Abstract
Background:
Kisspeptin peptides are critical in human reproductive physiology and are potential therapies for infertility. Kisspeptin-10 stimulates gonadotropin release in both male and female rodents. However, few studies have investigated the effects of kisspeptin-10 on gonadotropin release in humans, and none have investigated the effect in women. If kisspeptin is to be useful for treating reproductive disease, its effects in both men and women must be established.
Aim:
To compare the effects of kisspeptin-10 administration on reproductive hormone release in healthy men and women.
Methods:
Intravenous bolus kisspeptin-10 was administered to men and women (n = 4–5 per group). Subcutaneous bolus and iv infusion of kisspeptin-10 was also administered to female women (n = 4–5 per group). Circulating reproductive hormones were measured.
Results:
In healthy men, serum LH and FSH were elevated after iv bolus kisspeptin-10, at doses as low as 0.3 and 1.0 nmol/kg, respectively. In healthy women during the follicular phase of the menstrual cycle, no alterations in serum gonadotropins were observed after iv bolus, sc bolus, or iv infusion of kisspeptin-10 at maximal doses of 10 nmol/kg, 32 nmol/kg, and 720pmol/kg/min, respectively. In women during the preovulatory phase, serum LH and FSH were elevated after iv bolus kisspeptin-10 (10 nmol/kg).
Conclusion:
Kisspeptin-10 stimulates gonadotropin release in men as well as women during the preovulatory phase of menstrual cycle but fails to stimulate gonadotropin release in women during the follicular phase. The sexual dimorphism of the responsiveness of healthy men and women to kisspeptin-10 administration has important clinical implications for the potential of kisspeptin-10 to treat disorders of reproduction.
The kisspeptins are critical regulators of mammalian reproductive physiology (1, 2). In humans, inactivating mutations of the kisspeptin receptor (KISS1R) cause pubertal failure (3, 4), and activating mutations can lead to precocious puberty (5). The human kisspeptin peptides, kisspeptin-10, -13, -14, and -54, are named according to their number of constituent amino acids (6). All kisspeptin peptides share the C-terminal decapeptide sequence, kisspeptin-10, which is required for biological activity in vitro (7).
Central or peripheral administration of the shorter kisspeptin peptide, kisspeptin-10, has been demonstrated to stimulate gonadotropin release in several mammalian species (8–18). This effect is abolished by preadministration of an antagonist to the hypothalamic hormone GnRH (10, 15). Kisspeptin-10 is therefore thought to stimulate the release of GnRH from the hypothalamus, which then stimulates gonadotropin release from the pituitary gland. The longer kisspeptin peptide, kisspeptin-54, has also been shown to stimulate gonadotropin release in rodents (19–21). In addition, administration of kisspeptin-54 stimulates gonadotropin secretion in humans (22–25). Kisspeptin therefore has the potential to become a novel therapy for treatment of reproductive disorders in humans.
The shorter amino acid sequence of kisspeptin-10 makes it simpler and cheaper to synthesize than kisspeptin-54. Future kisspeptin therapies may therefore be based upon kisspeptin-10 rather than kisspeptin-54. It is therefore therapeutically important to determine whether kisspeptin-10 can stimulate reproductive hormone release in healthy men and women. Kisspeptin-10 stimulates gonadotropin release in male rhesus monkeys (13–15). Furthermore, two recent reports have suggested that kisspeptin-10 administration to healthy men stimulates gonadotropin release (26, 27). Although kisspeptin-10 is known to stimulate gonadotropin release in animals (8, 9, 12, 17), there are no published data examining the effects of administering kisspeptin-10 to female primates or humans.
This study aimed to determine the effects of kisspeptin-10 administration on reproductive hormone release in healthy men and, for the first time, in healthy women.
Subjects and Methods
Subjects
The study was conducted with Ethics Committee approval (reference 08/H0707/95) in accordance with The Declaration of Helsinki. Written informed consent was obtained from all subjects. Thirty-five healthy female subjects and 11 healthy male subjects were recruited, using criteria summarized in Supplemental Table 1 (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Study days
Subjects were admitted to our Clinical Investigation Unit and asked to lay supine for the duration of each study. Urine was tested to exclude pregnancy in women (Clearview Easy-HCG; Inverness Medical Innovations Inc., Waltham, MA). All blood samples were analyzed for measurement of serum LH, FSH, estradiol (in women) or testosterone (in men), and plasma kisspeptin immunoreactivity (IR). Heart rate, blood pressure, and the presence of adverse symptoms were recorded at regular intervals.
Study 1: effects of iv bolus injection of saline or kisspeptin-10 in healthy male volunteers
Intravenous bolus injection of 0.9% saline or kisspeptin-10 (at doses of 0.3, 1.0, 3.0, or 10 nmol/kg) was administered at time 0 min. Blood samples were taken at −30, 0, 10, 20, 30, 40, 50, 60, 75, 90, 120, 150, and 180 min (n = 4–5 per group).
Study 2: effects of iv bolus injection of kisspeptin-10 or kisspeptin-54 in healthy female volunteers
Follicular phase of the menstrual cycle
Women between d 2–10 of their menstrual cycle were administered an iv bolus injection of 0.9% saline, kisspeptin-54 (1.0 nmol/kg), or kisspeptin-10 (at doses of 1.0, 3.0, or 10 nmol/kg) at time 0 min, and blood samples were taken at −30, 0, 10, 20, 30, 40, 50, 60, 75, 90, 120, 150, and 180 min (n = 4–5 per group).
