Abstract
Context:
In many disorders requiring steroid therapy, there is substantial decrease in bone mineral density. The association between steroid use and 25-hydroxyvitamin D [25(OH)D] deficiency has not been confirmed in large population-based studies, and currently there are no specific vitamin D recommendations for steroid users.
Objective:
The aim of the study was to evaluate the association of serum 25(OH)D deficiency [defined as 25(OH)D <10 ng/ml] with oral steroid use.
Design:
Cross-sectional analysis was performed using NHANES 2001–2006.
Setting:
We analyzed a nationally representative sample of U.S. children and adults.
Participants:
The study sample consisted of children, adolescents, and adults from NHANES 2001–2006 (n = 22,650), representative of 286 million U.S. residents, with serum 25(OH)D levels and data on other potential confounders.
Main Outcome Measure:
We measured serum 25(OH)D levels below 10 ng/ml.
Results:
A total of 181 individuals (0.9% of the population) used steroids within the past 30 d. Overall, 5% of the population had 25(OH)D levels below 10 ng/ml. Among steroid users, 11% had 25(OH)D levels below 10 ng/ml, compared to 5% among steroid nonusers (P = 0.009). The odds of having 25(OH)D deficiency were 2-fold higher in those who reported steroid use compared to those without steroid use [odds ratio (OR), 2.36; 95% confidence interval (CI), 1.25, 4.45]. This association remained after multivariable adjustment (OR, 2.21; 95% CI, 1.01, 4.85) and in a multivariable model using NHANES III data (OR, 1.88; 95% CI, 1.01, 3.48).
Conclusion:
Steroid use is independently associated with 25(OH)D deficiency in this nationally representative cohort limited by cross-sectional data. It suggests the need for screening and repletion in patients on chronic steroids.
Vitamin D deficiency, variously defined as 25-hydroxyvitamin D [25(OH)D] levels below 20, 15, or 10 ng/ml, is common in the general population (1–3). Multiple studies have shown an association of low 25(OH)D with all-cause mortality, cardiovascular disease, autoimmune diseases, infectious diseases, and increased risk of fractures (2, 4–9). Glucocorticoid use has also been linked to many of these outcomes.
Glucocorticoid use is common in patients with chronic pulmonary, rheumatic, and kidney diseases, and in these patients glucocorticoid use is associated with osteoporosis and increased fractures. Glucocorticoids decrease intestinal calcium absorption and increase urinary excretion of calcium (10). Additionally, glucocorticoids are known to enhance bone resorption and decrease bone formation, thereby decreasing bone mass and increasing the risk of fractures (11). Laboratory studies reveal that steroids may increase 24-hydroxylase activity, thereby decreasing 25(OH)D levels (12–14). Recent literature in pulmonology, gastroenterology, and rheumatology has noted that patients treated with oral glucocorticoids have significantly lower levels of 25(OH)D (15–17).
This association between steroid use and 25(OH)D deficiency has not been confirmed in large population-based studies. In November 2010, the Institute of Medicine published new recommendations of 600 IU/d for children and adults (1–70 yr of age) and 800 IU/d for those more than 70 yr of age (18). There were no specific recommendations made for those who are treated with steroids.
The aim of the present study was to examine the association of serum 25(OH)D deficiency with oral steroid use in a large representative sample of U.S. residents using the National Health and Nutrition Examination Surveys (NHANES) from 2001–2006.
Subjects and Methods
Study population
NHANES is a nationally representative, cross-sectional survey designed to assess the health and nutritional status of civilian noninstitutionalized adults and children in the United States. Participants were selected through a complex, multistage probability design. All of the participants underwent standardized home interviews as well as physical examinations and laboratory testing at a mobile health center. The current study included all children, adolescents, and adults ages 1 yr and older with recorded 25(OH)D levels and data available on other covariates. A total of 31,509 participants were examined in NHANES 2001–2006, of which 8,859 participants were excluded from the analysis for having the following missing values: serum 25(OH)D (n = 3,417), poverty income ratio (PIR) (n = 1,913), obesity (n = 3,509), milk intake (n = 3), and days missed from school or work (n = 17). 25(OH)D levels were measured in all participants at least 6 yr of age from 2001–2002 and in all participants at least 1 yr of age from 2003–2006. NHANES 2001–2006 was approved by the National Center for Health Statistics Institutional Review Board. All of the participants at least 18 yr old provided informed consent, participants 12 to 17 yr old and their parents provided informed consent, participants 7 to 11 yr old provided assent and parental consent, and parents provided informed consent for those less than 7 yr old.
Participants in NHANES III, performed between 1988–1994, were examined to evaluate the robustness of the associations. A total of 33,994 participants were examined in NHANES III, of which 18,620 participants were excluded from the analysis for having the following missing values: serum 25(OH)D (n = 8,331), PIR (n = 3,410), obesity (n = 5,122), and supplement use (n = 1,757).
