Abstract
Context:
Results from animal studies suggest that consumption of large amounts of fructose can promote inflammation and impair fibrinolysis. Data describing the effects of fructose consumption on circulating levels of proinflammatory and prothrombotic markers in humans are unavailable.
Objective:
Our objective was to determine the effects of 10 wk of dietary fructose or glucose consumption on plasma concentrations of monocyte chemoattractant protein-1 (MCP-1), plasminogen activator inhibitor-1 (PAI-1), E-selectin, intercellular adhesion molecule-1, C-reactive protein, and IL-6.
Design and Setting:
This was a parallel-arm study with two inpatient phases (2 wk baseline, final 2 wk intervention), conducted in a clinical research facility, and an outpatient phase (8 wk) during which subjects resided at home.
Participants:
Participants were older (40–72 yr), overweight/obese (body mass index = 25–35 kg/m2) men (n = 16) and women (n = 15).
Interventions:
Participants consumed glucose- or fructose-sweetened beverages providing 25% of energy requirements for 10 wk. Blood samples were collected at baseline and during the 10th week of intervention.
Main Outcome Measures:
Fasting concentrations of MCP-1 (P = 0.009), PAI-1 (P = 0.002), and E-selectin (P = 0.048) as well as postprandial concentrations of PAI-1 (P < 0.0001) increased in subjects consuming fructose but not in those consuming glucose. Fasting levels of C-reactive protein, IL-6, and intercellular adhesion molecule-1 were not changed in either group.
Conclusions:
Consumption of fructose for 10 wk leads to increases of MCP-1, PAI-1, and E-selectin. These findings suggest the possibility that fructose may contribute to the development of the metabolic syndrome via effects on proinflammatory and prothrombotic mediators.
We recently reported that consumption of fructose-sweetened beverages for 10 wk, at 25% of energy requirements, promotes the development of three pathological characteristics associated with metabolic syndrome: dyslipidemia, decreased insulin sensitivity, and increased visceral adiposity in older, overweight adults compared with isocaloric consumption of glucose (1). Metabolic syndrome has also been shown to be associated with proinflammatory and prothrombotic conditions, and there is considerable evidence supporting a relationship between components of the metabolic syndrome and plasma concentrations of biological markers of inflammation and thrombosis (2).
To determine whether fructose-induced dyslipidemia, increases of visceral adiposity, and reductions of insulin sensitivity are accompanied by an increase of systemic inflammation and/or impaired fibrinolysis, we measured circulating concentrations of monocyte chemoattractant protein-1 (MCP-1), plasminogen activator inhibitor-1 (PAI-1), soluble leukocyte adhesion molecule-1 (E-selectin), soluble intercellular adhesion molecule-1 (ICAM), C-reactive protein (CRP) and IL-6 at baseline and after 10 wk of intervention in the subjects from our previous investigation (1). The results indicate that consumption of fructose, but not glucose, at 25% of energy requirements increases plasma concentrations of MCP-1, E-selectin, and PAI-1. Circulating concentrations of CRP, IL-6, and ICAM were unchanged during consumption of either sugar.
Subjects and Methods
Study design
This was a parallel-arm study with three phases: 1) a 2-wk inpatient baseline period, 2) an 8-wk outpatient intervention period, and 3) a 2-wk inpatient intervention period. During the baseline and inpatient intervention periods, subjects resided in the University of California-Davis Clinical and Translational Science Center's Clinical Research Center, and during the 8-wk outpatient intervention, subjects resided at home. During the intervention phases, subjects consumed either fructose-sweetened (n = 16) or glucose-sweetened (n = 15) beverages at 25% of energy requirements.
Subjects
Inclusion criteria included age 40–72 yr and body mass index (BMI) of 25–35 kg/m2 with a self-report of stable body weight during the previous 6 months. Women were categorized as postmenopausal based on self-reports of no menstruation for at least 1 yr. Exclusion criteria included evidence of diabetes, renal or hepatic disease, fasting serum triglyceride (TG) concentrations over 400 mg/dl, hypertension, and weight loss surgery. Individuals who smoked, exercised more than 3.5 h/wk at a level more vigorous than walking, or used thyroid, lipid-lowering, glucose-lowering, antihypertensive, antidepressant, or weight-loss medications were also excluded. The University of California-Davis Institutional Review Board approved the experimental protocol. Subjects provided informed consent to participate in the study. Thirty-nine subjects enrolled in the study, and experimental groups were matched for gender, BMI, and fasting TG and insulin concentrations. As reported by Stanhope et al. (1), baseline anthropomorphic and metabolic characteristics did not differ between the two experimental groups. Seven subjects did not complete the study because of inability or unwillingness to comply with protocol or due to personal or work-related conflicts. We were unable to measure inflammatory markers in one female subject in the fructose group due to a lack of plasma samples. Subjects were primarily Caucasian (n = 24); however, Hispanics (n = 5) and African-Americans (n = 3) were also included in the study.
