Abstract
Context:
Leptin is a potent modulator of the hypothalamic-pituitary-gonadal axis mediating the effect of energy deprivation on several hypothalamic-pituitary-peripheral axes. Activin A, inhibin B, and follistatin (FST) also regulate the hypothalamic-pituitary-gonadal axis in humans. It remains unknown whether energy deprivation affects these hormone levels in a leptin-dependent or -independent manner.
Objective:
We investigated 1) day-night variability patterns of activin, inhibin, and FST in the fed state, 2) whether their levels are affected by fasting, and 3) whether such an effect is mediated by leptin in physiological replacement or pharmacological doses.
Design:
We conducted two studies in healthy, eumenorrheic females, each comprising three separate admissions. In study 1, six women were maintained for 72 h 1) on isocaloric diet, 2) fasting while receiving placebo, or 3) fasting while receiving metreleptin in physiological replacement doses. In study 2, five women were administered physiological or pharmacological metreleptin doses (0.01, 0.1, or 0.3 mg/kg iv four times daily).
Results:
Neither activin A nor FST had a pulsatile or day-night variability pattern. Inhibin B levels were also nonpulsatile, but a trend toward a day-night pattern was noted. When compared with the fed state, inhibin B levels remained unchanged, whereas FST levels increased (P = 0.01) and activin A decreased (P = 0.01) in the fasting state. These changes were not corrected with metreleptin administered in replacement or pharmacological doses.
Conclusions:
Short-term energy deprivation alters levels of activin A and FST, but these effects are not mediated by leptin.
Activins, inhibins, and follistatin (FST) were discovered in the 1980s and found to regulate gonadotropin secretion from the anterior pituitary (1, 2). Activins and inhibins belong to the TGF-β family, whose members exert their effects on a variety of systems, including ovarian regulation, adult tissue homeostasis, bone formation, tissue remodeling, wound healing and repair, muscle development, and angiogenesis (3, 4). Activin primarily stimulates pituitary gonadotropin release, whereas inhibin binds and neutralizes activin by inhibiting ligand-receptor interactions and promoting activin internalization and degradation (5, 6). FST is a ubiquitous, monomeric, secreted glycoprotein that, despite its lack of structural homology with other TGF-β family members, interacts with several of them and is the main activin-binding protein. FST and inhibin down-regulate activin's stimulatory effect and act as potent modulators of the hypothalamic-pituitary-gonadal (HPG) axis (6).
Another powerful modulator of the HPG axis is leptin, the major signal of energy stores in the body. Leptin is a 167-amino-acid adipocyte-secreted protein coded by the leptin gene that was originally described in an obesity mouse model (7). Its role in human physiology has since been extensively studied, and leptin has been found to play a major role in the neuroendocrine regulation of energy homeostasis (8–10). During states of energy deprivation, leptin levels decrease rapidly and trigger the neuroendocrine response to starvation, including down-regulation of the HPG axis in humans with fasting-induced hypoleptinemia (8, 11–13). The latter translates into suppression of the overnight LH peak frequency in females (13) and males (12) and up to 40% decrease in testosterone levels in males (12). Conversely, leptin replacement results in resumption of LH pulsatility and ovulatory menstruation in leptin-deficient patients (14). Thus, in energy-insufficiency states, leptin acts as a signal of energy insufficiency to inhibit energy-requiring processes, such as reproduction, that are not crucial for survival (15, 16).
Despite the major roles that activin, inhibin, and FST play in ovarian function and reproductive physiology in humans, only limited data exist in terms of the secretion patterns of these hormones, and they apply mainly to pubertal girls and males (17, 18). The only study in young, healthy women measured circulating inhibin B over 6 h, which severely limits the interpretation of secretion pattern data (19). Moreover, no studies have been published on the effect of food deprivation on these hormone levels or on the potential role of leptin if such an effect does exist.
We designed two interventional studies to determine whether 1) the 24-h levels of these hormones display any pulsatile and/or day-night pattern in young, healthy females of reproductive age, 2) their levels are affected by short-term energy deprivation, 3) if such an effect exists, it is mediated by food deprivation-induced changes in circulating leptin, and 4) short-term energy deprivation and changes in these hormones alter reproductive hormone levels. Moreover, to fully assess the potential effect of leptin on these hormone levels in the fasting state, we further investigated whether meterleptin administration not only in physiological replacement but also in pharmacological doses would alter levels of these hormones in young, healthy, eumenorrheic women.
Materials and Methods
Study designs
Seventy-two-hour fed/fasting study
Following a previously described protocol (13), six healthy lean women [age 22.8 ± 3.4 yr; body mass index (BMI) = 21.7 ± 2.2 kg/m2] with regular menstrual cycles (length 26–32 d) and no history of oral contraceptive use for at least 6 months participated in a clinical study involving three separate 4-d-long admissions, separated by at least 8 wk, at the General Clinical Research Center (GCRC) of the Beth Israel Deaconess Medical Center. Starting at 0800 h on d 3, blood samples were drawn every 15 min for 24 h through an indwelling catheter. The timing of these frequent blood sampling admissions was scheduled within menstrual cycle d 6–11 and within 2 d of the menstrual cycle day on which subjects had participated in the other two studies.
For the baseline pulsatility study, subjects were admitted to the GCRC for 72 h and participated in a baseline study in the isocaloric fed state [20% of calories from breakfast (0800 h), 35% from lunch (1300 h), 35% from dinner (1800 h), and 10% from snack (2200 h)]. Once the initial analysis revealed a trend toward day-night secretion (see below), we enrolled an additional three healthy, lean, eumenorrheic subjects (total n = 9) and measured their inhibin B levels in the fed state.
