Abstract
Context:
Little information exists about longitudinal changes in body composition and physical function in relation to sex hormone levels in older men.
Objective:
The aim of the study was to determine associations of testosterone, estradiol, and SHBG with changes in body composition and physical function.
Design and Setting:
We conducted a prospective cohort study within the Osteoporotic Fractures in Men (MrOS) study at six U.S. clinical centers.
Participants:
A total of 5994 ambulatory men aged 65 yr or older enrolled in the MrOS. We examined 1183 men with complete measures of sex steroid hormones, body composition, and some measure of physical function.
Intervention:
There were no interventions.
Main Outcome Measure(s):
Sex steroids were measured by mass spectrometry in serum collected at baseline. Measurements of body composition using dual-energy x-ray absorptiometry and physical performance (grip strength, leg power, timed chair stands, narrow walk, and 6-m walk) were performed at baseline and repeated 4.5 yr later.
Results:
Overall, men lost 1.3 kg (±4.4 sd) weight between study visits. Lean mass, especially appendicular, declined less at higher baseline testosterone levels (P < 0.05). These associations were most evident in the 40% of men who lost more than 2.0 kg during follow-up. In weight losers, higher testosterone was associated with less decline in timed chair stands. Estradiol was not related to body composition or physical function changes. Higher SHBG was associated with less loss of appendicular lean mass and grip strength.
Conclusions:
Higher endogenous testosterone is associated with reduced loss of lean mass and lower extremity function in older men losing weight. Endogenous testosterone may contribute to healthy aging.
Sex steroid levels, lean mass, and physical function tend to decline with age (1–13), yet little information exists about the relation of endogenous sex hormone levels to changes in body composition and physical function in older men. Men lose more muscle mass (8, 14–17) and strength (3, 5, 18, 19) than women as they age, suggesting that endogenous sex steroids, and testosterone in particular, may contribute to body composition and physical function changes. Also, exogenous testosterone supplementation in older men is associated with increases in lean body mass, decreases in fat mass, and improved muscle strength (20–23).
Previous longitudinal studies of body composition were conducted mostly in young and middle-aged men (24, 25) who differ from older men in body composition and sex steroid levels (11, 26, 27). In 110 Japanese-American men aged 64 yr on average, total testosterone was not associated with change in total body fat but was inversely associated with changes in intraabdominal fat (28). Lean mass changes were not reported. Some (29, 30), but not all (31), longitudinal studies of physical function report that higher testosterone levels are associated with less decline in physical performance measures or mobility. Whether changes in body composition modify this possible association of testosterone with physical performance has not been reported.
Unraveling the association between sex steroids and changes in body composition and physical function in older men is an important public health issue. Loss of muscle mass and strength contribute to frailty (32–34) and are associated with falls, mobility limitations, incident disability, and fractures (9, 10, 35, 36). Increased adiposity is associated with overall ill health, disability, and cardiovascular disease in older men (37). Loss of lean mass and loss/gain of fat mass are associated with increased mortality in older men (38). To specifically address effects of sex steroids and body composition changes in elderly men, we used data from a large cohort of U.S. men aged 65 or older from the Osteoporotic Fractures in Men (MrOS) study. We tested the hypothesis that higher baseline measures of sex steroids or SHBG are associated with lesser declines in lean mass, less gain in fat mass, and maintenance of physical performance over an average follow-up of 4.5 yr.
Subjects and Methods
Subjects
MrOS is a multicenter prospective observational study of musculoskeletal health in older men. Its design is detailed elsewhere (39, 40). The MrOS cohort was recruited from six clinical sites using mass mailing and enrolled 5994 community-dwelling, ambulatory men aged 65 yr or older without bilateral hip replacements (40). Each center's Institutional Review Board approved the study, and participants gave written informed consent.
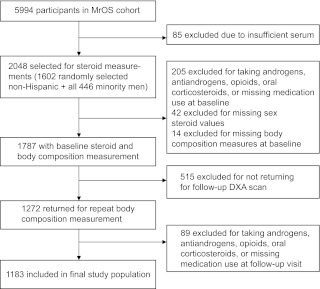
We selected 2048 men from the cohort (n = 5995) for steroid measurements; 1602 Caucasian men were chosen at random, and we included all 446 non-Caucasian men (Fig. 1). Those taking androgens, antiandrogens, opioids, or oral corticosteroids at baseline or those missing baseline medication data (n = 205), sex steroid values (n = 42), or body composition measures (n = 14) were excluded. Of those remaining, 1272 men had both baseline and repeat body composition measurements. At the follow-up visit, men taking androgens, antiandrogens, opioids, or oral corticosteroids or missing medication data were also excluded (n = 89). The remaining 1183 men are the subject of this report.
Fig. 1.
Outline of participant inclusion.
Questionnaire data
Participants completed two study visits, 4.5 yr apart. At baseline, race/ethnicity, smoking/alcohol consumption, self-reported health status, and medical history were determined by questionnaire. All prescription medications validated by the clinics were recorded in an electronic medications inventory database (San Francisco Coordinating Center, San Francisco, CA). Each medication was matched to its ingredient(s) based on the Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA) (41). Physical activity was assessed with the Physical Activity Score for the Elderly (PASE) (42). Mental functioning and physical health were measured with the Medical Outcomes Study 12-Item Short Form (SF-12) (43).
Body composition measures
Weight was measured in indoor clothing without shoes using a calibrated balance beam scale. Body composition was measured using dual-energy x-ray absorptiometry (DXA) (QDR 4500W; Hologic Inc., Bedford, MA) as previously described (39, 44). A central quality control laboratory, certification of DXA operators, and standardized procedures for scanning ensured reproducibility of DXA measurements. At baseline, a Hologic whole body phantom was circulated at the six clinical sites. Variability across clinics was within acceptable limits, and cross-calibration correction factors were not required. Each clinic scanned a Hologic whole body phantom throughout the study to monitor longitudinal changes, and correction factors were applied to participant data as appropriate. Validity of these DXA measures for assessing body composition in the elderly has been reported (45, 46).
