94.0 (86.0–100.0) | 94.0 (86.5–100.0) | 92.0 (85.0–99.0) | 0.69 |
| Insulin to glucose ratio | 0.12 (0.08–0.20) | 0.13 (0.08–0.21) | 0.10 (0.07–0.19) | 0.41 |
Values are means ± sd, counts (percentages), or medians (interquartile ranges).
Of the 64 participants from the PTH(1-84) group, 78% complied with at least 80% of their study injections. Of the 33 participants from the alendronate group, 82% complied with at least 80% of their study pills.
Changes in ucOC, by treatment group
Median total and ucOC levels increased with PTH(1-84) and decreased with alendronate (Fig. 1). In the PTH(1-84) group, the median 3-month increase in total OC was 31.1 ng/ml, a 177% increase [95% confidence interval (CI) 139–232%]; the median 3-month increase in ucOC was 11.2 ng/ml, a 242% increase (95% CI 184–312%). In the alendronate group, the median 3-month decrease in total OC was 5.7 ng/ml, a 32% decrease (95% CI −45 to −16%); the median 3-month decrease in ucOC was 1.2 ng/ml, a 29% decrease (95% CI −45 to −8%). These responses to therapy differed significantly between groups (P < 0.001 for both total OC and ucOC).
Fig. 1.
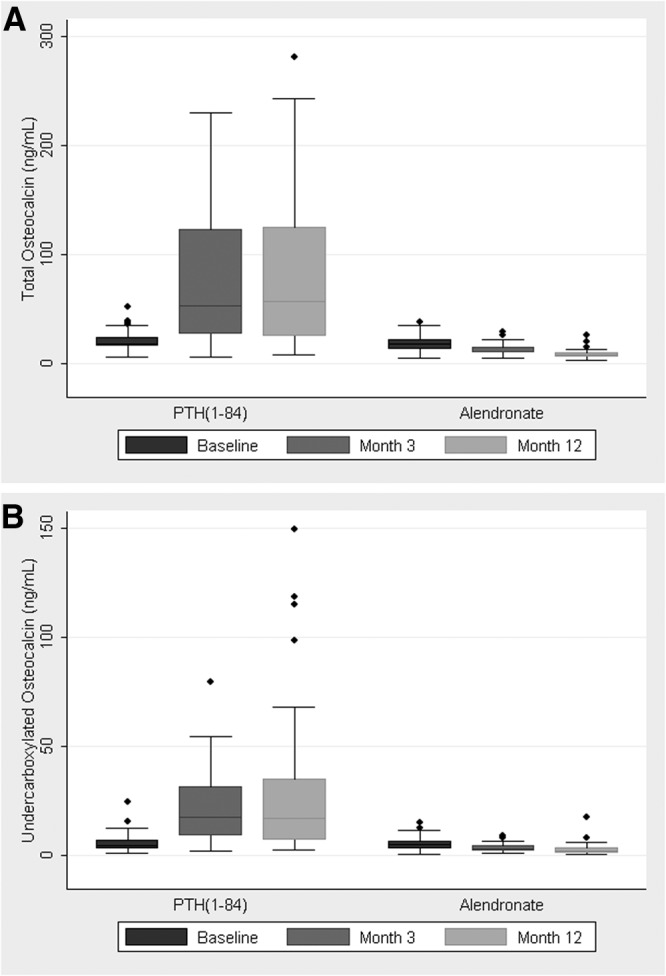
Serum levels of total osteocalcin (A) and ucOC (B) at baseline, month 3, and month 12 by treatment group. Boxes represent medians and interquartile ranges (IQR), and whiskers represent the lowest and highest points still within 1.5 IQR of the box. *, P < 0.01 compared with baseline.
The ratio of ucOC to total OC increased with PTH(1-84) therapy, from a median of 0.22 at baseline, to 0.27 at month 3 and 0.28 at month 12 (P < 0.01 for change from baseline for both time points). The ratio did not change with alendronate therapy. These responses to therapy differed significantly between treatment groups (P = 0.04 and P < 0.01 for 3 and 12 month changes, respectively).