Preovulatory phase of the menstrual cycle
Women 15–16 d before their next predicted period received iv bolus injection of 10 nmol/kg kisspeptin-10 as described for the follicular phase (n = 5).
Study 3: effects of sc bolus injection of saline or kisspeptin-10 in healthy female volunteers in the follicular phase of the menstrual cycle
Kisspeptin-10 (at doses 2, 4, 8, 16, or 32 nmol/kg) or 0.9% saline was administered sc at time 0 min, and blood samples were taken at −30, 0, 15, 30, 45, 60, 75, 90, 120, 150, 180, 210, and 240 min (n = 4–5 per group).
Study 4: effects of iv infusion of saline or kisspeptin-10 in healthy female volunteers in the follicular phase of the menstrual cycle
Kisspeptin-10 was dissolved in saline containing gelofusine (5% vol/vol) (B. Braun Medical, Sheffield, UK) and was infused iv over 90 min. During the first 30 min of infusion, the volunteers were administered 20, 50, 90, 180, 360, or 720 pmol/kg/min. The infusion rate for each volunteer was then halved for the remaining 60 min of each infusion. Blood samples were taken at −30, 0, 15, 30, 45, 60, 75, 90, 120, 150, 180, 210, and 240 min.
Study 5: determining the half-life of kisspeptin-10 in healthy female and male volunteers
To determine the plasma half-life of kisspeptin-10 in men, and in women during the follicular and preovulatory phases of the menstrual cycle, frequent blood sampling was performed during iv infusion of 360 pmol/kg/min kisspeptin-10. The protocol was identical to that used in study 4; except that detailed blood sampling was performed at 1-min (from 91–100 min) and 2-min (from 102–120 min) intervals immediately after stopping kisspeptin-10 infusion. Blood samples were assayed for plasma kisspeptin IR. The decay curve of kisspeptin IR was used to calculate the half-time of disappearance (t1/2) for infused kisspeptin-10 as described previously (22).
Data analysis
Data are presented as mean ± sem. Time profiles of hormone levels were compared using two-way ANOVA with Bonferroni's multiple-comparison test. Pairs of means were compared with unpaired t tests (or Mann-Whitney U test if nonparametric), and multiple means of area under curve (AUC) reproductive hormone release were compared using one-way ANOVA with Bonferonni's multiple-comparison test (or Kruskall-Wallis with Dunn's multiple-comparison tests if nonparametric). Slopes of linear regression curves were compared using an F test. In all cases, P < 0.05 was considered statistically significant. All data of serum reproductive hormones during treatment are presented as increases in serum levels after injection when compared with preinjection levels.
Results
Baseline characteristics of the subjects recruited to the study are summarized in Table 1.
Table 1.
Baseline characteristics of healthy male and female volunteers administered kisspeptin-10
| Baseline characteristic | Healthy male volunteers (n = 11) | Healthy female volunteers (n = 35) |
|---|---|---|
| Age (yr) | 28.8 ± 2.1 | 30.4 ± 0.19 |
| BMI (kg/m2) | 24.5 ± 0.5 | 22.9 ± 0.1 |
| Length of menstrual cycle (d) | 27 ± 0.1 | |
| LH (IU/liter) | 2.9 ± 0.2 | |
| Follicular | 3.8 ± 0.6 | |
| Preovulatory | 6.8 ± 1.6a | |
| FSH (IU/liter) | 2.6 ± 0.2 | |
| Follicular | 3.7 ± 0.4 | |
| Preovulatory | 6.8 ± 1.6b | |
| Testosterone (nmol/liter) | 21.6 ± 1.5 | |
| Estradiol (pmol/liter) | ||
| Follicular | 256 ± 43 | |
| Preovulatory | 562 ± 195a |
Female endocrine profiles are presented during the follicular and preovulatory phases of the menstrual cycle. Data are shown as mean ± sem.
P < 0.05 vs. follicular phase of the menstrual cycle.
P < 0.01 vs. follicular phase of the menstrual cycle.
Study 1: effects of iv bolus injection of saline or kisspeptin-10 in healthy male volunteers
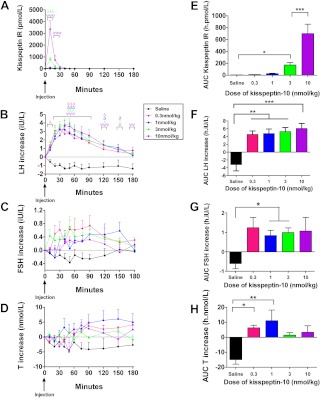
Plasma kisspeptin IR was elevated after iv bolus injection of kisspeptin-10 at all doses in healthy male volunteers (Fig. 1, A and E). The highest plasma kisspeptin IR was observed after 10 nmol/kg kisspeptin-10 (mean AUC kisspeptin IR was 700 ± 160 h·pmol/liter, P < 0.001 vs. saline). At this dose, mean peak kisspeptin IR (3350 ± 725 pmol/liter) was observed 10 min after injection, and plasma kisspeptin IR returned to undetectable levels 50 min after injection. Serum LH was elevated significantly after administration of each tested dose of iv bolus kisspeptin-10 injection compared with saline (Fig. 1, B and F). Peak stimulation of serum LH was observed 30–40 min after injection, and levels of serum LH gradually returned to baseline 180 min after injection (Fig. 1B). Maximal stimulation of LH was observed after iv bolus 10 nmol/kg kisspeptin-10 (mean AUC LH increase was 6.1 ± 1.3 h·IU/liter, P < 0.001 vs. saline) (Fig. 1F). Serum FSH was significantly increased compared with saline injection after iv bolus injection of 1.0 or 3.0 nmol/kg kisspeptin-10 (Fig. 1, C and G). Serum testosterone was significantly increased compared with saline injection after iv bolus injection of 0.3 or 1.0 nmol/kg kisspeptin-10 (Fig. 1, D and H). Serum levels of testosterone at these doses steadily increased to peak levels 150–180 min after injection (Fig. 1D).