Physical performance measures
Grip strength was assessed in each hand using a Jamar handheld dynamometer (47). The maximum score of two trials for the dominant hand was used for analysis. Lower extremity power was measured with the Nottingham Power Rig (48, 49), a very reliable measure of leg power in the MrOS cohort (50). Walking speed was determined by timing completion of a 6-m course performed at the participant's usual walking speed. The time to complete a tandem-walking course of 6 m × 20 cm was used to determine balance. A trial was considered successful if the participant had no more than two deviations from the lane. Participants' ability to rise from a chair without use of arms, along with their ability to complete five chair stands, and the time required to complete the task were determined. Participants who could not complete a physical function measure were classified as “unable” and were assigned a value from the lowest end of the cohort distribution. Participants missing a value were excluded from analyses. All participants had data for at least two physical function measures, and 98% had data for at least four physical function measurements at both visits.
Sex steroid measurements
Baseline fasting morning blood was collected: serum was prepared immediately after phlebotomy and stored at −70 C. Total serum testosterone (total T) and estradiol (total E) were measured using combined gas chromatographic negative ionization tandem mass spectrometry (GC/NCI/MS/MS) and liquid chromatographic electrospray tandem mass spectrometry (LC/ESI/MS/MS) bioanalytical methods (Taylor Technology, Princeton, NJ). A 1/(concentration)2 weighted least squares regression procedure was used to fit a linear function to the calibration data. The lower limit of detection for estradiol was 0.625 pg/ml (2.29 pmol/liter) and for testosterone was 25.0 pg/ml (0.09 nmol/liter). Duplicate aliquots from each participant were assayed and averaged. Intra- and interassay coefficients of variation (CV) were 2.5 and 6.0%, respectively, for testosterone and 6.4 and 10.1%, respectively, for estradiol. Serum SHBG concentrations were measured using an Immulite Analyzer with chemiluminescent substrate (Diagnostic Products Corp., Los Angeles, CA). The standard curve ranged from 0.2 to 180 nm/liter. The SHBG intraassay CV was 4.4%, and interassay CV was 6.0%. Albumin values for free hormone calculations were obtained from baseline serum using routine colorimetric methods (Roche COBAS Integra 800 automated analyzer; Roche Diagnostics, Indianapolis, IN; interassay CV, 2.0%). Bioavailable fractions of testosterone (bioT) and estradiol (bioE) were calculated using the method of Södergård et al. (51).
Statistical analysis
Baseline characteristics were compared by total T quartiles using χ2 for categorical variables and ANOVA F-test or Kruskal-Wallis tests for continuous variables.
Restricted cubic spline models were used to visualize the shape of the association between baseline measures of sex steroids and absolute change in body composition measures to test for nonlinearity in the associations (52). These assessments provided evidence that effects were linear. Therefore, linear regression models were used to compare change in body composition and physical function measures, with results presented as adjusted means and P values for trend across quartiles of total T, total E, and SHBG. Initial models were adjusted for study site, age group, and race only, and then for multiple potential confounders. Analyses were repeated using bioavailable sex steroid levels. Total and bioavailable fractions of the sex steroids were highly correlated (r = 0.88 for correlation between bioT and total T; r = 0.92 for correlation between bioE and total E).
We evaluated several potential confounding factors known to influence sex steroid measures, body composition, and physical function including demographic (age group, study clinic site, race/ethnicity), lifestyle (alcohol use/smoking), physical activity (PASE) (42), physical and mental health (SF-12) (53), and self-reported clinician-diagnosed medical conditions (diabetes, non-skin cancer) (39). During descriptive analyses, we determined which factors varied according to both sex steroid levels and body composition or physical function. These factors were then added into the model individually in the multivariable analysis, and those models were compared with the crude model that contained only baseline sex steroid quartiles. Variables were retained in the final model if they altered the association between any sex steroid and any body composition (for body composition models) or physical function (for physical function models) measure by 10% or more (54).
We also examined change in lean mass models adjusted for fat mass change and change in fat mass models adjusted for lean mass change. Finally, we tested whether the association between body composition measures and sex steroids depended significantly on age (>75 vs. ≤75 yr), self-reported health status (excellent/good vs. fair/poor/very poor), and weight change (gain = >2.0 kg body weight gain; stable = ≤2.0 kg change in body weight; loss = >2.0 kg body weight loss). An interaction term was included in the multivariate analysis of covariance, and stratification analysis was performed when the interaction term was significant (P < 0.05).
Statistical analyses were completed using SAS v9.2 (SAS Inc., Cary, NC).
Results
Baseline characteristics
We included 1183 men with an average age of 72.3 yr at baseline (Fig. 1). Almost three fourths were Caucasian. Men with higher total T levels had better self-rated health status (P = 0.0004), fewer (non-skin) cancer diagnoses (P = 0.005), better physical health (measured by the SF-12's physical summary score; P = 0.05), and were less likely to report diabetes (P < 0.0001) than those with lower total T levels (Table 1). Men in the analysis were younger (P < 0.0001), healthier (P < 0.0001 on several measures of health), more physically active (P < 0.0001), and more likely to be white (P = 0.006) and nonsmokers (P = 0.02), compared with those excluded from the analysis because they did not return for the second visit. Men in the analysis had higher baseline lean mass (57.1 vs. 55.7 kg; P = 0.0004) but not different baseline fat mass compared with those who did not return.
Table 1.