Changes in metabolic parameters by treatment group
Among all participants in the original PaTH study, mean 12-month changes in body weight were −0.4 kg (P = 0.14) and +0.2 kg (P = 0.65) in the PTH(1-84) and alendronate arms, respectively (P = 0.21 for between group difference in change). Mean changes in fat mass were −0.5 kg (P = 0.02) and +0.03 kg (P = 0.91) in the PTH(1-84) and alendronate arms, respectively (P = 0.13 for between group difference in change).
Among participants in this ancillary study, those in the PTH(1-84) group experienced a small but statistically significant decrease in mean body weight over 12 months (−0.8 kg, 95% CI −1.5 to −0.1, P = 0.03) and a trend toward a decrease in fat mass (−0.5 kg, −1.0 to 0.0, P = 0.06). Those in the alendronate group had no statistically significant change in weight (−0.1 kg, −1.0 to 0.8, P = 0.86) or fat mass (−0.5 kg, −1.1 to 0.2, P = 0.15). There were no differences in these changes between groups [mean differences +0.7 kg (−0.4 to +1.8) and +0.4 kg (−0.8 to +0.9) for weight and fat mass, respectively]. Mean change in adiponectin was +0.3 μg/ml (−0.2 to +0.9) with PTH(1-84) and −0.2 μg/ml (−1.2 to +0.9) with alendronate; the difference in change was −0.6 (−1.6 to +0.6). Leptin, insulin, and glucose levels and the insulin/ to glucose ratio did not change significantly in either group.
Change in ucOC and changes in body weight and fat mass
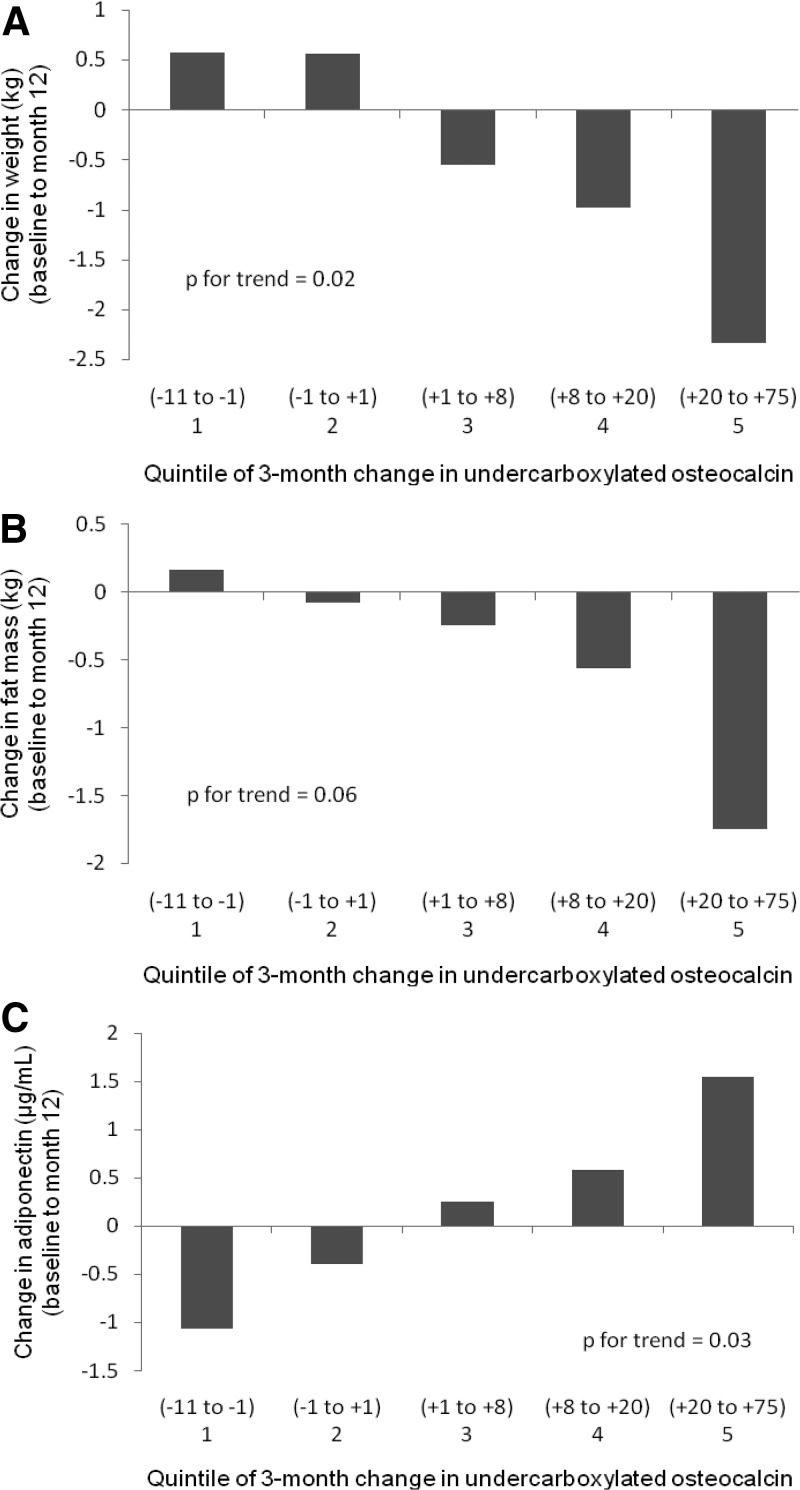
Among women treated with PTH(1-84), greater 3-month increases in ucOC were correlated with decreases in body weight and fat mass (Table 2). Among women treated with alendronate, these effects did not reach statistical significance but were in the same direction. There was no evidence for a differential effect of change in ucOC on change in weight or fat mass between the two treatment groups using linear models (P = 0.18 and P = 0.11, respectively). In the overall sample, the 3-month change in ucOC was inversely associated with the 12-month change in weight after adjustment for age and treatment group (Table 3). Weight loss increased across quintiles of change in ucOC (P = 0.02 for trend) (Fig. 2A). We found a trend toward a parallel effect on fat mass (P = 0.06) (Table 3 and Fig. 2B).