The 72-h fasting studies were performed in a double-blinded, randomized fashion. Subjects were admitted to the GCRC for 72 h and participated in two fasting studies: 1) a 72-h fasting study with administration of meterleptin (provided by Amylin, Inc., San Diego, CA; previously known as r-metHuLeptin, provided by Amgen, Inc., Thousand Oaks, CA) (replacement dose of 0.08 mg/kg · d on d 1, followed by 0.2 mg/kg · d on d 2–3, in four equal doses given sc every 6 h starting at 0800 h on d 1), and 2) a 72-h fasting study with administration of placebo (same schedule, volume, and mode of administration as the meterleptin dose).
Dose-dependent leptin replacement
Five healthy lean women (age 20.4 ± 0.7 yr; BMI = 21.9 ± 0.7 kg/m2) with regular menstrual cycles (length 26–32 d) and no history of oral contraceptive use for at least 6 months participated in one clinical study, involving three admissions separated by at least 3–10 wk (as previously described) (20): 1) a 72-h fasting study with administration of meterleptin (dose of 0.01 mg/kg once daily at 0800 h), 2) a 72-h fasting study with administration of meterleptin (dose of 0.1 mg/kg once daily at 0800 h), and 3) a 72-h fasting study with administration of meterleptin (dose of 0.3 mg/kg once daily at 0800 h). In all three studies, starting at 0800 h on d 3, blood samples were drawn and available at time 0, +10 min, +30 min, and +1, +2, +3, +4, +5, +6, +8, +10, +12, and +18 h after meterleptin administration.
All studies were approved by the Institutional Review Board of the Beth Israel Deaconess Medical Center and investigator-initiated Investigational New Drug approval was obtained by CSM from the U.S. Food and Drug Administration for use of meterleptin.
Hormone assays
FST levels were measured using a commercially available ELISA (R&D Systems, Minneapolis, MN), with a sensitivity of 29 pg/ml, intraassay coefficients of variation (CV) of 2.0–2.7% and interassay CV of 7.1–9.2%. Inhibin B levels were measured using a commercially available Gen II ELISA (Beckman Coulter, Inc., Brea, CA), with a sensitivity of 2.6 pg/ml, intraassay CV of 2.2–3.8%, and interassay CV of 3.7–5.6%. Activin A levels were measured using a commercially available ELISA (R&D Systems, Minneapolis, MN), with a sensitivity of 3.67 pg/ml, intraassay CV of 4.2–4.4%, and interassay CV of 4.7–7.9%. FSH levels were measured with the automated Immulite 1000 chemiluminescence assay system, with a sensitivity of 0.1 mIU/ml, intraassay CV of 2.6%, and interassay CV of 5.8%. Free fatty acids (FFA), estradiol, insulin, total testosterone, and SHBG levels were measured as previously described (12, 13). Total leptin levels (nanograms per milliliter) were measured using RIA [Linco Research (St. Louis, MO), now Millipore (Billerica, MA)], with a sensitivity of 0.5 ng/ml, intraassay CV of 7%, and interassay CV of 18–20%. This assay detected both endogenous leptin and exogenously administered meterleptin. LH levels were measured using an automated immunoassay system (Immulite 1000; Siemens, Deerfield, IL), with a sensitivity of 0.1 mIU/ml, intraassay CV of 4.8–6.5%, and interassay CV of 7.2–26.0%. All samples were run in dilutions as necessary for values to fall in the linear part of the standard curve and in duplicate within the same assay to decrease interassay variability.
Pulse analysis
Pulse_XP software (UVA Pulse Analysis Software, Charlottesville, VA) was used to analyze pulsatile (episodic) and day-night (periodic) secretion. Episodic secretion was examined using the previously evaluated Cluster8 computerized pulse-analysis algorithm (21). This algorithm uses the assay's measurement error, as expressed in sd between duplicate measurements, to detect clusters of statistically significant peaks or nadirs compared with preceding and following values, using a pooled t statistic. For the FST data that had a temporal resolution of 15 min, we used an MxN cluster of 1 × 2 and a T-score of 1.5. For inhibin B and activin A data that had a temporal resolution of 30 min a MxN cluster of 1 × 1 and a T-score of 1.5 was used. The periodic component of the time series was evaluated by the cosine algorithm that fits a four-parameter cosine function with a fixed period of 24 h at the time series and allows for evaluation of the amplitude, the residual variability, and the phase agreement between subjects. We also used a nonlinear ordinal least-squares trigonometric regression model, with a fixed period at 1 d, applied to all subjects to evaluate potential periodicity, and the adjusted nonlinear coefficient of determination (R2) was calculated.
Statistical analysis
Data are presented as mean ± sem and statistical analysis used Stata version 11.1 (Stata Corp., College Station, TX). Data were examined for normality using probability-probability plots and the Shapiro-Wilkes test. For study 1, we analyzed our data using hierarchical, mixed-effects linear regression models. Hormone levels were modeled as a linear function of time and condition (fed, fasting with placebo, or fasting with meterleptin) using dummy encoding. Time and condition were the fixed effects of the model, and we included random intercepts at both the subject and condition level. For study 2, we compared hormone levels in the three conditions (0.01, 0.1, and 0.3 mg/kg meterleptin) by repeated-measurements analysis of covariance across the evaluated time points. Both time and condition were used as within-subject factors in the repeated-measurements analysis of covariance model, because the subjects participating in the three studies were the same, whereas the baseline hormone levels were used as a covariate to adjust for baseline fluctuations between the subjects. Post hoc tests were performed, with Tukey's honestly significant difference test for multiple comparisons.