Study variables
Demographic variables in the current analysis of NHANES 2001–2006 included age, sex, race/ethnicity, and the PIR. Participants over age 85 yr of age were reported by NHANES as being 85 yr old to maintain anonymity. Race/ethnicity was self-reported and categorized as non-Hispanic white, non-Hispanic black, Mexican-American, or other. Poverty status was defined using the PIR, an index calculated by dividing family income by a poverty threshold specific to the family size. We defined PIR of 1 or less as below the poverty threshold. PIR categories were: 5.00 or greater, above 1.0 to 4.99, and 1.00 or less.
Steroid use and the use of any medication data were acquired from the medication questionnaire and pill-bottle review. Participants were asked whether they used any medications within the past 30 d and were classified as “yes” or “no” to medication use. If yes, they were asked to report the medication(s) used. We coded positive steroid use for anyone who reported the use of prednisone, prednisolone, methylprednisolone, or methylprednisolone acetate within the past 30 d. Those who did not report use of any of these medications were recorded as no steroid use. Steroid use data from NHANES III were acquired from the medication questionnaire with an affirmative response for the prescription drug class category adrenal corticosteroids coded as positive steroid use. Milk consumption data were obtained from the diet, behavior, and nutrition section of the NHANES 2001–2006 Sample Person Questionnaire and were categorized as daily, more than once per week but less than daily, less than once per week, and never. Vitamin D supplementation use was assessed by participant questionnaire and pill-bottle review. Supplementation use was categorized as none, more than zero and less than 400 IU/d, or at least 400 IU/d. In NHANES III, individual vitamin D intake was not assessed; therefore, we used the use of a multivitamin supplement.
As part of the physical exam, each participant's height and weight were measured. Obesity was categorized by weight in 1 yr olds and by body mass index (BMI) in those at least 2 yr old. In 1 yr olds, obesity was defined as weight exceeding the 95th percentile for gender-specific weight curves. BMI was calculated as weight in kilograms divided by height in meters squared. In those from 2 to <18 yr of age, obesity was defined using age- and gender-specific percentile curves of BMI. In those at least 18 yr old, the widely used adult cutoff point of 30 kg/m2 defined obesity.
Days missed from school or work were obtained from the medical conditions section of the NHANES 2001–2006 Sample Person Questionnaire. Those 6–19 yr of age were asked: how many days during the past 12 months did you miss from school because of injury or illness. Those 16 yr and older were asked: how many days during the past 12 months did you miss from work. Physical activity was assessed based on direct questioning of the participants regarding hours per day spent using computers, television, and video games.
25(OH)D was measured using the DiaSorin 25(OH)D assay (DiaSorin, Stillwater, MN). 25(OH)D samples from NHANES III were adjusted to make a valid comparison to the NHANES 2001–2006 survey years due to a reformulation of the DiaSorin RIA kit that resulted in a shift in assay results between the two time periods (19). Although there is no consensus as to the definition of 25(OH)D deficiency, we chose severe 25(OH)D deficiency (<10 ng/ml) as the main outcome measure, a value associated with clinical myopathy, osteomalacia, and rickets (20). Given that far fewer participants in NHANES III had 25(OH)D levels below 10 ng/ml (1%), too few for adequate statistical inferences, we used a cutoff of below 15 ng/ml because the prevalence of 25(OH)D deficiency with this cutoff was closer to that using the below 10 ng/ml cutoff in 2001–2006, and thus appeared more directly comparable. Previous research has shown differences in vitamin D levels over time, most likely due to changes in sunscreen use, outside activity, and obesity prevalence (21).
Serum creatinine was measured using the Beckman Synchron LX20 method. Serum creatinine was recorded in all participants 12 yr of age and older for NHANES 2001–2006. Estimated glomerular filtration rate (eGFR) was then calculated using the Schwartz formula in those participants less than 18 yr of age and the CKD-EPI equation in those 18 yr of age and older (22, 23). PTH levels were measured in NHANES 2003–2006 using the ECL/Origen-Electrohemiluminescent method. Serum albumin was measured via a bichromatic digital endpoint method.
Statistical analysis
All analyses were performed using sample weights that account for unequal probability of selection, nonresponse, and planned oversampling of non-Hispanic blacks and Mexican-Americans. Survey analysis was performed by creating a subpopulation of all participants with a recorded vitamin D level and any value for the chosen study variables. Participants were categorized by 25(OH)D levels below 10 ng/ml and at least 10 ng/ml. Statistical significance of participant characteristics between the two 25(OH)D subgroups was determined by univariate linear and logistic regression for continuous and dichotomous variables, respectively. Participants were also categorized by those who reported and those who did not report steroid use. Statistical significance was again determined using univariate linear and logistic regression.