Table 2.
Unadjusted correlations between 3-month change in ucOC and 12-month changes in measures of fat and glucose metabolism, stratified by treatment group
| Study group | 12-Month changes |
|||||||
|---|---|---|---|---|---|---|---|---|
| Body weight | Fat mass | Adiponectin | Leptin | Insulin | Glucose | Insulin to glucose ratio | ||
| 3-Month change in ucOC | All participants | −0.27 (P = 0.01) | −0.17 (P = 0.10) | 0.29 (P < 0.01) | <0.01 (P = 0.99) | −0.08 (P = 0.42) | −0.04 (P = 0.73) | −0.06 (P = 0.54) |
| PTH (1-84) | −0.32 (P = 0.01) | −0.30 (P = 0.02) | 0.29 (P = 0.02) | −0.02 (P = 0.86) | −0.21 (P = 0.09) | −0.14 (P = 0.26) | −0.16 (P = 0.22) | |
| Alendronate | −0.21 (P = 0.24) | −0.11 (P = 0.55) | 0.06 (P = 0.75) | 0.19 (P = 0.30) | <0.01 (P = 0.99) | 0.09 (P = 0.62) | −0.13 (P = 0.48) | |
Values are Spearman's coefficients of correlation, with associated P values.
Table 3.
Association between 3-month change in ucOC and 12-month changes in metabolic parameters in all participants
| Model | 12-Month change |
|||||
|---|---|---|---|---|---|---|
| Weight |
Fat mass |
Adiponectin |
||||
| β (95% CI) | P value | β (95% CI) | P value | β (95% CI) | P value | |
| Unadjusted | −0.24 (−0.44, −0.05) | 0.02 | −0.17 (−0.37, 0.03) | 0.10 | 0.26 (0.06, 0.46) | 0.01 |
| Adjusted for age + treatment group | −0.25 (−0.49, −0.01) | 0.04 | −0.23 (−0.47, 0.01) | 0.06 | 0.30 (0.07, 0.53) | 0.01 |
| Adjusted for age + treatment group + change in fat mass | 0.23 (0.00, 0.45) | 0.048 | ||||
Values for β are standardized regression coefficients.
Fig. 2.
Mean changes in body weight (A), fat mass (B), and adiponectin (C) by quintile of the 3-month change in ucOC, adjusted for age and treatment group [PTH(1-84) vs. alendronate]. Ranges for each quintile are in nanograms per milliliter.