Fig. 1.
Plasma kisspeptin IR and serum reproductive hormone levels after iv bolus injection of kisspeptin-10 to healthy male volunteers. A–D, Time profiles for plasma kisspeptin IR (A) and changes in serum LH (B), FSH (C), and testosterone (D) during 4 h after iv bolus injection of saline or kisspeptin-10 to healthy male volunteers (n = 4–5 per group). For 10 nmol/kg vs. saline: ψ, P < 0.05; ψψ, P < 0.01; ψψψ, P < 0.001. For 3 nmol/kg vs. saline: λ, P < 0.05; λλλ, P < 0.001. For 1 nmol/kg vs. saline: Δ, P < 0.05; ΔΔΔ, P < 0.001. E–H, AUC for plasma kisspeptin IR (E) and changes in serum LH (F), FSH (G), and testosterone (H) during 4 h after iv bolus injection of saline or kisspeptin-10 to healthy male volunteers (n = 4–5 per group). *, P < 0.05; **, P < 0.01; ***, P < 0.001. T, Testosterone. Data are shown as mean ± sem.
Study 2: effects of iv bolus injection of saline or kisspeptin-10 or kisspeptin-54 in healthy female volunteers
Follicular phase of the menstrual cycle
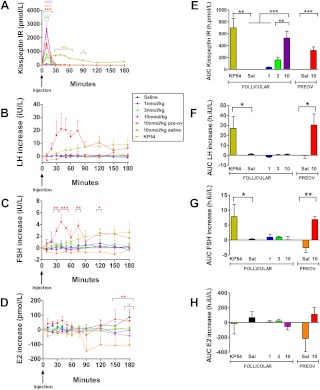
Plasma kisspeptin IR was elevated after iv bolus injection of kisspeptin-10 at all doses in healthy female volunteers during the follicular phase of menstrual cycle (Fig. 2, A and E). The highest plasma kisspeptin IR was observed after iv bolus injection of 10 nmol/kg kisspeptin-10 (mean AUC kisspeptin IR in women during follicular phase was 527 ± 108 h·pmol/liter, P < 0.001 vs. saline); this was lower when compared with kisspeptin IR after injection of the same dose of kisspeptin-10 to men, but this difference was not significant (P = 0.42 vs. men). At this dose, mean peak kisspeptin IR (2638 ± 302 pmol/liter) was observed 10 min after injection, and plasma kisspeptin IR returned to undetectable levels 50 min after injection. Gonadotropin release was elevated significantly after iv bolus injection of 1 nmol/kg kisspeptin-54 [mean AUC increase (in h·IU/liter): 27.0 ± 11.8 (LH), P < 0.05 vs. saline, and 7.9 ± 4.1 (FSH), P < 0.05 vs. saline). Unexpectedly, no significant changes in serum levels of reproductive hormones were observed after iv bolus injection of kisspeptin-10 at doses up to 10 nmol/kg (Fig. 2, B–D and F–H).
Fig. 2.
Plasma kisspeptin IR and serum reproductive hormone levels after iv bolus injection of kisspeptin-10 to healthy female volunteers. A–D, Time profiles for plasma kisspeptin IR (A) and changes in serum LH (B), FSH (C), and estradiol (D) during 4 h after iv bolus injection of saline, kisspeptin-10 (KP10), or kisspeptin-54 (KP54) to healthy female volunteers. For 10 nmol/kg KP10 vs. saline: ψ, P < 0.05; ψψ, P < 0.01; ψψψ, P < 0.001. For 3 nmol/kg KP10 vs. saline: λλλ, P < 0.001. For 1 nmol/kg KP54 vs. saline: κ, P < 0.05; κκ, P < 0.01; κκκ, P < 0.001. For 10 nmol/kg KP10 (Preov) vs. saline: *, P < 0.05; **, P < 0.01; ***, P < 0.001. E–H, AUC for plasma kisspeptin IR (E) and changes in serum LH (F), FSH (G), and estradiol (H) during 4 h after iv bolus injection of saline or kisspeptin-10 to healthy female volunteers. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Data are shown as mean ± sem. E2, Estradiol; Preov, preovulatory phase of the menstrual cycle.
Preovulatory phase of the menstrual cycle
Kisspeptin IR was elevated significantly in women during the preovulatory phase after kisspeptin-10 injection compared with saline, and this elevation was nonsignificantly different when compared with kisspeptin IR after the same dose of kisspeptin-10 in follicular-phase women or men (mean AUC kisspeptin IR in preovulatory phase was 320 ± 56 h·pmol/liter, P = 0.13 vs. follicular phase, and P = 0.06 vs. men) (Fig. 2E). Serum LH and FSH were elevated significantly after iv bolus injection of 10 nmol/kg kisspeptin-10 in women during the preovulatory phase of the menstrual cycle [mean AUC increase was 30.3 ± 7.7 h·IU/liter (LH), P < 0.05 vs. saline, and 6.9 ± 0.9 h·IU/liter (FSH), P < 0.01 vs. saline] (Fig. 2, B, C, F, and G); however, serum estradiol was not altered significantly (mean AUC estradiol increase was 111 ± 96 h·pmol/liter, P = 0.14 vs. saline) (Fig. 2, D and H).