Univariate logistic regression was performed to analyze predictors of 25(OH)D deficiency. Those variables that were significant at P < 0.25 on univariate testing were included in the analysis. Multivariable logistic regression analysis was then used to investigate the association between glucocorticoid use and 25(OH)D levels below10 ng/ml in an unadjusted model. We then created a model including the variables age, sex, and race/ethnicity determined a priori to be clinically meaningful and to remain in the model regardless of statistical significance. In the third model, variables that were significant at P < 0.25 on univariate testing and variables that were felt to be a priori confounders of the association were also considered for inclusion in the final multivariable model. A backward selection method was used to create the final model, which ultimately included the variables age, sex, race/ethnicity, PIR, obesity, milk intake, supplement use, days missed from school or work, and the use of any medication. Although we lost 4421 participants when adjusting for eGFR, it was felt to be an important variable, and therefore the primary analysis is presented both with and without adjustment for eGFR.
Additional multivariable analyses were performed in prespecified subgroups including age, sex, race/ethnicity, and obesity. Sensitivity analyses were performed using different definitions of 25(OH)D deficiency and adjusting for additional potential confounders. Associations between serum albumin and mortality have been shown in many disease states (24). To assess for possible confounding due to nutritional/health status, we performed a sensitivity analysis with serum albumin. Low levels of physical activity have been associated with vitamin D insufficiency. We examined whether adjustment for physical activity altered the association between low vitamin D levels and steroid use. Data on physical activity was available on 10,605 participants. We also examined whether adjustment for PTH levels altered the observed associations. PTH values were available in 13,803 of the initial population. Separate models were rerun adding albumin, physical activity, and PTH as adjustors.
All statistical analyses were performed in STATA 11.1 (StataCorp LP, College Station, TX).
Results
Low 25(OH)D levels
There were 22,650 children, adolescents, and adults in NHANES 2001–2006 with recorded 25(OH)D levels and complete data on other variables, representative of 286 million U.S. residents (Table 1). Overall, 32% of the population had levels less than 20 ng/ml, 15% less than 15 ng/ml, and 5% less than 10 ng/ml. Participants with 25(OH)D levels below 10 ng/ml were more likely to be female, non-Hispanic black, and obese; have lower PIR; and have lower levels of milk intake and supplement use. They were also less likely to use any medication but more likely to use steroids. On multivariable modeling, consistent with recent studies, females [odds ratio (OR), 1.71; 95% confidence interval (CI), 1.45, 2.02], non-Hispanic blacks (OR, 11.83; 95% CI, 9.58, 14.61), Mexican-Americans (OR, 2.49; 95% CI, 1.79, 4.54), obese individuals (OR, 1.87; 95% CI, 1.59, 2.20), and those who drank milk less than once per week or never (OR, 3.44; 95% CI, 2.73, 4.34; and OR, 4.66; 95% CI, 3.59, 6.05, respectively) were more likely to be 25(OH)D deficient, when controlling for steroid use, age, sex, race/ethnicity, PIR, obesity, milk intake, supplement use, days missed from school or work, and the use of any medication. Those who used either more than zero and less than 400 IU/d, or at least 400 IU/d of vitamin D supplementation per day were less likely to be 25(OH)D deficient (OR, 0.16; 95% CI, 0.07, 0.34; and OR, 0.25; 95% CI, 0.15, 0.43, respectively) compared with those who reported no vitamin D supplementation in the fully adjusted model. The use of any medication in the fully adjusted model was not statistically significant (OR, 0.92; 95% CI, 0.79, 1.07).
Table 1.