Diets
During inpatient metabolic phases, subjects consumed diets designed to maintain energy balance (15% of energy as protein, 30% as fat, and 55% as carbohydrate). Daily energy intake was calculated at baseline using the Mifflin equation to estimate resting energy expenditure and adjusted for activity using a multiplication factor (1.3–1.5). During baseline, the carbohydrate content consisted primarily of complex carbohydrates. For the final 2-wk inpatient intervention period, subjects consumed diets at baseline energy levels and macronutrient composition except that 30% of energy was from complex carbohydrates and 25% was provided by fructose- or glucose-sweetened beverages. Additional details about dietary composition and intake have been described previously (1).
Fasting and postprandial blood samples
The 24-h blood collections were conducted during baseline and after 10 wk of intervention. Three fasting blood samples were collected at 0800, 0830, and 0900 h. Thirty-three postprandial blood samples were collected at 30- to 60-min intervals from 0930 until 0800 h the next morning. Plasma from the three fasting samples (0800, 0830, and 0900 h) was pooled, as was plasma from the final three postprandial blood samples of the day (2200, 2300, and 2330 h); multiple aliquots of each pooled sample were stored at −80 C.
Measurement of inflammatory markers
MCP-1, PAI-1, ICAM, and IL-6 (high sensitivity) were quantified using commercially available ELISA kits from R&D Systems (Minneapolis, MN), E-selectin was measured using an ELISA from Bender Medsystems/eBioscience (San Diego, CA), and CRP levels were determined using a Polychem Chemistry Analyzer (PolyMedCo, Inc., Cortlandt Manor, NY).
Data analysis
Statistical tests were performed with SAS version 9.2. The absolute or percent change for each outcome was calculated for each subject, and these values were averaged for the two groups and then analyzed in a two-factor (type of sugar and gender) mixed procedures (PROC MIXED) ANOVA model with adjustment for differences in baseline values. Variability in responses between individuals accounts for the differences between the average changes calculated on a per-subject basis and the apparent changes based on the means for each group. Outcomes with least-squares means of the change (10 vs. 0 wk) significantly different from zero were identified. Statistical tests with P values <0.05 were considered significant. Data are presented as mean ± sem. Data were also adjusted for age and ethnicity in a separate analysis (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Results
MCP-1
Fasting plasma concentrations of MCP-1 were not significantly changed from baseline in subjects consuming glucose-sweetened beverages (P = 0.634) (Table 1). However, in subjects consuming fructose, MCP-1 levels increased significantly compared with baseline values (P = 0.0009) and compared with subjects in the glucose group (P = 0.03 for effect of sugar) (Table 1).
Table 1.
Fasting plasma concentrations of MCP-1, E-selectin, ICAM, IL-6, and CRP and postprandial concentrations of PAI-1 at baseline and after 10 wk of consumption of fructose- or glucose-sweetened beverages
| Fructose |
Glucose |
P value for effect of sugar | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 10 wk | % change at 10 wk | Baseline | 10 wk | % change at 10 wk | ||
| MCP-1 (pg/ml) | 144.7 ± 18.8 | 199.3 ± 19.4 | 83.5 ± 32.9c | 178.7 ± 10.5 | 163.2 ± 18.3 | −9.2 ± 9.9 | 0.03 |
| ppPAI-1 (ng/ml) | 3.8 ± 0.5 | 4.6 ± 0.5 | 48.5 ± 16.4c | 5.2 ± 0.9 | 5.0 ± 0.7 | 1.6 ± 7.8 | 0.03 |
| E-selectin (ng/dl) | 45.0 ± 5.5 | 51.5 ± 4.6 | 29.3 ± 13.7b | 50.0 ± 5.5 | 49.2 ± 4.6 | 5.9 ± 8.2 | 0.17 |
| ICAM (ng/ml) | 221.9 ± 6.3 | 228.4 ± 6.9 | 3.5 ± 2.8 | 190.1 ± 21.8 | 188.2 ± 22.0 | −0.5 ± 3.2 | 0.22 |
| IL-6 (pg/ml) | 3.5 ± 0.7 | 3.1 ± 0.5 | 7.2 ± 11.9 | 3.3 ± 0.7 | 3.9 ± 1.0 | 20.3 ± 15.8 | 0.31 |
| CRPa (mg/liter) | 3.7 ± 0.8 | 3.1 ± 0.6 | −5.0 ± 9.2 | 5.7 ± 2.4 | 4.4 ± 1.3 | 11.1 ± 18.1 | 0.33 |
Values are means ± sem; n = 31 (fructose group n = 16; glucose group n = 15). PROC MIXED two-factor (sugar and gender) ANOVA with adjustment for differences in baseline values was used. ppPAI-1, Postprandial PAI-1.
