Abstract
Context:
Adrenal tumors have a prevalence of around 2% in the general population. Adrenocortical carcinoma (ACC) is rare but accounts for 2–11% of incidentally discovered adrenal masses. Differentiating ACC from adrenocortical adenoma (ACA) represents a diagnostic challenge in patients with adrenal incidentalomas, with tumor size, imaging, and even histology all providing unsatisfactory predictive values.
Objective:
Here we developed a novel steroid metabolomic approach, mass spectrometry-based steroid profiling followed by machine learning analysis, and examined its diagnostic value for the detection of adrenal malignancy.
Design:
Quantification of 32 distinct adrenal derived steroids was carried out by gas chromatography/mass spectrometry in 24-h urine samples from 102 ACA patients (age range 19–84 yr) and 45 ACC patients (20–80 yr). Underlying diagnosis was ascertained by histology and metastasis in ACC and by clinical follow-up [median duration 52 (range 26–201) months] without evidence of metastasis in ACA. Steroid excretion data were subjected to generalized matrix learning vector quantization (GMLVQ) to identify the most discriminative steroids.
Results:
Steroid profiling revealed a pattern of predominantly immature, early-stage steroidogenesis in ACC. GMLVQ analysis identified a subset of nine steroids that performed best in differentiating ACA from ACC. Receiver-operating characteristics analysis of GMLVQ results demonstrated sensitivity = specificity = 90% (area under the curve = 0.97) employing all 32 steroids and sensitivity = specificity = 88% (area under the curve = 0.96) when using only the nine most differentiating markers.
Conclusions:
Urine steroid metabolomics is a novel, highly sensitive, and specific biomarker tool for discriminating benign from malignant adrenal tumors, with obvious promise for the diagnostic work-up of patients with adrenal incidentalomas.
Adrenal tumors are common, with a reported prevalence of around 2% in the general population, based on computed tomography (CT) and autopsy series (1–3). Prevalence increases with age, with 1% of 40-yr-olds and 7% of 70-yr-olds harboring an adrenal tumor (1–3). In an aging society, coupled with the widespread use of abdominal imaging, healthcare professionals are confronted with an increasingly large number of patients with incidentally discovered adrenal masses, i.e. adrenal incidentalomas, that require diagnostic work-up but with an optimal approach yet to be determined (1, 2, 4–7). Two key questions that need to be addressed in such a patient are, first, whether the tumor autonomously secretes hormones that could have a detrimental effect on health and, second, whether the adrenal mass is benign or malignant.
Results of previous studies indicate that up to 25% of adrenal nodules are hormonally active (1, 2, 8), which may require surgical or medical intervention. Exclusion of catecholamine excess caused by a pheochromocytoma arising from the adrenal medulla is a mandatory part of the diagnostic work-up (1, 2). Overt and autonomous cortisol and aldosterone secretion result in Cushing's syndrome and primary hyperaldosteronism, respectively; both may lead to premature cardiovascular death if left untreated. Overproduction of adrenal androgen precursors, dehydroepiandrosterone (DHEA) and androstenedione, is rarer but may pose diagnostic problems, in particular in women with a relatively small adrenal mass and a phenotype resembling polycystic ovary syndrome. Additionally, adrenal tumors are frequently associated with lower levels of autonomous cortisol secretion in patients lacking overt clinical features of Cushing's syndrome. There remains ongoing debate around the optimal treatment for these patients with mild or subclinical Cushing's syndrome (9–11).
Adrenocortical carcinoma (ACC) is a rare tumor but accounts for 2–11% of incidentally discovered adrenal masses undergoing diagnostic work-up in specialist referral centers (1, 2, 8). ACC carries a poor prognosis, and cure can be achieved only by complete surgical removal without capsule violation (12, 13). Even when basing the histopathological assessment on the entire tumor specimen, the differentiation between benign and malignant lesions represents a major diagnostic challenge (14, 15); molecular markers that may reliably indicate malignancy are being developed but require surgical tumor removal (16–18). The risk of malignancy increases with the diameter of the adrenal mass (1, 2, 8). However, size alone is a poor predictor, with, for example, sensitivity and specificity rates of 81 and 61%, respectively, for a 4-cm cutoff (19). Imaging provides some guidance, in particular tumor density assessed by CT and magnetic resonance imaging-based chemical shift analysis (12, 19). However, inhomogeneous tumors are difficult to assess, and although sensitivities of up to 100% have been reported in some imaging studies, specificity is generally poor (1, 2, 7, 19). [18F]Fluorodeoxyglucose positron emission tomography has been reported to be both highly sensitive (97%) and specific (91%) in differentiating benign from malignant adrenal tumors (20) but is expensive and not always available, thus does not lend itself for routine screening.
Although 60–70% of ACC are biochemically found to overproduce hormones, this is not clinically apparent in many cases. This may be explained by relatively inefficient steroid production in ACC, manifesting with increased steroid precursors, due to a dedifferentiated and thus incomplete pattern of steroidogenic enzyme expression. This appears to be supported by some case reports and a small series analyzing steroid excretion in ACC (21–24).