Study 3: effects of sc bolus injection of saline or kisspeptin-10 in healthy female volunteers in the follicular phase of the menstrual cycle
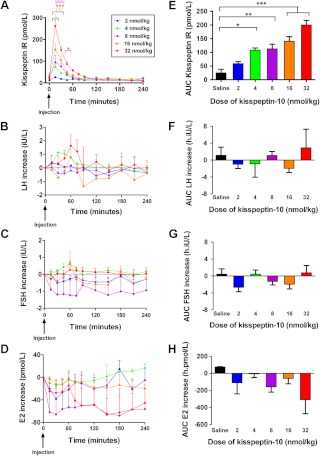
Plasma kisspeptin IR was significantly elevated during the 4 h after sc injection of kisspeptin-10 at doses of 4 nmol/kg and higher, when compared with saline (Fig. 3A). The highest plasma kisspeptin IR after sc injection was observed after injection of 32 nmol/kg kisspeptin-10 (mean AUC kisspeptin IR was 201 ± 16 h·pmol/liter, P < 0.001 vs. saline).
Fig. 3.
Plasma kisspeptin IR and serum reproductive hormone levels after sc bolus injection of kisspeptin-10 to healthy female volunteers in the follicular phase of the menstrual cycle. A–D, Time profiles for plasma kisspeptin IR (A) and changes in serum LH (B), FSH (C), and estradiol (D) during 4 h after sc bolus injection of kisspeptin-10 to healthy female volunteers in the follicular phase of the menstrual cycle (n = 4–5 per group). For 32 vs. 2 nmol/kg: *, P < 0.05; ***, P < 0.001. For 16 vs. 2 nmol/kg: ƒƒƒ, P < 0.001. For 8 vs. 2 nmol/kg: ψψψ, P < 0.001. For 4 vs. 2 nmol/kg: λ, P < 0.05. E–H, AUC for plasma kisspeptin IR (E) and changes in serum LH (F), FSH (G), and testosterone (H) during 4 h after sc bolus injection of kisspeptin-10 to healthy female volunteers in the follicular phase of the menstrual cycle (n = 4–5 per group). *, P < 0.05; **, P < 0.01; ***, P < 0.001. E2, Estradiol. Data are shown as mean ± sem.
No significant changes in serum reproductive hormone levels were observed after sc bolus injection of kisspeptin-10 at any dose (Fig. 3, B–H).
Study 4: effects of iv infusion of kisspeptin-10 in healthy female volunteers in the follicular phase of the menstrual cycle
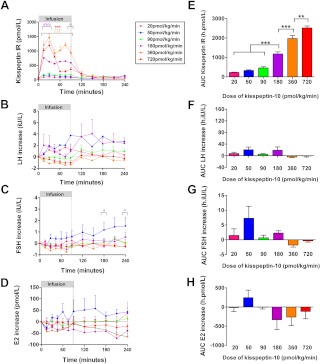
Intravenous infusion of peptides delivers the peptide directly into the circulation, avoiding possible degradation in sc tissue, and results in a sustained increase in circulating levels of the administered peptide.
Plasma kisspeptin IR increased during iv infusion of kisspeptin-10 at all doses (Fig. 4A). The highest plasma kisspeptin IR was observed during iv infusion of 720 pmol/kg/min kisspeptin-10 (mean AUC kisspeptin IR was 2518 ± 100 h·pmol/liter). All iv infusion doses of kisspeptin-10 were associated with a higher mean plasma kisspeptin IR than the highest studied sc dose of kisspeptin-10 (32 nmol/kg, mean AUC kisspeptin IR was 201 ± 16 h·pmol/liter). No significant changes in serum reproductive hormone levels were observed after iv infusion of any dose of kisspeptin-10 in healthy female volunteers in the follicular phase of the menstrual cycle (Fig. 4, B–H).
Fig. 4.
Plasma kisspeptin IR and serum reproductive hormone levels during iv infusion of kisspeptin-10 to healthy female volunteers in the follicular phase of the menstrual cycle. A–D, Time profiles for plasma kisspeptin IR (A) and changes in serum LH (B), FSH (C), and estrogen (D) during 4 h after commencement of a 90-min iv infusion of kisspeptin-10 to healthy female volunteers in the follicular phase of the menstrual cycle (n = 4–5 per group). For 720 vs. 20 pmol/kg · min: ***, P < 0.001. For 360 vs. 20 pmol/kg · min: ƒƒƒ, P < 0.001. For 180 vs. 20 pmol/kg · min: ψ, P < 0.05; ψψψ, P < 0.001. For 50 vs. 20 pmol/kg · min: Δ, P < 0.05. E–H, AUC for plasma kisspeptin IR (E) and changes in serum LH (F), FSH (G), and testosterone (H) during 4 h after commencement of a 90-min iv infusion of kisspeptin-10 to healthy female volunteers in the follicular phase of the menstrual cycle (n = 4–5 per group). **, P < 0.01; ***, P < 0.001. E2, Estradiol. Data are shown as mean ± sem.
