Abstract
Context and Objective:
Dietary intake of animal proteins is associated with an increase in urinary calcium and nephrolithiasis risk. We tested the hypothesis that the acid load imposed by dietary proteins causes this hypercalciuria.
Design and Setting:
In a short-term crossover metabolic study, an alkali salt was provided with a high-protein diet (HPD) to neutralize the acid load imparted by dietary proteins.
Participants and Interventions:
Eleven healthy volunteers were evaluated at the end of each of four phases while consuming metabolic diets with fixed calcium and sodium content. Phases 1 and 3 consisted of a control diet (CD). Phases 2 and 4 consisted of a eucaloric HPD (60 g/d animal proteins added to CD). Along with HPD in phases 2 and 4, subjects ingested 30 mEq twice daily of either potassium citrate (KCitrate, alkaline salt) or potassium chloride (KCl, control neutral salt).
Results:
KCitrate completely neutralized the acid load imparted by HPD (based on changes in urine pH and net acid excretion) and increased urinary citrate. Urinary calcium increased during both HPD phases compared with CD but was not significantly different between the HPD + KCl and HPD + KCitrate phases (182 ± 85 vs. 170 ± 85 mg/d; P = 0.28). Increased urinary saturation with respect to calcium oxalate and uric acid with HPD was abrogated by KCitrate.
Conclusions:
This study suggests that, at least in the short-term, mechanism(s) other than acid load account for hypercalciuria induced by HPD. The beneficial effect of KCitrate on nephrolithiasis risk with HPD is through correction of declines in urine pH and citrate.
Dietary protein intake is a well-recognized determinant of urinary calcium excretion. On average, 24-h urine calcium rises by approximately 1 mg for every 1 g increase in daily protein intake (1, 2). This hypercalciuria accounts in part for the increased risk of kidney stone formation associated with excess protein consumption (3, 4). Persistent hypercalciuria may also lead to a negative calcium balance and bone loss (5, 6).
Several mechanisms have been postulated to explain the relationship between dietary protein intake and urinary calcium. The production of acid during metabolism of proteins may be responsible for hypercalciuria through mobilization of bone mineral for buffering of this acid load (7, 8) and/or via a reduction in renal tubular calcium reabsorption (9–11). Additionally, protein intake may enhance urinary calcium excretion through increased intestinal calcium absorption (12, 13) and/or through a rise in glomerular filtration rate (14). The goal of the present metabolic study was to evaluate the “acid load” hypothesis by assessing urinary calcium excretion and risk of nephrolithiasis in healthy subjects on a high animal protein diet given an alkali salt to neutralize the acid load imparted by dietary proteins.
Subjects and Methods
Study subjects
Study subjects were normal healthy adult volunteers of either gender and of any ethnicity who were recruited through advertisement. Exclusion criteria were history of nephrolithiasis, peptic ulcer disease, chronic diarrhea, intestinal surgery, or treatment with drugs that alter acid-base or potassium balance. Subjects were also excluded if any of the following abnormalities were present on screening laboratory studies: anemia, metabolic acidosis or alkalosis, hyper- or hypokalemia, hyper- or hypocalcemia, or decreased endogenous creatinine clearance (estimated glomerular filtration rate <90 ml/min). The study was approved by the University of Texas Southwestern (UTSW) Medical Center Institutional Review Board, and all subjects provided written informed consent.
Study protocol
Volunteers were studied in the UTSW Medical Center inpatient Clinical and Translational Research Center (CTRC; formerly General Clinical Research Center) at the end of each of the following 2-wk phases: phases 1 and 3, control diet (CD; 15% of calories from proteins; 1.0 g/kg · d); phases 2 and 4, high-protein diet (HPD; in which 60 g/d of animal protein was added to CD while maintaining constant total caloric intake) (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). During the first week of each phase, subjects consumed a diet at home under the direction of the CTRC dietitian to match the recommended protein, sodium, and calcium intake. Fixed metabolic diets (see Table 2 for composition) were prepared by the UTSW CTRC dietitian and consumed by the subjects during the second week of each phase, with calcium and sodium intake kept constant throughout all four phases.
Table 2.
Macronutrient and micronutrient composition of diets
| CD | HPD | P value | |
|---|---|---|---|
| Total calories (kcal/d) | 2245 ± 523 | 2179 ± 625 | 0.16 |
| Protein (g/d) | 86 ± 24 | 157 ± 41 | <0.001 |
| Fat (g/d) | 90 ± 21 | 132 ± 44 | <0.001 |
| Carbohydrates (g/d) | 273 ± 71 | 92 ± 89 | <0.001 |
| % calories from protein | 15 ± 1 | 29 ± 4 | <0.001 |
| % calories from fat | 36 ± 3 | 54 ± 10 | <0.001 |
| % calories from carbohydrates | 48 ± 4 | 17 ± 13 | <0.001 |
| Na (mEq/d) | 135 ± 28 | 135 ± 28 | 0.88 |
| Ca (mg/d) | 849 ± 218 | 850 ± 196 | 0.74 |
| P (mg/d) | 1363 ± 426 | 1865 ± 502 | <0.001 |
| Mg (mg/d) | 296 ± 101 | 256 ± 75 | 0.10 |
| Oxalate (mg/d) | 184 ± 229 | 136 ± 239 | 0.62 |
Data are expressed as mean ± sd.
During phase 2, subjects supplemented their diet with potassium citrate (KCitrate) or potassium chloride (KCl) (depending on the assignment determined by the CTRC statistician) in three 10-mEq tablets twice daily (with breakfast and dinner) for a total of 60 mEq/d. During phase 4, subjects took the opposite potassium salt at the same dose schedule. The dose of KCitrate of 60 mEq/d was chosen to neutralize the expected excess acid conferred by the HPD. KCitrate was a commercially available preparation (10 mEq per tablet in wax matrix; Mission Pharmacal Company, San Antonio, TX), and KCl tablets (10 mEq per tablet in wax matrix, Mission Pharmacal Company) were formulated to be identical in appearance to KCitrate.
