Abstract
Context:
Anomalous venous drainage can lead to false-negative inferior petrosal sinus sampling (IPSS) results. Baseline inferior petrosal sinus to peripheral (IPS/P) prolactin ratio higher than 1.8 ipsilateral to the highest ACTH ratio has been proposed to verify successful catheterization. Prolactin-normalized ACTH IPS/P ratios may differentiate Cushing's disease (CD) from ectopic ACTH syndrome (EAS).
Objective:
Our objective was to examine the utility of prolactin measurement during IPSS.
Design, Setting, and Participants:
We conducted a retrospective analysis of prolactin levels in basal and CRH-stimulated IPSS samples in ACTH-dependent Cushing's syndrome (2007–2010).
Results:
Twenty-five of 29 patients had a pathologically proven diagnosis (17 CD and eight EAS). IPSS results were partitioned into true positive for CD (n = 16), true negative (n = 7), false negative (n = 1), and false positive (n = 1). Prolactin IPS/P ratio suggested successful IPSS in eight of 11 with abnormal venograms. Baseline prolactin IPS/P ratio was helpful in two patients with abnormal venograms and false-negative (catheterization unsuccessful) or true-negative (catheterization successful) IPSS results; the normalized ratio correctly diagnosed their disease. Normalized ACTH IPS/P ratio was at least 1.3 in all with CD, but prolactin IPS/P ratios were misleadingly low in two. One patient with cyclic EAS had a false-positive IPSS when eucortisolemic (baseline prolactin IPS/P = 1.7; normalized ratio = 5.6). All other EAS patients had normalized ratios no higher than 0.7.
Conclusion:
Prolactin measurement and evaluation of the venogram can improve diagnostic accuracy when IPSS results suggest EAS but is not necessary with positive IPSS results. Confirmation of hypercortisolemia remains a prerequisite for IPSS. A normalized ratio of 0.7–1.3 was not diagnostic.
Inferior petrosal sinus sampling (IPSS) is considered the gold standard test for differentiating between a pituitary and an ectopic source of ACTH-dependent Cushing's syndrome. A petrosal sinus to peripheral ACTH gradient of at least 2.0 at baseline or at least 3.0 after CRH administration suggests a pituitary source of ACTH (1). However, false-negative results have been reported in 1–10% of cases (2–4). These false-negative results have been attributed to anomalous venous drainage, hypoplastic petrosal sinuses, and lack of expertise and/or technical problems leading to unsuccessful inferior petrosal sinus catheterization.
Measurement of other pituitary hormones, including GH, TSH, α-subunit, and prolactin, has been advocated to evaluate catheter placement during IPSS (5–9). Prolactin is the most abundant pituitary hormone, and the location of normal lactotrophs in the anterior pituitary gland is different from the corticotropes, making it a reliable reference hormone (10). Moreover, TSH, GH, and α-subunit levels can be suppressed in hypercortisolemia, especially in cases with ectopic ACTH syndrome (EAS) (6). Therefore, recent studies have focused on prolactin measurement as a marker of successful catheterization during IPSS (7–9), Findling et al. (8) reported on the utility of prolactin in three index cases, 44 patients with proven Cushing's disease and five patients with proven EAS. They concluded that an inferior petrosal sinus to peripheral (IPS/P) prolactin ratio of greater than 1.8 before CRH administration indicated successful catheterization when calculated on the same side as the peak ACTH IPS/P ratio. In another recent series, Mulligan et al. (9) evaluated 35 patients with Cushing's disease and one with EAS who underwent IPSS and found that false-negative IPSS ACTH results had a prolactin IPS/P ratio of less than 1.3. Furthermore, in the Findling series, after normalizing the peak ACTH IPS/P ratio by dividing it by this prolactin IPS/P ratio, values greater than 0.8 suggested Cushing's disease, whereas a ratio less than 0.6 indicated EAS.
Our aim in this retrospective study was to further examine the utility of prolactin measurement as a marker of successful catheterization during IPSS in 29 consecutive patients with ACTH-dependent Cushing's syndrome.
Subjects and Methods
Subjects
We conducted a retrospective review of 29 consecutive patients with ACTH-dependent Cushing's syndrome who underwent IPSS between 2007 and 2010 at the National Institutes of Health Warren Grant Magnuson Clinical Center in Bethesda, MD. After providing written informed consent, patients participated in protocols approved by the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Diagnosis of Cushing's syndrome
All patients were hypercortisolemic (except one, False-positive IPSS: one patient) with nonsuppressed plasma ACTH levels and underwent testing to differentiate between Cushing's disease and EAS. They underwent IPSS because responses to an 8-mg overnight dexamethasone suppression test and CRH stimulation test were discordant and/or the pituitary magnetic resonance imaging (MRI) was normal or equivocal (mass less than 6 mm).