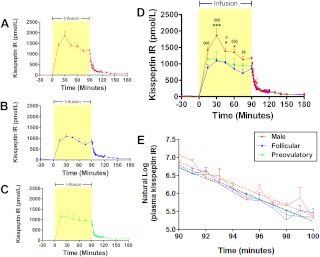
Study 5: pharmacokinetic profile of kisspeptin IR during iv infusion of kisspeptin-10 in healthy male and female volunteers
To determine the plasma half-life of kisspeptin-10, frequent blood sampling was performed in women during the follicular and preovulatory phases of menstrual cycle, and in men, after cessation of an iv infusion of 360 pmol/kg/min kisspeptin-10 (Fig. 5). When plotted on a natural log scale, linear regression slopes were not significantly different among the three groups (F = 0.099; degrees of Freedom numerator = 2; degrees of Freedom denominator = 111; P = 0.91), and plasma half-lives of kisspeptin-10 were calculated as 3.8 ± 0.3 min (men), 4.1 ± 0.4 min (follicular-phase women), and 4.1 ± 0.4 min (preovulatory-phase women) (Fig. 5E).
Fig. 5.
Detailed time profiles of plasma kisspeptin IR after iv infusion of kisspeptin-10 to healthy male and female volunteers. A–D, Blood sampling was performed for measurement of plasma kisspeptin IR during 4 h after commencement of a 90-min iv infusion of kisspeptin-10 (360 pmol/kg · min) to healthy male volunteers (A and D) and female volunteers during the follicular (B and D) and preovulatory (C and D) phases of the menstrual cycle (n = 4–5 per group). For men vs. women during follicular phase: φφφ, P < 0.001; φ, P < 0.05. For men vs. women during preovulatory phase: ***, P < 0.001; *, P < 0.05. E, Blood sampling was performed at 1-min intervals immediately after stopping infusion of kisspeptin-10. Linear curve fits of natural log plasma kisspeptin IR (dotted lines) were similar for all three groups (P = 0.91). Data are shown as mean ± sem.
Discussion
These studies reveal a previously unknown sexual dimorphism in responsiveness to kisspeptin-10 administration in healthy men and women. Numerous studies in animals have demonstrated that kisspeptin-10 robustly stimulates gonadotropin release, thus implicating kisspeptin signaling as a potential therapeutic target for treating female patients with infertility (8, 9, 12, 17). Kisspepin-10 stimulates gonadotropin release in male rhesus monkeys (13–15), and recent reports have suggested that kisspepeptin-10 administration stimulates gonadotropin release in healthy men (26, 27). However, the effects of administration of kisspeptin-10 in female primates or women have not previously been studied.
We observed that iv bolus injection of kisspeptin-10 robustly stimulated LH release in healthy men at all doses tested. These results are consistent with our previous observation that administration of the longer form of kisspeptin, kisspeptin-54, stimulates reproductive hormone release in men (22). Furthermore, two recent reports have shown that iv injection of kisspeptin-10 potently stimulates LH release in healthy male volunteers at doses similar to those used in this study (26, 27) and that prolonged infusion of kisspeptin-10 significantly stimulates LH pulsatility in healthy male volunteers (27).
Studies in rodents suggest that kisspeptin-10 stimulates gonadotropin release in both males and females (8, 9). Furthermore, we observed that the kisspeptin-10 peptide used in this study stimulated LH release in female adult mice (Supplemental Fig. 1), although with a much lower potency when compared with kisspeptin-54. We were therefore surprised to observe that doses of kisspeptin-10 identical to those used in men failed to stimulate reproductive hormone release in healthy female volunteers during the follicular phase of the menstrual cycle when administered as an iv bolus injection. It is possible that the lack of effect of iv administration of kisspeptin-10 to stimulate reproductive hormone release in women in the follicular phase of their menstrual cycle was due to the short period of time that circulating kisspeptin-10 levels were elevated after iv bolus administration. Subcutaneous bolus administration of kisspeptin-10 is likely to result in a longer period of elevated circulating levels of kisspeptin-10 than iv bolus administration (22, 23). Furthermore, we have previously determined that sc bolus injection of kisspeptin-54 at doses as low as 0.4 nmol/kg potently stimulates serum LH in healthy women during the follicular phase of the menstrual cycle (23). However, serum gonadotropin levels were not elevated by sc bolus injection of kisspeptin-10 in healthy women in the follicular phase of the menstrual cycle, despite elevations in plasma kisspeptin IR for up to 90 min after injection.
The lack of effect of sc bolus administration of kisspeptin-10 to stimulate reproductive hormone release in women in the follicular phase of their menstrual cycle may have been due to breakdown of kisspeptin-10 in the sc tissue. Intravenous infusion of peptides delivers the peptide directly into the circulation, avoiding possible degradation in sc tissue, and results in a sustained increase in circulating levels of the administered peptide. However, serum gonadotropin levels were not elevated during iv infusion of kisspeptin-10 to healthy female volunteers in the follicular phase of the menstrual cycle, despite markedly raised plasma levels of kisspeptin IR up to concentrations of 2000 pmol/liter. Our data therefore suggest that women in the follicular phase of the menstrual cycle are markedly less responsive to kisspeptin-10 administration than men. The underlying mechanism for this observation is unclear. However, sexual dimorphism of hypothalamic kisspeptin signaling pathways has been demonstrated in rodents (28–30).