Demographics by baseline total T quartiles
| Baseline characteristics | Quartiles of total T |
||||
|---|---|---|---|---|---|
| 1 (lowest) | 2 | 3 | 4 (highest) | P valuea | |
| n | 296 | 296 | 296 | 295 | |
| Total T range (nmol/liter)b | 5.5–303.0 | 304.0–394.0 | 395.0–495.0 | 496.0–1340.0 | |
| Age (yr) | 0.78 | ||||
| 65–69 | 106 (35.8) | 121 (40.9) | 103 (34.8) | 117 (39.7) | |
| 70–74 | 86 (29.1) | 82 (27.7) | 86 (29.1) | 88 (29.8) | |
| 75–79 | 74 (25.0) | 65 (22.0) | 76 (25.7) | 58 (19.7) | |
| 80+ | 30 (10.1) | 28 (9.5) | 31 (10.5) | 32 (10.9) | |
| Study site | 0.25 | ||||
| Birmingham | 47 (15.9) | 45 (15.2) | 29 (9.8) | 34 (11.5) | |
| Minneapolis | 46 (15.5) | 44 (14.9) | 39 (13.2) | 39 (13.2) | |
| Palo Alto | 67 (22.6) | 54 (18.2) | 70 (23.7) | 67 (22.7) | |
| Pittsburgh | 48 (16.2) | 43 (14.5) | 48 (16.2) | 38 (12.9) | |
| Portland | 47 (15.9) | 63 (21.3) | 57 (19.3) | 54 (18.3) | |
| San Diego | 41 (13.9) | 47 (15.9) | 53 (17.9) | 63 (21.4) | |
| Caucasian race | 213 (72.0) | 211 (71.3) | 217 (73.3) | 203 (68.8) | 0.67 |
| Alcohol consumption | |||||
| None | 95 (32.1) | 91 (30.7) | 90 (30.4) | 94 (31.9) | 0.52 |
| <14 drinks/wk | 170 (57.4) | 165 (55.7) | 169 (57.1) | 177 (60.0) | |
| ≥14 drinks/wk | 31 (10.5) | 40 (13.5) | 37 (12.5) | 24 (8.1) | |
| Ever smoked | 191 (64.5) | 178 (60.1) | 182 (61.5) | 168 (57.0) | 0.30 |
| Self-rated health | 0.0004 | ||||
| Excellent | 89 (30.1) | 104 (35.1) | 118 (40.0) | 128 (43.4) | |
| Good | 159 (53.7) | 156 (52.7) | 159 (53.7) | 143 (48.5) | |
| Fair/poor/very poor | 48 (16.2) | 36 (12.2) | 19 (6.4) | 24 (8.1) | |
| Non-skin cancer | 61 (20.6) | 48 (16.2) | 37 (12.5) | 32 (10.9) | 0.005 |
| Self-reported history of diabetes | 51 (17.2) | 30 (10.1) | 24 (8.1) | 17 (5.8) | <0.0001 |
| Physical summary scale, median (IQR)c | 53.0 (45.3–56.6) | 55.4 (48.5–56.8) | 55.5 (48.4–56.8) | 54.5 (46.7–56.7) | 0.05 |
| Mental summary scale, median (IQR)c | 57.5 (53.9–59.5) | 57.9 (54.3–59.8) | 57.9 (55.2–59.8) | 57.9 (55.2–59.9) | 0.11 |
| Physical activity (PASE), mean (sd)d | 152.0 (68.6) | 152.3 (65.8) | 154.7 (66.9) | 156.6 (65.1) | 0.82 |
Data are expressed as number of subjects (%), unless described otherwise.
P values for categorical variables were compared using χ2 tests for categorical variables; P values for continuous variables with normal distributions were calculated using one-way ANOVA; P values for variables with skewed distributions were calculated using Kruskal-Wallis tests.
To convert metric units (ng/dl) to SI (nmol/liter), multiply the concentrations of testosterone by 0.0347.
Physical health and mental functioning were measured with the Medical Outcomes Study 12-Item Short Form (SF-12). A higher score indicates better functioning (43).
Total PASE score was computed by multiplying the amount of time spent in each activity (hours/week) or participation (yes/no) in an activity by the empirically derived item weights and summing over all activities. Therefore, higher PASE score means more physical activity participation (42).
Overall change in body composition
On average, participants in the MrOS study lost 1.0 kg (±4.2 sd) of body weight during the 4.5 yr of study follow-up; 426 (36%) lost more than 2 kg, 241 (20%) gained more than 2 kg, and 516 (44%) had stable weight (≤2 kg weight loss). Lean body mass declined [−1.16 (±2.12) kg] without a significant change in fat mass [0.17 (±3.03) kg].
Baseline sex hormones and change in weight
Neither total T, nor total E, nor SHBG was associated with subsequent change in weight (Table 2).
Table 2.
Change in body composition measures in relation to baseline sex steroid and SHBG quartiles in older men
| Quartiles |
Fully adjusteda | Adjusted for lean or fat changeb | ||||
|---|---|---|---|---|---|---|
| 1 (Lowest) | 2 | 3 | 4 (Highest) | |||
| Total T quartiles | ||||||
| Range (ng/dl)c | 5.5–303.0 | 304.0–394.0 | 395.0–495.0 | 496.0–1340.0 | ||
| n | 296 | 296 | 296 | 295 | ||
| Absolute change in weight | −1.41 (−2.09, −0.73) | −0.80 (−1.51, −0.09) | −0.59 (−1.32, 0.14) | −0.75 (−1.50, 0.01) | 0.09 | |
| Absolute change in lean mass | −1.42 (−1.75, −1.10) | −0.99 (−1.33, −0.65) | −1.05 (−1.39, −0.70) | −0.99 (−1.35, −0.63) | 0.04 | 0.07 |
| Absolute change in fat mass | 0.34 (−0.13, 0.80) | 0.53 (0.05, 1.02) | 0.83 (0.33, 1.33) | 0.58 (0.06, 1.10) | 0.29 | 0.65 |
| Total E quartiles | ||||||
| Range (pg/dl)c | 1.3–17.7 | 17.8–21.9 | 22.0–27.0 | 27.1–97.6 | ||
| n | 296 | 297 | 295 | 295 | ||
| Absolute change in weight | −0.57 (−1.28, 0.13) | −1.03 (−1.75, −0.32) | −1.19 (−1.90, −0.47) | −1.10 (−1.82, −0.38) | 0.16 | |
| Absolute change in lean mass | −1.02 (−1.36, −0.68) | −1.15 (−1.50, −0.81) | −1.24 (−1.58, −0.90) | −1.23 (−1.57, −0.89) | 0.21 | 0.32 |
| Absolute change in fat mass | 0.78 (0.29, 1.26) | 0.41 (−0.08, 0.90) | 0.45 (−0.04, 0.94) | 0.48 (−0.01, 0.97) | 0.32 | 0.52 |
| Baseline SHBG quartiles | ||||||
| Range (nmol/liter)c | 13.5–34.2 | 34.3–44.8 | 44.9–57.3 | 57.4–179.0 | ||
| n | 295 | 297 | 296 | 295 | ||
| Absolute change in weight | −1.18 (−1.86, −0.49) | −0.98 (−1.69, −0.26) | −0.85 (−1.59, −0.10) | −0.69 (−1.43, 0.04) | 0.19 | |
| Absolute change in lean mass | −1.19 (−1.52, −0.87) | −1.26 (−1.60, −0.92) | −1.05 (−1.40, −0.69) | −1.06 (−1.41, −0.71) | 0.28 | 0.40 |
| Absolute change in fat mass | 0.38 (−0.09, 0.84) | 0.71 (0.22, 1.20) | 0.39 (−0.12, 0.90) | 0.71 (0.21, 1.21) | 0.38 | 0.56 |
Data are expressed as adjusted mean in kilograms (95% CI). Means are fully adjusted.
