Abstract
Context:
Factors that influence long-term weight loss after Roux-en Y gastric bypass (RYGB) surgeries are poorly defined. The melanocortin system plays an important role in regulating energy homeostasis, satiety, and glucose metabolism. Variations of the MC4R comprise the most prevalent monogenetic obesity disorder.
Objective:
The objective of the study was to examine the role of MC4R variants and diabetic status in long-term weight loss after RYGB.
Participants and Methods:
In 1433 extremely obese patients who underwent RYGB, we sequenced for genetic variants of MC4R. We examined the MC4R genotype and its relationship with weight loss profile, and clinical phenotypes accumulated during a 48-month period before and after surgery.
Results:
We found 80 subjects with rare and common variants of MC4R in the RYGB cohort. Among these, 26 and 36 patients carry the I251L and V103I variants, respectively. These common alleles are negatively associated with obesity. Remarkably, after the 12-month presurgery caloric restriction and RYGB, I251L allele carriers lost 9% more weight (∼9 kg) compared with the noncarriers, continued rapid weight loss longer, regained less weight, and had lower presurgery homeostatic model assessment for insulin resistance values. Normoglycemic, I251L allele carriers lost more weight compared with their diabetic and prediabetic counterparts and maintained their weight loss. Among noncarriers, normoglycemic individuals initially lost more weight compared with dysglycemics, but this difference was not maintained in the long term.
Conclusions:
Individuals carrying the I251L common allele are predisposed to better clinical outcome, reduced risk of type 2 diabetes, and better weight loss during diet and surgical interventions. Diabetic status has only a small, short -term effect on weight loss after RYGB.
Obesity poses increased risks of cardiovascular diseases, metabolic diseases, and cancer. It is estimated that life expectancy for an obese individual can be reduced by as much as 20 yr (1). For some individuals, dieting, medicine, and exercise fail to control long-term weight loss. For severely obese individuals and those who do not respond to other treatments, surgical interventions are increasingly recommended.
Gastric banding, Roux-en Y gastric bypass (RYGB), and biliopancreatic diversion are the major bariatric procedures, with the first two accounting for 90% of these surgeries (2). The benefits of surgical interventions with respect to metabolic improvement of diabetes and reduction of risks of other comorbidities in obese individuals are increasingly appreciated (3). However, despite the recent utility of bariatric procedures, 36% of patients who underwent bariatric surgery are unsatisfied with the achieved weight loss (4).
Adiposity is a highly heritable trait (5). Common variants in genes such as FTO are found in large portions of the population. However, each individual variant has a small effect on body weight, and the mechanism for their involvement in metabolism is not fully clear (6). Monogenic obesity disorders are less common; however, the effects of monogenic variants are large and the mechanisms by which they contribute to obesity and metabolic disorder are better understood. Variants in a number of genes have been associated with extreme obesity, including nearly 100 rare variants in the MC4R gene (7). The melanocortin-4-receptor (MC4R) protein responds to the natural ligands α- and β-MSH and agouti gene-related peptide in the hypothalamus to regulate energy homeostasis and satiety (8). In contrast to those associated with obesity, two nonsynonymous common alleles (V103I rs2229616, c.307G > A; and I251L rs5282087, c.751A > C), which occur at a frequency of 1–3% in Caucasian populations, have been associated with a decreased risk for obesity. The I251L variant exhibits strong negative association with childhood and adult severe obesity with a 48% reduction in risk of obesity and 0.7 kg/m2 reduction in body mass index (BMI) (9). A single V103I allele confers a 0.48 kg/m2 reduction in BMI (10, 11). MC4R(I251L) expressed in human embryonic kidney 293 cells have increased intrinsic activity and MC4R(V103I) has reduced sensitivity to the natural antagonist agouti gene-related peptide (12). Although these changes would be consistent with a lean phenotype, the mechanism for negative association of these alleles with obesity is not fully clear.
We and others have reported on the effect of common obesity alleles, including the intronic noncoding MC4R allele, on weight loss after various forms of bariatric surgery (13, 14). Previous studies that have examined the association of nonsynonymous exonic MC4R variants with weight loss in bariatric surgery patients were limited by very small samples sizes, arbitrary grouping of different variants with unknown phenotypes, and short follow-up periods (15–18). Due to these limitations, little was reported on the metabolic status of those patients. These studies also focused on rare variants that are positively associated with weight gain. In the current study, we genotyped 1433 RYGB patients for MC4R mutations and examined the weight loss profiles and metabolic phenotypes of carriers for the I251L and V103I common alleles that are negatively associated with obesity. We found those subjects who carry the I251L alleles show better weight loss during caloric restriction as well as after RYGB surgery. These patients also had better metabolic indicators, including insulin sensitivity. This is the first large-scale, comprehensive, longitudinal study to examine the role of common variants in MC4R and to identify a genetic marker that is associated with improved weight loss after RYGB surgery. In addition, we report on the role of diabetic status in both the short- and long-term weight loss (up to 36 months after surgery).
Materials and Methods
Study populations
A cohort of 1433 patients who underwent primary RYGB was examined in the study. Extensive clinical and survey data have been described (19). A cohort of 451 nonobese patients was selected from the MyCode population at Geisinger Clinic. The MyCode study collects blood samples from patients on a volunteer basis after obtaining consent during internist or family practitioner visits (20). DNA was extracted from these blood samples by the Geisinger Clinic Genomic Core (GCGC) and was used to determine allelic frequency. The MyCode governing board granted access to patients' DNA on a deidentified manner.