Participant characteristics by 25(OH)D levels within children ages 1–18 yr and adults older than 18 yr from NHANES 2001–2006 (n = 22,650)
| Age 1–18 yr |
Age >18 yr |
|||||
|---|---|---|---|---|---|---|
| <10 ng/ml (n = 536) | ≥10 ng/ml (n = 8,926) | P valueb | <10 ng/ml (n = 1,133) | ≥10 ng/ml (n = 12,055) | P valueb | |
| Age (yr)a | 15 ± 0.20 | 11 ± 0.10 | <0.001 | 44 ± 0.77 | 46 ± 0.35 | 0.01 |
| Female (%) | 65 | 48 | <0.001 | 64 | 51 | <0.001 |
| Race/ethnicity (%) | ||||||
| Non-Hispanic white | 8 | 62 | <0.001 | 31 | 75 | <0.001 |
| Non-Hispanic black | 69 | 13 | <0.001 | 50 | 8 | <0.001 |
| Mexican-American | 17 | 18 | 0.58 | 13 | 12 | 0.41 |
| Other, multiracial | 7 | 6 | 0.63 | 6 | 5 | 0.45 |
| PIR (%) | ||||||
| ≥5 | 8 | 15 | 0.03 | 14 | 26 | <0.001 |
| >1–4.99 | 57 | 63 | 0.05 | 65 | 62 | 0.15 |
| 0–1 | 36 | 22 | <0.001 | 21 | 12 | <0.001 |
| Obese (%) | 34 | 18 | <0.001 | 50 | 31 | <0.001 |
| Milk intake (%) | ||||||
| ≥1/d | 39 | 78 | <0.001 | 21 | 47 | <0.001 |
| ≥1/wk but <1/d | 33 | 14 | <0.001 | 29 | 26 | 0.07 |
| <1/wk or varied | 16 | 5 | <0.001 | 22 | 14 | <0.001 |
| Never | 12 | 3 | <0.001 | 28 | 13 | <0.001 |
| Supplement use (%) | ||||||
| None | 96 | 87 | 0.01 | 96 | 80 | <0.001 |
| <400 IU/d | 2 | 3 | 0.41 | 1 | 6 | <0.001 |
| ≥400 IU/d | 2 | 10 | <0.001 | 4 | 14 | <0.001 |
| Days missed from school or worka | 3 ± 0.30 | 3 ± 0.12 | 0.89 | 5 ± 1.11 | 4 ± 0.26 | 0.26 |
| eGFR (ml/min/1.73 m2)a,c | 134 ± 2.00 | 140 ± 0.77 | 0.02 | 101 ± 1.19 | 94 ± 0.52 | <0.001 |
| Any medications used (%) | 19 | 27 | 0.02 | 51 | 55 | 0.03 |
| Steroid use (%) | 1 | 0.4 | 0.1 | 2 | 1 | 0.04 |
Participants over 85 yr of age are reported as being 85 yr old in order to protect anonymity.
Values are expressed as mean ± se.
P value from linear or logistic regression.
eGFR only available in participants at least 12 yr of age; number of observations = 18,229.
Steroid use
Overall, 0.9% of the population used steroids within the past 30 d. Among steroid users, 11% had 25(OH)D levels below 10 ng/ml, compared with 5% among steroid nonusers (P = 0.009) (Table 2). Steroid use compared with no steroid use was more common in those who were older, obese, and used less than 400 IU/d of vitamin D supplements (Table 2). Steroid use was more common (2%) in patients with 25(OH)D levels less than 10 ng/ml, compared with 0.9% in patients with 25(OH)D levels above 10 ng/ml (P = 0.009).
Table 2.
Participant characteristics by steroid use within the past 30 d of 22,650 children and adults aged 1 to 85 yr of age from NHANES 2001–2006
| Steroid use (n = 181) | No steroid use (n = 22,469) | P valueb | |
|---|---|---|---|
| 25(OH)D levels (%) | |||
| <10 ng/ml | 11 | 5 | 0.009 |
| <15 ng/ml | 21 | 15 | 0.05 |
| <20 ng/ml | 36 | 32 | 0.39 |
| <30 ng/ml | 75 | 76 | 0.85 |
| Age (yr)a | 52 ± 1.98 | 37 ± 0.35 | <0.001 |
| Female (%) | 46 | 51 | 0.32 |
| Race/ethnicity (%) | |||
| Non-Hispanic white | 77 | 70 | 0.08 |
| Non-Hispanic black | 14 | 12 | 0.39 |
| Mexican-American | 7 | 13 | 0.01 |
| Other, multiracial | 2 | 5 | 0.18 |
| PIR (%) | |||
| ≥5 | 22 | 23 | 0.94 |
| >1–4.99 | 63 | 62 | 0.94 |
| 0–1 | 15 | 15 | 0.99 |
| Obese (%) | 38 | 29 | 0.04 |
| Milk intake (%) | |||
| ≥1/d | 50 | 53 | 0.44 |
| ≥1/wk but <1/d | 27 | 23 | 0.26 |
| <1/wk or varied | 9 | 12 | 0.36 |
| Never | 14 | 12 | 0.54 |
| Supplement use (%) | |||
| None | 77 | 82 | 0.09 |
| <400 IU/d | 11 | 5 | 0.002 |
| ≥400 IU/d | 12 | 13 | 0.88 |
| Days missed from school or worka | 10 ± 4.90 | 4 ± 0.20 | 0.01 |
| eGFR (ml/min/1.73m2)a,c | 76 ± 2.68 | 100 ± 0.56 | <0.001 |
| Any medications used (%) | 100 | 48 | d |
Participants over 85 yr of age are reported as being 85 yr old in order to protect anonymity.
Values are expressed as mean ± se.
P value from linear or logistic regression.
eGFR was only available in participants ≥12 yr of age; number of observations = 18,229.