P value for model adjusted for clinic site, age group, race, serum selection method, self-reported history of diabetes, self-reported history of non-skin cancer, and alcohol.
Full model + further adjusted for change in opposite body composition measure (i.e. for change in lean mass, model adjusted for change in fat mass; for change in fat mass, model adjusted for change in lean mass).
To convert metric units (ng/dl) to SI (nmol/liter), multiply the concentrations of testosterone by 0.0347 and multiply the concentrations of estradiol by 3.671.
Baseline sex hormones and change in lean body mass
Men with higher total T levels had less loss of total lean body mass compared with men with lower total T levels (−0.99 vs. −1.42 kg for highest vs. lowest total T quartile; P = 0.04; Table 2). However, this association was no longer significant after adjusting for change in fat mass (P = 0.07). Higher testosterone levels were more clearly related to reduced loss of appendicular lean mass (−0.63 vs. −0.96 kg for highest vs. lowest quartile; P = 0.003); this association persisted after adjustment for fat mass (P = 0.006). BioT had similar associations with lean mass measures. Neither total E nor bioE was associated with change in lean body mass. SHBG was not associated with total lean mass, but higher SHBG was associated with less loss of appendicular lean mass (−0.66 vs. −0.82 for highest vs. lowest SHBG quartile; P = 0.04).
Baseline sex hormones and change in fat mass
Neither total T, nor total E, nor SHBG was associated with change in total fat or trunk fat mass (Table 2).
Association between sex steroids and change in body composition by age, health status, and weight change
We examined whether the observed associations between sex steroids and change in body composition varied by age (>75 yr vs. ≤75 yr), health status (excellent/good vs. fair/poor/very poor), or body weight change category [gain (>2.0 kg increase), stable (≤2.0 kg change), loss (>2.0 kg decrease)]. Neither age nor health status influenced the association between sex steroids and body composition (P > 0.07 for each).
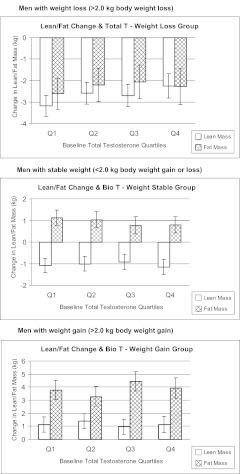
There was a significant interaction of weight change category with the association between total T and change in total lean mass (P = 0.03) and total fat mass (P = 0.04). An association between total T and change in total lean and appendicular lean mass occurred only in the 426 men who lost more than 2.0 kg of body weight (−2.26 vs. −3.18 kg lean mass loss in highest vs. lowest total T quartile; P = 0.001; Fig. 2). No significant associations between total T and change in lean mass were detected in men who either gained weight or had stable weight (P > 0.20 for each). Among men with stable weight, those in the highest total T quartile gained slightly less total fat mass (0.80 vs. 1.12 kg fat mass gain in highest vs. lowest total T quartile; P = 0.05; Fig. 2). Total T was not associated with fat mass change in those who lost or gained weight.
Fig. 2.
Association between total T and body composition changes by weight change category. Models are adjusted for clinic site, age group, race, serum selection method, self-reported history of diabetes, self-reported history of non-skin cancer, and alcohol use.
There were no significant associations between total E and body composition by weight change category (P > 0.07 for each). Among men losing weight, higher SHBG was associated with less loss of appendicular lean mass (P = 0.03), but not total lean mass (P = 0.07). However, the SHBG association with appendicular lean mass change was not independent of sex hormone levels. There was no association between fat mass change and SHBG in any weight change group.
Baseline sex hormones and change in physical function
Overall, total T was not related to change in any physical function measure. However, when we limited our analysis to those men who had lost weight (where interaction between lean mass and total T was strongest), higher total T was associated with a smaller increase in chair stands time (P = 0.02; Table 3). However, total T was not associated with any of the remaining four physical function measures in men who lost weight. Although SHBG was not associated with any physical function measures in the entire cohort, higher SHBG was associated with a smaller decline in grip strength (P = 0.04) in those men who lost weight. However, the SHBG association with decline in grip strength was not independent of sex hormone levels. Further adjusting the models for change in lean mass attenuated the associations that had been noted between testosterone and chair stands (adjusted P = 0.05). Total E was not associated with change in any physical function measure (P > 0.15 for each).
Table 3.