Catheterization/IPSS protocol
An experienced interventional radiologist performed bilateral, simultaneous catheterization of the inferior petrosal sinuses as described by Miller and Doppman (11). Venography was obtained to evaluate venous anatomy and catheter placement. Retrograde flow of contrast dye into the contralateral cavernous sinuses was used as a marker of adequate sampling. After the correct placement of catheters, blood samples were obtained from each of three ports (peripheral, left inferior petrosal sinus, and right inferior petrosal sinus). Two sets of samples were obtained at baseline. Ovine CRH was then peripherally injected at 1 μg/kg (maximum dose of 100 μg), and samples were obtained 3, 5, and 10 min later.
Hormone assays
Upon collection, IPSS samples were placed in an ice-water bath. At the end of the procedure, they were taken to the Department of Laboratory Medicine, where plasma was separated and used for immediate measurement of ACTH; rarely, samples were centrifuged, frozen, and assayed within 24 h. Plasma ACTH was measured in an automated chemiluminescence immunoassay (Siemens Healthcare Diagnostics, Deerfield, IL) (12). The intraassay coefficient of variation (CV) was 1.7–2.3%, and the interassay CV was 2.8–8.4%; the functional detection limit was 5 pg/ml (1.1 pmol/liter). Prolactin was subsequently measured in research plasma samples (stored initially at −20 C and later at −80 C in the research laboratory) at baseline (time zero) and at the time point with the peak ACTH IPS/P ratio after CRH administration (Aurora St. Luke's Medical Center) using an immunoradiometric assay (Diagnostic Products Corp., Los Angeles, CA) with a functional assay sensitivity of 0.1 ng/ml (0.004 nmol/liter), intraassay CV of 1.1–2.7%, and interassay CV of 1.6–6.3%. In one patient, prolactin levels were measured at the National Institutes of Health using a chemiluminescent immunometric assay (intraassay CV of 2.7–3.4% and interassay CV of 4.6–5.3%).
Study analysis
A diagnosis of Cushing's disease or EAS was assigned based on surgical pathology results. A peak ACTH IPS/P value of at least 2 before and/or at least 3 after CRH administration was considered to be consistent with Cushing's disease, with lower values suggestive of EAS. These IPSS results were classified based on whether they were consistent with the final pathological diagnosis, as true positive (IPSS results and pathology indicating Cushing's disease), true negative (IPSS results and pathology indicating EAS), false negative (IPSS results indicating EAS in a patient with a pathology diagnosis of Cushing's disease), or false positive (IPSS results indicating Cushing's disease in a patient with a pathology diagnosis of EAS). If IPSS results suggested EAS but a source could not be localized, patients were categorized as having occult ACTH-dependent Cushing's syndrome.
Baseline prolactin IPS/P ratio was calculated ipsilateral to the dominant ACTH IPS/P ratio, which was defined as the highest ACTH IPS/P ratio after CRH administration. Values higher than 1.8 were considered to indicate adequate catheterization (8). We then calculated the normalized ACTH/prolactin IPS/P ratio by dividing the post-CRH dominant ACTH IPS/P ratio by the basal ipsilateral IPS/P prolactin ratio as previously described (8). Values lower than 0.6 were considered to indicate EAS, and those higher than 0.8 to indicate Cushing's disease.
The strategy proposed previously uses the prolactin IPS/P ratio ipsilateral to the dominant ACTH IPS/P to evaluate venous effluent. Because patients might have bilateral aberrant venous drainage, we also evaluated the prolactin IPS/P ratio on the nondominant side.
Continuous variables were described with means and sd. Categorical variables were described using frequencies and percentages. No formal statistical analyses were performed.
Results
The mean age of the 29 patients (19 women) was 42.8 yr (range, 23–66 yr). Four had occult ACTH-dependent Cushing's syndrome. Among the others, pathology results confirmed Cushing's disease in 17 and EAS in eight (seven pulmonary carcinoids and one thymic neuroendocrine tumor).
Evaluation of the dominant ACTH IPS/P ratio
Known Cushing's disease
True-positive IPSS: 16 patients.
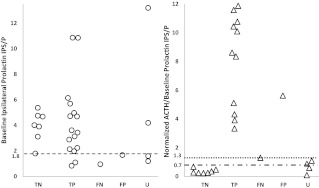
Sixteen patients with surgically proven Cushing's disease had a peak ACTH IPS/P ratio higher than 3, consistent with a pituitary source (true positives). Baseline ipsilateral prolactin IPS/P ratio higher than 1.8 indicated successful catheterization in 14 (range, 1.9–6.2), whereas failed catheterization was suggested in two (0.8, 1.1). The normalized ACTH/prolactin IPS/P ratio in all 16 patients was higher than 0.8 (3.3–40.9), consistent with Cushing's disease (Fig. 1).
Fig. 1.