It is important to consider whether the contrasting effects of kisspeptin-10 in men and women may have been attributable to factors other than differential sensitivity to the peptide. We observed that levels of plasma kisspeptin IR appeared slightly lower in women when compared with men when identical weight-adjusted doses of kisspeptin-10 were administered. It is possible that levels of kisspeptin-10 may have been modified by factors known to differ between the sexes, such as body fat content or clearance of peptide from the circulation (19). However, it is noteworthy that only a marginal elevation of plasma kisspeptin IR (approximately 10 h·pmol/liter) was necessary to stimulate significant LH secretion in men after injection of kisspeptin-10 (0.3 nmol/kg iv bolus); by contrast, a 200-fold greater elevation in plasma kisspeptin IR failed to stimulate LH release in women during the follicular phase of the menstrual cycle. Furthermore, we observed no significant differences in the plasma half-lives of kisspeptin-10 between men and women. It is therefore unlikely that the striking contrast between male and female responsiveness to kisspeptin-10 administration reflects differences in metabolism of kisspeptin-10. We observed nonsignificant reductions in serum LH (P = 0.19) and FSH (P = 0.91) after saline injection in men when compared with saline injection in women. Although both men and women underwent identical study protocols, it is possible that minor differences in factors inhibiting reproductive function such as stress may be greater in the male group. However, such a difference would not explain why men are more responsive to kisspeptin-10 than women in the follicular phase of the menstrual cycle.
In the current study, we observed that exogenous kisspeptin-54 stimulated significant gonadotropin secretion in women during the follicular phase of the menstrual cycle, despite a failure of exogenous kisspeptin-10 to stimulate gonadotropin secretion at a 10-fold higher molar dose. Furthermore, kisspeptin-54 injection stimulated LH in female mice more potently when compared with kisspeptin-10, and it has been previously observed that kisspeptin-52 (31) and kisspeptin-54 (21) stimulate LH more potently when compared with kisspeptin-10 in male rats. Collectively, these data suggest that the greater potency of kisspeptin-54 when compared with kisspeptin-10 may explain why exogenous kisspeptin-54 injection (but not exogenous kisspeptin-10) stimulates reproductive hormone release in women during the follicular phase of the menstrual cycle. The differences between kisspeptin-10 and kisspeptin-54 may be a consequence of the rapid breakdown of kisspeptin-10 in the circulation. The in vivo plasma half-life of iv kisspeptin-10 in this study was calculated to approximately 4 min, which is 7-fold shorter than the calculated in vivo plasma half-life of kisspeptin-54 (22).
It is interesting to consider whether men and women have differential responses to kisspeptin-54 (as they appear to be to kisspeptin-10) based on previous studies. Intravenous infusion of kisspeptin-54 at doses as low as 1.2 nmol (0.25 pmol/kg/min) stimulate LH secretion in healthy men (22). By comparison, sc bolus injection of kisspeptin-54 at doses of 28 nmol (0.4 nmol/kg) or more stimulate LH secretion in healthy women during the follicular phase of the menstrual cycle (23); furthermore, this study suggests that iv bolus injection of approximately 60 nmol (1 nmol/kg) kisspeptin-54 stimulates significant LH secretion in these women. These data demonstrate that, unlike kisspeptin-10, kisspeptin-54 can stimulate reproductive hormone release in women in the follicular phase of their menstrual cycle.
In the current study, gonadotropin responses during kisspeptin-10 infusion appeared more variable than after iv or sc bolus injections. These alterations in gonadotropin secretion did not appear to be related to either the dose or to the onset of kisspeptin infusion; however, it is possible that subtle effects of kisspeptin-10 infusion on gonadotropin release were not detected in the current study protocol.
We have previously demonstrated that women in the preovulatory phase of the menstrual cycle are significantly more sensitive to the effects of kisspeptin-54 on gonadotropin release when compared with women in the follicular phase of the menstrual cycle (23). In keeping with these observations, iv bolus injection of kisspeptin-10 significantly stimulated LH and FSH release in women during the preovulatory phase of the menstrual cycle in the current study. Because we observed that kisspeptin-10 has a similar pharmacokinetic profile in both follicular and preovulatory phases of the menstrual cycle, our results suggest that women have heightened sensitivity to kisspeptin-10 during the preovulatory phase of the menstrual cycle. In female rodents, c-fos expression within kisspeptin neurons and levels of kiss1 expression are increased within the anteroventral periventricular nucleus of the hypothalamus immediately before ovulation (35). Furthermore, levels of kiss1r expression are increased in rat hypothalamic fragments at diestrus when compared with proestrus (36). It is therefore possible that women in the preovulatory phase of the menstrual cycle have heightened responsiveness to kisspeptin-10 administration when compared with women during the follicular phase of the menstrual cycle.
In the current study, we observed that kisspeptin-10 stimulated LH secretion more potently than FSH. This is consistent with our previous studies of kisspeptin-54 administration in healthy men and women. After iv bolus kisspeptin-10 injection, peak FSH secretion was observed 45–150 min after injection, which was later than peak LH secretion (30 min after injection); this phenomenon was also observed after sc bolus injection of kisspeptin-54 in healthy women (23). Our results therefore suggest that both kisspeptin-10 and -54 stimulate LH secretion more potently and more rapidly than FSH.