The bariatric cohort
The patients underwent a preoperative assessment and preparation program of monthly visits during which a comprehensive set of clinical and laboratory measures were obtained along with blood samples for DNA isolation. The preoperative assessment and preparation period included a diet-induced weight loss target of 10% of body weight. Patients were placed on a hypocaloric diet with a 500- to 700-kcal deficit with behavioral support. Patients who lost less than 3% of their body weight after 4–6 months on the hypocaloric diet were prescribed a liquid diet of approximately1000 calories per day until surgery. After completion of the preoperative program, all patients underwent a RYGB procedure. Height was obtained at the initial visit and weight measurements obtained at each visit. The BMI was calculated as the weight (kilograms) divided by the square of the height (square meters). Patients with pregnancies in the postoperative period were excluded from the analysis. The DNA was obtained from the GCGC in a deidentified manner. All DNA samples were coded by the GCGC before providing them for this study. The clinical variables as well as weight and height measurements for the coded samples were provided to this study by a data broker who was not part of the study and did not have access to the genotyping data.
Genotyping and sequencing
DNA was extracted from blood or tissue samples using standard methods as described previously (19). The single-exon MC4R gene was amplified from genomic DNA using primers: MC4R forward (ATCAATTCAGGGGGACACTG) and MC4R reverse (TGCATGTTCCTATATTGCGTG) using high-fidelity Pfu polymerase. The PCR products were cleaned and analyzed using automated DNA sequencing. Sequencing was performed using two reactions with the following primers: MC4R-seq forward (TGTAGCTCCTTGCTTGCATC) and MC4R-seq reverse (GGCCATCAGGAACATGTGGA) to cover the entire coding region of MC4R. Mutation Surveyor software (GeneSoft, State College, PA) was used to detect base changes in the coding region. A second independent PCR reaction was performed on all identified mutants. The repeat samples were sequenced and the data were analyzed using Mutation Surveyor and verified manually.
Curve fitting and statistical analysis
Body weight is expressed as percentage of body weight in kilograms at surgery or as percentage excess body weight (EBW) loss. Body weights at the time of surgery are set at 100% and weights at other times are set accordingly. The EBW is defined as the amount of excess weight above an idealized BMI of 25 kg/m2. For example, someone who is 2 m tall (78.7 in.) and weights 200 kg (∼440 lb) has a BMI of 50 kg/m2. This person is therefore initially 100 kg over the BMI of 25 kg/m2. Loss of 10 kg of weight constitutes 10% loss of initial EBW. Body weight curves were best fit using the four-parameter logistic equation (sigmoidal curve with variable slope):
Statistical differences between the top, bottom, and slope of the curves were compared via GraphPad global fit using an F test (GraphPad Inc., San Diego, CA). The months to the weight nadir calculation (Table 1) is defined as the time at which the change in the trajectory of the curve fits is 0.1 or less.
Table 1.
Parameters for pre- and postsurgery weight loss profiles, duration of weight loss, and late-onset weight regain (means ± sem)
| Reference | I251L | V103I | P value (one way ANOVA) | |
|---|---|---|---|---|
| Weight at initial visit (top plateau) (%)a | 108 ± 0.1 | 109 ± 0.7 | 107 ± 0.7 | 0.2 |
| Weight at nadir (bottom plateau) (%)a | 72 ± 0.1 | 65 ± 0.5c | 73 ± 0.6 | <0.0001 |
| Total weight loss (% kilograms at surgery) | 36 | 44 | 34 | |
| Months to weight nadira | 10 | 13 | 9 | |
| Slope from weight nadir (9–13 to 36 months)b | 0.018 | −0.072 | 0.158 | 0.4 |
Differences were determined using one-way ANOVA.
Parametes are from Hill regression global-fitting parameters.
Parametes are from linear fit.
Significance levels after Bonferroni's multiple comparisons post hoc, P < 0.0001 vs. reference.
Subjects were divided based on the MC4R allele. Those carrying the ancestral allele, or no variants, are referred to as reference or noncarrier. Mean values for the clinical parameters of the reference and I251L allele carriers were analyzed using an unpaired t test between genotypes or between groups of the same genotype with different diabetic status, using Welch's correction for unequal variances whenever necessary (Prism 5; GraphPad). Subjects were subdivided into normoglycemic [glycated hemoglobin (HbA1c) <6.0 and not taking any diabetic medications] or dysglycemic (HbA1c ≥6.0 or taking one of four diabetic medications: biguanides, sulfonylureas, insulin, and insulin sensitizing agents) groups. For 8 h fasting plasma glucose and fasting plasma insulin, patients on diabetes medications were excluded. Baseline homeostatic model assessment for insulin resistance (HOMAIR) was calculated as HOMAIR = (fasting plasma insulin × fasting plasma glucose)/22.5. For liver and lipid panel indices, subjects who were on cholesterol absorption medicines (statins) were excluded from analyses.
For the bivariate analysis of mutation rates, a Fisher's exact test was used.