Baseline ipsilateral prolactin IPS/P ratios (left panel) and normalized ACTH/prolactin IPS/P ratios (right panel) in 29 patients with ACTH-dependent Cushing's syndrome (25 with a surgically proven diagnosis and four with occult Cushing's syndrome). Patients were divided into true negatives (TN: pathology and IPSS results consistent with EAS, n = 7), true positives (TP: pathology and IPSS results consistent with CD, n = 16), false negatives (FN: negative IPSS results in a patient with surgically proven CD, n = 1), false positive (FP: positive IPSS results in a patient with surgically proven EAS, n = 1), and occult (U: biochemical testing consistent with EAS, but a source could not be localized). Normalized ACTH/prolactin IPS/P ratios in five cases were higher than 12 and are not shown in the above figure. All patients with surgically proven Cushing's disease had a normalized ACTH IPS/P ratio of 1.3 or higher (dotted line). Patients with surgically proven EAS (except for the false-positive case) had a normalized ACTH/prolactin IPS/P ratio of 0.7 or lower (dashed line). CD, Cushing's disease.
False-negative IPSS: one patient.
One patient had a peak ACTH IPS/P ratio of 1.3 (false negative). The pituitary MRI showed a 7-mm left pituitary microadenoma (Table 1). Although IPSS angiography revealed a small right petrosal vein and a very large left petrosal vein, the radiologist felt that the catheters accurately sampled both petrosal veins. However, baseline ipsilateral prolactin IPS/P ratio was 1.0, indicating unsuccessful catheterization. The prolactin-normalized peak ACTH IPS/P ratio of 1.3 was higher than 0.8, accurately diagnosing Cushing's disease (Fig. 1).
Table 1.
Clinical features of two patients with erroneous IPSS results (false negative and false positive)
| Characteristic | False negative (IPSS, ectopic; pathology, CD) | False positive (IPSS, CD; pathology, ectopic) |
|---|---|---|
| Age at diagnosis (yr) | 23 | 54 |
| Sex | Male | Female |
| Symptoms/signs | Weight gain, facial swelling, muscle weakness, violaceous striae, easy bruising, dorsocervical and supraclavicular fat pads | Weight gain, proximal muscle weakness, easy bruising, memory problems, dorsocervical and supraclavicular fat pads |
| ACTH (pg/ml) (normal, <46) | 65.7 | 34.5 |
| 24-h UFC (μg/24 h) (normal, 3.5–45) | 3030 | 27 (eucortisolemic) 755 (hypercortisolemic) |
| CRH stimulation test results suggest | Pituitary | Ectopic (hypercortisolemic) |
| HD DST results suggest | Ectopic | Ectopic (hypercortisolemic) |
| MRI pituitary | 7 mm left pituitary adenoma | Normal |
| IPSS | ||
| Peak ACTH IPS/Pa | 1.3 (347/275) | 9.5 (212/22.4) |
| Baseline PRL IPS/Pa | 1.0 (14.8/15.4) | 1.7 (4.2/2.5) |
| ACTH/PRL IPS/P | 1.3 | 5.6 |
| Surgical pathology | Left 7-mm pituitary adenoma, staining positive for ACTH | Left lower pulmonary carcinoid, staining positive for ACTH |
To convert ACTH level to picomoles per liter, multiply by 0.2202. To convert 24-h UFC level to nanomoles per day, multiply by 2.759. CD, Cushing's disease; HD DST, high-dose dexamethasone suppression test; PRL, prolactin; ectopic, ectopic ACTH secretion.
Actual ACTH or prolactin values are shown in parentheses.
Known ectopic ACTH syndrome
True-negative IPSS: seven patients.
Seven of the eight patients with surgically proven EAS had ACTH IPS/P ratios consistent with an ectopic source of ACTH (true negatives). In six, baseline prolactin IPS/P was higher than 1.8 (3.1–5.4), indicating successful catheterization. Normalized ACTH/prolactin IPS/P ratios in these patients were lower than 0.6 (0.2–0.5), consistent with EAS. Baseline prolactin IPS/P ratio in one of the seven true negatives was borderline (1.8) with a normalized ACTH/prolactin IPS/P ratio of 0.7, an indeterminate result (Fig. 1).
False-positive IPSS: one patient.
One of the patients with surgically proven EAS had a peak ACTH IPS/P ratio of 9.5, a false-positive result. Baseline ipsilateral prolactin IPS/P ratio was 1.7, suggesting unsuccessful catheterization, and normalized ACTH/prolactin IPS/P ratio was 5.6, indicative of a pituitary source (Fig. 1). Laboratory results subsequently revealed cyclic Cushing's syndrome; the catheterization had been performed when she was eucortisolemic with a morning serum cortisol of 5.1 μg/dl (140.8 nmol/liter; normal range, 5–25 μg/dl, 138–690 nmol/liter), a 24-h urine free cortisol (UFC) of 27 μg/24 h (74.5 nmol/d; normal range, 3.5–45 μg/24 h, 9.7–124.2 nmol/d), and a plasma ACTH level of 18.3 pg/ml (4 pmol/liter; normal <46 pg/ml, <10.1 pmol/liter). Three weeks earlier, the UFC was 8116 μg/24 h (22,400 nmol/d). CRH stimulation test and a high-dose dexamethasone suppression test, performed 1 wk later when she was hypercortisolemic (UFC 755 μg/24 h, 2083.8 nmol/d), were consistent with EAS. Imaging revealed a 1.4-cm retrocardiac lesion. Surgical pathology showed an ACTH-producing pulmonary carcinoid (Table 1). Postoperatively, ACTH levels were undetectable, and hypercortisolemia remitted. On the 10th postoperative day, she had normal diurnal cortisol levels and did not require glucocorticoid replacement therapy, consistent with unsuppressed normal corticotropes in cyclic Cushing's syndrome.