Change in physical function measures in relation to baseline sex steroid and SHBG quartiles in men losing weight (≥2.0 kg body weight loss)
| Quartiles |
P valuea | ||||
|---|---|---|---|---|---|
| 1 (lowest) | 2 | 3 | 4 (highest) | ||
| Total T quartiles | |||||
| Range (ng/dl)b | 5.5–303.0 | 304.0–394.0 | 395.0–495.0 | 496.0–1340.0 | |
| n | 121 | 102 | 104 | 99 | |
| Absolute change in grip strength (kg) | −5.1 (−6.7, −3.4) | −3.9 (−5.6, −2.1) | −3.9 (−5.7, −2.0) | −3.4 (−5.4, −1.5) | 0.11 |
| Absolute change in leg power (watts) | −57.9 (−76.4, −39.4) | 46.3 (−660, −26.5) | −41.4 (−61.8, −20.9) | −49.6 (−70.7, −28.5) | 0.36 |
| Absolute change in time to complete five chair stands (sec) | 4.6 (3.2, 6.0) | 2.9 (1.4, 4.4) | 3.1 (1.5, 4.6) | 2.5 (0.9, 4.1) | 0.02 |
| Absolute change in 6-m normal walk pace (m/sec) | −0.17 (−0.23, −0.11) | −0.12 (−0.19, −0.06) | −0.11 (−0.17, −0.04) | −0.12 (−0.19, −0.05) | 0.17 |
| Absolute change in 20-cm narrow walk (m/sec) | −0.35 (−0.46, −0.25) | −0.29 (−0.40, −0.17) | −0.32 (−0.43, −0.20) | −0.25 (−0.37, −0.12) | 0.10 |
| Total E quartiles | |||||
| Range (pg/dl)b | 1.3–17.7 | 17.8–21.9 | 22.0–27.0 | 27.1–97.6 | |
| n | 97 | 111 | 109 | 109 | |
| Absolute change in grip strength (kg) | −4.2 (−6.0, −2.3) | −4.8 (−6.5, −3.0) | −4.0 (−5.8, −2.2) | −3.8 (−5.7, −1.9) | 0.49 |
| Absolute change in leg power (watts) | −51.3 (−71.8, −30.7) | −52.3 (−71.4, −33.2) | −48.9 (−68.4, −29.4) | −48.6 (−69.6, −27.7) | 0.69 |
| Absolute change in time to complete five chair stands (sec) | 4.3 (2.8, 5.9) | 3.6 (2.1, 5.0) | 2.8 (1.3, 4.3) | 3.4 (1.8, 4.9) | 0.20 |
| Absolute change in 6-m normal walk pace (m/sec) | −0.19 (−0.26, −0.12) | −0.11 (−0.17, −0.05) | −0.11 (−0.17, −0.04) | −0.16 (−0.23, −0.09) | 0.61 |
| Absolute change in 20-cm narrow walk (m/sec) | −0.33 (−0.45, −0.21) | −0.34 (−0.45, −0.23) | −0.28 (−0.39, −0.16) | −0.30 (−0.42, −0.18) | 0.53 |
| Baseline SHBG quartiles | |||||
| Range (nmol/liter)b | 13.5–34.2 | 34.3–44.8 | 44.9–57.3 | 57.4–179.0 | |
| n | 107 | 109 | 109 | 101 | |
| Absolute change in grip strength (kg) | −5.5 (−7.2, −3.8) | −4.0 (−5.7, −2.2) | −3.2 (−5.0, −1.3) | −3.5 (−5.4, −1.6) | 0.04 |
| Absolute change in leg power (watts) | −55.0 (−74.2, −35.8) | −52.5 (−71.6, −33.3) | −42.7 (−62.9, −22.5) | −46.4 (−67.7, −25.1) | 0.25 |
| Absolute change in time to complete 5 chair stands (sec) | 4.4 (2.9, 5.8) | 3.5 (2.0, 4.9) | 2.7 (1.2, 4.2) | 2.8 (1.2, 4.5) | 0.06 |
| Absolute change in 6-m normal walk pace (m/sec) | −0.17 (−0.23, −0.11) | −0.12 (−0.19, −0.06) | −0.11 (−0.17, −0.04) | −0.13 (−0.20, −0.06) | 0.31 |
| Absolute change in 20 cm narrow walk (m/sec) | −0.32 (−0.43, −0.21) | −0.36 (−0.47, −0.25) | −0.29 (−0.41, −0.18) | −0.23 (−0.35, −0.11) | 0.07 |
Data are expressed as adjusted mean (95% CI). Means are fully adjusted.
P value for model adjusted for clinic site, age group, race, serum selection method, self-reported history of diabetes, self-rated health, and physical summary scale.
To convert metric units (ng/dl) to SI (nmol/liter), multiply the concentrations of testosterone by 0.0347 and multiply the concentrations of estradiol by 3.671.
Discussion
Men aged 65 and older with higher testosterone levels had less loss of lean body mass over 4.5 yr compared with men with lower testosterone levels, but testosterone was less clearly related to preservation of physical function. The associations did not differ by age or self-reported health status but were most apparent in those who lost more than 2.0 kg of body weight. Thus, in the 40% of MrOS men who lost weight over 4.5 yr, higher testosterone levels were associated with relative preservation of lean mass and some attenuation in lower extremity functional decline.
This report provides new details about the association between sex steroids and body composition changes in a large cohort of community-dwelling older men. In younger men, lower endogenous testosterone has been associated with a greater increase in body mass index, waist circumference, and intraabdominal fat (24, 25, 28). However, body composition changes are different in older than younger men. Older men tend to lose lean mass as they age, but the magnitude of decline varies (8–10). Although lean mass decreases in older men who lose weight as well as in those whose weight is stable, the losses are greatest in men who lose weight (9, 10, 16). When we stratified our results by weight change category, the association between testosterone and loss of lean mass was only significant in men who had lost weight. We may have been able to detect the association between testosterone and lean mass only in this subgroup because they were the men who lost the most lean mass. Alternatively, testosterone may be associated only with changes in lean mass during negative energy balance.