Here we undertook steroid metabolite excretion analysis by mass spectrometry followed by computational analysis and tested the performance of this novel biomarker tool in detecting malignancy and hormone excess in a large cohort of patients with adrenal tumors.
Patients and Methods
Study population
The 24-h urine samples from adrenal tumor patients were collected between 2006 and 2009 in six clinical specialist referral centers participating in the European Network for the Study of Adrenal Tumors (ENS@T; www.ensat.org), with approval of local ethical review boards and informed consent. Only 24-h urines from patients with adrenal tumors identified as either benign [adrenocortical adenoma (ACA)] or malignant (ACC) as conclusively as possible were included in the analysis. Underlying diagnosis had been ascertained by histology and evidence of metastasis in ACC and by imaging, biochemical, and clinical follow-up showing no evidence of metastasis in ACA patients. Exclusion criteria were pregnancy and treatment with steroids or drugs known to induce expression and activity of hepatic cytochrome P450 enzymes (e.g. mitotane) or to alter steroid secretion in any other way (e.g. cytotoxic chemotherapy, ketoconazole, metyrapone, mifepristone, spironolactone, or eplerenone).
Biochemical analysis
Patients underwent a comprehensive adrenal tumor work-up comprising clinical assessment including standardized biochemical testing to detect glucocorticoid excess (24-h urinary free cortisol), mineralocorticoid excess (paired plasma renin and serum aldosterone measurements), and adrenal androgen excess [serum DHEA sulfate (DHEAS)] with the additional measurement of serum 17-hydroxyprogesterone (17-HP) and 17β-estradiol. Autonomous glucocorticoid excess was defined as increased urinary free cortisol excretion. Aldosterone excess was ascertained by a serum aldosterone/plasma renin activity ratio higher than 750 pmol/liter/ng/ml·h where the absolute serum aldosterone was higher than 450 pmol/liter. Adrenal androgen excess was defined as serum DHEAS above the normal reference range. Increased serum 17β-estradiol in men or postmenopausal women was accepted as evidence of tumor-related estrogen excess. In all patients, pheochromocytoma had been excluded by analysis of 24-h urinary catecholamine or metanephrine excretion or measurement of plasma metanephrines.
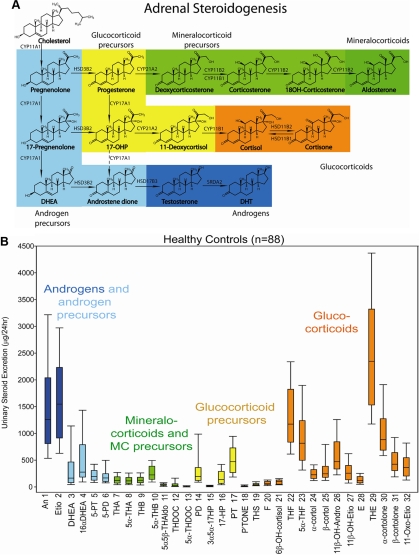
Measurement of 24-h urinary steroid metabolite excretion in ACA and ACC patients and in a healthy control cohort (26 men, 62 women, age range 18–60 yr) was carried out by a long-established method using gas chromatography/mass spectrometry (GC/MS). Urine samples had been stored at −20 C before analysis, which was carried out within 2 yr of collection. A detailed description of this methodology has been published previously (25, 26). In summary, free and conjugated steroids were extracted from 1 ml urine by solid-phase extraction. Steroid conjugates were enzymatically hydrolyzed, reextracted, and chemically derivatized to form methyloxime trimethyl silyl ethers. GC/MS was carried out on an Agilent 5973 instrument operating in selected-ion-monitoring (SIM) mode to achieve sensitive and specific detection and quantification of 32 selected steroid metabolites. These were chosen to include important representatives of steroid groups such as androgen metabolites, glucocorticoid metabolites, mineralocorticoid metabolites, and 3β-hydroxy-Δ5 steroid precursors as shown in Fig. 1 (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org, gives a detailed description of quantified steroids).
Fig. 1.
A, Schematic representation of steroidogenesis depicting the major products of adrenocortical steroid synthesis, the mineralocorticoid aldosterone (dark green) and its precursors (light green), glucocorticoid precursors (yellow), the active glucocorticoid cortisol (orange) and its metabolite cortisone, and the adrenal androgens and their precursors (light blue). Synthesis of active androgens (dark blue) mainly takes place in the gonads. B, The 24-h urinary steroid metabolite excretion in healthy controls (n = 88). Box plots represent median and interquartile ranges; the whiskers represent 5th and 95th percentile, respectively. Color coding of steroid metabolites mirrors that used for depicting the major adrenal corticosteroid classes in A. CYP, Cytochrome P450; HSD, hydroxysteroid dehydrogenase; DHT, 5α-dihydrotestosterone.
Computational analysis of steroid metabolite excretion
All numerical steroid excretion values were log-transformed and subsequently normalized by subtracting the respective mean values obtained in healthy controls with a similar age and sex distribution (n = 88) and dividing by the corresponding sd, which yielded 32 log-transformed steroid excretion values for each patient, expressed on a scale set by the control group.