Laboratory testing and analytical procedures
Subjects had fasting blood drawn and collected two 2-h fasting urine samples and two 24-h urine samples on the last 2 d of each phase. Serum chemistries (Na, K, Cl, CO2, glucose, creatinine, phosphorus, liver function tests) and certain urine tests (Na, K, phosphorus, and creatinine) were run by an autoanalyzer (Synchron CX9ALX; Beckman, Brea, CA). Serum and urine Ca and Mg were analyzed by atomic absorption spectrophotometry (Instrumentation Laboratory, Bedford, MA). Urine uric acid was analyzed by the uricase method, urine citrate by a citrate lyase procedure (Cobas Fara, Roche, NJ), and urine pH using a digital pH electrode. Urine ammonium was determined by the glutamate dehydrogenase method, whereas urine bicarbonate was calculated from urine pH and pCO2. Oxalate was measured by ion chromatography (Dionex, Sunnyvale, CA), urine titratable acidity was measured directly using automated burette end-point titration system (Radiometer, Copenhagen, Denmark), and net acid excretion (NAE) was calculated as (titratable acidity + ammonium) − (bicarbonate + citrate), all expressed in milliequivalents. For the remaining assays described below, serum and urine samples from all four phases were batched for each volunteer and run in a single run; urine prostaglandin E2 (PGE2) was measured by ELISA (Cayman Chemical Company, Ann Arbor, MI). Intact PTH was assessed by immunoradiometric assay kit (Allegro intact PTH IRMA; Nichols Institute Diagnostics, San Juan Capistrano, CA). Serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D were assayed by kits, with initial immunoextraction followed by quantitation by enzyme immunoassay (Immunodiagnostics Systems, Scottsdale, AZ). Serum cross-linked carboxyterminal telopeptide of type I collagen (CTX, Serum Crosslaps; Nordic Bioscience Diagnostics, Herlev, Denmark) and urine cross-linked aminoterminal telopeptide of type I collagen (NTX; Osteomark; Ostex International, Seattle, WA) were assessed by ELISA. Serum procollagen I amino-terminal propeptide (PINP) was assayed by RIA kit (UniQ PINP; Orion Diagnostica, Espoo, Finland).
Supersaturation index (SI) of calcium oxalate (CaOx), brushite, and uric acid were calculated by dividing the ionic activity product in actual urine samples by the respective thermodynamic solubility product, using the Joint Expert Speciation System (JESS) (15). A value of 1 represents saturation; greater than 1, supersaturation; and less than 1, undersaturation.
Serum ultrafilterable calcium (UFCa) was estimated from serum total calcium, albumin, globulin, and pH (calculated from serum bicarbonate using the Henderson-Hasselbach equation) as previously described (16): UFCa in mmol/liter = (0.6626 × [serum total calcium]) − (0.097 × [serum albumin]) − (0.040 × [serum globulin]) + (0.0068 × [H+]) + 0.054. Fractional excretion of calcium was calculated as: (fasting urine calcium/UFCa)/(fasting urine creatinine/fasting serum creatinine) × 100%.
Fractional intestinal calcium absorption was measured using a dual-tracer stable isotope technique. On the morning of the second to last day in each phase, 20.0 μg of 42Ca was administered iv before the start of a 24-h urine collection. Then oral calcium was administered as 46Ca, 1.0 mg, mixed in 250 ml of a standardized liquid synthetic diet containing 100 mg of elemental calcium as a carrier. The receptacle containing the liquid synthetic diet and 46Ca was rinsed with 50 ml of distilled water that was swallowed by the study subject. Urine samples collected for the subsequent 24 h were used for the measurement of stable isotopes and calculation of fractional intestinal calcium absorption as previously described (17).
Statistical analysis
The four study phases were compared with mixed-effects model repeated measures (MMRM) analysis. Multiple comparisons were tested with least square means differences from the models, applying the Bonferroni adjustment for multiple testing. Data with skewed distributions were log-transformed to meet analysis assumptions. The effect of the order in which the potassium supplements were given was evaluated with mixed models. Statistical tests were two-sided at the 0.05 level of significance. Statistical analysis was conducted using SAS 9.2 (SAS Institute, Cary, NC).
Results
Demographics
A total of 11 subjects participated in this study. One subject withdrew before completion of the fourth phase of study, and 10 subjects completed the entire study. Demographics of study subjects are shown in Table 1. Participants were predominantly women of Caucasian ethnicity. The mean (± sd) age was 43 (±17) yr, and mean body mass index was 29.2 (± 6.7) kg/m2.
Table 1.
Demographic characteristics of study subjects
| Characteristic | |
|---|---|
| n | 11 |
| Gender distribution (female/male) | 7/4 |
| Ethnicity (African-American/Asian/Caucasian) | 1/2/8 |
| Age (yr) | 43 ± 17 |
| Height (cm) | 168 ± 10 |
| Weight (kg) | 82 ± 21 |
| Body mass index (kg/m2) | 29.2 ± 6.7 |
Data are expressed as number or mean ± sd.
Diet composition and adherence to diet
The composition of the two different metabolic diets for each subject was estimated using a database generated by the UTSW CTRC of analyzed contents of individual food components and by using U.S. Department of Agriculture food tables (18). Diet composition during the CD and HPD phases is shown in Table 2. Compared with the CD, the HPD had nearly 2-fold higher protein content, with higher fat and lower carbohydrate content. Sodium and calcium content was not different between the two diets by design.
The adherence of study subjects to the diets provided is reflected in the higher blood urea nitrogen and serum uric acid levels (Table 3) and the higher urinary sulfate and phosphorus (Table 4) in the HPD phases compared with the CD phases. None of the urinary variables was significantly different between CD1 and CD2 phases, indicative of adequate washout after the first HPD phase. Urine sodium and magnesium excretion was also not different across the four phases, further demonstrating compliance with the provided diets. The order in which the subjects received the potassium supplements did not affect the serum or urine results as assessed by MMRM analysis.