Change in ucOC and changes in adiponectin, leptin, insulin, and glucose levels
The 3-month change in ucOC was positively correlated with the 12-month change in adiponectin (Table 2). This association remained statistically significant after adjustment for age and treatment group (Table 3 and Fig. 2C). It was attenuated but remained statistically significant after further adjustment for change in fat mass. There was no evidence for an interaction between change in ucOC and treatment group (P = 0.90 for interaction).
We found no evidence for an association between the 3-month change in ucOC and the 12-month change in leptin, the other adipokine measured (P = 0.92 after adjustment for age and treatment group).
The 3-month change in ucOC was not correlated with the 12-month change in insulin, glucose, or insulin to glucose ratio, overall or within the treatment groups (Table 2). Results were unchanged after adjustment for age and treatment group (data not shown). There was no evidence for an interaction between the 3-month change in ucOC and treatment group for those metabolic measures. Exclusion of participants with an insulin level with CV greater than 20% yielded similar results. In assessing the effects of the 12-month change in ucOC, we found weak evidence for an inverse relationship with the 12-month change in insulin (P = 0.08 after adjustment for age and treatment group). Results for the 12-month change in ucOC were otherwise similar to the findings for the 3-month change in ucOC.
Discussion
In recent studies, the administration of ucOC to mice has been shown to decrease fat pad mass, increase expression of the insulin-sensitizing adipokine adiponectin, and improve glucose handling (2). To translate these animal studies to human subjects, we took advantage of the effects of osteoporosis medications on OC and its precursor protein, ucOC. We investigated whether metabolic changes similar to those seen in mice accompany osteoporosis medication-induced changes in ucOC. By sampling women treated with PTH(1-84), which is known to increase osteoblastic activity, or alendronate, which targets osteoclastic activity but also decreases osteoblastic activity, we increased our power to detect these associations.
This is the first longitudinal study in human subjects of the effect of changes in ucOC on weight, fat mass, and adiponectin. We found that 3-month increases in ucOC were associated with decreases in body weight and fat. In addition, 3-month increases in ucOC were associated with increases in adiponectin, independent of change in fat mass.
Our observations are consistent with the findings of animal studies of ucOC (1, 2) as well as with the cross-sectional findings of other human subjects studies. In the published human studies to date, total OC has been inversely associated with fat mass (4, 5), percentage fat (3–5, 7), and waist circumference (6) in men. ucOC was inversely associated with percentage fat in men (9). In postmenopausal women, total OC was positively associated with adiponectin (3, 9).
An important strength of our longitudinal study is the broad range of ucOC change resulting from the osteoporosis therapies compared in the PaTH study. Although our observational analysis cannot establish a causal relationship between ucOC and weight loss, fat mass loss, or adiponectin secretion, the changes in ucOC we witnessed were primarily driven by the pharmacological interventions of the PaTH trial. Thus, the associations between changes in ucOC and metabolic parameters found in our study suggest there may be a link between the skeleton and energy metabolism in humans.
Recent experiments by Foresta et al. (17) raised the possibility that adipose tissue itself can produce and secrete ucOC. In light of that study, in which fat mass was associated with a reduced level of ucOC, it is conceivable that a reduction in fat mass in our PTH(1-84) group could contribute to a rise in ucOC, above and beyond the treatment-driven increase in osteoblast-secreted ucOC. We lacked adequate power to implement more complex analyses to disentangle potential bidirectional effects between changes in ucOC and fat mass, so such analyses proved uninformative with our data.
Adiponectin increases when fat mass decreases (18, 19). We found that greater increases in ucOC were associated with greater increases in adiponectin, even after adjustment for loss of fat mass. This suggestion of a direct effect of ucOC on adiponectin via a pathway other than fat mass is consistent with previous cell culture experiments, in which OC induced expression of adiponectin by adipocytes (1, 2). In previous cell culture and animal experiments, other adipokines including leptin were not related to OC (1), and in our study, change in ucOC was similarly unassociated with change in leptin.
A limitation of this study is that participants were not required to fast before their morning blood draws. Thus, the serum glucose and insulin levels may be a poor measure of glucose handling. This may have contributed to our failure to find an association between change in ucOC and changes in glucose and insulin.