Results
Day-night variation and pulsatility of FST, inhibin B, and activin A (Fig. 1)
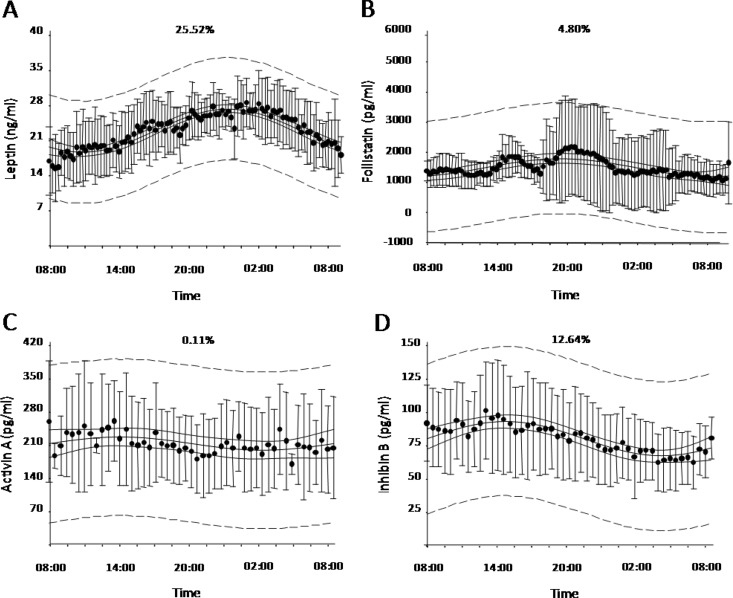
Fig. 1.
Average 24-h (0800–0800 h) leptin (n = 6) (A), FST (n = 6) (B), activin A (n = 6) (C), and inhibin B (n = 9) (D) levels on d 3 of baseline fed state (nonlinear adjusted R2 is displayed on top center of each panel; dashed lines represent 95% prediction band; solid lines represent 95% confidence bands).
The Cluster8 pulse detection algorithm failed to detect any statistically significant pulses in the FST, inhibin B, or activin A secretion patterns. Nonlinear ordinal least-squares regression revealed no day-night pattern for FST (adjusted R2 = 4.80%) or activin A (adjusted R2 = 0.11%). Analysis of inhibin B secretion revealed a trend toward a day-night pattern, which was confirmed by the analysis of three additional subjects (n = 9; adjusted R2 = 12.64%), with higher levels occurring at midday (peak at 1545 h) and lower levels occurring in the early morning (nadir at 0630 h; peak-to-nadir level difference of 48.74%). However, when we tried to replicate these results in the fasting plus placebo and fasting plus meterleptin states, we failed to demonstrate a day-night variability pattern.
Effects of 72-h fasting on FFA and hormone levels (Figs. 2 and 3)
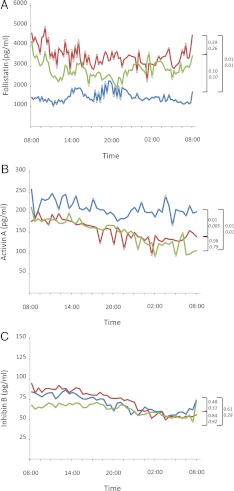
Fig. 2.
Average (n = 6) 24-h profile (0800–0800 h) of FST (A), activin A (B), and inhibin B (C) levels on d 3 of baseline fed state (blue), 72 h fasting with placebo (red), and 72 h fasting with replacement-dose meterleptin (green) (P values are shown next to brackets, lower P values shown in italics are adjusted for age and BMI).
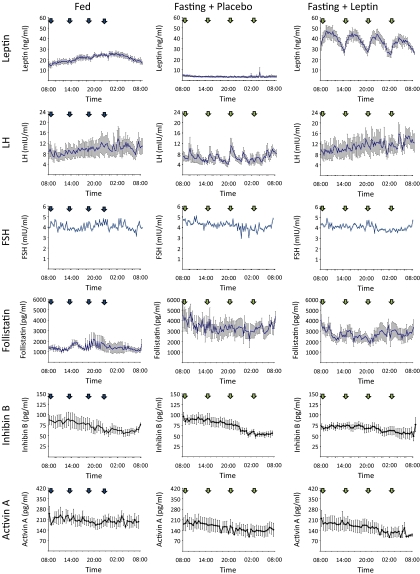
Fig. 3.
Average (n = 6) 24-h profile (0800–0800 h) of leptin, LH (12, 13), FSH, FST, inhibin B, and activin A levels on d 3 of baseline fed state (left), 72 h fasting with placebo (center), and 72 h fasting with replacement-dose meterleptin (right) (blue arrows indicate meals; green arrows indicate iv placebo/leptin).
As previously described (12, 13), 72-h fasting led to a significant decrease of serum leptin values (2.8 ± 0.3 ng/ml) from the baseline fed state (16.7 ± 1.2 ng/ml), but these levels fully recovered to the physiological range upon meterleptin administration (28.8 ± 2.0 ng/ml, overall P = 0.002). Similarly, overnight LH peak frequency had a statistically significant decrease during 72-h fasting (7.3 ± 0.4 vs. 4.5 ± 1.1 peaks per 12 h, P < 0.05), with complete recovery after meterleptin replacement (6.4 ± 0.5 peaks per 12 h, P = 0.04). Furthermore, 72-h fasting significantly increased FFA levels (0.04 ± 0.0 vs. 0.9 ± 0.2 mEq/liter, P = 0.03), significantly decreased insulin levels (8.1 ± 1.1 vs. 1.2 ± 0.2 μIU/ml, P < 0.01), but did not alter estradiol, total testosterone, or SHBG levels (all P > 0.05). Meterleptin administration in the fasting state did not change significantly any of these hormone levels (all P > 0.05).