Further data analysis was carried out by learning vector quantization (LVQ), which identifies typical representatives of the different classes from a set of example data (27). Matrix relevance LVQ incorporates the data-driven identification of a suitable distance measure distinguishing the steroid excretion profile of ACA from ACC patients. The specific technique used was generalized matrix relevance LVQ (GMLVQ), a variant that optimizes the discriminative power of the method (28).
Data classification by LVQ is based on prototypes, i.e. typical representatives of the classes, here ACA and ACC, obtained from labeled example data in a computerized training process. The training aims to efficiently discriminate the classes by assigning data points according to their distances from the prototypes. GMLVQ additionally incorporates the data-driven identification of a suitable distance measure in the learning process. Mathematically, the data points and prototypes are vectors of the 32 steroid excretion values; the dissimilarity or distance measure is parameterized by a symmetric 32 × 32 matrix λ = ΩTΩ, where Ω is also 32 × 32. Diagonal values of the relevance matrix add up to 1 and correspond to the importance of individual steroids in separating the classes; off-diagonal values give the importance of pairs of steroids in the classification scheme. For additional details of the application of GMLVQ on the steroid metabolite data and preceding data handling and transformation, see Supplemental Methods.
For comparison with GMLVQ analysis, we also employed statistical modeling techniques, linear discriminant analysis (LDA) and a standard implementation of logistic regression (29) that was performed employing a standard algorithm (G02GB; Numerical Algorithm Group, Oxford, UK).
Statistical analysis
Steroid excretion data were represented as median and interquartile ranges, the latter being calculated using the Cleveland algorithm implemented in SigmaPlot (Systat Software Inc., Chicago, IL). Total excretion by individual steroid and steroid subgroup (androgens, mineralocorticoids, glucocorticoids, and respective precursor steroids) were compared between control, ACA, and ACC groups using the Kruskal-Wallis nonparametric test; for significance values of P < 0.05, pair-wise comparisons were carried out using Dunn's post hoc test.
Results
Patient characteristics, clinical presentation, and routine biochemistry findings
Demographic and clinical characteristics of 102 patients with ACA and 45 patients with ACC. are summarized in Table 1.
Table 1.
Demographic and clinical characteristics of the adrenal tumor patients
| ACA group (n = 102) | ACC group (n = 45) | |
|---|---|---|
| Median age (range) at time of urine collection (yr) | 60 (19–84) | 55 (20–80) |
| Sex (male, female) | 39, 63 | 24, 21 |
| Tumor load at time of urine collection | Adrenal tumor (n = 102) | Adrenal tumor, no metastasis (n = 9) |
| Adrenal tumor plus metastasis (n = 26) | ||
| ACC metastasis after removal of primary tumor (n = 10) | ||
| Maximum diameter of adrenal tumor at time of urine collection (median and range) | 26 (9–78) mm | 90 (14–230) mm |
| Surgical removal of adrenal tumor | 24/102 (24%) | 30/45 (67%) |
| Median Weiss scorea | 1 (0–2) (n = 15) | 6 (3–9) (n = 21) |
| Duration of follow-up (median and range) | 52 (26–201) months since diagnosis (all patients alive) (n = 102) | 14 (1–187) months from diagnosis to death due to metastatic ACC in deceased patients (n = 35) |
| 45 (30–100) months since diagnosis in alive patients (n = 10)b |
The Weiss system scores the presence or absence of nine histopathological features (Weiss score range 0–9); scores under 3 are indicative of a benign adrenal tumor, scores of 3 are borderline, and scores of 4 and above are indicative of malignancy (14).
Seven of the 10 surviving patients suffer from metastatic disease. The three remaining patients have not shown evidence of recurrence yet (all three initially presented with early-stage disease [ENS@T II (13); primary tumor diameters 80, 89, and 160 mm; histology indicative of ACC with Weiss scores of 5, 7, and 4, respectively; current survival times 45, 51, and 42 months, respectively].
According to routine biochemistry results, 33 of the 102 ACA patients (32%) showed evidence of hormone excess. Of those, 14 patients had isolated glucocorticoid excess and 13 patients isolated mineralocorticoid excess. Four additional patients had combined gluco- and mineralocorticoid excess. Two patients had mildly elevated DHEAS levels. Routine biochemistry revealed no evidence of hormone excess in the remaining 69 ACA patients (68%). Serum 17-HP was measured in 87 ACA patients and increased in five (6%), in two with glucocorticoid excess and in three as an isolated finding.
Among the 45 ACC patients, routine biochemistry showed evidence of hormone excess in 33 patients (73%). Isolated glucocorticoid and adrenal androgen excess was documented in 11 and seven patients, respectively. Twelve ACC patients had combined glucocorticoid and adrenal androgen excess, with additional aldosterone excess in two of them. Three additional patients had 17β-estradiol excess, in combination with glucocorticoid and adrenal androgen excess in two and one, respectively. Twelve ACC patients (27%) showed no evidence of glucocorticoid, mineralocorticoid, or adrenal androgen excess; however, three of them had a raised serum 17-HP. Overall, serum 17-HP was increased above the reference range in 22 of 39 ACC patients (56%).