We did not find statistically significant differences between the PTH(1-84) and alendronate groups in 12-month changes in metabolic parameters, even though PTH(1-84) increased ucOC and alendronate decreased it. This may in part have been due to the substantial variability in the ucOC responses within the PTH(1-84) group. This variability motivated our study design, and by focusing directly on the changes in ucOC, rather than treatment, we were able to detect the influence of the changes on metabolic parameters. Variability in the effects of teriparatide PTH[1-34] on ucOC may also help explain results reported by Anastasilakis et al. (20). In that small study of 25 postmenopausal women with osteoporosis, without a placebo control group, no changes in glucose or insulin levels were seen in oral glucose tolerance testing after 6 months of treatment. These results suggest that the effects of PTH, alendronate, and other osteoporosis medications on fat and glucose metabolism should be evaluated in larger, placebo-controlled trials. It is possible that, even if ucOC plays a role in the skeletal regulation of energy metabolism, interventions that modulate ucOC could ultimately prove inadequate at improving metabolism in clinically meaningful ways.
A strength of this study is that we measured ucOC as well as total OC. Lee et al. (1) demonstrated that it was the undercarboxylated form of the protein which has metabolic effects, and thus, we used change in ucOC as our predictor of interest. By measuring total OC and ucOC, we were also able to investigate whether treatment with PTH(1-84) or alendronate changes the proportion of OC that is undercarboxylated. Alendronate therapy did not change this proportion, consistent with the findings of Hirao et al. (10). In contrast, PTH(1-84) therapy increased the ucOC to total OC ratio. This may be due to an inability of the γ-carboxylation reaction to keep up with the dramatic rise in OC secretion during the anabolic therapy.
In conclusion, we demonstrated that change in ucOC induced by the osteoporosis medications PTH(1-84) and alendronate is associated with metabolic changes. Participants with greater increases in ucOC had greater decreases in body weight and fat mass and greater increases in the insulin-sensitizing adipokine adiponectin. These associations correspond with observations from animal models and may support a role for ucOC in the skeletal regulation of energy metabolism in humans.
Acknowledgments
The PaTH study was supported by a contract with the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS-045, N01-AR-9-2245; to D.M.B.). The study drugs were supplied by NPS Pharmaceuticals, Inc. [PTH(1-84)] and Merck & Co., Inc. (alendronate). The authors have full control of all primary data. This ancillary study was supported by the Veterans Affairs Research Enhancement Award Program. Additional support for A.L.S. came from the Department of Veterans Affairs, through a Health Issues of Women Veterans Fellowship and a Career Development Award.
Disclosure Summary: A.L.S., C.J.R., E.V., J.P.B., and D.M.S. have nothing to disclose. D.E.S. has received research support from Amgen and Novartis. A.V.S. has received research support from Merck. L.P. has consulted for NPS Pharmaceuticals, Inc. and Nycomed. D.M.B. has received research support from Amgen, Merck, Novartis, and Roche and has consulted for Nycomed.
Footnotes
- BMD
- Bone mineral density
- CI
- confidence interval
- CV
- coefficient of variation
- OC
- osteocalcin
- PaTH
- Parathyroid Hormone and Alendronate study
- ucOC
- undercarboxylated osteocalcin.