Table 3.
Serum biochemistry
| Variable | CD1 | HPD + KCl | CD2 | HPD + KCitrate | P value (by MMRM) |
|---|---|---|---|---|---|
| Electrolytes | |||||
| Na (mEq/liter) | 138 ± 2 | 137 ± 3a | 139 ± 1 | 138 ± 2 | 0.01 |
| K (mEq/liter) | 4.2 ± 0.3 | 4.3 ± 0.2 | 4.3 ± 0.2 | 4.3 ± 0.3 | 0.64 |
| Cl (mEq/liter) | 104 ± 2 | 105 ± 3 | 104 ± 2 | 104 ± 2 | 0.13 |
| CO2 (mEq/liter) | 27.9 ± 1.7 | 25.8 ± 2.3a | 28.4 ± 1.3 | 26.5 ± 1.5 | 0.0002 |
| BUN (mg/dl) | 10 ± 2 | 16 ± 3a | 10 ± 2 | 16 ± 3b | <0.0001 |
| Cr (mg/dl) | 0.85 ± 0.18 | 0.91 ± 0.20 | 0.87 ± 0.15 | 0.92 ± 0.17 | 0.04 |
| UA (mg/dl) | 5.8 ± 0.9 | 7.3 ± 1.9a | 5.7 ± 0.8 | 7.0 ± 1.6b | <0.0001 |
| Ca (mg/dl) | 9.4 ± 0.3 | 9.4 ± 0.3 | 9.4 ± 0.3 | 9.4 ± 0.4 | 0.86 |
| P (mg/dl) | 3.1 ± 0.4c | 3.4 ± 0.4 | 3.4 ± 0.3 | 3.4 ± 0.4 | 0.02 |
| Mg (mg/dl) | 2.2 ± 0.2 | 2.2 ± 0.2 | 2.2 ± 0.2 | 2.3 ± 0.1 | 0.14 |
| Lipids | |||||
| Total cholesterol (mg/dl) | 217 ± 26 | 210 ± 24 | 223 ± 26 | 215 ± 26 | 0.33 |
| HDL-cholesterol (mg/dl) | 57 ± 17 | 55 ± 15 | 55 ± 13 | 58 ± 16 | 0.19 |
| Triglycerides (mg/dl) | 131 ± 79 | 91 ± 37a | 141 ± 87 | 97 ± 37 | 0.002 |
| LDL-cholesterol (mg/dl) | 133 ± 16 | 136 ± 22 | 140 ± 25 | 138 ± 29 | 0.78 |
| Calcium/bone metabolism | |||||
| Serum UFCa (mmol/liter) | 1.32 ± 0.05 | 1.33 ± 0.04 | 1.32 ± 0.04 | 1.32 ± 0.04 | 0.71 |
| PTH (pg/ml) | 61 ± 34 | 52 ± 27 | 60 ± 24 | 66 ± 30 | 0.23 |
| 25-Hydroxyvitamin D (ng/ml) | 29 ± 12 | 34 ± 15 | 31 ± 13 | 34 ± 13 | 0.03 |
| 1,25-Dihydroxyvitamin D (pg/ml) | 51 ± 36 | 42 ± 13 | 46 ± 16 | 58 ± 35 | 0.25 |
| Ca absorption (%) | 42.5 ± 13.9 | 45.6 ± 11.5 | 42.8 ± 8.9 | 42.1 ± 12.7 | 0.48 |
| BAP (U/liter) | 21.8 ± 10.8 | 21.8 ± 10.3 | 22.4 ± 11.8 | 21.3 ± 11.0 | 0.43 |
| CTX (ng/ml) | 0.65 ± 0.50 | 0.69 ± 0.53 | 0.67 ± 0.46 | 0.62 ± 0.44 | 0.49 |
| PINP (ng/ml) | 42 ± 22 | 41 ± 17 | 43 ± 20 | 43 ± 25 | 0.93 |
Data are expressed as mean ± sd. BUN, Blood urea nitrogen; Cr, creatinine; UA, uric acid; HDL, high-density lipoprotein; LDL, low-density lipoprotein; BAP, bone-specific alkaline phosphatase.
Statistical significance at P < 0.0083 level is indicated by:
for HPD + KCl vs. CD (similar results are achieved whether HPD + KCl is compared to CD1 alone, CD2 alone, or mean of the two CD);
for HPD + KCitrate vs. CD (similar results are achieved whether HPD + KCitrate is compared to CD1 alone, CD2 alone, or mean of the two CD);
for CD1 vs. CD2 and CD1 vs. HPD + KCl.
Table 4.