On d 3 of the 72-h fasting, FST levels increased significantly compared with levels in the fed state (3499.7 ± 706.8 vs. 1522.4 ± 316.7 pg/ml, P = 0.01). Leptin replacement during fasting resulted in FST levels (2785.1 ± 537.6 pg/ml) that were not statistically different from the fasting plus placebo (P = 0.39) or fed states (P = 0.10). Conversely, fasting significantly decreased activin A levels compared to the fed state (154.5 ± 36.9 vs. 206.5 ± 34.3 ng/ml, P = 0.01). However, in this case, treatment of fasting subjects with replacement-dose meterleptin did not cause a statistically significant change in activin A levels (154.0 ± 38.1 ng/ml) compared with fasting plus placebo (P = 0.98), and levels remained statistically higher compared with the fed state (P = 0.01). After short-term energy deprivation, inhibin B levels did not change compared with the fed state, with and without leptin replacement (69.5 ± 10.5 vs. 73.0 ± 7.7 vs. 62.0 ± 6.4 ng/ml, all P > 0.05), and neither did FSH levels (3.9 ± 0.6 vs. 4.2 ± 0.8 vs. 4.0 ± 0.2 mIU/ml, all P > 0.05). These findings remained unchanged after controlling for age and BMI (italicized P values in Fig. 2).
Effects of physiological and pharmacological leptin replacement on activin A, inhibin B, and FST levels
As previously described (20), on the third day of a 72-h fast, circulating levels of leptin were decreased from the baseline fed state. However, these levels were progressively restored with increasing doses of leptin replacement and eventually eliminated at the 0.3 mg/kg meterleptin dose.
After meterleptin administration, there was no effect of dose on any of the levels of FST (P = 0.84), activin A (P = 0.30), or inhibin B (P = 0.09), after adjusting for baseline values.
Discussion
We demonstrate for the first time that FST, inhibin B, and activin A are not secreted in a pulsatile manner in young, healthy females, nor do they display a day-night pattern of secretion. Only inhibin B exhibited a clinically nonsignificant trend toward day-night secretion with peak levels in midday in the first six subjects studied, and despite increasing the sample size by 50%, we were unable to demonstrate a significant day-night pattern. Another novel finding of this study is that during periods of short-term energy deprivation, activin A levels decrease significantly, whereas FST levels increase significantly compared with the fed state, but inhibin B and FSH levels are not altered. Our interventional studies involving physiological and pharmacological leptin replacement demonstrated that the underlying mechanism for these changes is not leptin dependent and thus remains to be elucidated.
These findings are consistent with the teleological theory of decreased reproductive capacity during hypocaloric states, when the limited energy stores are shifted toward functions that are essential for survival. They also suggest that, in addition to leptin, hormones such as activin A and FST may also play a role in down-regulating the HPG axis during states of energy deprivation, but the mechanisms through which they exert their effects warrant further investigation. It remains to be conclusively demonstrated by interventional studies involving administration of activin A or inhibitors of FST, when these reach a clinical stage of development, whether these changes may act in parallel and/or in concert with leptin to regulate the HPG axis in healthy, lean females.
Currently, very little is known about the secretion pattern of the hormones studied herein. In animal studies, rams exhibited a nonpulsatile secretion pattern for FST (22), and male rhesus monkeys were found to have no diurnal rhythm of inhibin secretion (23). In humans, the majority of data are derived from studies of healthy male volunteers in the fed state. The earliest study, by Carlsen et al. (18), measured inhibin B levels every 30 min for 24 h in 13 healthy male volunteers (21–36 yr old) and identified a diurnal pattern with peak values in the early morning and nadirs in the late afternoon and evening hours. Similar patterns of secretion were noted when measuring immunoreactive inhibin concentrations in 24- to 27-yr-old healthy males every 15 min (24) and every hour for 24 h (25) as well as after FSH or testosterone injections to 18- to 50-yr-old male volunteers (26, 27). In a study of seven pubertal girls that underwent serum sampling every 15 min for 24 h, FST levels were highest between 0500 and 1100 h, activin A decreased during the night, and inhibin B decreased during the day, reaching a nadir between 1700 and 2245 h (17). Finally, in the only study of young women, where midfollicular inhibin B levels are measured every 10 min for 6 h in 10 women with polycystic ovary syndrome vs. five controls, the pattern of peaks every 60–70 min seen in controls was abolished in polycystic ovary syndrome patients (19).
Our results thus represent the first study to examine the secretion pattern of all three hormones in healthy, regularly menstruating females, using samples drawn every 15 (for FST) to 30 (for inhibin B and activin A) minutes for 24 h. Interestingly, inhibin B was the only hormone where a nonsignificant tendency toward a day-night pattern was noted. In contrast to the previously published data in healthy men or pubertal girls described above, we demonstrate a statistically nonsignificant pattern of inhibin B peaks in midday and nadirs in the early hours of the morning. This variation in secretion patterns could be explained by a gender-specific mechanism in addition to the HPG axis maturation process that occurs during and after puberty, but we feel confident about our findings, especially because they were replicated with the additional three subjects.
Another important component of our interventional studies was examining the effect of energy deprivation on these hormones, a question that has also not been studied in the past. No studies exist comparing activin, inhibin, and FST levels in fed vs. fasting men or women, although such data would have clinical and therapeutic implications. Clinically, energy deprivation is manifested mainly in the syndromes of anorexia nervosa (AN) and/or hypothalamic amenorrhea (HA). In a study of 23 women with HA, inhibin levels did not differ compared with controls, but their activin A levels were significantly higher (28). Because these patients start to regain their body weight and leptin levels increase, so do inhibin B levels, ultimately leading to restoration of their menstrual function (29). These observational studies cannot prove causality and thus cannot prove with certainty whether the observed restoration of inhibin B levels was due to the increase in leptin levels with refeeding and/or to a third confounding factor that also changed during refeeding, thus driving hormonal and reproductive changes. Our interventional data built upon and expanded on these observations.