Unable to calculate P value secondary to cell with no participants.
Association of steroid use and low 25(OH)D levels
The odds of having 25(OH)D deficiency were 2-fold higher in those who reported steroid use compared with those without steroid use (OR, 2.36; 95% CI, 1.25, 4.45). This association remained after multivariable adjustment for age, sex, and race/ethnicity (OR, 2.21; 95% CI, 1.06, 4.62) and in the fully adjusted multivariable model (OR, 2.21; 95% CI, 1.01, 4.85) (Table 3). When adding eGFR to the multivariable analysis, the OR for steroid use was 2.34 (95% CI, 1.02, 5.34).
Table 3.
Logistic regression models, OR of low 25(OH)D associated with steroid use
| OR | 95% CI | |
|---|---|---|
| 25(OH)D levels <10 ng/ml | ||
| Unadjusted model | 2.36 | 1.25, 4.45 |
| Partially adjusted modela | 2.21 | 1.06, 4.62 |
| Multivariable adjusted modelb | 2.21 | 1.01, 4.85 |
| 25(OH)D levels <15 ng/ml | ||
| Unadjusted model | 1.43 | 1.00, 2.04 |
| Partially adjusted modela | 1.23 | 0.86, 1.77 |
| Multivariable adjusted modelb | 1.36 | 0.92, 2.00 |
| 25(OH)D levels <20 ng/ml | ||
| Unadjusted model | 1.17 | 0.82, 1.67 |
| Partially adjusted modela | 1.01 | 0.70, 1.45 |
| Multivariable adjusted modelb | 1.18 | 0.78, 1.78 |
Adjusted for age, sex, race/ethnicity.
Adjusted for age, sex, race/ethnicity, PIR, obesity, milk intake, vitamin D supplementation use, any days missed from school or work, and any medication use.
Subgroup analysis
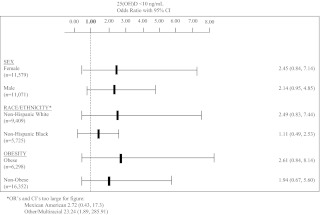
The association of steroid use with 25(OH)D deficiency was examined within categories of age, sex, race/ethnicity, and obesity. Among participants no older than 18 yr, the OR for steroid use was 14.05 (95% CI, 1.22, 161.98), whereas among participants more than 18 yr old, the OR was 2.13 (95% CI, 0.92, 4.90). Other groups are presented in Fig. 1.
Fig. 1.
Fully adjusted OR and 95% CI for subgroup analyses of sex, race/ethnicity, and obesity.
Sensitivity analysis
Sensitivity analyses were performed to test whether adjustments for physical activity, PTH levels, or albumin levels altered the observed associations between steroid use and 25(OH)D deficiency. When adjusting the main analysis for physical activity, the OR for steroid use was 2.31 (95% CI, 0.66, 7.99). Adding PTH to the main multivariable model, the OR for steroid use was 3.32 (95% CI, 1.41, 7.82). When adjusting the main multivariable model for serum albumin as a measure of overall nutritional/health status, the OR for steroid use was 2.18 (95% CI, 0.99, 4.80).
Several sensitivity analyses were also performed to explore the association of steroid use with other definitions of 25(OH)D deficiency (Table 3). When defining 25(OH)D deficiency as levels below 15 ng/ml, the unadjusted OR for steroid users was 1.43 (95% CI, 1.00, 2.04). The association lost significance when adjusting for other variables. When defining 25(OH)D deficiency as levels below 20 ng/ml, none of the models had statistically significant results.
A sensitivity analysis was performed using data from NHANES III, with the adjusted 25(OH)D assay values. We analyzed 15,374 participants representative of 166 million U.S. residents. Similar to NHANES 2001–2006, 1% of the population reported steroid use. Unlike NHANES 2001–2006, only 1% of the participants had 25(OH)D levels below 10 ng/ml; 9% had 25(OH)D levels below 15 ng/ml and 24% below 20 ng/ml. Among steroid users, 14% had 25(OH)D levels below 15 ng/ml, compared with 9% among steroid nonusers (P = 0.04). Steroid use was more common in participants with 25(OH)D levels less than 15 ng/ml, 2% compared with 1% in patients with 25(OH)D levels above 15 ng/ml (P = 0.04). The odds of having 25(OH)D deficiency, defined as 25(OH)D level below 15 ng/ml, were approximately 2-fold higher in those who reported steroid use compared with those without steroid use (OR, 1.75; 95% CI, 1.03, 3.00). This association remained after multivariable adjustment with age, sex, race/ethnicity, obesity, PIR, and supplement use (OR, 1.88; 95% CI, 1.01, 3.48).