Urinary steroid metabolite profiling
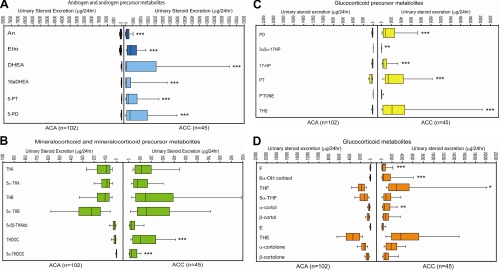
Urinary steroid profiling revealed distinct differences between ACA and ACC patients (Fig. 2). ACC patients had a significantly higher excretion of androgen precursor metabolites [pregnenediol (5-PD), pregnenetriol (5-PT), DHEA, 16α-OH-DHEA; see Supplemental Table 1] and also of metabolites of active androgens (androsterone and etiocholanolone; Fig. 2A and Supplemental Table 2). Although the deoxycorticosterone metabolites tetrahydro-11-deoxycorticosterone (THDOC) and 5α-THDOC were significantly increased in ACC (Fig. 2B and Supplemental Table 2), other mineralocorticoid precursor metabolites, such as those of corticosterone, did not differ between ACA and ACC. Glucocorticoid precursor metabolites PD, PT, 17-HP, and tetrahydro-11-deoxycortisol (THS) were significantly higher in ACC, as were free cortisol, 6βOH-cortisol, tetrahydrocortisol, and α-cortol (Fig. 2, C and D, and Supplemental Table 2). Representation of all steroid excretion data in the form of a heat map suggested increased excretion of THS (steroid 19) in many ACA and ACC patients and high excretion of the androgen precursor metabolites 5-PD and 5-PT (steroids 5 and 6) in most of the ACC patients (Supplemental Fig. 1).
Fig. 2.
Steroid metabolite excretion in ACA (n = 102) and ACC (n = 45) according to steroid classes. A, Metabolites of adrenal androgen precursors and active androgens; B, metabolites of mineralocorticoids and their precursors; C, metabolites of glucocorticoid precursors; D, cortisol and cortisone metabolites. Box plots represent median and interquartile ranges; the whiskers represent 5th and 95th percentile, respectively. *, P < 0.05; **, P < 0.01; ***, P < 0.001 comparing ACA with ACC.
Statistical comparison of total excretion per steroid subclass revealed that ACC patients exhibited significantly increased excretion of metabolites derived from the precursors of androgen, mineralocorticoid, and glucocorticoid synthesis (all P < 0.001 vs. controls; Table 2). Even when analyzing the 12 ACC patients without evidence of hormone excess on routine biochemistry, urinary excretion of androgen and glucocorticoid precursor metabolites remained significantly increased (P = 0.015 and P = 0.002, respectively; Supplemental Table 3). By contrast, steroid precursor metabolite excretion in ACA did not differ from healthy controls (Table 2).
Table 2.
Urinary excretion of steroid metabolites by steroid subclass in healthy controls and patients with ACA and ACC
| Metabolite subclass | Urinary excretion of steroid metabolite subclasses (μg/24 h) |
||
|---|---|---|---|
| Controls (n = 88) | ACA (n = 102) | ACC (n = 45) | |
| Androgen metabolites (steroids 1–2) | |||
| Median | 2,787 | 1,426 | 4,679 |
| IQR | 1,808–4,305 | 724–2,292 | 1,936–8,807 |
| P = 4 × 10−9a | P = 0.31a | ||
| P = 1 × 10−10b | |||
| Androgen precursor metabolites (steroids 3–6) | |||
| Median | 493 | 717 | 8,417 |
| IQR | 320–944 | 389–1,115 | 2,543–57,453 |
| P = 0.24 | P = 6 × 10−16 | ||
| P = 8 × 10−12 | |||
| Mineralocorticoid metabolites (steroids 7–11) | |||
| Median | 598 | 568 | 667 |
| IQR | 355–941 | 381–824 | 301–1,149 |
| P = 0.84 | P = 0.84 | ||
| P = 0.84 | |||
| Mineralocorticoid precursor metabolites (steroids 12–13) | |||
| Median | 25 | 20 | 122 |
| IQR | 13–44 | 14–36 | 52–333 |
| P = 1.00 | P = 3 × 10−4 | ||
| P = 5 × 10−11 | |||
| Glucocorticoid precursor metabolites (steroids 14–19) | |||
| Median | 973 | 879 | 7,646 |
| IQR | 570–1,317 | 545–1521 | 3,704–19,103 |
| P = 1.0 | P = 6 × 10−15 | ||
| P = 3 × 10−17 | |||
| Glucocorticoid metabolites (steroids 20–32) | |||
| Median | 7,763 | 11,655 | 14,526 |
| IQR | 5,639–11,382 | 8,454–15,906 | 8,587–37,802 |
| P = 2 × 10−5 | P = 2 × 10−7 | ||
| P = 0.18 | |||
Steroid subclasses comprise the sum of individual steroids as specified, with numbering of individual steroids referring to Fig. 1. Statistical analysis was performed employing Kruskal-Wallis nonparametric testing and Dunn's post hoc test. IQR, Interquartile range.