References
- 1. Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. 2007. Endocrine regulation of energy metabolism by the skeleton. Cell 130:456–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferron M, Hinoi E, Karsenty G, Ducy P. 2008. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci USA 105:5266–5270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kanazawa I, Yamaguchi T, Yamamoto M, Yamauchi M, Kurioka S, Yano S, Sugimoto T. 2009. Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J Clin Endocrinol Metab 94:45–49 [DOI] [PubMed] [Google Scholar]
- 4. Pittas AG, Harris SS, Eliades M, Stark P, Dawson-Hughes B. 2009. Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab 94:827–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kindblom JM, Ohlsson C, Ljunggren O, Karlsson MK, Tivesten A, Smith U, Mellström D. 2009. Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men. J Bone Miner Res 24:785–791 [DOI] [PubMed] [Google Scholar]
- 6. Yeap BB, Chubb SA, Flicker L, McCaul KA, Ebeling PR, Beilby JP, Norman PE. 2010. Reduced serum total osteocalcin is associated with metabolic syndrome in older men via waist circumference, hyperglycemia, and triglyceride levels. Eur J Endocrinol 163:265–272 [DOI] [PubMed] [Google Scholar]
- 7. Zhou M, Ma X, Li H, Pan X, Tang J, Gao Y, Hou X, Lu H, Bao Y, Jia W. 2009. Serum osteocalcin concentrations in relation to glucose and lipid metabolism in Chinese individuals. Eur J Endocrinol 161:723–729 [DOI] [PubMed] [Google Scholar]
- 8. Im JA, Yu BP, Jeon JY, Kim SH. 2008. Relationship between osteocalcin and glucose metabolism in postmenopausal women. Clin Chim Acta 396:66–69 [DOI] [PubMed] [Google Scholar]
- 9. Kanazawa I, Yamaguchi T, Yamauchi M, Yamamoto M, Kurioka S, Yaho S, Sugimoto T. 2011. Serum undercarboxylated osteocalcin was inversely associated with plasma glucose level and fat mass in type 2 diabetes mellitus. Osteoporos Int 22:187–194 [DOI] [PubMed] [Google Scholar]
- 10. Hirao M, Hashimoto J, Ando W, Ono T, Yoshikawa H. 2008. Response of serum carboxylated and undercarboxylated osteocalcin to alendronate monotherapy and combined therapy with vitamin K2 in postmenopausal women. J Bone Miner Metab 26:260–264 [DOI] [PubMed] [Google Scholar]
- 11. Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, Garnero P, Bouxsein ML, Bilezikian JP, Rosen CJ. 2003. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 349:1207–1215 [DOI] [PubMed] [Google Scholar]
- 12. Sellmeyer DE, Black DM, Palermo L, Greenspan S, Ensrud K, Bilezikian J, Rosen CJ. 2007. Heterogeneity in skeletal response to full-length parathyroid hormone in the treatment of osteoporosis. Osteoporos Int 18:973–979 [DOI] [PubMed] [Google Scholar]
- 13. Vergnaud P, Garnero P, Meunier PJ, Bréart G, Kamihagi K, Delmas PD. 1997. Undercarboxylated osteocalcin measured with a specific immunoassay predicts hip fracture in elderly women: the EPIDOS study. J Clin Endocrinol Metab 82:719–724 [DOI] [PubMed] [Google Scholar]
- 14. Gundberg CM, Nieman SD, Abrams S, Rosen H. 1998. Vitamin K status and bone health: an analysis of methods for determination of undercarboxylated osteocalcin. J Clin Endocrinol Metab 83:3258–3266 [DOI] [PubMed] [Google Scholar]
- 15. Kaptoge S, Dalzell N, Folkerd E, Doody D, Khaw KT, Beck TJ, Loveridge N, Mawer EB, Berry JL, Shearer MJ, Dowsett M, Reeve J. 2007. Sex hormone status may modulate rate of expansion of proximal femur diameter in older women alongside other skeletal regulators. J Clin Endocrinol Metab 92:304–313 [DOI] [PubMed] [Google Scholar]
- 16. Conway SP, Wolfe SP, Brownlee KG, White H, Oldroyd B, Truscott JG, Harvey JM, Shearer MJ. 2005. Vitamin K status among children with cystic fibrosis and its relationship to bone mineral density and bone turnover. Pediatrics 115:1325–1331 [DOI] [PubMed] [Google Scholar]
- 17. Foresta C, Strapazzon G, De Toni L, Gianesello L, Calcagno A, Pilon C, Plebani M, Vettor R. 2010. Evidence for osteocalcin production by adipose tissue and its role in human metabolism. J Clin Endocrinol Metab 95:3502–3506 [DOI] [PubMed] [Google Scholar]
- 18. Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL, Chen CL, Tai TY, Chuang LM. 2001. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab 86:3815–3819 [DOI] [PubMed] [Google Scholar]
- 19. Faraj M, Havel PJ, Phélis S, Blank D, Sniderman AD, Cianflone K. 2003. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab 88:1594–1602 [DOI] [PubMed] [Google Scholar]
- 20. Anastasilakis AD, Efstathiadou Z, Plevraki E, Koukoulis GN, Slavakis A, Kita M, Avramidis A. 2008. Effect of exogenous intermittent recombinant human PTH 1-34 administration and chronic endogenous parathyroid hormone excess on glucose homeostasis and insulin sensitivity. Horm Metab Res 40:702–707 [DOI] [PubMed] [Google Scholar]