Discussion
Recent research has confirmed that vitamin D deficiency is a global problem affecting an estimated 1 billion people worldwide (2, 4, 25–27). Multiple large-scale studies have estimated a prevalence of vitamin D deficiency, using various definitions of vitamin D deficiency, to vary between 24 and 70% in the general pediatric population and between 36 and 57% in an adult population (1–4, 25, 26, 28–31). Studies in vitamin D receptor (VDR) knockout mice demonstrate that VDR-deficient mice have increased sensitivity to autoimmune diseases, are more prone to oncogene-induced tumors, and develop high renin hypertension and cardiac hypertrophy (32, 33). In addition, recent studies in humans reveal that there are potential benefits of adequate vitamin D levels not only on bone, but also on blood pressure, rate of kidney disease progression, and prevention of cardiovascular disease, autoimmune diseases, cancer, and all-cause mortality (5, 6, 8, 9, 34–37).
This is the first study, to our knowledge, that shows a significant association between steroid use and vitamin D deficiency in a large, nationally representative sample of children and adults. In 2001–2006, approximately 0.9% (2.1 million) of U.S. children and adults reported steroid use within the past 30 d. Of those who used steroids, a statistically significant greater percentage had 25(OH)D levels less than 10 ng/ml compared with those who did not report steroid use.
The present findings are consistent with results from recent smaller studies in the fields of pulmonology, gastroenterology, and rheumatology that found an association between steroid use and low levels of 25(OH)D. In a cohort study of 124 women with systemic lupus erythematosus, Toloza et al. (17) demonstrated by multivariable logistic regression that cumulative glucocorticoid exposure was significantly associated with low levels of 25(OH)D (P = 0.03) when adjusting for ethnicity, season, and serum creatinine. Sentongo et al. (16) demonstrated that among 112 children, adolescents, and young adults with Crohn's disease, vitamin D deficiency was associated with winter season, African-American ethnicity, Crohn's disease confined to the upper gastrointestinal tract, and magnitude of lifetime exposure to glucocorticoid therapy (23.7 ± 13.5 compared with 17.5 ± 12.2 mg/d glucocorticoids; P = 0.05). In a study of 100 asthmatic children, the use of inhaled steroids (P = 0.05), oral steroids (P = 0.02), and total steroid dose (P = 0.001) all showed significant inverse correlations with serum vitamin D levels (15).
The mechanism of action of glucocorticoids and its association with vitamin D deficiency is not completely understood. Glucocorticoids are known to enhance bone resorption, decrease bone formation, decrease intestinal calcium absorption, and increase urinary calcium excretion (2). The administration of glucocorticoids to vitamin D-deficient rats does not affect the rate of conversion of a physiological dose of 25(OH)D to 1,25-dihydroxyvitamin D [1,25(OH)2D] (38, 39). In 2000, Akeno et al. (12) demonstrated that dexamethasone increased renal expression of vitamin D-24-hydroxylase, which in turn degrades vitamin D metabolites such as 25(OH)D and 1,25(OH)2D. This same group then used an established renal cell line, LLC-PK1 cells, and UMR-106 osteoblast-like cells, in which they demonstrated that cells treated with 1,25(OH)2D expressed 24-hydroxylase mRNA and that treatment with dexamethasone for 24 h significantly enhanced the abundance of 24-hydroxylase mRNA (13). This increased glucocorticoid-stimulated expression of 24-hydroxylase mRNA was completely abolished with the addition of cycloheximide, a protein synthesis inhibitor (13). Most recently, in 2010, Dhawan and Christakos (14) demonstrated that via a novel mechanism of functional cooperation of glucocorticoid receptor, C/EBPβ, and VDR, glucocorticoids directly enhance 24-hydroxylase transcription. In summary, it appears that steroids may enhance inactivation of 25(OH)D by up-regulating 24-hydroxylase activity.
Extending current clinical and experimental data supporting an association between glucocorticoid use and vitamin D deficiency, we showed in a nationally representative sample of U.S. children and adults that there is a statistically significant association between the two. It is likely that those participants who reported steroid use suffered from chronic illness that may be associated with poor nutrition, decreased consumption of vitamin D and calcium-rich foods, as well as decreased sun exposure from lack of outside physical activity, all of which place these participants at increased risk for vitamin D deficiency. However, even when adjusting for days missed from school or work, use of any medications, and eGFR, glucocorticoid use remained independently associated with severe vitamin D deficiency.
Although the results of this study from the analysis of NHANES 2001–2006 and replicated in NHANES III, representative samples of the U.S. population, could have broad implications, our study has several important limitations. We are limited by a lack of information on several potential important confounders, including the season of measurement of 25(OH)D, the latitude of the participants' homes, the reason for glucocorticoid use, as well as the cumulative dose of glucocorticoid. Although NHANES 2001–2006 provides some of the best and most recent estimates of the prevalence of chronic disease, this study uses a cross-sectional study design, and therefore, caution should be taken when considering the direction of associations, and, as in any observational study, causality cannot be established. Despite the cross-sectional nature of the study, the sample size of the study cohort is larger than any previously reported study investigating steroid use and vitamin D levels. This allowed us to control for a variety of confounders including demographics, dietary factors, and obesity scores, which were all collected in a standardized fashion.