Comparison of controls vs. ACA and ACC, respectively.
Comparison of ACA vs. ACC.
Urinary excretion of androgen metabolites was significantly decreased in ACA (P < 0.001; Table 2), as also suggested by the ACA heat map (steroids 1–6 in Supplemental Fig. 1). However, the subgroup of ACA patients with evidence of hormone excess on routine biochemistry showed normal androgen metabolite excretion (P = 0.13; Supplemental Table 3). Compared with controls, urinary excretion of active glucocorticoid metabolites was significantly increased in both ACA and ACC (both P < 0.001; Table 2). Among ACC patients, only those with abnormal routine biochemistry had increased glucocorticoid metabolite excretion (P < 0.001; Supplemental Table 3). In contrast, increased glucocorticoid metabolite excretion was similarly present in ACA patients with and without abnormal routine biochemistry (both P < 0.001 vs. controls; Supplemental Table 3).
When analyzed by sum of steroid subclass metabolites, 82% of ACC showed increased excretion of at least one steroid subclass, compared with only 29% in ACA and 16% in controls. The majority of ACC patients (69%) showed androgen precursor excess, either combined with increased excretion of glucocorticoid precursors (36%) or both glucocorticoid and mineralocorticoid precursors (33%). Isolated glucocorticoid precursor excess was found in 9% of ACC and 1% of ACA patients. Combined mineralocorticoid and glucocorticoid precursor excess was found in 4% of ACA and 2% of ACC. None of the patients had isolated androgen metabolite excess; isolated androgen precursor metabolite excess was found in 2% of both ACA and ACC patients.
We did not detect any effect of age, sex, tumor size, or presence of metastasis on the above described characteristics of the urine steroid metabolomes in ACC and ACA patients.
Machine learning classification performance and selection of differentiating steroid markers
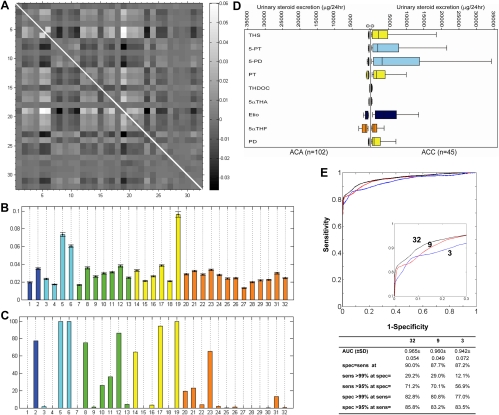
GMLVQ analysis was applied to the steroid data to achieve classification of the samples by determining a discriminative distance measure that characterizes the differences between ACA and ACC. Figure 3A depicts the corresponding relevance matrix, achieved by averaging 1000 analysis runs with random splits of the steroid excretion data into training set (90%) and test set (10%). Analysis of the diagonal elements of the relevance matrix revealed that three steroid metabolites, THS, 5-PT, and 5-PD, were most informative in discriminating ACC from ACA (Fig. 3B). In addition, we identified the six next most discriminative markers (Fig. 3C). The selection of fewer than 10 steroid markers was arbitrarily decided to facilitate future transfer of our biomarker tool to a high-throughput measurement platform, i.e. tandem mass spectrometry, which is limited by the number of steroids that can be analyzed in a single run. The excretion values of the identified nine most discriminative steroid metabolites in ACA and ACC patients are depicted in Fig. 3D.
Fig. 3.
Results of GMLVQ analysis. A, Relevance matrix as obtained by GMLVQ as an average of 1000 randomized training runs. The panel provides a gray-scale representation of the off-diagonal elements in the average relevance matrix. Both the x- and the y-axes correspond to the numbering of individual steroids (n = 32) in Fig. 1 and Supplemental Table 1; each square corresponds to the combination of two steroids. Large positive and negative values as represented by bright and dark squares, respectively, indicate that the corresponding pair of steroid markers is highly relevant for the discrimination of ACC from ACA. The gray scale on the right defines the numerical values of the matrix elements. For clarity, the diagonal elements of the relevance matrix have been omitted, as indicated by the white line. B, All 32 diagonal elements of the relevance matrix, quantifying the significance of each single steroid marker for the discrimination of ACC from ACA (color code as in Fig. 1B), with all significances adding up to the sum of 1. Error bars correspond to the observed sd over the 1000 randomized training runs. C, Respective percentages of the 1000 randomized training runs in which single steroid features were identified as being among the nine most relevant features for the differentiation of ACC from ACA. D, Steroid biomarkers selected after GMLVQ analysis as the nine most relevant markers for differentiating ACC from ACA. Box plots represent median and interquartile ranges; the whiskers represent 5th and 95th percentile, respectively (color code as in Fig. 1B). E, ROC curve for all steroid metabolites (n = 32) and the three and nine steroid markers identified as most discriminating. The inset represents a magnification of the upper left-hand corner of the ROC curves, provided for visual clarity. Numerical characteristics are shown of the threshold-average ROC curves for all 32 steroids and the subsets of three steroids (THS, 5-PT, and 5-PD) and nine steroids [THS, 5-PT, 5-PD, PT, THDOC, 5αTHA Etio, 5αTHF, and PD; for explanation of steriod metabolite abbreviations see Supplemental Table 1] identified as most discriminative after GMLVQ analysis. ROC curves and all values for areas under the curve (AUC) and sensitivity (sens) and specificity (spec) correspond to average test set performances over 1000 random splits of the data set into 90% training data and 10% test data.