We examined directly the effects of short-term (72-h) energy deprivation on the hormones activin A, inhibin B, and FST to test our underlying hypothesis that inhibin B and FST levels would increase, whereas activin A levels would decrease in response to caloric restriction. We also examined whether such effects could be mediated by leptin by physiologically and pharmacologically replacing it. We demonstrated that only activin A and FST levels changed in response to fasting, whereas inhibin B levels remained unchanged. We speculate that the effects of caloric restriction on inhibin B, if any, occur after at least 4 d and were therefore not detected during our study. Whether this is indeed the case would remain to be investigated by long-term energy-deprivation studies.
In recent years, leptin has emerged as one of the primary signals of energy homeostasis with a key role in the neuroendocrine adaptation to both over- and undereating, acting as a metabolic gatekeeper for reproductive function (16). The primary site of leptin's action is on the GnRH neurons of the hypothalamus where it stimulates orexigenic and anorexigenic pathways that in turn adjust food intake and body weight (30–32). In addition to this central mechanism, evidence exists that peripheral tissues, namely the ovary, express not only leptin but also its receptor, therefore providing an additional mechanism for leptin's effect on folliculogenesis and ovulation (31, 33–35). During states of energy deprivation, leptin concentrations decrease much more dramatically and out of proportion to fat mass changes, compared with states of energy excess (12, 36). During caloric restriction, leptin levels fall and down-regulate not only the HPG axis but also the hypothalamic-pituitary-thyroid and -GH axes, ultimately leading to energy conservation for immediate survival (37). Similarly, in patients with AN, falling leptin levels correlate closely with decreasing LH levels and menstrual aberrations, but both levels normalize in response to refeeding and weight gain (15). Several studies of HA subjects who were treated with meterleptin have demonstrated improvement in LH pulse frequency as well as resumption of follicle growth, ovulation, menstrual cyclicity, and bone mineral density (14, 38, 39).
Based on these established data supporting leptin's key role in the neuroendocrine adaptation to starvation, we hypothesized that meterleptin administration to our subjects would lead to restoration of altered activin A and FST levels after short-term energy deprivation. Leptin replacement tended to restore FST values to those seen in the fed state but did not lead to complete return to fed baseline levels. In contrast, activin A levels were not at all affected by meterleptin administration. Furthermore, varying doses of meterleptin (from replacement to supraphysiological) also did not confer a statistical change in any of the hormone levels studied, despite changes in leptin values. The only existing evidence for a potential interplay between leptin (specifically its soluble receptor, sOB-R) and FST is derived from follicular fluid of infertile women where soluble leptin receptor correlated with FST and activin levels (40, 41), but association studies cannot prove causality. Furthermore, the two interventional studies of meterleptin replacement in HA patients also support a lack of change in inhibin B levels over the 3-month treatment period (14, 39). Our interventional study does not provide any support to the hypothesis that leptin accounts for starvation-induced changes in circulating levels of FST, activin A, and inhibin B. It is very possible that mechanisms other than leptin, which mainly regulates the HPG axis centrally, may also be important in regulating peripheral molecules that feed back to regulate gonadotropin secretion by the pituitary (41).
We also measured FSH levels every 15 min during each one of the three conditions and demonstrated that they remain unchanged despite short-term energy deprivation or leptin replacement. Studies of energy deprivation in animals have almost uniformly demonstrated a decrease in FSH levels after fasting (42–47) or altered FSH signaling (48). The data from men are not as conclusive, with some studies demonstrating a decrease in FSH levels after fasting (49–51) and others showing no change at all (52–54). Chan et al. (12) showed that 72-h fasting in lean, healthy males resulted in a significant decrease in leptin levels from 2.24 ± 0.77 to 0.27 ± 0.08 ng/ml, accompanied by a significant decrease of insulin and testosterone levels (55) as well as a significant increase in levels of FFA and SHBG. In contrast, 72-h fasting in lean, healthy females, which decreased circulating leptin levels from 14.7 ± 2.6 to 2.8 ± 0.3 ng/ml, demonstrated no change in any of these hormone levels, including FSH (13). This finding is further supported by other studies of short-term energy deprivation in lean reproductive-age women (56–58). We speculate that the changes in FSH, if any, in lean healthy women might occur after the 72 h of fasting that we investigated here. It is also possible that a more significant decrease in leptin levels, below 2.8 ng/ml, achieved herein could possibly be necessary for activation of leptin-dependent mechanisms. This remains to be studied further. Finally, it is possible that because activin A and FST act primarily in a paracrine/autocrine manner to exert their effects at the local tissue level (e.g. the ovary), these effects may be differentially regulated by fasting and/or leptin, as opposed to their central effect to alter gonadotropin levels.
Our study also has several strengths over some of the already published ones: 1) our day-night pattern analysis had a high temporal resolution of every 15–30 min over 24 h, 2) our study was fully standardized for meal timing, caloric content, and menstrual cycle days between and within subjects, and 3) we used state-of-the art assays, including a well-validated second-generation inhibin B assay (59). Future studies need to evaluate whether more long-term caloric deprivation leads to similar changes in healthy women and whether chronic caloric deprivation in the context of disease states, such as AN and HA, is also associated with similar changes. In addition, important information could be derived from studies elucidating the underlying mechanisms of these changes as well as their effect on menstrual regulation, fertility, and overall health.