In our study, higher testosterone levels were even more strongly associated with preservation of appendicular lean mass, which may be highly related to health outcomes such as strength and frailty. However, it was less clear that higher testosterone levels translated into less decline in physical function in our cohort. Testosterone was not associated with change in physical function in the entire cohort. This is consistent with previous studies which found that total T was not associated with decline in physical performance (walking speed, chair stands, tandem stand) or strength (handgrip strength) or development of frailty (29, 30, 55). Lower free testosterone levels have been associated with higher frailty and a greater risk of mobility limitations 5 to 6 yr after the sex steroid measurement (29, 30). However, free testosterone measurements may be less accurate and reproducible than total T measures (56). We did find that in men who were losing weight (where the association between testosterone and lean mass was strongest), higher testosterone levels were associated with less loss of lower extremity function, as evidenced by a smaller increase in time to complete five chair stands. Adjustment for lean mass change slightly attenuated this association, suggesting that effects of testosterone on lean muscle mass may mediate the positive association between testosterone and lower extremity function in men losing weight. Consistent with previous reports (3, 5), our results suggest that physical function decline is likely a multifactorial process. The association between testosterone and change in physical function may depend on how body composition is changing in the population being studied. Further research should examine these associations more closely because preserving physical function could ultimately have important health implications through decreased risk of falls, risk of mobility limitations, incident disability, and fractures (9, 35).
Our study has several strengths. It is the first well-powered study to prospectively address the longitudinal association between body composition, physical function, and sex steroids in older men. We used gas chromatography/mass spectrometry to measure sex steroids; gas chromatography/mass spectrometry is accurate at the low sex hormone concentrations seen in older men (57, 58). Body composition was carefully measured with DXA, and potential confounding variables, including age, race/ethnicity, medications, medical history, mental functioning, alcohol, physical activity, and physical health, were evaluated. Many men were more than 80 yr old, a population sector that is increasing steadily but whose sex hormone distribution and body composition changes have not been well studied.
There are also several limitations. We made multiple comparisons and examined five sex steroid measures and numerous body composition outcomes. We could not determine whether weight loss was intentional or unintentional. This distinction may be important and should be further evaluated in future studies. Although we evaluated many factors known to influence sex steroid measures and body composition or physical function, we may not have controlled for every comorbid condition or medication that might lower testosterone levels. We also did not examine diurnal variations in testosterone levels, but all samples were obtained in the morning and diurnal variation is limited in older men (59). Sex hormones were measured only at baseline. Studies with longitudinal measurements of sex steroid hormones are needed to establish an association between longitudinal changes in sex steroids and changes in body composition and physical function.
In conclusion, higher testosterone levels were associated with less lean mass loss in older men, especially those who were losing weight. This was accompanied by evidence of attenuation in the decline in lower extremity performance in those losing weight. Maintaining lean mass, particularly appendicular lean mass, is an important component of healthy aging. The association between higher endogenous testosterone and less lean mass and lower extremity performance loss is consistent with our previous reports that higher testosterone levels are associated with a lower incidence of falls and less frailty (32, 60). Our study adds evidence to the growing body of literature that higher levels of endogenous testosterone may be favorably associated with some components of healthy aging in men.
Acknowledgments
We thank Martie Sucec for her editorial assistance and Desirée Pheister and Terresa Fair for their assistance with manuscript preparation.
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Institute on Aging, the National Center for Research Resources, and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140. In addition, E.S.L. is supported by K23-RR020049, and L.M.M. is supported by R01HL084184 and P60AR05473101.
Disclosure Summary: E.B.-C. has received grants from Merck, Arena, Roche, and Pfizer pharmaceuticals. J.A.C. has received research grants and consulting fees from Novartis, Inc. These financial supports do not represent a conflict of interest. The remaining authors have no financial disclosures.
Footnotes
- bioE
- Bioavailable estrogen
- bioT
- bioavailable testosterone
- CV
- coefficients of variation
- DXA
- dual-energy x-ray absorptiometry
- total E
- total estradiol
- total T
- total testosterone.
References
- 1. Koster A, Visser M, Simonsick EM, Yu B, Allison DB, Newman AB, van Eijk JT, Schwartz AV, Satterfield S, Harris TB. 2010. Association between fitness and changes in body composition and muscle strength. J Am Geriatr Soc 58:219–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Forrest KY, Zmuda JM, Cauley JA. 2005. Patterns and determinants of muscle strength change with aging in older men. Aging Male 8:151–156 [DOI] [PubMed] [Google Scholar]
- 3. Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, Fiatarone Singh MA. 2001. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci 56:B209–B217 [DOI] [PubMed] [Google Scholar]
- 4. Bassey EJ. 1998. Longitudinal changes in selected physical capabilities: muscle strength, flexibility and body size. Age Ageing 27(Suppl 3):12–16 [DOI] [PubMed] [Google Scholar]