Results
MC4R genotyping
We studied a large RYGB cohort from a single clinic (19) approved by the Geisinger Clinic Institutional Review Board. All patients provided written informed consent. This cohort of 1433 patients was 99% Caucasian and 80% female, with a mean age 45.8 yr and mean BMI of 46.6 kg/m2 (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). Approximately one third of these patients failed to achieve a target of 60% excess body weight loss at 24 months after surgery (Supplemental Fig. 1).
To address the relationship between the MC4R variants and weight loss, we sequenced the coding region of the MC4R gene in each patient. Thirty-six patients (2.5%) were carriers of the V103I allele and 26 (1.8%) were carriers of the I251L allele (Supplemental Table 2). The allele frequencies in 451 lean (mean BMI 23.1 kg/m2); sex- and age-matched controls were 4.0 and 1.3% for V103I and I251L, respectively. The differences in the frequency of V103I and I251L in the obese and lean cohorts were not statistically significant (P = 0.1 and P = 0.7, respectively; Fisher's exact test), consistent with previous single population reports (9). Eighteen individuals (1.3%) in the obese group carried rare MC4R variants. Rare MC4R variants have exhibited vast differences in functional activity; from no binding or functional activity to as much as 40-fold increase in functional activity (12), depending on the mutation and the assay used as well as the ligand probe. Because different rare variants can have unique effects on MC4R function with distinct clinical phenotypes (21), the 18 patients carrying any of these rare variants were excluded from the following analysis to eliminate potential confounding factors.
Baseline and post-RYGB surgery body weight profiles
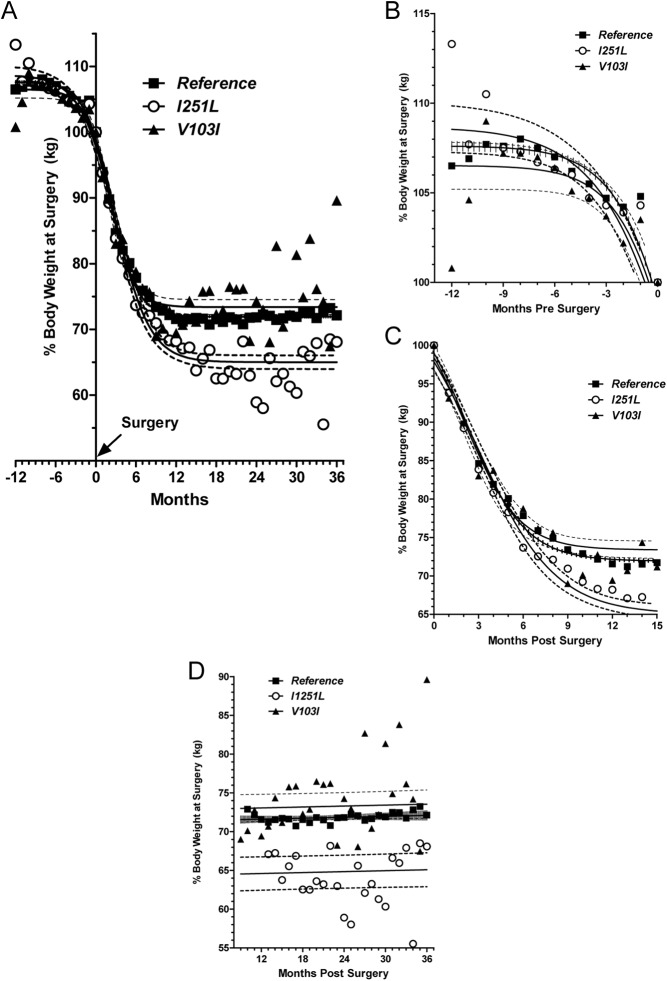
Stratification of the RYGB cohort by I251L and V103I genotype showed no differences in age, sex, or BMI at the time of surgery. Cohort statistics at time of surgery is presented in Supplemental Table 3. The lack of difference in BMI among the reference and I251L and V103I carriers in the RYGB cohort is expected, given the selection criteria for surgery and complex genetic and environmental factors that lead to severe obesity. Change in body weight as a function of time before and after surgery is shown in Fig. 1A, with weight displayed as percentage of kilograms at surgery. Nonlinear regression analysis was used to fit data for reference, I251L, and V103I allele carriers (Hill plot curve fit R2 values were 0.67, 0.89, and 0.73, respectively). Analysis of body weight change expressed as percentage BMI (data not shown) or percentage excess body weight loss (Supplemental Fig. 2) produced similar results. Figure 1A shows three distinct phases of weight change over time. The first phase included a 12-month presurgery preparation period that reflects weight loss due to dietary restriction (required for all RYGB cohort patients before surgery). The second phase covered the time from RYGB surgery to approximately 9–13 months after surgery and reflected rapid weight loss caused by the dramatic, immediate effects of surgery. The third phase was the period beginning at approximately 13 months after surgery and reflected the new homeostatic set point that is established. Figure 1, B–D, shows details of weight loss data for each phase.
Fig. 1.