Occult Cushing's syndrome
A tumor could not be localized in four patients with IPSS consistent with EAS (occult Cushing's syndrome). Baseline ipsilateral prolactin IPS/P ratio in occult patients 1 and 2 (Table 2 and Fig. 1) indicated successful catheterization, with normalized ACTH/prolactin ratios suggesting EAS (0.6 and 0.1). Occult patient 2 had conflicting biochemical test results (Table 2). The cortisol response to CRH, a possible 4-mm right-sided pituitary adenoma on MRI, and an 86% decrease in cortisol after 8 mg dexamethasone suggested Cushing's disease, but subsequent CRH stimulation results and a peak ACTH IPS/P ratio of 2.3 suggested EAS. The validity of the IPSS results was questioned because the petrosal veins were small and difficult to cannulate. In light of this and with biochemical tests suggesting a pituitary source, she underwent transsphenoidal surgery (TSS). However, pathology was unremarkable, and she remained hypercortisolemic.
Table 2.
Clinical features of four patients with occult Cushing's syndrome
| Characteristic | Occult Cushing's syndrome |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Age at diagnosis (yr) | 26 | 44 | 50 | 62 |
| Sex | Male | Female | Male | Female |
| ACTH (pg/ml) (normal, <46) | 639 | 74.8 | 167 | 123 |
| 24-h UFC (μg/24 h) (Normal: 3.5–45) | 11,651 | 565 | 507 | 112 |
| CRH stimulation test results suggest | Ectopic | Done twice: discordant results first, then ectopic | Pituitary | Ectopic |
| HD DST results suggest | Ectopic | Pituitary | Ectopic | Ectopic |
| MRI Pituitary | 4-mm pituitary adenoma | 4-mm possible right pituitary adenoma | 4-mm possible right pituitary adenoma | No evidence of adenoma |
| IPSS venogram | Normal | Bilateral small petrosal veins, difficult to cannulate | Small left petrosal vein | Small left petrosal vein, large right petrosal vein |
| IPSS | ||||
| Peak ACTH IPS/Pa | 1.3 (685/527) | 2.3 (188/82) | 1.3 (285/235) | 1.4 (185/132) |
| Baseline PRL IPS/Pa | 13.2 (104/7.9) | 4.2 (71.6/17.1) | 1.2 (10.8/9.3) | 1.6 (34/21.8) |
| ACTH/PRL IPS/P | 0.1 | 0.6 | 1.1 | 0.9 |
| Other Imaging | ||||
| CT C/A/P | Negative | Negative | 8-mm lung nodule | Left lung nodules |
| MRI C/A/P, cardiac | Possible pericardiac lesion | Negative | Negative | Negative |
| Octreotide scan | Negative | Negative | Negative | Negative |
| Surgery | Underwent TSS at outside facility, pathology with no evidence of adenoma, persistent postoperative hypercortisolism | Underwent TSS at NIH, pathology with no evidence of adenoma, persistent postoperative hypercortisolism | Underwent TSS at NIH, pathology with no evidence of adenoma, persistent postoperative hypercortisolism | Left adnexal mass, status post bilateral salpingo-oopherectomy at NIH, pathology showed fibrothecoma, persistent postoperative hypercortisolism |
| Current status | Medical treatment with ketoconazole and metyrapone, being followed for tumor localization | Medical treatment with Mifepristone, being followed for tumor localization | Underwent bilateral adrenalectomy, being followed for tumor localization | Died |
C/A/P, chest, abdomen, and pelvis; CT, computerized tomography; PRL, prolactin; TSS, transsphenoidal surgery. To convert ACTH level to picomoles per liter, multiply by 0.2202. To convert 24-h UFC level to nanomoles per day, multiply by 2.759.
Actual ACTH or prolactin values are shown in parentheses.
Prolactin was then measured in stored research samples of this patient. Baseline prolactin IPS/P ratio, ipsilateral to the dominant right ACTH IPS/P ratio, was 4.2, suggesting successful catheterization despite abnormal venography. The normalized ratio was indeterminate (0.6). On the left side, all prolactin IPS/P ratios were below 1.8, indicating unsuccessful catheterization, and the normalized ACTH/prolactin IPS/P ratio (1.4) suggested Cushing's disease (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Occult patients 3 and 4 had baseline prolactin IPS/P ratios of 1.2 and 1.6, suggesting failed catheterization (Table 2 and Fig. 1). Patient 3 had a normalized ACTH/prolactin IPS/P ratio suggesting Cushing's disease but had a previously unsuccessful TSS. Patient 4 had a normalized ACTH/prolactin IPS/P ratio suggesting a pituitary source, but she died of sepsis caused by a perforated gastric ulcer before undergoing additional evaluation. An autopsy was not performed.