- 5. Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. 2006. The loss of skeletal muscle strength, mass, and quality in older adults: the Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci 61:1059–1064 [DOI] [PubMed] [Google Scholar]
- 6. Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB, Goodpaster BH. 2009. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr 90:1579–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Araujo AB, Travison TG, Bhasin S, Esche GR, Williams RE, Clark RV, McKinlay JB. 2008. Association between testosterone and estradiol and age-related decline in physical function in a diverse sample of men. J Am Geriatr Soc 56:2000–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Visser M, Pahor M, Tylavsky F, Kritchevsky SB, Cauley JA, Newman AB, Blunt BA, Harris TB. 2003. One- and two-year change in body composition as measured by DXA in a population-based cohort of older men and women. J Appl Physiol 94:2368–2374 [DOI] [PubMed] [Google Scholar]
- 9. Fantin F, Di Francesco V, Fontana G, Zivelonghi A, Bissoli L, Zoico E, Rossi A, Micciolo R, Bosello O, Zamboni M. 2007. Longitudinal body composition changes in old men and women: interrelationships with worsening disability. J Gerontol A Biol Sci Med Sci 62:1375–1381 [DOI] [PubMed] [Google Scholar]
- 10. Newman AB, Lee JS, Visser M, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Nevitt M, Harris TB. 2005. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr 82:872–878; quiz 915–916 [DOI] [PubMed] [Google Scholar]
- 11. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. 2001. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 86:724–731 [DOI] [PubMed] [Google Scholar]
- 12. Travison TG, Araujo AB, Kupelian V, O'Donnell AB, McKinlay JB. 2007. The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. J Clin Endocrinol Metab 92:549–555 [DOI] [PubMed] [Google Scholar]
- 13. Morley JE, Kaiser FE, Perry HM, 3rd, Patrick P, Morley PM, Stauber PM, Vellas B, Baumgartner RN, Garry PJ. 1997. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism 46:410–413 [DOI] [PubMed] [Google Scholar]
- 14. Gallagher D, Visser M, De Meersman RE, Sepúlveda D, Baumgartner RN, Pierson RN, Harris T, Heymsfield SB. 1997. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol 83:229–239 [DOI] [PubMed] [Google Scholar]
- 15. Kirchengast S, Huber J. 2009. Gender and age differences in lean soft tissue mass and sarcopenia among healthy elderly. Anthropol Anz 67:139–151 [DOI] [PubMed] [Google Scholar]
- 16. Hughes VA, Frontera WR, Roubenoff R, Evans WJ, Singh MA. 2002. Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J Clin Nutr 76:473–481 [DOI] [PubMed] [Google Scholar]
- 17. Dey DK, Bosaeus I, Lissner L, Steen B. 2009. Changes in body composition and its relation to muscle strength in 75-year-old men and women: a 5-year prospective follow-up study of the NORA cohort in Goteborg, Sweden. Nutrition 25:613–619 [DOI] [PubMed] [Google Scholar]
- 18. Rantanen T, Era P, Heikkinen E. 1997. Physical activity and the changes in maximal isometric strength in men and women from the age of 75 to 80 years. J Am Geriatr Soc 45:1439–1445 [DOI] [PubMed] [Google Scholar]
- 19. Lindle RS, Metter EJ, Lynch NA, Fleg JL, Fozard JL, Tobin J, Roy TA, Hurley BF. 1997. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J Appl Physiol 83:1581–1587 [DOI] [PubMed] [Google Scholar]
- 20. Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, Holmes JH, Dlewati A, Santanna J, Rosen CJ, Strom BL. 1999. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab 84:2647–2653 [DOI] [PubMed] [Google Scholar]
- 21. Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, Yarasheski KE, Sinha-Hikim I, Dzekov C, Dzekov J, Magliano L, Storer TW. 2005. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab 90:678–688 [DOI] [PubMed] [Google Scholar]
- 22. Sattler FR, Castaneda-Sceppa C, Binder EF, Schroeder ET, Wang Y, Bhasin S, Kawakubo M, Stewart Y, Yarasheski KE, Ulloor J, Colletti P, Roubenoff R, Azen SP. 2009. Testosterone and growth hormone improve body composition and muscle performance in older men. J Clin Endocrinol Metab 94:1991–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Borst SE. 2004. Interventions for sarcopenia and muscle weakness in older people. Age Ageing 33:548–555 [DOI] [PubMed] [Google Scholar]
- 24. Gapstur SM, Gann PH, Kopp P, Colangelo L, Longcope C, Liu K. 2002. Serum androgen concentrations in young men: a longitudinal analysis of associations with age, obesity, and race. The CARDIA male hormone study. Cancer Epidemiol Biomarkers Prev 11:1041–1047 [PubMed] [Google Scholar]
- 25. Khaw KT, Barrett-Connor E. 1992. Lower endogenous androgens predict central adiposity in men. Ann Epidemiol 2:675–682 [DOI] [PubMed] [Google Scholar]
- 26. Ferrini RL, Barrett-Connor E. 1998. Sex hormones and age: a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol 147:750–754 [DOI] [PubMed] [Google Scholar]
- 27. Kaufman JM, Vermeulen A. 2005. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev 26:833–876 [DOI] [PubMed] [Google Scholar]
- 28. Tsai EC, Boyko EJ, Leonetti DL, Fujimoto WY. 2000. Low serum testosterone level as a predictor of increased visceral fat in Japanese-American men. Int J Obes Relat Metab Disord 24:485–491 [DOI] [PubMed] [Google Scholar]
- 29. Krasnoff JB, Basaria S, Pencina MJ, Jasuja GK, Vasan RS, Ulloor J, Zhang A, Coviello A, Kelly-Hayes M, D'Agostino RB, Wolf PA, Bhasin S, Murabito JM. 2010. Free testosterone levels are associated with mobility limitation and physical performance in community-dwelling men: the Framingham Offspring Study. J Clin Endocrinol Metab 95:2790–2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hyde Z, Flicker L, Almeida OP, Hankey GJ, McCaul KA, Chubb SA, Yeap BB. 2010. Low free testosterone predicts frailty in older men: the Health in Men Study. J Clin Endocrinol Metab 95:3165–3172 [DOI] [PubMed] [Google Scholar]
- 31. Schaap LA, Pluijm SM, Deeg DJ, Penninx BW, Nicklas BJ, Lips P, Harris TB, Newman AB, Kritchevsky SB, Cauley JA, Goodpaster BH, Tylavsky FA, Yaffe K, Visser M. 2008. Low testosterone levels and decline in physical performance and muscle strength in older men: findings from two prospective cohort studies. Clin Endocrinol (Oxf) 68:42–50 [DOI] [PubMed] [Google Scholar]