Urine biochemistry
| Variable | CD1 | HPD + KCl | CD2 | HPD + KCitrate | P value (by MMRM) |
|---|---|---|---|---|---|
| 24-h Urine | |||||
| Electrolytes | |||||
| Volume (liters/d) | 3.39 ± 0.73 | 3.08 ± 0.52 | 3.18 ± 0.51 | 3.36 ± 0.70 | 0.41 |
| pH | 6.32 ± 0.37 | 5.67 ± 0.53a | 6.24 ± 0.33 | 6.41 ± 0.41c | <0.0001 |
| Ca (mg/d) | 147 ± 84 | 182 ± 85a | 134 ± 66 | 170 ± 85 | 0.002 |
| Mg (mg/d) | 95 ± 42 | 87 ± 33 | 86 ± 31 | 92 ± 36 | 0.34 |
| P (mg/d) | 745 ± 314 | 992 ± 372a | 692 ± 232 | 1034 ± 326b | <0.0001 |
| Cr (mg/d) | 1598 ± 731 | 1631 ± 565 | 1457 ± 536 | 1717 ± 636 | 0.0047 |
| Na (mEq/d) | 111 ± 54 | 100 ± 25 | 112 ± 37 | 102 ± 31 | 0.62 |
| K (mEq/d) | 59 ± 25 | 99 ± 19a | 55 ± 18 | 102 ± 17b | <0.0001 |
| Cl (mEq/d) | 107 ± 41 | 141 ± 25a | 106 ± 34 | 94 ± 32c | 0.0011 |
| Oxalate (mg/d) | 30 ± 11 | 28 ± 14 | 29 ± 9 | 31 ± 23 | 0.89 |
| Sulfate (mmol/d) | 19 ± 10 | 27 ± 11a | 18 ± 6 | 31 ± 9b | <0.0001 |
| Uric acid (mg/d) | 607 ± 185 | 690 ± 256 | 571 ± 130 | 808 ± 186b | 0.0015 |
| Acid-base variables | |||||
| Ammonium (mEq/d) | 36 ± 12 | 64 ± 26a | 35 ± 13 | 39 ± 17c | <0.0001 |
| Citrate (mg/d) | 705 ± 241 | 423 ± 267a | 667 ± 225 | 978 ± 272b,c | <0.0001 |
| Citrate (mEq/d) | 8.0 ± 2.6 | 5.2 ± 3.1a | 7.8 ± 2.7 | 11.4 ± 3.0b,c | <0.0001 |
| Bicarbonate (mEq/d) | 8.4 ± 11.2 | 4.7 ± 6.1 | 9.4 ± 13.0 | 18.0 ± 9.6b,c | 0.0009 |
| TA (mEq/d) | 22 ± 6 | 49 ± 12a | 26 ± 11 | 30 ± 11c | <0.0001 |
| NAE (mEq/d) | 39 ± 15 | 104 ± 40a | 41 ± 27 | 37 ± 30c | <0.0001 |
| Bone turnover markers | |||||
| NTX (nm BCE/d) | 512 ± 554 | 436 ± 268 | 457 ± 362 | 420 ± 297 | 0.56 |
| NTX/Cr (nm BCE/mg) | 0.30 ± 0.23 | 0.26 ± 0.12 | 0.31 ± 0.19 | 0.25 ± 0.14 | 0.31 |
| PGE2 (ng/d) | 193 ± 73 | 207 ± 148 | 207 ± 89 | 197 ± 53 | 0.98 |
| PGE2/Cr (ng/mg) | 0.13 ± 0.06 | 0.13 ± 0.07 | 0.15 ± 0.07 | 0.13 ± 0.04 | 0.61 |
| DPD (nm/d) | 56 ± 28 | 55 ± 29 | 56 ± 25 | 57 ± 32 | 0.98 |
| DPD/Cr (nm/mg) | 0.04 ± 0.02 | 0.03 ± 0.01 | 0.04 ± 0.02 | 0.04 ± 0.02 | 0.094 |
| Fasting urine | |||||
| Fasting pH | 6.33 ± 0.41 | 5.49 ± 0.35a | 6.41 ± 0.37 | 6.18 ± 0.42c | <0.0001 |
| Fasting Ca/Cr (mg/mg) | 0.064 ± 0.040 | 0.086 ± 0.046a | 0.059 ± 0.027 | 0.071 ± 0.038 | 0.018 |
| FE calcium (%) | 1.00 ± 0.65 | 1.52 ± 1.00a | 0.94 ± 0.41 | 1.22 ± 0.75 | 0.010 |
Data are expressed as mean ± sd. TA, Titratable acidity; Cr, creatinine; BCE, bone collagen equivalents; FE, fractional excretion.
Statistical significance at P < 0.0083 level is indicated by:
for HPD + KCl vs. CD (similar results are achieved whether HPD + KCl is compared to CD1 alone, CD2, or the mean of two CD);
for HPD + KCitrate vs. CD (similar results are achieved whether HPD + KCitrate is compared to CD1 alone, CD2, or the mean of two CD);
for HPD + KCl vs. HPD + KCitrate.
Acid-base variables
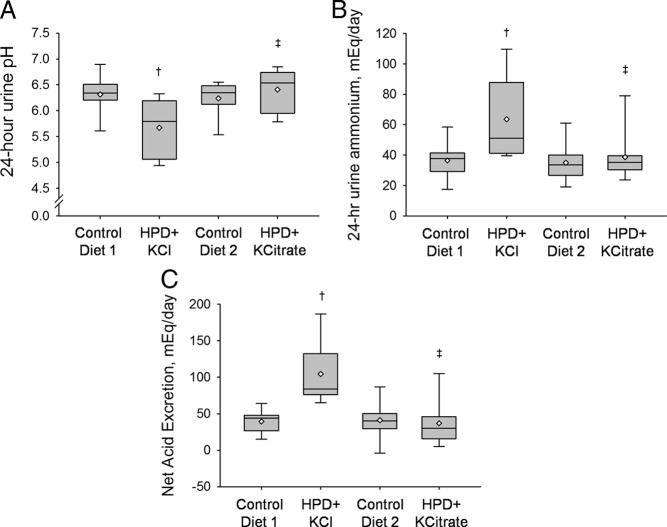
Ingestion of the HPD along with the neutral potassium salt, KCl, delivered a significantly greater acid load than the CD as demonstrated by the lower 24-h urine pH (Fig. 1A) and 24-h urine citrate and by the higher 24-h urine ammonium (Fig. 1B) and NAE (Fig. 1C). Serum bicarbonate concentration and fasting urine pH were also lower during the HPD + KCl phase compared with the CD phases (Tables 3 and 4). On the other hand, treatment with the alkaline potassium salt, KCitrate, neutralized the acid load delivered by the HPD as indicated by restoration of 24-h urine pH, urine ammonium, and NAE to the level of the CD phases (Fig. 1). Serum bicarbonate concentration and fasting urine pH were also not different between the HPD + KCitrate and the CD phases (Tables 3 and 4). Compared with the HPD + KCl, HPD + KCitrate treatment resulted in higher 24-h urine pH (6.41 ± 0.41 vs. 5.67 ± 0.53; P < 0.0001), fasting urine pH (6.18 ± 0.42 vs. 5.49 ± 0.35; P < 0.0001), and 24-h urine citrate (978 ± 272 vs. 423 ± 267 mg/d; P < 0.0001). Compared with HPD + KCl, HPD + KCitrate was also associated with lower urine ammonium (39 ± 17 vs. 64 ± 26 mEq/d; P < 0.0001) and NAE (37 ± 30 vs. 104 ± 40 mEq/d; P < 0.0001) (Fig. 1).