It is interesting to consider why we did not observe any consistent stimulation of testosterone secretion after iv bolus kisspeptin-10 injection in healthy men when compared with the robust increases in serum LH and FSH observed at all tested doses. Significant increases in serum testosterone were observed only at 0.3 and 1.0 nmol/kg iv bolus kisspeptin-10, but these rises were marginal (no more than 10 h·nmol/liter above baseline). Furthermore, iv bolus injection of kisspeptin-10 stimulated gonadotropin release in women during the follicular phase of the menstrual cycle but did not increase serum estradiol during 3 h after injection. Our previous data suggest that at least 4 h are required for serum levels of sex steroids to peak after a sc bolus injection of kisspeptin-54 (23–25). A longer period of blood sampling after injection may have revealed more pronounced alterations in sex steroid secretion in subjects after injection of kisspeptin-10. We observed that serum gonadotropins almost returned to baseline levels within 3 h after iv bolus injection of kisspeptin-10; it is possible that iv bolus injection of kisspeptin-10 has a duration of action inadequate to stimulate significant gonadal sex steroid release.
In the current study, all tested iv bolus doses of kisspeptin-10 (0.3–10 nmol/kg) were associated with similar degrees of gonadotropin secretion in healthy male subjects. A recent study by George et al. (27) suggests that kisspeptin-10 stimulates serum LH secretion at doses as low as 0.01 μg/kg (equivalent to 0.008 nmol/kg), with a maximal response seen at 1 μg/kg (0.8 nmol/kg) in men. Taking these data into consideration, all doses of kisspeptin-10 selected during our study may have stimulated near-maximal levels of gonadotropin secretion in healthy male volunteers. Interestingly, George et al. (27) observed that the increase in serum LH at 3 μg/kg (2.4 nmol/kg) was lower than the increase in serum LH at 1 μg/kg, which they speculated was attributable to tachyphylaxis, a phenomenon that we have previously observed after chronic kisspeptin-54 injections (24, 25, 27). We therefore cannot exclude that the similarity between LH responses at doses between 0.3 and 10 nmol/kg kisspeptin-10 in men might be explained in part by tachyphylaxis to kisspeptin-10 at the higher tested doses. Additional studies are required to investigate these observations.
In summary, this is the first clinical study to report the effects of kisspeptin-10 administration on gonadotropin release in women and to compare the effects of kisspeptin-10 between men and women. Kisspeptin-10 robustly stimulates gonadotropin release in men but fails to stimulate gonadotropin release in healthy female volunteers in the follicular phase of the menstrual cycle when administered by iv bolus injection, sc bolus injection, or iv infusion. These experiments reveal sexual dimorphism in the responsiveness of healthy human volunteers to kisspeptin-10 administration. These findings have important clinical implications for the potential therapeutic use of kisspeptin-10 to treat disorders of reproduction.
Supplementary Material
Acknowledgments
We are grateful to the Wellcome Trust and Sir John McMichael Centre for providing infrastructure for this study.
The Section of Investigative Medicine is funded by grants from the Medical Research Council (MRC), Biotechnology and Biological Sciences Research Council, National Institute of Health Research (NIHR), an Integrative Mammalian Biology Capacity Building Award, and an FP7-HEALTH-2009-241592 EurOCHIP Grant and is supported by the NIHR Imperial Biomedical Research Centre Funding Scheme. This work was also funded by an MRC project grant. C.N.J. is supported by an NIHR Clinical Lectureship, Society for Endocrinology Early Career Grant, and Academy of Medical Sciences/Wellcome Starter Grant for Clinical Lecturers. G.M.K.N. is supported by a Wellcome Clinical Training Fellowship. A.N.C. is supported by an Imperial College Healthcare NHS Trust Charity Award. W.S.D. is supported by an NIHR Career Development Fellowship.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- Area under the curve
- IR
- immunoreactivity.
References
- 1. Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. 2001. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 411:613–617 [DOI] [PubMed] [Google Scholar]
- 2. Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CG, Wilson S, Bergsma DJ, Emson P, Faull R, Philpott KL, Harrison DC. 2001. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem 276:28969–28975 [DOI] [PubMed] [Google Scholar]
- 3. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. 2003. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. 2003. The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 5. Teles MG, Bianco SD, Brito VN, Trarbach EB, Kuohung W, Xu S, Seminara SB, Mendonca BB, Kaiser UB, Latronico AC. 2008. A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med 358:709–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bilban M, Ghaffari-Tabrizi N, Hintermann E, Bauer S, Molzer S, Zoratti C, Malli R, Sharabi A, Hiden U, Graier W, Knöfler M, Andreae F, Wagner O, Quaranta V, Desoye G. 2004. Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. J Cell Sci 117:1319–1328 [DOI] [PubMed] [Google Scholar]
- 7. Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brézillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. 2001. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem 276:34631–34636 [DOI] [PubMed] [Google Scholar]
- 8. Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. 2004. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 80:264–272 [DOI] [PubMed] [Google Scholar]