In conclusion, our results demonstrate for the first time that levels of FST, activin A, and inhibin B do not display a significant day-night or pulsatile pattern in young healthy women in the fed state. Short-term energy deprivation in female subjects leads to a significant decrease in activin A and increase in FST levels. This effect could not be explained through food deprivation-induced changes in leptin levels, suggesting that alternative mechanisms do exist. Future studies could investigate how these hormones are affected by both short- and long-term energy restriction and evaluate additional outcome measures, such as menstrual patterns and fertility potential.
Acknowledgments
This work was supported by National Institutes of Diabetes and Digestive and Kidney Diseases Grant 58785 as well as the National Institutes of Health National Center for Research Resources Grant M01-RR-01032 (Harvard Clinical and Translational Science Center) and Grant UL1 RR025758. Additional funding was provided by the Endocrine Fellows Foundation Fellows Development Research Grant in Diabetes, Obesity, and Fat Cell Biology. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The Mantzoros Laboratory is also supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grants 79929 and 81913 and AG032030. Amylin Pharmaceuticals, Inc., supplied meterleptin for this study but had no role in the study design; conduct of the study; collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Clinical trial registration numbers at www.clinicaltrials.gov are NCT00140231 and NCT00140205.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AN
- Anorexia nervosa
- BMI
- body mass index
- CV
- coefficient of variation
- FFA
- free fatty acids
- FST
- follistatin
- GCRC
- General Clinical Research Center
- HA
- hypothalamic amenorrhea
- HPG
- hypothalamic-pituitary-gonadal.
References
- 1. Vale W, Rivier C, Hsueh A, Campen C, Meunier H, Bicsak T, Vaughan J, Corrigan A, Bardin W, Sawchenko P, et al. 1988. Chemical and biological characterization of the inhibin family of protein hormones. Recent Prog Horm Res 44:1–34 [DOI] [PubMed] [Google Scholar]
- 2. Ying SY. 1988. Inhibins, activins, and follistatins: gonadal proteins modulating the secretion of follicle-stimulating hormone. Endocr Rev 9:267–293 [DOI] [PubMed] [Google Scholar]
- 3. Bilezikjian LM, Blount AL, Donaldson CJ, Vale WW. 2006. Pituitary actions of ligands of the TGF-β family: activins and inhibins. Reproduction 132:207–215 [DOI] [PubMed] [Google Scholar]
- 4. Lin SY, Morrison JR, Phillips DJ, de Kretser DM. 2003. Regulation of ovarian function by the TGF-β superfamily and follistatin. Reproduction 126:133–148 [DOI] [PubMed] [Google Scholar]
- 5. Tsuchida K, Nakatani M, Hitachi K, Uezumi A, Sunada Y, Ageta H, Inokuchi K. 2009. Activin signaling as an emerging target for therapeutic interventions. Cell Commun Signal 7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bernard DJ, Fortin J, Wang Y, Lamba P. 2010. Mechanisms of FSH synthesis: what we know, what we don't, and why you should care. Fertil Steril 93:2465–2485 [DOI] [PubMed] [Google Scholar]
- 7. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. 1994. Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432 [DOI] [PubMed] [Google Scholar]
- 8. Kelesidis T, Kelesidis I, Chou S, Mantzoros CS. 2010. Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Intern Med 152:93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sinha MK, Sturis J, Ohannesian J, Magosin S, Stephens T, Heiman ML, Polonsky KS, Caro JF. 1996. Ultradian oscillations of leptin secretion in humans. Biochem Biophys Res Commun 228:733–738 [DOI] [PubMed] [Google Scholar]
- 10. Blüher S, Mantzoros CS. 2009. Leptin in humans: lessons from translational research. Am J Clin Nutr 89:991S–997S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boden G, Chen X, Mozzoli M, Ryan I. 1996. Effect of fasting on serum leptin in normal human subjects. J Clin Endocrinol Metab 81:3419–3423 [DOI] [PubMed] [Google Scholar]
- 12. Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. 2003. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest 111:1409–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan JL, Matarese G, Shetty GK, Raciti P, Kelesidis I, Aufiero D, De Rosa V, Perna F, Fontana S, Mantzoros CS. 2006. Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. Proc Natl Acad Sci USA 103:8481–8486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. 2004. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med 351:987–997 [DOI] [PubMed] [Google Scholar]
- 15. Mantzoros CS. 2000. Role of leptin in reproduction. Ann NY Acad Sci 900:174–183 [DOI] [PubMed] [Google Scholar]
- 16. Chan JL, Mantzoros CS. 2005. Role of leptin in energy-deprivation states: normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. Lancet 366:74–85 [DOI] [PubMed] [Google Scholar]
- 17. Foster CM, Olton PR, Padmanabhan V. 2005. Diurnal changes in FSH-regulatory peptides and their relationship to gonadotrophins in pubertal girls. Hum Reprod 20:543–548 [DOI] [PubMed] [Google Scholar]