Fig. 1.
Acid-base variables during the four study phases. A, 24-h urine pH. B, 24-h urine ammonium. C, 24-h urine NAE. Data shown as box plots, with the bottom and top of each box representing the 25th and 75th percentiles, the band near the middle representing the median, and the diamond representing the mean. †, P < 0.0083 for HPD + KCl vs. CD1 and CD2. ‡, P < 0.0083 for HPD + KCl vs. HPD + KCitrate.
Calcium metabolism and bone turnover
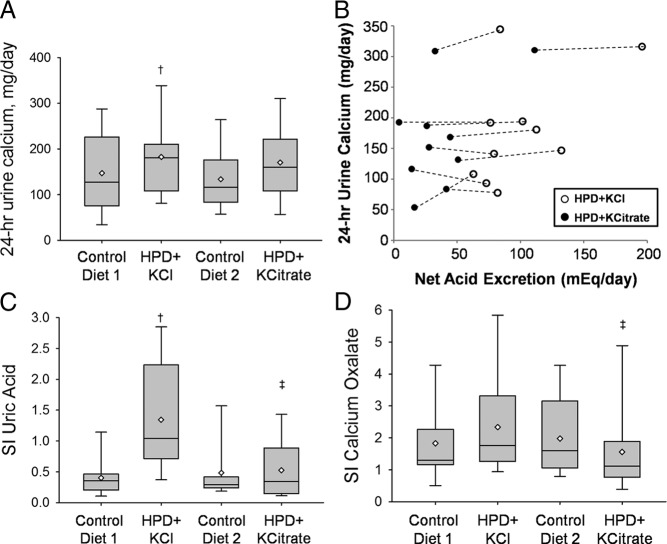
Compared with the CD phases, ingestion of the HPD with KCl was associated with a significant increase in 24-h urine calcium (HPD + KCl, 182 ± 85 mg/d, P = 0.007; vs. CD1, 147 ± 84 mg/d, P < 0.001; vs. CD2, 134 ± 66 mg/d; Fig. 2A). Ingestion of the HPD with KCitrate was associated with a smaller increase in 24-h urine calcium (HPD + KCitrate, 170 ± 85 mg/d, P = 0.097 vs. CD1 and P = 0.01 vs. CD2; Fig. 2A). The 24-h urine calcium was not significantly different between the HPD + KCl and HPD + KCitrate phases (182 ± 85 vs. 170 ± 85 mg/d; P = 0.28). The estimated model difference in 24-h urine calcium for HPD + KCl vs. HPD + KCitrate was 12.8 ± 22 mg/d (95% confidence interval, −12 to 39 mg/d). Figure 2B displays the relationship between 24-h urine calcium and NAE in the HPD + KCl and HPD + KCitrate phases in individual subjects. Although provision of KCitrate significantly reduced NAE in all subjects compared with KCl, in most subjects it did not alter urine calcium to a substantial extent. Although serum UFCa was not different between the four phases of study (Table 3), the fractional excretion of calcium was significantly higher in the HPD + KCl phase compared with the CD phases.
Fig. 2.
Urine calcium, its relationship with NAE, and SI. Data are shown as box plots, with the bottom and top of each box representing the 25th and 75th percentiles, the band near the middle representing the median, and the diamond representing the mean. A, 24-h urine calcium during the four study phases. †, P < 0.0083 for HPD + KCl vs. CD1 and CD2. B, Relationship between NAE and urinary calcium during the HPD + KCl and the HPD + KCitrate phases. C, SI for uric acid. †, P < 0.0083 for HPD + KCl vs. CD1 and CD2. ‡, P < 0.0083 for HPD + KCl vs. HPD + KCitrate. D, SI for CaOx. ‡, P < 0.0083 for HPD + KCl vs. HPD + KCitrate.
Fractional intestinal calcium absorption measured by stable dual calcium isotope method did not change significantly across the four phases (ranging between 42.1 and 45.6%). In addition, no significant difference was noted across the four phases in bone formation (serum PINP and bone-specific alkaline phosphatase) or bone resorption [serum CTX, urine NTX, and urine deoxypyridinoline (DPD)] markers (Tables 3 and 4). Similarly, there were no significant changes in serum 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, or urine PGE2 excretion (Tables 3 and 4).
Urinary saturation of stone-forming salts
Urinary saturation of uric acid estimated by SI was significantly higher in the HPD + KCl phase (1.3 ± 0.9) compared with the CD (CD1, 0.4 ± 0.3; CD2, 0.5 ± 0.5) and HPD + KCitrate (0.5 ± 0.5) phases (Fig. 2C). Urine oxalate excretion was stable throughout the four phases. Ingestion of the HPD with KCitrate was associated with a significantly lower SI of CaOx than ingestion of HPD with KCl (1.6 ± 0.4 vs. 2.3 ± 1.6; P = 0.0028; Fig. 2D).
Discussion
Improved understanding of the mechanism responsible for greater calcium excretion with dietary protein intake is vital because hypercalciuria is a risk factor for kidney stone formation (19) and may lead to a negative calcium balance and bone loss (5, 6). In this metabolic study, we evaluated the role of the acid load imparted by dietary proteins on calcium excretion and the risk of kidney stone formation. We found that whereas KCitrate neutralized the acid load delivered by a HPD, it did not prevent the hypercalciuria associated with this diet. These findings suggest that, at least in the short-term, mechanism(s) other than acid loading account for increased urinary calcium excretion during ingestion of HPD. On the other hand, the increased urinary saturation with respect to CaOx and uric acid induced by the HPD was abrogated by KCitrate.