Receiver-operating characteristics (ROC) analysis revealed an excellent performance of GMLVQ (Fig. 3E). The use of three and nine markers, selected individually in each training run, was only slightly inferior to the ROC curve generated from the entire data set of all 32 steroids, with similar sensitivities and specificities all at or slightly below sensitivity = specificity = 90% (Fig. 3E).
Compared with GMLVQ, LDA performed relatively poorly when applied to the total set of 32 steroid markers, due to strong overfitting effects. However, overfitting was not present anymore when LDA was applied to the subsets of the three and nine most discriminative markers, respectively, after those had been selected by GMLVQ. Similarly, logistic regression fitting failed to converge when applied to the full data set, but when applied to the two steroid marker subsets, logistic regression produced results that were slightly inferior but comparable to GMLVQ. Thus, both LDA and logistic regression confirmed the validity of the steroid marker selection by GMLVQ.
Discussion
Urinary steroid profiling with subsequent machine learning analysis of steroid excretion data differentiates benign and malignant adrenocortical tumors with high sensitivity and specificity. Our sensitivity and specificity of 90% is superior to the diagnostic value of imaging techniques such as CT, magnetic resonance imaging, and positron emission tomography, currently employed in the differential diagnosis of adrenal incidentalomas (19, 30–34). In particular, the high specificity of our method renders it invaluable in the detection of adrenocortical malignancy, a diagnosis that is virtually impossible to achieve with any single imaging technique.
In contrast to a random blood sample, the analysis of urinary steroid metabolite excretion also comes with the added advantage of noninvasive assessment of the 24-h net steroid production and thus a higher sensitivity in detecting abnormal steroid secretion. Our results indicate that combined androgen and glucocorticoid excess is a characteristic feature in ACC, identified by urinary steroid profiling in 69% of cases but in only 27% of cases by routine biochemistry. Routine serum biochemistry found that the glucocorticoid precursor 17-HP was elevated in 56% of ACC and 6% of ACA patients. However, mass spectrometry-based steroid excretion profiling much more strikingly demonstrated a characteristic accumulation of precursor steroids rather than end products of adrenal steroidogenesis in 85% of ACC patients, across all steroid classes, thereby indicating early-stage, immature steroidogenesis as a characteristic of malignant adrenal tumors. The high incidence of steroid precursor excess supports the concept of dedifferentiation of ACC cells, resulting in a shift from full steroidogenic potential toward proliferative capacity. Of note, steroid profiling also detected significantly increased steroid precursor excretion in those ACC (27%) that had been classified as endocrine inactive based on the results of routine biochemical work-up.
We identified the 11-deoxycortisol metabolite THS as the most discriminative steroid in differentiating ACC from ACA. THS was significantly increased in both ACA and ACC patients compared with controls, but excretion levels were significantly higher in ACC than in ACA, suggesting inhibition or lack of expression of 11β-hydroxylase, the enzyme that converts the glucocorticoid precursor 11-deoxycortisol to active cortisol. Increased secretion of 11-deoxycortisol by adrenal tumors has been previously described (22) but has not been used for differentiating benign from malignant adrenal tumors.
Adrenal-derived androgen excess was a characteristic feature of ACC and detected with a much higher sensitivity by our biomarker tool that demonstrated urinary androgen precursor excess in 71% of ACC and 2% of ACA, whereas serum DHEAS was increased in only 42% and 2%, respectively. By contrast, low urinary androgen metabolite excretion was a characteristic feature in ACA, in particular in those classified as endocrine inactive by routine biochemistry. Interestingly, steroid profiling revealed significantly elevated glucocorticoid metabolite excretion in all ACA patients, i.e. also in those with no evidence of hormone excess in the routine biochemical work-up. This would support the concept of a continuum of hormone secretion in benign adrenal adenomas from increased urinary metabolite excretion to clinically overt disease. Urinary steroid profiling may represent an exquisitely sensitive tool for the quantification of steroid excess in the follow-up of patients with subclinical Cushing's syndrome (9, 10) and also borderline primary hyperaldosteronism. Similarly, steroid profiling could be employed to detect the tumor-specific steroid fingerprint when monitoring for recurrence or treatment response in ACC.