Evaluation of Post-CRH prolactin values and basal prolactin values from the petrosal sinus with lower ACTH IPS/P ratio
Two EAS patients with nondominant ACTH IPS/P gradients consistent with EAS had baseline prolactin IPS/P values on that side suggesting inadequate catheterization (1.0 and 1.3). Normalized ACTH/prolactin ratio suggested Cushing's disease in one (1.0) and was borderline in the other (0.8). The cyclic EAS patient had misleading results in both dominant and nondominant IPS samples (Table 3).
Table 3.
IPSS ACTH (pg/ml) and prolactin (ng/ml) data in 29 patients with ACTH-dependent Cushing's syndrome with baseline prolactin IPS/P ratios and normalized ACTH/prolactin IPS/P ratios shown for both inferior petrosal sinuses
| Type | IPSS result | Peak ACTH IPS/P | Contralateral Peak ACTH IPS/P | Ipsilateral BL PRL IPS/P | Contralateral BL PRL IPS/P | Ipsilateral normalized peak ACTH/BL PRL IPS/P | Contralateral normalized peak ACTH/BL PRL IPS/P | Concordant normalized ratios |
|---|---|---|---|---|---|---|---|---|
| EAS | TN | 1.2 | 1.1 | 1.8 | 3 | 0.7 | 0.4 | Yes |
| EAS | TN | 1.5 | 1.4 | 3.1 | 2.4 | 0.5 | 0.6 | Yes |
| EAS | TN | 1.1 | 1.1 | 4 | 4.1 | 0.3 | 0.3 | Yes |
| EAS | TN | 1.4 | 1.3 | 5.4 | 2.1 | 0.3 | 0.6 | Yes |
| EAS | TN | 1.1 | 1.1 | 4.8 | 1.3 | 0.2 | 0.8 | No |
| EAS | TN | 1.2 | 1.1 | 4.7 | 2.4 | 0.3 | 0.5 | Yes |
| EASa | TN | 1.4 | 1.0 | 3.9 | 1.0 | 0.4 | 1.0 | No |
| EAS | FP | 9.5 | 5.7 | 1.7 | 1.5 | 5.6 | 3.8 | Yes |
| CD | TP | 20.4 | 1.0 | 4.7 | 1 | 4.3 | 1 | Yes |
| CD | TP | 113.4 | 100.2 | 10.9 | 8 | 10.4 | 12.5 | Yes |
| CD | TP | 53.6 | 11.9 | 5 | 4.1 | 10.8 | 2.9 | Yes |
| CDa | TP | 131.7 | 15.6 | 6.2 | 2.2 | 21.4 | 7.1 | Yes |
| CD | TP | 20 | 17.7 | 2 | 2.4 | 10.1 | 7.4 | Yes |
| CDa | TP | 49.2 | 6.7 | 5.7 | 3.8 | 8.6 | 1.8 | Yes |
| CD | TP | 87.5 | 1.6 | 2.1 | 1.4 | 40.9 | 1.1 | Yes |
| CD | TP | 9.7 | 1.0 | 0.8 | 0.8 | 11.6 | 1.3 | Yes |
| CD | TP | 18.5 | 12.1 | 3.6 | 2.9 | 5.1 | 4.2 | Yes |
| CDa | TP | 167.3 | 6.2 | 4.7 | 3.9 | 35.5 | 1.6 | Yes |
| CDa | TP | 37.7 | 3.5 | 2.2 | 1.2 | 16.8 | 2.9 | Yes |
| CDa | TP | 36.3 | 26.8 | 10.9 | 2.7 | 3.3 | 9.9 | Yes |
| CD | TP | 9.2 | 1.5 | 1.1 | 1.0 | 8.4 | 1.5 | Yes |
| CDa | TP | 11.3 | 0.9 | 2.9 | 1.0 | 3.9 | 0.9 | Yes |
| CD | TP | 40.9 | 2.8 | 3.4 | 1.9 | 11.9 | 1.5 | Yes |
| CD | TP | 78.2 | 1.2 | 3.1 | 1.7 | 24.9 | 0.7 | No |
| CDa | FN | 1.3 | 1.2 | 1 | 1.6 | 1.3 | 0.8 | No |
| Occ 1 | 1.3 | 1.2 | 13.2 | 1.1 | 0.1 | 1.1 | No | |
| Occ 2a | 2.3 | 1.1 | 4.2 | 0.8 | 0.6 | 1.4 | No | |
| Occ 3a | 1.3 | 1.2 | 1.2 | 1.2 | 1.1 | 1 | Yes | |
| Occ 4a | 1.4 | 1.2 | 1.6 | 1.1 | 0.9 | 1.1 | Yes |
The ipsilateral calculations represent the side with the highest ACTH IPS/P value; contralateral results reflect the opposite side. Discordant results for normalized ACTH/prolactin IPS/P ratio were seen in six of the 29 patients and are shown in bold. BL, Baseline; CD, Cushing's disease; FN, false negative; FP, false positive; Occ, occult; PRL, prolactin; TP, true positive; TN, true negative.