Intake of high animal protein diets confers a large acid load, due to the generation of protons during the oxidation of sulfur-containing amino acids in animal proteins to sulfuric acid (20). The hypercalciuria induced by dietary proteins has been attributed in part to this acid load because there is a high degree of correlation between the increase in urinary calcium and the increase in urinary NAE after higher intake of proteins (7). In addition, the ingestion of ammonium chloride, a source of acid load (21), or of sulfur-containing amino acids alone (22) also leads to hypercalciuria. Furthermore, short- and long-term studies in healthy postmenopausal women (17, 23, 24), elderly men (24), and postmenopausal women with osteopenia (25) have shown that potassium alkali treatment lowers urinary calcium excretion. Although these studies suggested that lower urinary calcium excretion was related to a reduction in net acid intake, all were performed under habitual protein intake of approximately 1 mg protein/kg body weight/d. In this study, we found that provision of potassium alkali in the setting of HPD (2 mg/kg · d) does not result in a comparable hypocalciuric effect. Our results are similar to those by Ceglia et al. (26) who found no significant difference in urinary calcium excretion, whether a HPD was given with an alkaline load (potassium bicarbonate) or with placebo. These findings suggest that the hypercalciuria induced by HPD cannot be attributed solely to an acid load.
Some studies have suggested that the hypercalciuria associated with HPD may be related to enhanced intestinal calcium absorption (12, 13). We did not detect any significant change between the HPD and CD phases in intestinal calcium absorption measured by the dual isotope calcium technique (Table 4), a finding similar to that reported in the study by Ceglia et al. (26). Possible explanations for the discrepancy in the change in intestinal calcium absorption across studies include differences in dietary calcium content and/or methodological differences in the measurement of intestinal calcium absorption. The impact of dietary proteins on intestinal calcium absorption is more evident when dietary calcium is limited (12, 13) and less prominent when dietary calcium is abundant (13, 26). Furthermore, in our study, the stable oral calcium isotope was provided mixed in a standardized liquid synthetic diet in the morning, as previously described (17, 26). This technique may potentially miss postprandial differences in intestinal calcium absorption, which may in part explain the discrepancy between our results and those of other studies (12, 13) in which the calcium isotope was provided with three separate meals daily. Aromatic amino acids more abundantly present in the HPD may also augment urinary calcium excretion through activation of the calcium-sensing receptor (27). Another potential mechanism suggested to contribute to protein-induced hypercalciuria is an increase in urinary PGE2 levels, which may regulate calcium excretion (28). No significant change in urinary PGE2 excretion was seen between the HPD and CD phases in our study (Table 4).
It is unlikely that the changes in urinary calcium observed during the HPD phases were due to dietary factors other than protein intake. We maintained the same dietary sodium and calcium intake between the CD and HPD phases (Table 2), and isocaloric substitution of carbohydrates for fats does not alter urinary calcium (29). A higher dietary phosphorus load naturally accompanies a HPD (Tables 1 and 3). Several studies (using different phosphate sources and calcium intakes) have reported no change in net calcium balance because increased fecal calcium loss was offset by a reduction in urinary calcium excretion (30).
One long-term potential deleterious effect of HPD is an increase in nephrolithiasis risk, as suggested by epidemiological, clinical, and experimental studies. Intake of protein was directly associated with the risk of nephrolithiasis in a prospective study of over 45,000 men followed for more than 4 yr (3). Conversely, a diet restricted in protein and sodium was effective in preventing recurrent stone formation among patients with hypercalciuric nephrolithiasis (31). In a short-term metabolic study, a HPD (2 g protein/kg body weight · d) conferred an increased risk for CaOx and uric acid stones compared with a diet with usual protein intake (1.1 g protein/kg · d) (32). In our study, KCitrate provision along the HPD prevented the increase in urinary saturation with respect to CaOx and uric acid observed during the HPD + KCl phase (Table 4 and Fig. 2). Markedly acidic urine is a key risk factor in uric acid stone formation (33), and KCitrate reduced the risk of uric acid stones primarily by preventing the reduction in urinary pH associated with HPD ingestion. The significant difference in CaOx saturation between the two HPD phases occurred despite similar urinary calcium or oxalate excretion and was likely due to a combination of higher urine citrate and pH during the HPD + KCitrate phase (34). Both urine citrate and urine pH are key determinants of CaOx saturation (34). Citrate in urine is a key inhibitor of nephrolithiasis and acts chiefly through the formation of a complex with calcium, causing a reduction in the ionic calcium concentration and in the urinary saturation of CaOx (35). The decline in urinary citrate in the face of the metabolic acid load imparted by the HPD results in increased saturation of CaOx, an effect that is corrected by provision of the alkaline salt KCitrate. The higher 24-h urine pH with KCitrate also contributes to lower CaOx saturation via formation of more soluble calcium complexes (such as calcium phosphocitrate and dicalcium dihydrogen phosphate) instead of CaOx (34).
The long-term effects of HPD on calcium metabolism and bone status have been controversial. Although some studies have associated HPD with negative calcium balance (5), lower bone mineral density (6), and increased fractures (36), others have reported improved bone mineral density (37) and lower fracture risk (38, 39). A recent systematic review and meta-analysis of the relation between protein and bone health in healthy human adults found no definite effect of protein intake on fracture risk in the long term (40). In our metabolic study, no significant changes in bone turnover markers were noted with the HPD, although the 2-wk duration of observation may have been too short to detect such alteration.
Limitations of our study include the short-term duration, which may have prevented the detection of changes in bone turnover markers. Furthermore, although urine calcium excretion increased during the HPD phases, we have not identified a mechanism underlying such an increase. Nevertheless, we were able to rule out an effect of the acid component of protein. Another potential limitation is the lack of synchronization of the study phases with menstrual cycle in the four premenopausal women evaluated in this study. Since one main finding of this study is the lack of significant difference in 24-h urine calcium between the HPD + KCl and HPD + KCitrate phases, it is possible that the study was underpowered and that a larger sample size may have detected a significant difference. However, using the mean difference and sd from the available data, over 30 subjects would be needed for 80% power to detect a small effect size of 13 mg/d, which is not likely to be clinically significant. Finally, in this study we compared the effects of HPD + KCitrate to those of HPD + KCl rather than HPD + placebo. Although a placebo phase can evaluate the effects of HPD on calciuria, a comparison of KCitrate to placebo cannot separate the effects of potassium from those of alkali therapy. On the other hand, our comparison of KCitrate to KCl provides information on the effects of alkali alone, which is valuable to evaluate the role of acid load in HPD-induced calciuria. Furthermore, studies have shown that KCl does not have hypocalciuric effects on its own (25, 41), making it an adequate control for KCitrate in the HPD setting.