P values reported are for absolute change rather than percent change due to the high variability in CRP responses (see Supplemental Fig. 1 for individual responses for all variables).
Changes significantly different from baseline: b P < 0.05; c P < 0.001.
PAI-1
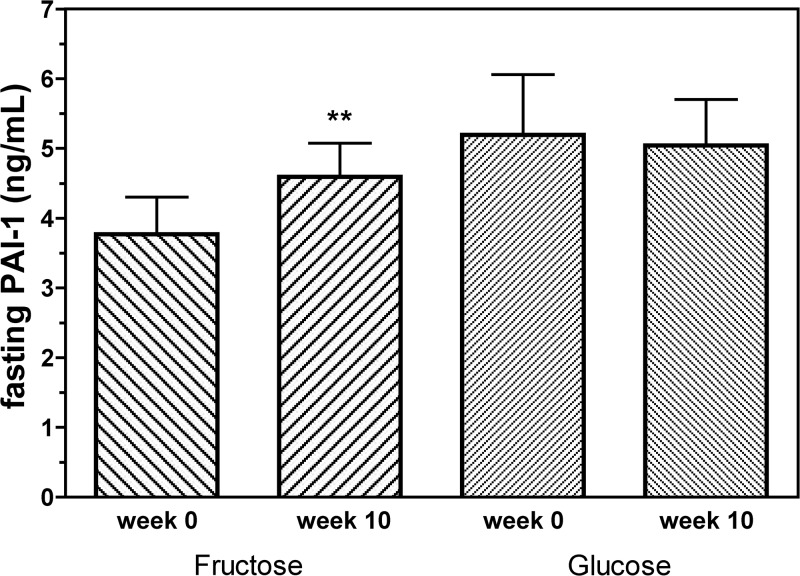
Fasting plasma PAI-1 concentrations increased significantly from baseline in subjects consuming fructose (P = 0.002) but not in those consuming glucose (P = 0.53) (P = 0.07 for effect of sugar) (Fig. 1). As with fasting PAI-1 levels, postprandial plasma PAI-1 levels also increased significantly in the fructose group, both compared with baseline (+1.76 ± 0.39 ng/ml; P < 0.0001) and compared with subjects consuming glucose (P = 0.03 for effect of sugar) (Table 1). Postprandial PAI-1 levels were unchanged in subjects consuming glucose. (+0.57 ± 0.28 ng/ml; P = 0.06) (Table 1).
Fig. 1.
Fasting concentrations of PAI-1 (nanograms per milliliter) before and after 10 wk of consuming fructose- or glucose-sweetened beverages. **, P < 0.01 compared with baseline values using PROC mixed two-factor (sugar and gender) ANOVA with adjustment for differences in baseline values; P = 0.07 for effect of sugar. Fructose group, n = 16; glucose group, n = 15.
E-selectin and ICAM
Fasting plasma concentrations of E-selectin were significantly increased from baseline in subjects consuming fructose-sweetened beverages (P = 0.048) but not in subjects consuming glucose-sweetened beverages (P = 0.95); however, the difference in response between the groups did not reach significance (P = 0.17 for effect of sugar) (Table 1). Although fasting plasma ICAM levels tended to increase slightly in subjects consuming fructose and remain unchanged in subjects consuming glucose, the responses were not statistically significant (Table 1).
CRP and IL-6
Fasting plasma concentrations of CRP were unchanged in subjects consuming fructose (−0.63 ± 0.48 mg/liter; P = 0.75) or glucose (−1.32 ± 1.16 mg/liter; P = 0.29) (P = 0.33 for effect of sugar) (Table 1). The high variability of CRP responses (>75% of mean) led to discrepancies between the absolute and percent changes calculated, particularly for the glucose group (Table 1). Fasting plasma concentrations of IL-6 were not changed in either experimental diet group (Table 1).