Weight loss in patients undergoing RYGB stratified by MC4R genotype. Weight at the time of surgery for each patient was set at 100%, and weights at other times are expressed accordingly. Individual points represent the mean percentage weight at surgery for patients for whom data were collected at the given time point. Solid lines represent the fitted curves. The dashed lines and hash marks represent 95% confidence intervals. A, Hill plot of weight loss data expressed as percentage of weight at surgery for 12 months before surgery up to 36 months after surgery. B, Weight loss 12 months preceding surgery. Dietary restriction during this period resulted in weight loss in patients in all groups, with the I251L carriers losing more weight. C, Weight loss in the 13 months immediately after surgery. The strong effects of surgery resulted in rapid weight loss; however, the response in the I251L allele carriers appears to be slightly faster. D, Because the Hill plot does not reflect the rebound from maximal weight loss, the weight loss from weight nadir out to 36 months was fitted using linear regression. I251L allele carriers showed a slight negative slope, indicating that they continued to lose weight during this period.
During the presurgery dietary restriction phase (Fig. 1B), the reference and V103I allele carriers lost approximately 7% body weight (3.0 kg/m2), whereas the carriers of the I251L allele lost 9% body weight (3.7 kg/m2). Figure 1C shows the weight loss immediately after surgery. During the first 6 months after surgery, the weight loss trajectories for the three groups were indistinguishable (no difference in slopes of the Hill analysis). Beginning at approximately 6 months after surgery, the weight loss curves for the V103I, reference, and I125L groups diverged. At 13 months after surgery, weight dropped to 73, 72, and 65% of the weight at surgery for the V103I, reference, and I251L groups, respectively (one way ANOVA, P < 0.0001, reference vs. I251L, Bonferroni post hoc test). This equates to a mean loss of 15.8, 16.2, and 18.8 kg/m2 for the V103I, reference, and I251L groups, respectively (P = 0.0001, F test). Furthermore, reference and V103I carriers reached maximum body weight loss at 10 and 9 months, respectively, whereas I251L carriers continued to lose weight until 13 months after surgery. The weight loss curve fit data are summarized in Table 1.
The data in Fig. 1A identify a plateau in weight loss between approximately 11.5 and 36 months after surgery, which suggests a new homeostatic steady state. Relative weight loss during this period was analyzed by linear regression (Fig. 1D). Weights for the reference group remained constant. The weight profile for the V103I allele carriers showed a positive slope, indicating weight regain. The profile for I251L carriers showed a negative slope, indicating continued weight loss (Table 1).
Effects of MC4R(I251L) allele on glucose and insulin metabolism
Mice lacking Mc4r and obese individuals with monogenic MC4R obesity are hyperinsulinemic. Because the I251L allele seems to improve weight loss outcomes, we asked whether glucose metabolism is also affected by the I251L variants. HbA1c levels were not significantly different between the allele carriers and the noncarriers. Baseline HOMAIR was used to determine insulin resistance between the two groups. HOMAIR values were significantly lower in I251L allele carriers compared with noncarriers (Fig. 2). Accordingly, both baseline glucose and insulin levels were also lower in I251L carriers. Insulin resistance is known to be associated with obesity. Insulin sensitivity in I251L allele carriers is consistent with a protective effect of this allele on metabolic changes associated with obesity. Average baseline HOMAIR value for V103I carriers [excluding those on type 2 diabetes (T2D) therapy] was 5.4, which was similar to the reference group. Diabetes had no effect on weight loss outcomes among the V103I carriers.
Fig. 2.
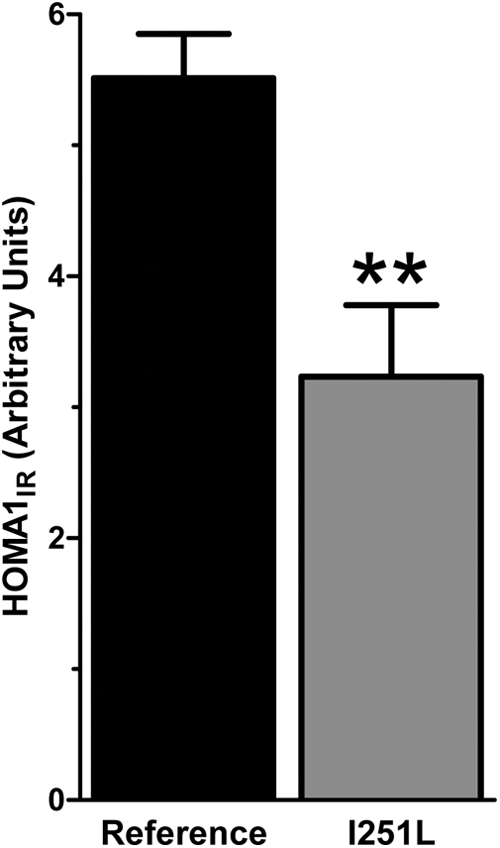
Presurgery HOMA1IR values of reference and I251L allele carriers. HOMAIR values were calculated with those taking any medications for T2D excluded from analysis (reference, n = 598; I251L, n = 15; **, P = 0.01, unpaired t test using Welch's correction for unequal variances).