- 9. Navarro VM, Castellano JM, Fernández-Fernández R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. 2004. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology 145:4565–4574 [DOI] [PubMed] [Google Scholar]
- 10. Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. 2004. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077 [DOI] [PubMed] [Google Scholar]
- 11. Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. 2005. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caraty A, Smith JT, Lomet D, Ben Saïd S, Morrissey A, Cognie J, Doughton B, Baril G, Briant C, Clarke IJ. 2007. Kisspeptin synchronizes preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology 148:5258–5267, [DOI] [PubMed] [Google Scholar]
- 13. Plant TM, Ramaswamy S, Dipietro MJ. 2006. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology 147:1007–1013 [DOI] [PubMed] [Google Scholar]
- 14. Ramaswamy S, Seminara SB, Pohl CR, DiPietro MJ, Crowley WF, Jr, Plant TM. 2007. Effect of continuous iv administration of human metastin 45–54 on the neuroendocrine activity of the hypothalamic-pituitary-testicular axis in the adult male rhesus monkey (Macaca mulatta). Endocrinology 148:3364–3370 [DOI] [PubMed] [Google Scholar]
- 15. Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. 2005. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA 102:2129–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Greives TJ, Mason AO, Scotti MA, Levine J, Ketterson ED, Kriegsfeld LJ, Demas GE. 2007. Environmental control of kisspeptin: implications for seasonal reproduction. Endocrinology 148:1158–1166 [DOI] [PubMed] [Google Scholar]
- 17. Lents CA, Heidorn NL, Barb CR, Ford JJ. 2008. Central and peripheral administration of kisspeptin activates gonadotropin but not somatotropin secretion in prepubertal gilts. Reproduction 135:879–887 [DOI] [PubMed] [Google Scholar]
- 18. Kadokawa H, Matsui M, Hayashi K, Matsunaga N, Kawashima C, Shimizu T, Kida K, Miyamoto A. 2008. Peripheral administration of kisspeptin-10 increases plasma concentrations of GH as well as LH in prepubertal Holstein heifers. J Endocrinol 196:331–334 [DOI] [PubMed] [Google Scholar]
- 19. Tovar S, Vázquez MJ, Navarro VM, Fernández-Fernández R, Castellano JM, Vigo E, Roa J, Casanueva FF, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. 2006. Effects of single or repeated intravenous administration of kisspeptin upon dynamic LH secretion in conscious male rats. Endocrinology 147:2696–2704 [DOI] [PubMed] [Google Scholar]
- 20. Patterson M, Murphy KG, Thompson EL, Patel S, Ghatei MA, Bloom SR. 2006. Administration of kisspeptin-54 into discrete regions of the hypothalamus potently increases plasma luteinising hormone and testosterone in male adult rats. J Neuroendocrinol 18:349–354 [DOI] [PubMed] [Google Scholar]
- 21. Thompson EL, Murphy KG, Patterson M, Bewick GA, Stamp GW, Curtis AE, Cooke JH, Jethwa PH, Todd JF, Ghatei MA, Bloom SR. 2006. Chronic subcutaneous administration of kisspeptin-54 causes testicular degeneration in adult male rats. Am J Physiol Endocrinol Metab 291:E1074–E1082 [DOI] [PubMed] [Google Scholar]
- 22. Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR. 2005. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab 90:6609–6615 [DOI] [PubMed] [Google Scholar]
- 23. Dhillo WS, Chaudhri OB, Thompson EL, Murphy KG, Patterson M, Ramachandran R, Nijher GK, Amber V, Kokkinos A, Donaldson M, Ghatei MA, Bloom SR. 2007. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab 92:3958–3966 [DOI] [PubMed] [Google Scholar]
- 24. Jayasena CN, Nijher GM, Chaudhri OB, Murphy KG, Ranger A, Lim A, Patel D, Mehta A, Todd C, Ramachandran R, Salem V, Stamp GW, Donaldson M, Ghatei MA, Bloom SR, Dhillo WS. 2009. Subcutaneous injection of kisspeptin-54 acutely stimulates gonadotropin secretion in women with hypothalamic amenorrhea, but chronic administration causes tachyphylaxis. J Clin Endocrinol Metab 94:4315–4323 [DOI] [PubMed] [Google Scholar]
- 25. Jayasena CN, Nijher GM, Abbara A, Murphy KG, Lim A, Patel D, Mehta A, Todd C, Donaldson M, Trew GH, Ghatei MA, Bloom SR, Dhillo WS. 2010. Twice-weekly administration of kisspeptin-54 for 8 weeks stimulates release of reproductive hormones in women with hypothalamic amenorrhea. Clin Pharmacol Ther 88:840–847 [DOI] [PubMed] [Google Scholar]
- 26. Chan YM, Butler JP, Pinnell NE, Pralong FP, Crowley WF, Jr, Ren C, Chan KK, Seminara SB. 2011. Kisspeptin resets the hypothalamic GnRH clock in men. J Clin Endocrinol Metab 96:E908–E915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. George JT, Veldhuis JD, Roseweir AK, Newton CL, Faccenda E, Millar RP, Anderson RA. 2010. Kisspeptin 10 rapidly and potently stimulates LH secretion in men. J Clin Endocrinol Metab 96:E1228–E1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clarkson J, Herbison AE. 2006. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147:5817–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. 2007. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 53:367–378 [DOI] [PubMed] [Google Scholar]
- 30. Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. 2007. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 148:1774–1783 [DOI] [PubMed] [Google Scholar]
- 31. Soldin OP, Chung SH, Mattison DR. 2011. Sex differences in drug disposition. J Biomed Biotechnol 2011:187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. 2006. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 26:6687–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roa J, Vigo E, Castellano JM, Navarro VM, Fernández-Fernández R, Casanueva FF, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. 2006. Hypothalamic expression of KiSS-1 system and gonadotropin-releasing effects of kisspeptin in different reproductive states of the female Rat. Endocrinology 147:2864–2878 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.