Discussion
MCP-1 and PAI-1
In humans, visceral adipose mass has been shown to be a primary determinant of PAI-1 levels (3), and both PAI-1 (4) and MCP-1 (5) have been shown to be preferentially secreted from visceral adipose tissue compared with sc adipose tissue. Thus, it is possible that the increased deposition of visceral adipose tissue in subjects consuming fructose may help to explain the observed increases of PAI-1 and MCP-1 in subjects consuming fructose; however, we did not detect any significant correlations between increases of VAT and increases of MCP-1 and PAI-1 (1).
Elevated levels of TG-rich lipoproteins have also been shown to be associated with increased PAI-1 concentrations (6). Moreover, it has been demonstrated that very-low-density lipoprotein-derived TG particles can induce PAI-1 expression in vascular endothelial cells (7). Considering that levels of TG-rich lipoproteins are measurably higher postprandially and that the increases of lipids and lipoproteins exhibited by subjects consuming fructose were also more pronounced during the postprandial state (1), we hypothesized that increases of PAI-1 would also be greater in the postprandial state. Although the difference between fasting and postprandial PAI-1 responses was not statistically significant, the absolute increase in postprandial PAI-1 levels was more than twice as large as the increase of fasting levels (see Results and Fig. 1).
It has been suggested that activation of protein kinase C (PKC) is responsible for the increased levels of PAI-1 observed in insulin resistance (8). PKC also appears to be involved in the stimulation of MCP-1 production, and secretion of MCP-1 from human liver fat-storing cells (stellate cells) is dependent on PKC activation (9). We have suggested that the reductions of insulin sensitivity and glucose tolerance previously reported in the subjects from our study were primarily the result of fructose-induced increases of hepatic de novo lipogenesis leading to accumulation of hepatic lipid and subsequent diacylglycerol-mediated PKC activation (1, 10, 11). The evidence linking expression of MCP-1 and PAI-1 to PKC activation suggests the possibility that the increases of MCP-1 and PAI-1 exhibited in response to fructose consumption may also be mediated by this mechanism, although we acknowledge that evidence for this mechanism is speculative because we were unable to obtain liver biopsies to measure PKC activity.
Cellular adhesion molecules, IL-6, and CRP
It is interesting that prolonged fructose consumption led to significant increases of E-selectin but not ICAM. Although there is evidence supporting a relationship between indices of insulin resistance and increased concentrations of various cellular adhesion molecules, several studies have reported that levels of E-selectin and/or ICAM are correlated with visceral fat volume (12) and reduced insulin sensitivity (13). The reductions of insulin sensitivity and increases of visceral adiposity that we have reported previously in these same subjects (1), together with the selective increase of E-selectin, support the relationships identified in previous investigations.
There is a growing body of evidence that the inflammatory mediators CRP and IL-6 are associated with proatherogenic conditions (14). Therefore, it is interesting that concentrations of CRP and IL-6 were unaffected by prolonged fructose consumption, despite other proinflammatory changes. These findings suggest that fructose consumption has a pleiotropic effect on markers of inflammation such that certain mediators are preferentially up-regulated (MCP-1, PAI-1, and E-selectin), whereas others (IL-6 and CRP) are not.
Conclusions
Consumption of fructose at 25% of energy requirements for 10 wk leads to increases of fasting MCP-1, PAI-1, and E-selectin as well as postprandial PAI-1 but not IL-6, CRP, or ICAM concentrations. These results suggest the possibility that prolonged consumption of fructose may contribute to the development of metabolic syndrome via induction of specific proinflammatory (MCP-1 and E-selectin) and prothrombotic (PAI-1) mediators. Additional studies examining larger groups of subjects are needed to confirm these findings.
Supplementary Material
Acknowledgments
We thank Marinelle Nuñez, Brandi Bair, Rebecca Stewart, Sara Wuehler, Barbara Gale, Artem Dyachenko, and Patrick Lam for their excellent technical support and Nicole Mullen and the nursing staff at University of California-Davis Clinical and Translational Science Center's Clinical Research Center for their dedicated nursing support. We also thank Janet Peerson for expert advice on the statistical analysis of the data.