- 32. Cawthon PM, Ensrud KE, Laughlin GA, Cauley JA, Dam TT, Barrett-Connor E, Fink HA, Hoffman AR, Lau E, Lane NE, Stefanick ML, Cummings SR, Orwoll ES. 2009. Sex hormones and frailty in older men: the osteoporotic fractures in men (MrOS) study. J Clin Endocrinol Metab 94:3806–3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ensrud KE, Ewing SK, Taylor BC, Fink HA, Cawthon PM, Stone KL, Hillier TA, Cauley JA, Hochberg MC, Rodondi N, Tracy JK, Cummings SR. 2008. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med 168:382–389 [DOI] [PubMed] [Google Scholar]
- 34. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. 2001. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56:M146–M156 [DOI] [PubMed] [Google Scholar]
- 35. Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. 2010. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int 21:543–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Janssen I, Heymsfield SB, Ross R. 2002. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 50:889–896 [DOI] [PubMed] [Google Scholar]
- 37. Ramsay SE, Whincup PH, Shaper AG, Wannamethee SG. 2006. The relations of body composition and adiposity measures to ill health and physical disability in elderly men. Am J Epidemiol 164:459–469 [DOI] [PubMed] [Google Scholar]
- 38. Lee CG, Boyko EJ, Nielson CM, Stefanick ML, Bauer DC, Hoffman AR, Dam TT, Lapidus JA, Cawthon PM, Ensrud KE, Orwoll ES. 2011. Mortality risk in older men associated with changes in weight, lean mass, and fat mass. J Am Geriatr Soc 59:233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K. 2005. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials 26:569–585 [DOI] [PubMed] [Google Scholar]
- 40. Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR. 2005. Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials 26:557–568 [DOI] [PubMed] [Google Scholar]
- 41. Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. 1994. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol 10:405–411 [DOI] [PubMed] [Google Scholar]
- 42. Washburn RA, Smith KW, Jette AM, Janney CA. 1993. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 46:153–162 [DOI] [PubMed] [Google Scholar]
- 43. Ware J, Kosinski M, Keller S. 1998. Modified physical and mental function summary scales were reported based on the 12-Item Medical Outcomes Study (MOS) Short Form 12 (SF-12). A higher score indicates better functioning. How to score the SF-12 Physical and Mental Health Summary Scores. Lincoln, RI: Quality Metric Inc [Google Scholar]
- 44. Strauss BJ, Gibson PR, Stroud DB, Borovnicar DJ, Xiong DW, Keogh J. 2000. Total body dual x-ray absorptiometry is a good measure of both fat mass and fat-free mass in liver cirrhosis compared to “gold-standard” techniques. Melbourne Liver Group. Ann NY Acad Sci 904:55–62 [DOI] [PubMed] [Google Scholar]
- 45. Heymsfield SB, Gallagher D, Visser M, Nunez C, Wang ZM. 1995. Measurement of skeletal muscle: laboratory and epidemiological methods. J Gerontol A Biol Sci Med Sci 50 Spec No:23–29 [DOI] [PubMed] [Google Scholar]
- 46. Baumgartner RN, Stauber PM, McHugh D, Wayne S, Garry PJ, Heymsfield SB. 1993. Body composition in the elderly using multicompartmental methods. Basic Life Sci 60:251–254 [DOI] [PubMed] [Google Scholar]
- 47. Härkönen R, Harju R, Alaranta H. 1993. Accuracy of the Jamar dynamometer. J Hand Ther 6:259–262 [DOI] [PubMed] [Google Scholar]
- 48. Bassey EJ, Short AH. 1990. A new method for measuring power output in a single leg extension: feasibility, reliability and validity. Eur J Appl Physiol Occup Physiol 60:385–390 [DOI] [PubMed] [Google Scholar]
- 49. Bassey EJ, Fiatarone MA, O'Neill EF, Kelly M, Evans WJ, Lipsitz LA. 1992. Leg extensor power and functional performance in very old men and women. Clin Sci (Lond) 82:321–327 [DOI] [PubMed] [Google Scholar]
- 50. Blackwell T, Cawthon PM, Marshall LM, Brand R. 2009. Consistency of leg extension power assessments in older men: the Osteoporotic Fractures in Men (MrOS) Study. Am J Phys Med Rehabil 88:934–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Södergård R, Bäckström T, Shanbhag V, Carstensen H. 1982. Calculation of free and bound fractions of testosterone and estradiol-17 β to human plasma proteins at body temperature. J Steroid Biochem 16:801–810 [DOI] [PubMed] [Google Scholar]
- 52. Heinzl H, Kaider A. 1997. Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Comput Methods Programs Biomed 54:201–208 [DOI] [PubMed] [Google Scholar]
- 53. Ware J, Jr, Kosinski M, Keller SD. 1996. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 34:220–233 [DOI] [PubMed] [Google Scholar]
- 54. Kleinbaum DG, Kupper LL, Morgenstern H. 1982. Confounding. In: Kleinbaum DG, Kupper LL, Morgenstern H. eds. Epidemiologic research: principles and quantitative methods. Belmont, CA: Lifetime Learning Publications; 242–265 [Google Scholar]
- 55. Schaap LA, Pluijm SM, Smit JH, van Schoor NM, Visser M, Gooren LJ, Lips P. 2005. The association of sex hormone levels with poor mobility, low muscle strength and incidence of falls among older men and women. Clin Endocrinol (Oxf) 63:152–160 [DOI] [PubMed] [Google Scholar]
- 56. Wartofsky L, Handelsman DJ. 2010. Standardization of hormonal assays for the 21st century. J Clin Endocrinol Metab 95:5141–5143 [DOI] [PubMed] [Google Scholar]
- 57. Taieb J, Mathian B, Millot F, Patricot MC, Mathieu E, Queyrel N, Lacroix I, Somma-Delpero C, Boudou P. 2003. Testosterone measured by 10 immunoassays and by isotope-dilution gas chromatography-mass spectrometry in sera from 116 men, women, and children. Clin Chem 49:1381–1395 [DOI] [PubMed] [Google Scholar]
- 58. Stanczyk FZ, Cho MM, Endres DB, Morrison JL, Patel S, Paulson RJ. 2003. Limitations of direct estradiol and testosterone immunoassay kits. Steroids 68:1173–1178 [DOI] [PubMed] [Google Scholar]
- 59. Brambilla DJ, Matsumoto AM, Araujo AB, McKinlay JB. 2009. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab 94:907–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Orwoll E, Lambert LC, Marshall LM, Blank J, Barrett-Connor E, Cauley J, Ensrud K, Cummings SR. 2006. Endogenous testosterone levels, physical performance, and fall risk in older men. Arch Intern Med 166:2124–2131 [DOI] [PubMed] [Google Scholar]