This current study indicates that steroid use is associated with severe 25(OH)D deficiency. It is generally recognized that serum 25(OH)D levels less than 10 ng/ml are associated with clinical myopathy, osteomalacia, and rickets (20). Therefore, it is prudent that we address the issue of vitamin D deficiency in the general population, but even more so in those who use glucocorticoids and are therefore at higher risk of vitamin D deficiency and its possible sequelae.
Acknowledgments
A.L.S. is supported by Grant T32 DK07110-27; J.K. is supported by Grant K23 DK084339; F.J.K. is supported by Grants T32 DK07110-27 and U01 DK066174-01; and M.L.M. is supported by Grants K23 DK078774, R01 DK080123, and R01 DK087783, all from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (NIH). Additionally, this publication was made possible by the Clinical and Translational Science Awards Grants UL1 RR025750, KL2 RR025749, and TL1 RR025748 from the National Center for Research Resources (NCRR), a component of the NIH, and the NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH.
Disclosure Summary: All who have contributed to this manuscript have been named as authors. None of the authors have any potential conflicts of interest to report.
Footnotes
- BMI
- Body mass index
- CI
- confidence interval
- eGFR
- estimated glomerular filtration rate
- OR
- odds ratio
- 1,25(OH)2D
- 1,25-dihydroxyvitamin D
- 25(OH)D
- 25-hydroxyvitamin D
- PIR
- poverty income ratio
- VDR
- vitamin D receptor.
References
- 1. Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML. 2009. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001–2004. Pediatrics 124:e362–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holick MF. 1987. Vitamin D and the kidney. Kidney Int 32:912–929 [DOI] [PubMed] [Google Scholar]
- 3. Binkley N, Novotny R, Krueger D, Kawahara T, Daida YG, Lensmeyer G, Hollis BW, Drezner MK. 2007. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab 92:2130–2135 [DOI] [PubMed] [Google Scholar]
- 4. Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. 2006. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 84:18–28 [DOI] [PubMed] [Google Scholar]
- 5. Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K. 2007. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the third National Health and Nutrition Examination Survey. Arch Intern Med 167:1159–1165 [DOI] [PubMed] [Google Scholar]
- 6. Scragg R, Sowers M, Bell C. 2007. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the third National Health and Nutrition Examination Survey. Am J Hypertens 20:713–719 [DOI] [PubMed] [Google Scholar]
- 7. Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. 2005. Estimates of optimal vitamin D status. Osteoporos Int 16:713–716 [DOI] [PubMed] [Google Scholar]
- 8. Autier P, Gandini S. 2007. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med 167:1730–1737 [DOI] [PubMed] [Google Scholar]
- 9. Melamed ML, Michos ED, Post W, Astor B. 2008. 25-Hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 168:1629–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Canalis E. 1996. Clinical review 83: mechanisms of glucocorticoid action in bone: implications to glucocorticoid-induced osteoporosis. J Clin Endocrinol Metab 81:3441–3447 [DOI] [PubMed] [Google Scholar]
- 11. Lukert BP, Raisz LG. 1990. Glucocorticoid-induced osteoporosis: pathogenesis and management. Ann Intern Med 112:352–364 [DOI] [PubMed] [Google Scholar]
- 12. Akeno N, Matsunuma A, Maeda T, Kawane T, Horiuchi N. 2000. Regulation of vitamin D-1α-hydroxylase and -24-hydroxylase expression by dexamethasone in mouse kidney. J Endocrinol 164:339–348 [DOI] [PubMed] [Google Scholar]
- 13. Kurahashi I, Matsunuma A, Kawane T, Abe M, Horiuchi N. 2002. Dexamethasone enhances vitamin D-24-hydroxylase expression in osteoblastic (umr-106) and renal (llc-pk1) cells treated with 1α,25-dihydroxyvitamin D3. Endocrine 17:109–118 [DOI] [PubMed] [Google Scholar]
- 14. Dhawan P, Christakos S. 2010. Novel regulation of 25-hydroxyvitamin D3 24-hydroxylase (24(oh)ase) transcription by glucocorticoids: cooperative effects of the glucocorticoid receptor, c/ebp β, and the vitamin C receptor in 24(oh)ase transcription. J Cell Biochem 110:1314–1323 [DOI] [PubMed] [Google Scholar]
- 15. Searing DA, Zhang Y, Murphy JR, Hauk PJ, Goleva E, Leung DY. 2010. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Allergy Clin Immunol 125:995–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sentongo TA, Semaeo EJ, Stettler N, Piccoli DA, Stallings VA, Zemel BS. 2002. Vitamin D status in children, adolescents, and young adults with Crohn disease. Am J Clin Nutr 76:1077–1081 [DOI] [PubMed] [Google Scholar]