Importantly, our approach combining mass spectrometry-based steroid profiling and GMLVQ analysis identified nine steroids selected as the most discriminatory in differentiating ACC from ACA, with similar classification performance of this subset in comparison to all quantified steroids. These results will facilitate the transfer of this profiling approach from the time-consuming GC/MS platform to high-throughput liquid chromatography/tandem mass spectrometry. Rapid profiling of up to 10 steroids by liquid chromatography/tandem mass spectrometry is entirely feasible (35–37) and will allow the development of a diagnostic screening test that is sensitive, specific, fast, and cost effective. It would be an exciting prospect for the clinical management of patients with ACC if urine steroid metabolomics would also be proven as a sensitive tool for the early detection of tumor recurrence, thus potentially obviating the need for frequent follow-up imaging if similarly sensitive as imaging. Furthermore, future studies will need to determine whether the steroid metabolome fingerprint of a tumor correlates with clinical behavior and prognosis.
Our study has the limitation of being retrospective. However, through collaboration with many centers across Europe, here we have already studied a large number of patients with ACC and ACA in whom tumor dignity, i.e. the definition of benign vs. malignant, had been ascertained as conclusively as possible, including a long period of follow-up that ensured detection of recurrence/metastasis (ACC) or lack of recurrence (ACA). It is possible that a small minority of the 102 ACA represent early-stage ACC that have been successfully removed but did not recur. However, this is unlikely and to a degree unavoidable given the inherent difficulties in the histopathological assessment of adrenal tumors (15). The availability of these unique and well characterized gold standard reference cohorts of ACA and ACC patients, facilitated by European collaboration, were a mandatory precondition for providing proof of concept for our method.
In conclusion, our data provide strong evidence for the discriminative power of the selected steroid markers, clearly indicating that urine steroid metabolomics, i.e. the combination of mass spectrometry-based steroid profiling and subsequent computational data analysis, represents a novel and highly promising biomarker approach to the differential diagnosis of adrenal tumors. Before implementation of our novel approach as a diagnostic test in routine clinical practice, prospective validation in large cohorts of patients with adrenal tumors is warranted, which we are currently embarking on.
Supplementary Material
Acknowledgments
We are grateful to the European Network for the Study of Adrenal Tumors (ENS@T; www.ensat.org) for invaluable help with the collection of samples for this study. We thank Jon Deeks (Professor of Biostatistics, University of Birmingham, Birmingham, UK) and Richard Riley (Senior Lecturer in Medical Statistics, University of Birmingham) for their critical review of the manuscript and helpful discussions.
This work was supported by the Medical Research Council UK (MRC Strategic Grant G0801473 to W.A. and P.M.S.) and the ENS@T. ENS@T gratefully acknowledges support by the European Science Foundation (Research Networking Program ESF-ENS@T), the European Commission (FP7 Collaborative Research Project ENS@T-CANCER 259735), and the Claire Khan Adrenal Trust Fund.
Disclosure Summary: W.A. and P.M.S. are inventors and M.B. is a contributor to a patent application on the use of steroid profiling as a biomarker tool in the differential diagnosis of steroid-producing and steroid-dependent tumors (PCT/GB2010/000274). All other authors did not declare a conflict of interest.
Footnotes
- ACA
- Adrenocortical adenoma
- ACC
- adrenocortical carcinoma
- CT
- computed tomography
- DHEA
- dehydroepiandrosterone
- DHEAS
- DHEA sulfate
- GC/MS
- gas chromatography/mass spectrometry
- GMLVQ
- generalized matrix relevance LVQ
- LDA
- linear discriminant analysis
- LVQ
- learning vector quantization
- 17-HP
- 17-hydroxyprogesterone
- 5-PD
- pregnenediol
- 5-PT
- pregnenetriol
- ROC
- receiver-operating characteristics
- THDOC
- tetrahydro-11-deoxycorticosterone
- THS
- tetrahydro-11-deoxycortisol.
References
- 1. Grumbach MM, Biller BM, Braunstein GD, Campbell KK, Carney JA, Godley PA, Harris EL, Lee JK, Oertel YC, Posner MC, Schlechte JA, Wieand HS. 2003. Management of the clinically inapparent adrenal mass (“incidentaloma”). Ann Intern Med 138:424–429 [DOI] [PubMed] [Google Scholar]
- 2. Mansmann G, Lau J, Balk E, Rothberg M, Miyachi Y, Bornstein SR. 2004. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev 25:309–340 [DOI] [PubMed] [Google Scholar]
- 3. Barzon L, Sonino N, Fallo F, Palu G, Boscaro M. 2003. Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol 149:273–285 [DOI] [PubMed] [Google Scholar]