Effects of T2D on postsurgery weight loss
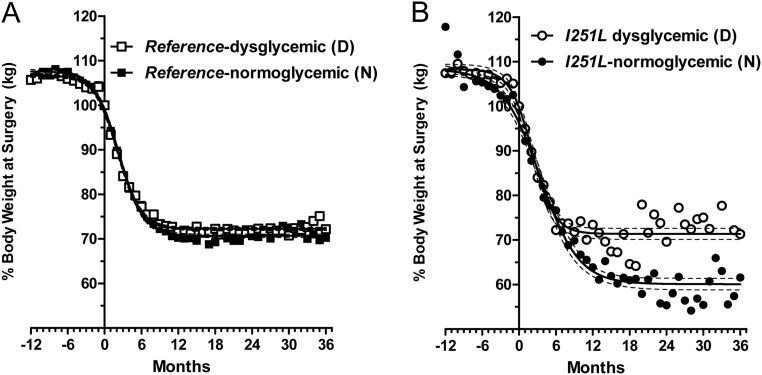
Although the effects of RYGB on resolution of diabetes have attracted significant attention (22), less is known about the effect of diabetic status on weight loss outcomes. Individuals diagnosed with T2D were reported to perform worse compared with nondiabetics 1 yr after gastric bypass, although long-term data were not reported (23). Given the difference in presurgery HOMAIR values among the two groups, we asked whether the presurgery dysglycemia affected weight loss outcomes. We stratified the patients based on the MC4R genotype. We defined diabetics based on presurgery HbA1c levels and use of diabetes therapy. Specifically, subjects with HbA1c of 6.0 or greater or subjects who were on any diabetes therapy were classified as dysglycemic. Given that the patient population is obese and at higher risk for diabetes, we included those with HbA1c levels of 6.0% to encompass prediabetics. Subjects who were not on diabetes therapy and with HbA1c less than 6.0 were classified as normoglycemic. The number of dysglycemic subjects was 4% higher among the reference than I251L carriers (Supplemental Table 4). Weight loss profiles between dysglycemics and normoglycemics of both groups were predictable in that dysglycemics lost less weight than their normoglycemic counterparts (Fig. 3). All groups had fairly similar starting points as indicated by the top plateau of the sigmoidal curves. Among the reference group, maximum weight loss of dysglycemic subjects was approximately 2% less than normoglycemic subjects (Fig. 3A). Among the I251L carriers, dysglycemic subjects lost 13% less weight than normoglycemics (Fig. 3B). The weight loss curve fit data are summarized in Table 2. Overall, normoglycemic (nondiabetic) I251L carriers (I251L N) lost the most weight, 50% of their weight from their initial visit to the nadir of their weight loss (Table 2). Individuals with dysglycemia or diabetes, on the other hand, performed the worst, regardless of genotype (Supplemental Fig. 3). The normoglycemic (nondiabetic) reference subjects (reference N) did not maintain a long-term weight loss advantage over their dysglycemic counterparts (Fig. 3A). By contrast, weight profiles for the normoglycemic I251L carriers showed no rebound (Fig. 3B).
Fig. 3.
Post-RYGB weight loss stratified by genotype and diabetic status. Weight loss reported as percentage weight at surgery is shown for the different groups. Longitudinal weight loss for noncarriers (A) and I251L carriers (B), divided into dysglycemic [diabetic (D)] and normoglycemic [nondiabetic (N)] subgroups. The nondiabetic I251L carriers lost significantly more weight compared with all other groups. Summary of the values and statistics for the different groups are presented in Table 2.
Table 2.
Parameters for pre- and postsurgery weight loss profiles (means ± sem are from Hill regression global fitting parameters) of each genotype stratified into dysglycemic (D) and normoglycemic (N)
| Reference (N) | Reference (D) | I251L (N) | I251L (D) | |
|---|---|---|---|---|
| Weight at initial visit (top plateau) (%) | 108 ± 0.2 | 107 ± 0.1a | 110 ± 1 | 108 ± 0.6 |
| Weight at nadir (bottom plateau) (%) | 71 ± 0.2 | 72 ± 0.1a | 60 ± 0.8b | 71 ± 0.5a |
| Total weight loss (%) | 37 | 35 | 50 | 37 |
Within-genotype differences of N and D groups were determined by t test with Welch's correction for unequal variances when necessary (P < 0.0001 vs. corresponding normoglycemic group).
Between-genotype differences were determined using an unpaired t test with Welch's correction for unequal variances when necessary (P < 0.0001 vs. reference).
A summary of different clinical variables between the two groups as stratified by genotypes and subdivided by diabetic status are presented in Table 3 and Supplemental Table 5. These data show an overall trend for better metabolic status for normoglycemics compared with the dysglycemics within each group. The differences in clinical parameters associated with glucose metabolism among individuals carrying the I251L allele are also apparent and show a trend toward improvement (Table 3).
Table 3.