Patients with abnormal venous anatomy.
Prolactin increased after CRH administration by at least 10% in most patients with Cushing's disease (10 of 17) and ectopic ACTH secretion (six of eight). Use of post-CRH prolactin values to calculate the normalized ACTH IPS/P ratios led to indeterminate results in four cases (Supplemental Data).
IPSS venogram and baseline prolactin IPS/P
The venous angiogram reports were reviewed by the interventional radiologist at the time of the procedure (Supplemental Table 2). Eleven patients (38%) had anomalous venous drainage or hypoplastic petrosal sinuses (six with Cushing's disease, one with EAS, and three with occult tumor). Difficult cannulation was documented in four of these cases, and samples were obtained with a smaller gauge catheter or from presumed draining veins. Of the 11 patients with anomalous venous drainage/anatomy, only three had a baseline prolactin IPS/P ratio <1.8 suggestive of inadequate sampling (two with occult Cushing's syndrome and one with EAS and false negative IPSS result). On the other hand, three cases with normal venous anatomy/drainage and no reported difficulty in cannulation had a baseline prolactin IPS/P ratio <1.8 (two with Cushing's disease and one with cyclic EAS and false positive IPSS result) (Supplemental Table 2).
Discussion
IPSS is the most reliable test to differentiate between an ACTH-secreting pituitary adenoma and an ectopic ACTH-secreting tumor (2, 13, 14). False-negative results have been attributed to anomalous venous drainage, hypoplastic petrosal sinuses, technical problems, and/or lack of expertise leading to improper catheterization (2, 3). Prolactin levels during IPSS have been proposed to identify unsuccessful catheterization (7–9), followed by normalized ACTH/prolactin IPS/P ratios to distinguish between Cushing's disease and EAS.
The diagnostic criteria using the prolactin-normalized ACTH ratio have been proposed based on a relatively small number of patients. Findling et al. concluded that a normalized ACTH/prolactin IPS/P ratio below 0.6 was indicative of EAS (n = 5), whereas a ratio higher than 0.8 was suggestive of Cushing's disease (n = 44) (8). They also described three cases with erroneous IPSS results where normalized ACTH/prolactin IPS/P ratios (>1.3) accurately diagnosed Cushing's disease (8).
This report expands the number of patients evaluated with prolactin during IPSS and suggests that the previously proposed normalized ACTH/prolactin IPS/P criterion for the diagnosis of Cushing's disease should be increased from higher than 0.8 to 1.3. We report two patients with occult Cushing's syndrome and one with ectopic ACTH secretion in this intermediate zone who would be falsely diagnosed with Cushing's disease based on a criterion of higher than 0.8. Thus, taken with previous reports, our data suggest that a normalized ACTH/prolactin IPS/P ratio of no more than 0.7 is consistent with EAS and a ratio of 1.3 or higher is consistent with Cushing's disease. Ratios between 0.7 and 1.3 are indeterminate. It will be important to expand the number of patients in future studies to determine the optimal diagnostic threshold.
Measurement of prolactin levels does not appear to be useful or necessary in patients with an ACTH IPS/P ratio suggestive of Cushing's disease. In this series, all but one of these patients was properly diagnosed without prolactin measurement. However, one EAS patient with a positive ACTH IPS/P ratio had a false diagnosis of Cushing's disease with both ACTH and prolactin-normalized ratios. We speculate that this reflects lack of inhibition of the normal corticotropes because of cyclic hypercortisolism, a confounder that would not be corrected by prolactin normalization.
We also evaluated whether the baseline or post-CRH prolactin value is better for normalization (Supplemental Data), because a potential drawback of using the baseline sample to assess adequacy of catheterization is the possibility that the catheter might not be in proper position at the time of post-CRH sampling. Most patients, regardless of the diagnosis, had a greater than 10% increase in IPS prolactin levels after CRH, consistent with previous reports (6, 15, 16). However, the post-CRH values did not improve diagnostic accuracy.
What is the role of venography? Demonstration of retrograde flow in the cavernous sinuses is thought to indicate successful catheterization during IPSS. Improper catheterization can lead to false-negative results, and review of the IPSS venous angiogram is an integral part of the interpretation of IPSS, especially with a negative central to peripheral ACTH gradient. In our series, IPSS venogram results did not always parallel the baseline prolactin IPS/P ratios. Eight of 11 patients who had anomalous venous anatomy or drainage had a baseline prolactin IPS/P ratio higher than 1.8, consistent with successful catheterization, including one with Cushing's disease and a false-negative IPSS. These results are in agreement with those reported in a review of 501 earlier patients with surgically proven Cushing's disease who underwent IPSS. Four patients had false-negative IPSS results presumably due to the presence of hypoplastic or plexiform inferior petrosal sinus on the side of the microadenoma and related anomalous drainage. However, 25 of 100 consecutive control patients with proven Cushing's disease had unilateral or bilateral atrophic inferior petrosal sinuses. Despite this, none had false-negative sampling results (3).