- 18. Carlsen E, Olsson C, Petersen JH, Andersson AM, Skakkebaek NE. 1999. Diurnal rhythm in serum levels of inhibin B in normal men: relation to testicular steroids and gonadotropins. J Clin Endocrinol Metab 84:1664–1669 [DOI] [PubMed] [Google Scholar]
- 19. Lockwood GM, Muttukrishna S, Groome NP, Matthews DR, Ledger WL. 1998. Mid-follicular phase pulses of inhibin B are absent in polycystic ovarian syndrome and are initiated by successful laparoscopic ovarian diathermy: a possible mechanism regulating emergence of the dominant follicle. J Clin Endocrinol Metab 83:1730–1735 [DOI] [PubMed] [Google Scholar]
- 20. Chan JL, Wong SL, Mantzoros CS. 2008. Pharmacokinetics of subcutaneous recombinant methionyl human leptin administration in healthy subjects in the fed and fasting states: regulation by gender and adiposity. Clin Pharmacokinet 47:753–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Licinio J, Mantzoros C, Negrão AB, Cizza G, Wong ML, Bongiorno PB, Chrousos GP, Karp B, Allen C, Flier JS, Gold PW. 1997. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nat Med 3:575–579 [DOI] [PubMed] [Google Scholar]
- 22. Gallinelli A, Gallo R, Genazzani AD, Matteo ML, Caruso A, Woodruff TK, Petraglia F. 1996. Episodic secretion of activin A in pregnant women. Eur J Endocrinol 135:340–344 [DOI] [PubMed] [Google Scholar]
- 23. Winters SJ, Medhamurthy R, Gay VL, Plant TM. 1991. A comparison of moment to moment and diurnal changes in circulating inhibin and testosterone concentrations in male rhesus monkeys (Macaca mulatta). Endocrinology 129:1755–1761 [DOI] [PubMed] [Google Scholar]
- 24. Brennemann W, Sommer L, Stoffel-Wagner B, Bidlingmaier F, Klingmüller D. 1994. Secretion pattern of immunoreactive inhibin in men. Eur J Endocrinol 131:273–279 [DOI] [PubMed] [Google Scholar]
- 25. Yamaguchi M, Mizunuma H, Miyamoto K, Hasegawa Y, Ibuki Y, Igarashi M. 1991. Immunoreactive inhibin concentrations in adult men: presence of a circadian rhythm. J Clin Endocrinol Metab 72:554–559 [DOI] [PubMed] [Google Scholar]
- 26. Walton MJ, Anderson RA, Kicman AT, Elton RA, Ossowska K, Baird DT. 2007. A diurnal variation in testicular hormone production is maintained following gonadotrophin suppression in normal men. Clin Endocrinol (Oxf) 66:123–129 [DOI] [PubMed] [Google Scholar]
- 27. Kamischke A, Simoni M, Schrameyer K, Lerchl A, Nieschlag E. 2001. Is inhibin B a pharmacodynamic parameter for FSH in normal men? Eur J Endocrinol 144:629–637 [DOI] [PubMed] [Google Scholar]
- 28. Petraglia F, Hartmann B, Luisi S, Florio P, Kirchengast S, Santuz M, Genazzani AD, Genazzani AR. 1998. Low levels of serum inhibin A and inhibin B in women with hypergonadotropic amenorrhea and evidence of high levels of activin A in women with hypothalamic amenorrhea. Fertil Steril 70:907–912 [DOI] [PubMed] [Google Scholar]
- 29. Popovic V, Djurovic M, Cetkovic A, Vojvodic D, Pekic S, Spremovic S, Petakov M, Damjanovic S, Milic N, Dieguez C, Casanueva FF. 2004. Inhibin B: a potential marker of gonadal activity in patients with anorexia nervosa during weight recovery. J Clin Endocrinol Metab 89:1838–1843 [DOI] [PubMed] [Google Scholar]
- 30. Leshan RL, Louis GW, Jo YH, Rhodes CJ, Münzberg H, Myers MG., Jr 2009. Direct innervation of GnRH neurons by metabolic- and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. J Neurosci 29:3138–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tena-Sempere M. 2007. Roles of ghrelin and leptin in the control of reproductive function. Neuroendocrinology 86:229–241 [DOI] [PubMed] [Google Scholar]
- 32. Sahu A. 2004. Minireview: A hypothalamic role in energy balance with special emphasis on leptin. Endocrinology 145:2613–2620 [DOI] [PubMed] [Google Scholar]
- 33. Sarkar M, Schilffarth S, Schams D, Meyer HH, Berisha B. 2010. The expression of leptin and its receptor during different physiological stages in the bovine ovary. Mol Reprod Dev 77:174–181 [DOI] [PubMed] [Google Scholar]
- 34. Cioffi JA, Shafer AW, Zupancic TJ, Smith-Gbur J, Mikhail A, Platika D, Snodgrass HR. 1996. Novel B219/OB receptor isoforms: possible role of leptin in hematopoiesis and reproduction. Nat Med 2:585–589 [DOI] [PubMed] [Google Scholar]
- 35. Löffler S, Aust G, Köhler U, Spanel-Borowski K. 2001. Evidence of leptin expression in normal and polycystic human ovaries. Mol Hum Reprod 7:1143–1149 [DOI] [PubMed] [Google Scholar]
- 36. Wolfe BE, Jimerson DC, Orlova C, Mantzoros CS. 2004. Effect of dieting on plasma leptin, soluble leptin receptor, adiponectin and resistin levels in healthy volunteers. Clin Endocrinol (Oxf) 61:332–338 [DOI] [PubMed] [Google Scholar]
- 37. Kolaczynski JW, Considine RV, Ohannesian J, Marco C, Opentanova I, Nyce MR, Myint M, Caro JF. 1996. Responses of leptin to short-term fasting and refeeding in humans: a link with ketogenesis but not ketones themselves. Diabetes 45:1511–1515 [DOI] [PubMed] [Google Scholar]
- 38. Sienkiewicz E, Magkos F, Aronis KN, Brinkoetter M, Chamberland JP, Chou S, Arampatzi KM, Gao C, Koniaris A, Mantzoros CS. 2011. Long-term metreleptin treatment increases bone mineral density and content at the lumbar spine of lean hypoleptinemic women. Metabolism 60:1211–1221 [DOI] [PubMed] [Google Scholar]