Clinical parameters at time of surgery: mean ± sem (n)
| Reference | I251L | P value | |
|---|---|---|---|
| HbA1c (%) | |||
| Total | 6.4 ± 0.04 (1209) | 6.5 ± 0.3 (24) | 0.7 |
| Normoglycemica | 5.5 ± 0.01 (492) | 5.6 ± 0.07 (10) | 0.2 |
| Dysglycemica | 6.9 ± 0.05 (747)b | 7.1 ± 0.4 (14)c | 0.7 |
| Glucose (mg/dl)d | |||
| Total | 92 ± 1 (611) | 87 ± 3 (15) | 0.1 |
| Normoglycemic | 90 ± 0.6 (462) | 86 ± 4 (10) | 0.3 |
| Dysglycemic | 101 ± 2 (149)b | 90 ± 2 (5)e | 0.006 |
| Insulin (μIU/ml)d | |||
| Total | 22 ± 1 (598) | 15 ± 2 (16)f | 0.01 |
| Normoglycemic | 20 ± 1 (453) | 13 ± 2 (10)f | 0.04 |
| Dysglycemic | 28 ± 3 (145)g | 18 ± 5 (5) | 0.09 |
| HOMAIR (arbitrary units)d | |||
| Total | 5.4 ± 0.3 (598) | 3.3 ± 0.6 (15)f | 0.01 |
| Normoglycemic | 4.7 ± 0.3 (453) | 2.9 ± 0.7 (10)f | 0.049 |
| Dysglycemic | 7.5 ± 1.2 (145)g | 3.9 ± 1 (5)f | 0.03 |
| REE (kcal/d) | |||
| Total | 2506 ± 28 (676) | 2481 ± 228 (12) | 0.9 |
| Normoglycemic | 2429 ± 48 (257) | 2724 ± 297 (7) | 0.3 |
| Dysglycemic | 2553 ± 34 (419)g | 2140 ± 326 (5) | 0.2 |
REE, Resting energy expenditure.
Subjects were stratified by genotype and subdivided into normoglycemic (HbA1c <6.0 and not taking any diabetic medications) or dysglycemic (HbA1c ≥6.0 or taking one of four diabetic medications) groups.
Unpaired t test with Welch's corrections for unequal variances whenever necessary was used to compare within genotype between dysglycemic and normoglycemic groups, P < 0.001 vs. normoglycemic group of the same genotype.
Unpaired t test with Welch's corrections for unequal variances whenever necessary was used to compare within genotype between dysglycemic and normoglycemic groups, P < 0.01.
Subjects taking diabetic medicines (biguanides, sulfonylureas, insulin, and insulin sensitizing agents) are excluded from calculations.
Unpaired t test between genotypes with Welch's correction for unequal variances whenever necessary, P < 0.01 vs. corresponding reference group.
Unpaired t test between genotypes with Welch's correction for unequal variances whenever necessary, P < 0.050 vs. corresponding reference group.
Unpaired t test with Welch's corrections for unequal variances whenever necessary was used to compare within genotype between dysglycemic and normoglycemic groups, P < 0.05 vs. normoglycemic group of the same genotype.
Discussion
Insufficient weight loss after gastric bypass surgery is a significant clinical problem. Our data in a cohort of 1433 RYGB patients followed up for 48 months before and after surgery showed that patients carrying the MC4R(I251L) common allele are predisposed to better weight loss during diet and surgical interventions. During the presurgery dietary restriction period, I251L allele carriers lost 1.9 kg (∼2%) more weight than the MC4R (V103I) carriers and noncarriers. During the rapid weight loss period after surgery, I251L carriers lost 7.2 kg more weight (∼7% initial weight) and continued to lose weight 3–4 months longer than the latter two groups. Long-term postsurgery weight regain was not evident with the I251L carriers at 36 months after surgery. Therefore, I251L allele carriers can expect to lose 19 BMI units of weight with little or no rebound 3 yr later. In addition to more favorable weight loss, the I251L allele carriers had better insulin resistance and lower liver enzyme profiles than the noncarriers. These factors are consistent with a protective effect of this allele on metabolic changes associated with obesity.
Obesity is a complex disease in which many gene variants and environmental factors contribute to body weight. Obese individuals likely carry variants in genes that cumulatively overcome the inhibitory influences of lean alleles, e.g. the I125L, on steady-state body weight. Therefore, individuals carrying the I251L or V103I allele alone would not necessarily have a lean phenotype; however, 2% of individuals who carry either allele are protected from obesity (9). The observation that V103I allele carriers did not lose as much weight as I251L carriers may be due to a more modest protective effect of the I103 allele (9, 10).
Previous studies that examined the association of MC4R variants with weight loss in bariatric surgery patients examined the association of rare variants on weight loss. One recent study, for example, examined weight loss in 92 RYGB patients of which four had rare MC4R mutations (18). However, that study did not include a caloric restriction period, reported only 12 months of follow-up data, and therefore did not address the multiphasic response to RYGB. The strong short-term weight loss effects of the surgery may also mask the effects of genotypic differences that are apparent only after a new homeostatic steady state is achieved.
We find improvement in metabolic status among the I251L carriers after RYGB surgery. In addition, reference and I251L carriers with better presurgery HbA1c levels lose significantly more weight. This is especially pronounced and long lasting among the I251L carriers. Better weight loss by nondiabetics after bariatric surgery compared with diabetics have been reported; however, the differences were reported at 1-yr follow-up, which could overlook some of the rebound weight gain observed in the majority of patients (23). We found a 2% increase in weight loss at the nadir among normoglycemic subjects with the reference MC4R allele (Fig. 3A); however, this difference dissipates after the patients have rebound weight gain. Among the I251L allele carriers, normoglycemics lost 13% more weight compared with those with dysglycemia (Fig. 3B) and maintained the weight loss out to 3 yr. Because carrying the I251L allele improves both metabolic status and weight loss outcomes, it is possible that the better metabolic status is the prerequisite for improved weight loss outcome. Alternatively, I251L could influence both factors independently. Given that T2D is likely influenced by several genetic and environmental factors, the influence of I251L alone on diabetic status may be masked by additional factors. Nevertheless, these data point to a critical role for I251L in determining the metabolic status of these patients and their relative weight loss. What remains unclear is how these factors interact to influence postsurgical clinical outcomes.