Conclusions
In summary, this metabolic study suggests that, at least in the short term, mechanism(s) other than acid loading account for increased urinary calcium during ingestion of HPD. On the other hand, the increased urinary saturation with respect to CaOx and uric acid induced by HPD may be prevented by coingestion of potassium alkali through correction of declines in urine pH and citrate. Long-term studies are needed to definitively determine the effects of HPD on the risk of fracture and nephrolithiasis and what the proper countermeasure(s) should be.
Supplementary Material
Acknowledgments
The authors were supported by National Institutes of Health Grants K23-RR21710, R01DK081523, R01 DK081423, M01-RR-00633, and UL1-RR024982.
Disclosure Summary: The authors have no relevant disclosures to make.
Footnotes
- CaOx
- Calcium oxalate
- CD
- control diet
- CTX
- cross-linked carboxyterminal telopeptide of type I collagen
- DPD
- deoxypyridinoline
- HPD
- high-protein diet
- KCitrate
- potassium citrate
- KCl
- potassium chloride
- MMRM
- mixed-effects model repeated measures
- NAE
- net acid excretion
- NTX
- cross-linked amino terminal telopeptide of type I collagen
- PGE2
- prostaglandin E2
- PINP
- procollagen I amino-terminal propeptide
- SI
- supersaturation index
- UFCa
- ultrafilterable calcium.
References
- 1. Ginty F. 2003. Dietary protein and bone health. Proc Nutr Soc 62:867–876 [DOI] [PubMed] [Google Scholar]
- 2. Kerstetter JE, O'Brien KO, Insogna KL. 2003. Low protein intake: the impact on calcium and bone homeostasis in humans. J Nutr 133:855S–861S [DOI] [PubMed] [Google Scholar]
- 3. Curhan GC, Willett WC, Rimm EB, Stampfer MJ. 1993. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med 328:833–838 [DOI] [PubMed] [Google Scholar]
- 4. Robertson WG, Heyburn PJ, Peacock M, Hanes FA, Swaminathan R. 1979. The effect of high animal protein intake on the risk of calcium stone-formation in the urinary tract. Clin Sci (Lond) 57:285–288 [DOI] [PubMed] [Google Scholar]
- 5. Hegsted M, Schuette SA, Zemel MB, Linkswiler HM. 1981. Urinary calcium and calcium balance in young men as affected by level of protein and phosphorus intake. J Nutr 111:553–562 [DOI] [PubMed] [Google Scholar]
- 6. Metz JA, Anderson JJ, Gallagher PN., Jr 1993. Intakes of calcium, phosphorus, and protein, and physical-activity level are related to radial bone mass in young adult women. Am J Clin Nutr 58:537–542 [DOI] [PubMed] [Google Scholar]
- 7. Lemann J., Jr 1999. Relationship between urinary calcium and net acid excretion as determined by dietary protein and potassium: a review. Nephron 81(Suppl 1):18–25 [DOI] [PubMed] [Google Scholar]
- 8. Bushinsky DA, Parker WR, Alexander KM, Krieger NS. 2001. Metabolic, but not respiratory, acidosis increases bone PGE(2) levels and calcium release. Am J Physiol Renal Physiol 281:F1058–F1066 [DOI] [PubMed] [Google Scholar]
- 9. Sutton RA, Wong NL, Dirks JH. 1979. Effects of metabolic acidosis and alkalosis on sodium and calcium transport in the dog kidney. Kidney Int 15:520–533 [DOI] [PubMed] [Google Scholar]
- 10. Yeh BI, Sun TJ, Lee JZ, Chen HH, Huang CL. 2003. Mechanism and molecular determinant for regulation of rabbit transient receptor potential type 5 (TRPV5) channel by extracellular pH. J Biol Chem 278:51044–51052 [DOI] [PubMed] [Google Scholar]
- 11. Moe OW, Huang CL. 2006. Hypercalciuria from acid load: renal mechanisms. J Nephrol 19(Suppl 9):S53–S61 [PubMed] [Google Scholar]
- 12. Kerstetter JE, O'Brien KO, Caseria DM, Wall DE, Insogna KL. 2005. The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women. J Clin Endocrinol Metab 90:26–31 [DOI] [PubMed] [Google Scholar]
- 13. Hunt JR, Johnson LK, Fariba Roughead ZK. 2009. Dietary protein and calcium interact to influence calcium retention: a controlled feeding study. Am J Clin Nutr 89:1357–1365 [DOI] [PubMed] [Google Scholar]
- 14. Pullman TN, Alving AS, Dern RJ, Landowne M. 1954. The influence of dietary protein intake on specific renal functions in normal man. J Lab Clin Med 44:320–332 [PubMed] [Google Scholar]
- 15. May PM, Murray K. 1991. JESS, a joint expert speciation system-I. Raison d'etre. Talanta 38:1409–1417 [DOI] [PubMed] [Google Scholar]
- 16. Cochran M, Rumbelow B, Allen G. 1998. The relation between the ultrafiltrable calcium fraction and blood pH and concentrations of total plasma calcium, albumin, and globulin. Clin Chem 44:1559–1562 [PubMed] [Google Scholar]
- 17. Sakhaee K, Maalouf NM, Abrams SA, Pak CY. 2005. Effects of potassium alkali and calcium supplementation on bone turnover in postmenopausal women. J Clin Endocrinol Metab 90:3528–3533 [DOI] [PubMed] [Google Scholar]
- 18. Agricultural Research Service 1976–1987. Composition of foods. Agricultural handbook no. 8, Series 8–1 to 8–16. Washington DC: US Department of Agriculture [Google Scholar]
- 19. Bushinsky DA. 1998. Nephrolithiasis. J Am Soc Nephrol 9:917–924 [DOI] [PubMed] [Google Scholar]