- 39. Chou SH, Chamberland JP, Liu X, Matarese G, Gao C, Stefanakis R, Brinkoetter MT, Gong H, Arampatzi K, Mantzoros CS. 2011. Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci USA 108:6585–6590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Welt CK, Schneyer AL, Heist K, Mantzoros CS. 2003. Leptin and soluble leptin receptor in follicular fluid. J Assist Reprod Genet 20:495–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mantzoros CS, Cramer DW, Liberman RF, Barbieri RL. 2000. Predictive value of serum and follicular fluid leptin concentrations during assisted reproductive cycles in normal women and in women with the polycystic ovarian syndrome. Hum Reprod 15:539–544 [DOI] [PubMed] [Google Scholar]
- 42. Alexander BM, Kiyma Z, McFarland M, Van Kirk EA, Hallford DM, Hawkins DE, Kane KK, Moss GE. 2007. Influence of short-term fasting during the luteal phase of the estrous cycle on ovarian follicular development during the ensuing proestrus of ewes. Anim Reprod Sci 97:356–363 [DOI] [PubMed] [Google Scholar]
- 43. Kosior-Korzecka U, Bobowiec R, Lipecka C. 2006. Fasting-induced changes in ovulation rate, plasma leptin, gonadotropins, GH, IGF-I and insulin concentrations during oestrus in ewes. J Vet Med A Physiol Pathol Clin Med 53:5–11 [DOI] [PubMed] [Google Scholar]
- 44. Bergendahl M, Perheentupa A, Huhtaniemi I. 1989. Effect of short-term starvation on reproductive hormone gene expression, secretion and receptor levels in male rats. J Endocrinol 121:409–417 [DOI] [PubMed] [Google Scholar]
- 45. Bergendahl M, Huhtaniemi I. 1993. Acute fasting is ineffective in suppressing pituitary-gonadal function of pubertal male rats. Am J Physiol 264:E717–E722 [DOI] [PubMed] [Google Scholar]
- 46. Gruenewald DA, Matsumoto AM. 1993. Reduced gonadotropin-releasing hormone gene expression with fasting in the male rat brain. Endocrinology 132:480–482 [DOI] [PubMed] [Google Scholar]
- 47. Bergendahl M, Perheentupa A, Huhtaniemi I. 1991. Starvation-induced suppression of pituitary-testicular function in rats is reversed by pulsatile gonadotropin-releasing hormone substitution. Biol Reprod 44:413–419 [DOI] [PubMed] [Google Scholar]
- 48. Yamamoto Y, Adam Luckenbach J, Goetz FW, Young G, Swanson P. 2011. Disruption of the salmon reproductive endocrine axis through prolonged nutritional stress: changes in circulating hormone levels and transcripts for ovarian genes involved in steroidogenesis and apoptosis. Gen Comp Endocrinol 172:331–343 [DOI] [PubMed] [Google Scholar]
- 49. Hoffer LJ, Beitins IZ, Kyung NH, Bistrian BR. 1986. Effects of severe dietary restriction on male reproductive hormones. J Clin Endocrinol Metab 62:288–292 [DOI] [PubMed] [Google Scholar]
- 50. Cameron JL, Weltzin TE, McConaha C, Helmreich DL, Kaye WH. 1991. Slowing of pulsatile luteinizing hormone secretion in men after forty-eight hours of fasting. J Clin Endocrinol Metab 73:35–41 [DOI] [PubMed] [Google Scholar]
- 51. Bogdan A, Bouchareb B, Touitou Y. 2001. Ramadan fasting alters endocrine and neuroendocrine circadian patterns. Meal-time as a synchronizer in humans? Life Sci 68:1607–1615 [DOI] [PubMed] [Google Scholar]
- 52. Röjdmark S. 1987. Influence of short-term fasting on the pituitary-testicular axis in normal men. Horm Res 25:140–146 [DOI] [PubMed] [Google Scholar]
- 53. Veldhuis JD, Iranmanesh A, Evans WS, Lizarralde G, Thorner MO, Vance ML. 1993. Amplitude suppression of the pulsatile mode of immunoradiometric luteinizing hormone release in fasting-induced hypoandrogenemia in normal men. J Clin Endocrinol Metab 76:587–593 [DOI] [PubMed] [Google Scholar]
- 54. Tegelman R, Lindeskog P, Carlstrom K, Pousette A, Blomstrand R. 1986. Peripheral hormone levels in healthy subjects during controlled fasting. Acta Endocrinol (Copenh) 113:457–462 [DOI] [PubMed] [Google Scholar]
- 55. Mantzoros CS, Liolios AD, Tritos NA, Kaklamani VG, Doulgerakis DE, Griveas I, Moses AC, Flier JS. 1998. Circulating insulin concentrations, smoking, and alcohol intake are important independent predictors of leptin in young healthy men. Obes Res 6:179–186 [DOI] [PubMed] [Google Scholar]
- 56. Berga SL, Loucks TL, Cameron JL. 2001. Endocrine and chronobiological effects of fasting in women. Fertil Steril 75:926–932 [DOI] [PubMed] [Google Scholar]
- 57. Alvero R, Kimzey L, Sebring N, Reynolds J, Loughran M, Nieman L, Olson BR. 1998. Effects of fasting on neuroendocrine function and follicle development in lean women. J Clin Endocrinol Metab 83:76–80 [DOI] [PubMed] [Google Scholar]
- 58. Soules MR, Merriggiola MC, Steiner RA, Clifton DK, Toivola B, Bremner WJ. 1994. Short-term fasting in normal women: absence of effects on gonadotrophin secretion and the menstrual cycle. Clin Endocrinol (Oxf) 40:725–731 [DOI] [PubMed] [Google Scholar]
- 59. Kalra B, Kumar A, Patel K, Patel A, Khosravi MJ. 2010. Development of a second generation Inhibin B ELISA. J Immunol Methods 362:22–31 [DOI] [PubMed] [Google Scholar]