This research was supported with funding from National Institutes of Health (NIH) Grant RO1 HL-075675. The project also received support from Grant UL1 RR024146 from the National Center for Research Resources, a component of the NIH, and NIH Roadmap for Medical Research. P.J.H.'s research program also receives support from NIH Grants HL-091333, AT-003545, and DK-097307. N.L.K.'s research is supported by intramural USDA-ARS CRIS 5306-51530-016-00D.
This study is registered at www.ClinicalTrials.gov, identifier NCT01165853.
Disclosure Summary: S.C.G. receives income from Bristol-Myers Squibb. None of the other authors has anything to disclose.
Footnotes
- BMI
- Body mass index
- CRP
- C-reactive protein
- ICAM
- intercellular adhesion molecule-1
- MCP-1
- monocyte chemoattractant protein-1
- PAI-1
- plasminogen activator inhibitor-1
- PKC
- protein kinase C
- TG
- triglyceride.
References
- 1. Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, McGahan JP, Seibert A, Krauss RM, Chiu S, Schaefer EJ, Ai M, Otokozawa S, Nakajima K, Nakano T, Beysen C, Hellerstein MK, Berglund L, Havel PJ. 2009. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 119:1322–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. 2005. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 111:1448–1454 [DOI] [PubMed] [Google Scholar]
- 3. Giltay EJ, Elbers JM, Gooren LJ, Emeis JJ, Kooistra T, Asscheman H, Stehouwer CD. 1998. Visceral fat accumulation is an important determinant of PAI-1 levels in young, nonobese men and women: modulation by cross-sex hormone administration. Arterioscler Thromb Vasc Biol 18:1716–1722 [DOI] [PubMed] [Google Scholar]
- 4. Shimomura I, Funahashi T, Takahashi M, Maeda K, Kotani K, Nakamura T, Yamashita S, Miura M, Fukuda Y, Takemura K, Tokunaga K, Matsuzawa Y. 1996. Enhanced expression of PAI-1 in visceral fat: possible contributor to vascular disease in obesity. Nat Med 2:800–803 [DOI] [PubMed] [Google Scholar]
- 5. Bruun JM, Lihn AS, Pedersen SB, Richelsen B. 2005. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab 90:2282–2289 [DOI] [PubMed] [Google Scholar]
- 6. Panahloo A, Mohamed-Ali V, Lane A, Green F, Humphries SE, Yudkin JS. 1995. Determinants of plasminogen activator inhibitor 1 activity in treated NIDDM and its relation to a polymorphism in the plasminogen activator inhibitor 1 gene. Diabetes 44:37–42 [DOI] [PubMed] [Google Scholar]
- 7. Allison BA, Nilsson L, Karpe F, Hamsten A, Eriksson P. 1999. Effects of native, triglyceride-enriched, and oxidatively modified LDL on plasminogen activator inhibitor-1 expression in human endothelial cells. Arterioscler Thromb Vasc Biol 19:1354–1360 [DOI] [PubMed] [Google Scholar]
- 8. Banfi C, Mussoni L, Risé P, Cattaneo MG, Vicentini L, Battaini F, Galli C, Tremoli E. 1999. Very low density lipoprotein-mediated signal transduction and plasminogen activator inhibitor type 1 in cultured HepG2 cells. Circ Res 85:208–217 [DOI] [PubMed] [Google Scholar]
- 9. Marra F, Valente AJ, Pinzani M, Abboud HE. 1993. Cultured human liver fat-storing cells produce monocyte chemotactic protein-1. Regulation by proinflammatory cytokines. J Clin Invest 92:1674–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stanhope KL, Havel PJ. 2008. Fructose consumption: potential mechanisms for its effects to increase visceral adiposity and induce dyslipidemia and insulin resistance. Curr Opin Lipidol 19:16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morino K, Petersen KF, Shulman GI. 2006. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55(Suppl 2):S9–S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pontiroli AE, Frigè F, Paganelli M, Folli F. 2009. In morbid obesity, metabolic abnormalities and adhesion molecules correlate with visceral fat, not with subcutaneous fat: effect of weight loss through surgery. Obes Surg 19:745–750 [DOI] [PubMed] [Google Scholar]
- 13. Targher G, Bonadonna RC, Alberiche M, Zenere MB, Muggeo M, Bonora E. 2001. Relation between soluble adhesion molecules and insulin sensitivity in type 2 diabetic individuals: role of adipose tissue. Diabetes Care 24:1961–1966 [DOI] [PubMed] [Google Scholar]
- 14. Meshkani R, Adeli K. 2009. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin Biochem 42:1331–1346 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.