It is noteworthy that most gastric bypass surgery patients show significant improvements in insulin levels before losing considerable amounts of weight. Mc4r knockout mice develop hyperinsulinemia before they gain significant weight (24). Finally, targeted reexpression of MC4R in specific cholinergic neurons of Mc4r-null mice attenuates hyperinsulinemia but only modestly reduces the body weight and hyperphagia (25). On the other hand, reexpression of MC4R in the hypothalamus in Mc4r-null mice attenuates weight gain by approximately 60% without affecting metabolic status (26). Together these data indicate that although both weight loss and metabolic statue are regulated by MC4R function, these effects originate from different regions in the nervous system. In addition, MC4R influence on weight loss and insulin occur at different time frames. The fact that having the I251L allele improved both metabolic status and weight loss suggests a role of MC4R in both processes; however, any interaction among these pathways remains to be determined. That long-term weight loss was not dependent on diabetic status argues that the effects of I251L are independent of the T2D factors.
The mechanism by which the I251L variant leads to improved outcome after RYGB is not known. Impaired MC4R function is associated with increased food intake and decreased energy metabolism in both humans and mice; therefore, we would anticipate people carrying the I251L allele may have increased energy expenditure and reduced food intake, giving rise to better weight loss. However, the resting energy expenditure for the I251L carriers was similar to those carrying the reference allele (Table 3). At the cellular level, we hypothesize that the change in MC4R structure and function alters downstream signaling that affects eating behavior. Xiang et al. (12) reported a modest increase in intrinsic signaling by MC4R(I251L) to the cAMP pathway when expressed in human embryonic kidney cells. Increased basal MC4R activity would be consistent with constitutive satiety signaling in the hypothalamus; however, additional studies are required to determine the exact mechanism by which this mutation alters cellular signaling that would lead to reduced food intake and/or increased energy expenditure. Although the role of MC4R in food intake and energy metabolism has been firmly established, other G protein-dependent pathways may also play roles in adipogenesis, metabolism, and eating behavior. It is not clear whether genetic variants in these pathways also play roles in the developments of obesity and eating disorders. Identifying these pathways would provide new insights into the neural mechanisms that control eating behavior and provide targets for novel drugs to treat obesity.
Supplementary Material
Acknowledgments
We acknowledge the extraordinary cooperation and support of the patients enrolled in the Geisinger Bariatric surgery program, without which these studies would not have been possible. We thank Dr. Xin Chu and the Geisinger Clinic Genomic Core for preparing the genomic DNA. We also thank G. Craig Wood and Ryan Colonie, who served as data brokers, and Amanda Styer, who carried out some of the preliminary genotyping experiments. We also thank Bryn Moore for helpful discussion and critical review of the manuscript.
This work was supported by funds from the Geisinger Clinic, the Weis Center for Research, the Geisinger Obesity Research Institute, and Grant DK072488 (to G.S.G. and C.D.S.) and Grant DK088231 (to G.S.G. and C.D.S.) from the National Institutes of Health.
Disclosure Summary: The authors declare no conflict of interest.
Footnotes
- BMI
- Body mass index
- EBW
- excess body weight
- GCGC
- Geisinger Clinic Genomic Core
- HbA1c
- glycated hemoglobin
- HOMAIR
- homeostatic model assessment for insulin resistance
- MC4R
- melanocortin-4-receptor
- RYGB
- Roux-en Y gastric bypass
- T2D
- type 2 diabetes.