This study's results support the selective use of prolactin measurements in patients with a peak IPS/P ACTH ratio suggestive of EAS. However, prolactin provided helpful additional information only in those cases that also had an abnormal venogram. However, Mulligan et al. (9) reported dissimilar results in two Cushing's disease patients with false-negative or equivocal IPSS results. Both cases had normal IPSS venous anatomy and successful catheterization, but prolactin IPS/P ratios suggested unsuccessful sampling. Therefore, although we found prolactin measurement valuable in cases with negative IPSS results and abnormal venogram, its utility in cases with normal venous anatomy needs further study at less specialized centers.
Previous reports use the IPS side of the highest ACTH ratio to evaluate adequate catheterization. If prolactin is secreted similarly from each IPS, each side might contribute useful information regarding abnormal catheterization, especially if the contralateral side had abnormal drainage that would otherwise reveal a tumor. Applying this strategy in our series, two patients with EAS and negative IPSS had contralateral prolactin IPS/P values, suggesting unsuccessful catheterization, and a resulting normalized ratio in the intermediate zone, possibly consistent with Cushing's disease (Table 3). The ipsilateral data predicted EAS. Although these data do not suggest routine use of the contralateral data, we recommend that future studies consider this possibility.
Results from this study emphasize the importance of suppression of normal corticotropes in the interpretation of IPSS. One patient with surgically proven EAS and cyclic hypercortisolism had a false-positive IPSS result when cortisol levels were normal only 3 wk after documented hypercortisolism. Normalized ACTH/prolactin IPS/P ratio did not correct for this result and suggested Cushing's disease. Thus, confirmation of consistent hypercortisolism remains a prerequisite for IPSS.
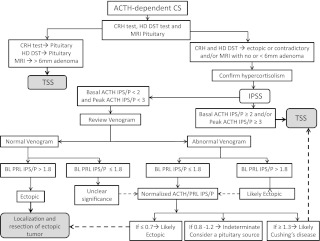
There are some limitations to our study, including its retrospective nature and the small number of patients with erroneous IPSS results. Although 38% of patients in our series had anomalous venous anatomy and drainage, only one had a false-negative result. Larger prospective studies at less specialized centers may be needed to further confirm these findings. In summary, measurement of prolactin levels during IPSS can increase accuracy when the ACTH IPS/P ratio suggests EAS but is not necessary with positive results. Reviewing the IPSS venogram is essential to identify cases with anomalous venous anatomy and drainage. We recommend that blood samples obtained during IPSS should be stored and prolactin levels measured at baseline in both right and left inferior petrosal sinus and peripheral samples if the baseline ACTH IPS/P ratio is less than 2.0 and the peak ACTH IPS/P ratio is less than 3.0 (Fig. 2). This is especially relevant if the IPSS venogram demonstrates anomalous venous anatomy or drainage. A baseline ipsilateral prolactin IPS/P ratio lower than 1.8 suggests inadequate catheterization; the use of contralateral ratios deserves additional evaluation. Prolactin-normalized ACTH IPS/P ratio can then be used to further distinguish between a pituitary and ectopic source of ACTH. Finally, our data suggest that a normalized ratio of 0.7 or below implies EAS, whereas that of 1.3 or higher suggests Cushing's disease, thus extending the zone of uncertainty in the interpretation of this ratio.
Fig. 2.
Suggested algorithm for evaluation of ACTH-dependent Cushing's syndrome. BL, Baseline; CS, Cushing's syndrome; HD DST, high-dose dexamethasone suppression test; PRL, prolactin; IPSS, inferior petrosal sinus sampling; IPS/P, inferior petrosal sinus to peripheral; TSS, transsphenoidal surgery.
Supplementary Material
Acknowledgments
This work was supported by the intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NIH).
Disclosure Summary: On behalf of the NIH, L.K.N. participates in a Cooperative Research and Development Agreement (CRADA) with HRA-Pharma to evaluate mifepristone treatment of ectopic ACTH secretion. H.R. is a consultant to Abbott Laboratories. S.T.S. has nothing to disclose.
Footnotes
- CD
- Cushing's disease
- CV
- coefficient of variation
- EAS
- ectopic ACTH syndrome
- IPS/P
- inferior petrosal sinus to peripheral
- IPSS
- inferior petrosal sinus sampling
- MRI
- magnetic resonance imaging
- TSS
- transsphenoidal surgery
- UFC
- urine free cortisol.