- 17. Toloza SM, Cole DE, Gladman DD, Ibañez D, Urowitz MB. 2010. Vitamin D insufficiency in a large female SLE cohort. Lupus 19:13–19 [DOI] [PubMed] [Google Scholar]
- 18. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. 2011. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 96:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yetley EA, Pfeiffer CM, Schleicher RL, Phinney KW, Lacher DA, Christakos S, Eckfeldt JH, Fleet JC, Howard G, Hoofnagle AN, Hui SL, Lensmeyer GL, Massaro J, Peacock M, Rosner B, Wiebe D, Bailey RL, Coates PM, Looker AC, Sempos C, Johnson CL, Picciano MF. 2010. NHANES monitoring of serum 25-hydroxyvitamin D: a roundtable summary. J Nutr 140:2030S–2045S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heaney RP. 2004. Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am J Clin Nutr 80:1706S–1709S [DOI] [PubMed] [Google Scholar]
- 21. Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. 2008. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr 88:1519–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. 2009. A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schwartz GJ, Brion LP, Spitzer A. 1987. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 34:571–590 [DOI] [PubMed] [Google Scholar]
- 24. Takata Y, Ansai T, Yoshihara A, Miyazaki H. 1 April 2011. Serum albumin (SA) levels and 10-year mortality in a community-dwelling 70-year-old population. Arch Gerontol Geriatr doi:10.1016/j.archger.2011.02.018 [DOI] [PubMed] [Google Scholar]
- 25. Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, Vamvakas EC, Dick IM, Prince RL, Finkelstein JS. 1998. Hypovitaminosis D in medical inpatients. N Engl J Med 338:777–783 [DOI] [PubMed] [Google Scholar]
- 26. Malabanan A, Veronikis IE, Holick MF. 1998. Redefining vitamin D insufficiency. Lancet 351:805–806 [DOI] [PubMed] [Google Scholar]
- 27. Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, Holick MF, Hollis BW, Lamberg-Allardt C, McGrath JJ, Norman AW, Scragg R, Whiting SJ, Willett WC, Zittermann A. 2007. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr 85:649–650 [DOI] [PubMed] [Google Scholar]
- 28. Tangpricha V, Pearce EN, Chen TC, Holick MF. 2002. Vitamin D insufficiency among free-living healthy young adults. Am J Med 112:659–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. 2004. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med 158:531–537 [DOI] [PubMed] [Google Scholar]
- 30. Gordon CM, Feldman HA, Sinclair L, Williams AL, Kleinman PK, Perez-Rossello J, Cox JE. 2008. Prevalence of vitamin D deficiency among healthy infants and toddlers. Arch Pediatr Adolesc Med 162:505–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. 2002. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone 30:771–777 [DOI] [PubMed] [Google Scholar]
- 32. Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M. 2008. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 29:726–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goltzman D. 2010. Vitamin D action: lessons learned from genetic mouse models. Ann NY Acad Sci 1192:145–152 [DOI] [PubMed] [Google Scholar]
- 34. Agarwal R, Acharya M, Tian J, Hippensteel RL, Melnick JZ, Qiu P, Williams L, Batlle D. 2005. Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int 68:2823–2828 [DOI] [PubMed] [Google Scholar]
- 35. Alborzi P, Patel NA, Peterson C, Bills JE, Bekele DM, Bunaye Z, Light RP, Agarwal R. 2008. Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: a randomized double-blind pilot trial. Hypertension 52:249–255 [DOI] [PubMed] [Google Scholar]
- 36. Mehrotra R, Kermah DA, Salusky IB, Wolf MS, Thadhani RI, Chiu YW, Martins D, Adler SG, Norris KC. 2009. Chronic kidney disease, hypovitaminosis D, and mortality in the United States. Kidney Int 76:977–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Melamed ML, Astor B, Michos ED, Hostetter TH, Powe NR, Muntner P. 2009. 25-Hydroxyvitamin D levels, race, and the progression of kidney disease. J Am Soc Nephrol 20:2631–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kimberg DV, Baerg RD, Gershon E, Graudusius RT. 1971. Effect of cortisone treatment on the active transport of calcium by the small intestine. J Clin Invest 50:1309–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Favus MJ, Kimberg DV, Millar GN, Gershon E. 1973. Effects of cortisone administration on the metabolism and localization of 25-hydroxycholecalciferol in the rat. J Clin Invest 52:1328–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]