- 20. Sabry ZI, Shadarevian SB, Cowan JW, Campbell JA. 1965. Relationship of dietary intake of sulphur amino-acids to urinary excretion of inorganic sulphate in man. Nature 206:931–933 [DOI] [PubMed] [Google Scholar]
- 21. Lemann J, Jr, Litzow JR, Lennon EJ. 1966. The effects of chronic acid loads in normal man: further evidence for the participation of bone mineral in the defense against chronic metabolic acidosis. J Clin Invest 45:1608–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zemel MB, Schuette SA, Hegsted M, Linkswiler HM. 1981. Role of the sulfur-containing amino acids in protein-induced hypercalciuria in men. J Nutr 111:545–552 [DOI] [PubMed] [Google Scholar]
- 23. Sebastian A, Harris ST, Ottaway JH, Todd KM, Morris RC., Jr 1994. Improved mineral balance and skeletal metabolism in postmenopausal women treated with potassium bicarbonate. N Engl J Med 330:1776–1781 [DOI] [PubMed] [Google Scholar]
- 24. Dawson-Hughes B, Harris SS, Palermo NJ, Castaneda-Sceppa C, Rasmussen HM, Dallal GE. 2009. Treatment with potassium bicarbonate lowers calcium excretion and bone resorption in older men and women. J Clin Endocrinol Metab 94:96–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jehle S, Zanetti A, Muser J, Hulter HN, Krapf R. 2006. Partial neutralization of the acidogenic Western diet with potassium citrate increases bone mass in postmenopausal women with osteopenia. J Am Soc Nephrol 17:3213–3222 [DOI] [PubMed] [Google Scholar]
- 26. Ceglia L, Harris SS, Abrams SA, Rasmussen HM, Dallal GE, Dawson-Hughes B. 2009. Potassium bicarbonate attenuates the urinary nitrogen excretion that accompanies an increase in dietary protein and may promote calcium absorption. J Clin Endocrinol Metab 94:645–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dawson-Hughes B, Harris SS, Rasmussen HM, Dallal GE. 2007. Comparative effects of oral aromatic and branched-chain amino acids on urine calcium excretion in humans. Osteoporos Int 18:955–961 [DOI] [PubMed] [Google Scholar]
- 28. Baggio B. 2004. Protein diet and hypercalciuria. Kidney Int 65:1970; author reply 1970 [DOI] [PubMed] [Google Scholar]
- 29. Garg A, Bonanome A, Grundy SM, Unger RH, Breslau NA, Pak CY. 1990. Effects of dietary carbohydrates on metabolism of calcium and other minerals in normal subjects and patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 70:1007–1013 [DOI] [PubMed] [Google Scholar]
- 30. Fenton TR, Lyon AW, Eliasziw M, Tough SC, Hanley DA. 2009. Phosphate decreases urine calcium and increases calcium balance: a meta-analysis of the osteoporosis acid-ash diet hypothesis. Nutr J 8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Borghi L, Schianchi T, Meschi T, Guerra A, Allegri F, Maggiore U, Novarini A. 2002. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 346:77–84 [DOI] [PubMed] [Google Scholar]
- 32. Reddy ST, Wang CY, Sakhaee K, Brinkley L, Pak CY. 2002. Effect of low-carbohydrate high-protein diets on acid-base balance, stone-forming propensity, and calcium metabolism. Am J Kidney Dis 40:265–274 [DOI] [PubMed] [Google Scholar]
- 33. Maalouf NM, Cameron MA, Moe OW, Sakhaee K. 2004. Novel insights into the pathogenesis of uric acid nephrolithiasis. Curr Opin Nephrol Hypertens 13:181–189 [DOI] [PubMed] [Google Scholar]
- 34. Pak CY, Maalouf NM, Rodgers K, Poindexter JR. 2009. Comparison of semi-empirical and computer derived methods for estimating urinary saturation of calcium oxalate. J Urol 182:2951–2956 [DOI] [PubMed] [Google Scholar]
- 35. Pak CY. 1991. Citrate and renal calculi: new insights and future directions. Am J Kidney Dis 17:420–425 [DOI] [PubMed] [Google Scholar]
- 36. Feskanich D, Willett WC, Stampfer MJ, Colditz GA. 1996. Protein consumption and bone fractures in women. Am J Epidemiol 143:472–479 [DOI] [PubMed] [Google Scholar]
- 37. Cooper C, Atkinson EJ, Hensrud DD, Wahner HW, O'Fallon WM, Riggs BL, Melton LJ., 3rd 1996. Dietary protein intake and bone mass in women. Calcif Tissue Int 58:320–325 [DOI] [PubMed] [Google Scholar]
- 38. Hannan MT, Tucker KL, Dawson-Hughes B, Cupples LA, Felson DT, Kiel DP. 2000. Effect of dietary protein on bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res 15:2504–2512 [DOI] [PubMed] [Google Scholar]
- 39. Wengreen HJ, Munger RG, West NA, Cutler DR, Corcoran CD, Zhang J, Sassano NE. 2004. Dietary protein intake and risk of osteoporotic hip fracture in elderly residents of Utah. J Bone Miner Res 19:537–545 [DOI] [PubMed] [Google Scholar]
- 40. Darling AL, Millward DJ, Torgerson DJ, Hewitt CE, Lanham-New SA. 2009. Dietary protein and bone health: a systematic review and meta-analysis. Am J Clin Nutr 90:1674–1692 [DOI] [PubMed] [Google Scholar]
- 41. Sakhaee K, Alpern R, Jacobson HR, Pak CY. 1991. Contrasting effects of various potassium salts on renal citrate excretion. J Clin Endocrinol Metab 72:396–400 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.