References
- 1. Newell-Price J, Bertagna X, Grossman AB, Nieman LK. 2006. Cushing's syndrome. Lancet 367:1605–1617 [DOI] [PubMed] [Google Scholar]
- 2. Swearingen B, Katznelson L, Miller K, Grinspoon S, Waltman A, Dorer DJ, Klibanski A, Biller BM. 2004. Diagnostic errors after inferior petrosal sinus sampling. J Clin Endocrinol Metab 89:3752–3763 [DOI] [PubMed] [Google Scholar]
- 3. Doppman JL, Chang R, Oldfield EH, Chrousos G, Stratakis CA, Nieman LK. 1999. The hypoplastic inferior petrosal sinus: a potential source of false-negative results in petrosal sampling for Cushing's disease. J Clin Endocrinol Metab 84:533–540 [DOI] [PubMed] [Google Scholar]
- 4. Lopez J, Barcelo B, Lucas T, Salame F, Alameda C, Boronat M, Salto L, Estrada J. 1996. Petrosal sinus sampling for diagnosis of Cushing's disease: evidence of false negative results. Clin Endocrinol (Oxf) 45:147–156 [DOI] [PubMed] [Google Scholar]
- 5. Zovickian J, Oldfield EH, Doppman JL, Cutler GB, Jr, Loriaux DL. 1988. Usefulness of inferior petrosal sinus venous endocrine markers in Cushing's disease. J Neurosurg 68:205–210 [DOI] [PubMed] [Google Scholar]
- 6. Allolio B, Günther RW, Benker G, Reinwein D, Winkelmann W, Schulte HM. 1990. A multihormonal response to corticotrophin-releasing hormone in inferior petrosal sinus blood of patients with Cushing's disease. J Clin Endocrinol Metab 71:1195–1201 [DOI] [PubMed] [Google Scholar]
- 7. McNally PG, Bolia A, Absalom SR, Falconer-Smith J, Howlett TA. 1993. Preliminary observations using endocrine markers of pituitary venous dilution during bilateral simultaneous inferior petrosal sinus catheterization in Cushing's syndrome: is combined CRF and TRH stimulation of value? Clin Endocrinol (Oxf) 39:681–686 [DOI] [PubMed] [Google Scholar]
- 8. Findling JW, Kehoe ME, Raff H. 2004. Identification of patients with Cushing's disease with negative pituitary adrenocorticotropin gradients during inferior petrosal sinus sampling: Prolactin as an index of pituitary venous effluent. J Clin Endocrinol Metab 89:6005–6009 [DOI] [PubMed] [Google Scholar]
- 9. Mulligan GB, Eray E, Faiman C, Gupta M, Pineyro MM, Makdissi A, Suh JH, Masaryk TJ, Prayson R, Weil RJ, Hamrahian AH. 2011. Reduction of false-negative results in inferior petrosal sinus sampling with simultaneous prolactin and corticotrophin measurement. Endocr Pract 17:33–40 [DOI] [PubMed] [Google Scholar]
- 10. Heaney AP, Melmed S. 2004. Molecular targets in pituitary tumors. Nat Rev Cancer 4:285–295 [DOI] [PubMed] [Google Scholar]
- 11. Miller DL, Doppman JL. 1991. Petrosal sinus sampling: Technique and rationale. Radiology 178:37–47 [DOI] [PubMed] [Google Scholar]
- 12. Raff H. 2008. Immulite vs scantibodies IRMA plasma ACTH assay. Clin Chem 54:1409–1410 [DOI] [PubMed] [Google Scholar]
- 13. Oldfield EH, Doppman JL, Nieman LK, Chrousos GP, Miller DL, Katz DA, Cutler GB, Jr, Loriaux DL. 1991. Petrosal sinus sampling with and without corticotrophin-releasing hormone for the differential diagnosis of Cushing's syndrome. N Engl J Med 325:897–905 [DOI] [PubMed] [Google Scholar]
- 14. Findling JW, Kehoe ME, Shaker JL, Raff H. 1991. Routine inferior petrosal sinus sampling in the differential diagnosis of aderenocorticotropin (ACTH)-dependent Cushing's syndrome. J Clin Endocrinol Metab 73:408–413 [DOI] [PubMed] [Google Scholar]
- 15. Loli P, Boccardi E, Branca V, Bramerio M, Barberis M, Losa M, Terreni MT, Lodrini S, Pollo B, Vignati F. 1998. Growth hormone and prolactin responses to corticotrophin-releasing–hormone in patients with Cushing's disease: a paracrine action of the adenomatous corticotroph cells? Clin Endocrinol (Oxf) 49:433–439 [DOI] [PubMed] [Google Scholar]
- 16. Schulte HM, Allolio B, Günther RW, Benker G, Winkelmann W, Ohnhaus EE, Reinwein D. 1988. Selective bilateral and simultaneous catheterization of the inferior petrosal sinus: CRF stimulates prolactin secretion from ACTH-producing microadenomas in Cushing's disease. Clin Endocrinol (Oxf) 28:289–295 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.