References
- 1. Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. 2003. Years of life lost due to obesity. JAMA 289:187–193 [DOI] [PubMed] [Google Scholar]
- 2. Robinson MK. 2009. Surgical treatment of obesity—weighing the facts. N Engl J Med 361:520–521 [DOI] [PubMed] [Google Scholar]
- 3. Goldfine AB, Shoelson SE, Aguirre V. 2009. Expansion and contraction: treating diabetes with bariatric surgery. Nat Med 15:616–617 [DOI] [PubMed] [Google Scholar]
- 4. Kruseman M, Leimgruber A, Zumbach F, Golay A. 2010. Dietary, weight, and psychological changes among patients with obesity, 8 years after gastric bypass. J Am Diet Assoc 110:527–534 [DOI] [PubMed] [Google Scholar]
- 5. Barsh GS, Farooqi IS, O'rahilly S. 2000. Genetics of body-weight regulation. Nature 404:644–651 [DOI] [PubMed] [Google Scholar]
- 6. Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, Shields B, Harries LW, Barrett JC, Ellard S, Groves CJ, Knight B, Patch AM, Ness AR, Ebrahim S, Lawlor DA, Ring SM, Ben-Shlomo Y, Jarvelin MR, Sovio U, Bennett AJ, Melzer D, Ferrucci L, Loos RJ, Barroso I, Wareham NJ, Karpe F, Owen KR, Cardon LR, Walker M, Hitman GA, Palmer CN, Doney AS, Morris AD, Smith GD, Hattersley AT, McCarthy MI. 2007. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316:889–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tao YX. 2010. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev 31:506–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cone RD. 2005. Anatomy and regulation of the central melanocortin system. Nat Neurosci 8:571–578 [DOI] [PubMed] [Google Scholar]
- 9. Stutzmann F, Vatin V, Cauchi S, Morandi A, Jouret B, Landt O, Tounian P, Levy-Marchal C, Buzzetti R, Pinelli L, Balkau B, Horber F, Bougnères P, Froguel P, Meyre D. 2007. Non-synonymous polymorphisms in melanocortin-4 receptor protect against obesity: the two facets of a janus obesity gene. Hum Mol Genet 16:1837–1844 [DOI] [PubMed] [Google Scholar]
- 10. Geller F, Reichwald K, Dempfle A, Illig T, Vollmert C, Herpertz S, Siffert W, Platzer M, Hess C, Gudermann T, Biebermann H, Wichmann HE, Schäfer H, Hinney A, Hebebrand J. 2004. Melanocortin-4 receptor gene variant I103 is negatively associated with obesity. Am J Hum Genet 74:572–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang D, Ma J, Zhang S, Hinney A, Hebebrand J, Wang Y, Wang HJ. 2010. Association of the MC4R V103I polymorphism with obesity: a Chinese case-control study and meta-analysis in 55,195 individuals. Obesity (Silver Spring) 18:573–579 [DOI] [PubMed] [Google Scholar]
- 12. Xiang Z, Litherland SA, Sorensen NB, Proneth B, Wood MS, Shaw AM, Millard WJ, Haskell-Luevano C. 2006. Pharmacological characterization of 40 human melanocortin-4 receptor polymorphisms with the endogenous proopiomelanocortin-derived agonists and the agouti-related protein (AGRP) antagonist. Biochemistry 45:7277–7288 [DOI] [PubMed] [Google Scholar]
- 13. Still CD, Wood GC, Chu X, Erdman R, Manney CH, Benotti PN, Petrick AT, Strodel WE, Mirshahi UL, Mirshahi T, Carey DJ, Gerhard GS. 2011. High allelic burden of four obesity SNPs is associated with poorer weight loss outcomes following gastric bypass surgery. Obesity (Silver Spring) 19:1676–1683 [DOI] [PubMed] [Google Scholar]
- 14. Sarzynski MA, Jacobson P, Rankinen T, Carlsson B, Sjostrom L, Bouchard C, Carlsson LM. 2011. Associations of markers in 11 obesity candidate genes with maximal weight loss and weight regain in the SOS bariatric surgery cases. Int J Obes (Lond) 35:676–683 [DOI] [PubMed] [Google Scholar]
- 15. Potoczna N, Steffen R, Horber FF. 2006. Surgical procedures for severely obese patients: impact and long-term results. Internist (Berl) 47:150–158 [DOI] [PubMed] [Google Scholar]
- 16. Peterli R, Peters T, von Flüe M, Hoch M, Eberle AN. 2006. Melanocortin-4 receptor gene and complications after gastric banding. Obes Surg 16:189–195 [DOI] [PubMed] [Google Scholar]
- 17. Aslan IR, Ranadive SA, Ersoy BA, Rogers SJ, Lustig RH, Vaisse C. 2011. Bariatric surgery in a patient with complete MC4R deficiency. Int J Obes (Lond) 35:457–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aslan IR, Campos GM, Calton MA, Evans DS, Merriman RB, Vaisse C. 2011. Weight loss after roux-en-Y gastric bypass in obese patients heterozygous for MC4R mutations. Obes Surg 21:930–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chu X, Erdman R, Susek M, Gerst H, Derr K, Al-Agha M, Wood GC, Hartman C, Yeager S, Blosky MA, Krum W, Stewart WF, Carey D, Benotti P, Still CD, Gerhard GS. 2008. Association of morbid obesity with FTO and INSIG2 allelic variants. Arch Surg 143:235–240; discussion 241 [DOI] [PubMed] [Google Scholar]
- 20. Gerhard GS, Langer RD, Carey DJ, Stewart WF. 2010. Electronic medical records in genomic medicine practice and research. In: Ginsburg GS, Willard HF. eds. Genomic and personalized medicine. San Diego: Academic Press/Elsevier; 142–151 [Google Scholar]
- 21. Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S. 2003. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med 348:1085–1095 [DOI] [PubMed] [Google Scholar]
- 22. Gill RS, Sharma AM, Al-Adra DP, Birch DW, Karmali S. 2011. The impact of bariatric surgery in patients with type-2 diabetes mellitus. Curr Diabetes Rev 7:185–189 [DOI] [PubMed] [Google Scholar]
- 23. Carbonell AM, Wolfe LG, Meador JG, Sugerman HJ, Kellum JM, Maher JW. 2008. Does diabetes affect weight loss after gastric bypass? Surg Obes Relat Dis 4:441–444 [DOI] [PubMed] [Google Scholar]
- 24. Fan W, Dinulescu DM, Butler AA, Zhou J, Marks DL, Cone RD. 2000. The central melanocortin system can directly regulate serum insulin levels. Endocrinology 141:3072–3079 [DOI] [PubMed] [Google Scholar]
- 25. Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB, Elmquist JK. 2011. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab 13:195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. 2005. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123:493–505 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.