- 4. Terzolo M, Reimondo G, Angeli A. 2009. Definition of an optimal strategy to evaluate and follow-up adrenal incidentalomas: time for further research. Eur J Endocrinol 161:529–532 [DOI] [PubMed] [Google Scholar]
- 5. Young WF., Jr 2007. Clinical practice. The incidentally discovered adrenal mass. N Engl J Med 356:601–610 [DOI] [PubMed] [Google Scholar]
- 6. Cawood TJ, Hunt PJ, O'Shea D, Cole D, Soule S. 2009. Recommended evaluation of adrenal incidentalomas is costly, has high false-positive rates and confers a risk of fatal cancer that is similar to the risk of the adrenal lesion becoming malignant: time for a rethink? Eur J Endocrinol 161:513–527 [DOI] [PubMed] [Google Scholar]
- 7. Nieman LK. 2010. Approach to the patient with an adrenal incidentaloma. J Clin Endocrinol Metab 95:4106–4113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mantero F, Terzolo M, Arnaldi G, Osella G, Masini AM, Alì A, Giovagnetti M, Opocher G, Angeli A. 2000. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J Clin Endocrinol Metab 85:637–644 [DOI] [PubMed] [Google Scholar]
- 9. Reincke M, Nieke J, Krestin GP, Saeger W, Allolio B, Winkelmann W. 1992. Preclinical Cushing's syndrome in adrenal “incidentalomas”: comparison with adrenal Cushing's syndrome. J Clin Endocrinol Metab 75:826–832 [DOI] [PubMed] [Google Scholar]
- 10. Terzolo M, Bovio S, Reimondo G, Pia A, Osella G, Borretta G, Angeli A. 2005. Subclinical Cushing's syndrome in adrenal incidentalomas. Endocrinol Metab Clin North Am 34:423–439, x [DOI] [PubMed] [Google Scholar]
- 11. Stewart PM. 2010. Is subclinical Cushing's syndrome an entity or a statistical fallout from diagnostic testing? Consensus surrounding the diagnosis is required before optimal treatment can be defined. J Clin Endocrinol Metab 95:2618–2620 [DOI] [PubMed] [Google Scholar]
- 12. Allolio B, Fassnacht M. 2006. Adrenocortical carcinoma: clinical update. J Clin Endocrinol Metab 91:2027–2037 [DOI] [PubMed] [Google Scholar]
- 13. Fassnacht M, Johanssen S, Quinkler M, Bucsky P, Willenberg HS, Beuschlein F, Terzolo M, Mueller HH, Hahner S, Allolio B. German Adrenocortical Carcinoma Registry Group; European Network for the Study of Adrenal Tumors 2009. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a Revised TNM Classification. Cancer 115:243–250 [DOI] [PubMed] [Google Scholar]
- 14. Weiss LM, Medeiros LJ, Vickery AL., Jr 1989. Pathologic features of prognostic significance in adrenocortical carcinoma. Am J Surg Pathol 13:202–206 [DOI] [PubMed] [Google Scholar]
- 15. Lau SK, Weiss LM. 2009. The Weiss system for evaluating adrenocortical neoplasms: 25 years later. Hum Pathol 40:757–768 [DOI] [PubMed] [Google Scholar]
- 16. de Fraipont F, El Atifi M, Cherradi N, Le Moigne G, Defaye G, Houlgatte R, Bertherat J, Bertagna X, Plouin PF, Baudin E, Berger F, Gicquel C, Chabre O, Feige JJ. 2005. Gene expression profiling of human adrenocortical tumors using complementary deoxyribonucleic Acid microarrays identifies several candidate genes as markers of malignancy. J Clin Endocrinol Metab 90:1819–1829 [DOI] [PubMed] [Google Scholar]
- 17. de Reyniès A, Assié G, Rickman DS, Tissier F, Groussin L, René-Corail F, Dousset B, Bertagna X, Clauser E, Bertherat J. 2009. Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. J Clin Oncol 27:1108–1115 [DOI] [PubMed] [Google Scholar]
- 18. Giordano TJ, Kuick R, Else T, Gauger PG, Vinco M, Bauersfeld J, Sanders D, Thomas DG, Doherty G, Hammer G. 2009. Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin Cancer Res 15:668–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hamrahian AH, Ioachimescu AG, Remer EM, Motta-Ramirez G, Bogabathina H, Levin HS, Reddy S, Gill IS, Siperstein A, Bravo EL. 2005. Clinical utility of noncontrast computed tomography attenuation value (Hounsfield units) to differentiate adrenal adenomas/hyperplasias from nonadenomas: Cleveland Clinic experience. J Clin Endocrinol Metab 90:871–877 [DOI] [PubMed] [Google Scholar]
- 20. Boland GW, Dwamena BA, Jagtiani Sangwaiya M, Goehler AG, Blake MA, Hahn PF, Scott JA, Kalra MK. 2011. Characterization of adrenal masses by using FDG PET: a systematic review and meta-analysis of diagnostic test performance. Radiology 259:117–126 [DOI] [PubMed] [Google Scholar]
- 21. Minowada S, Kinoshita K, Hara M, Isurugi K, Uchikawa T, Niijima T. 1985. Measurement of urinary steroid profile in patients with adrenal tumor as a screening method for carcinoma. Endocrinol Jpn 32:29–37 [DOI] [PubMed] [Google Scholar]
- 22. Doerr HG, Sippell WG, Drop SL, Bidlingmaier F, Knorr D. 1987. Evidence of 11β-hydroxylase deficiency in childhood adrenocortical tumors. The plasma corticosterone/11-deoxycorticosterone ratio as a possible marker for malignancy. Cancer 60:1625–1629 [DOI] [PubMed] [Google Scholar]
- 23. Grondal S, Eriksson B, Hagenas L, Werner S, Curstedt T. 1990. Steroid profile in urine: a useful tool in the diagnosis and follow up of adrenocortical carcinoma. Acta Endocrinol (Copenh) 122:656–663 [DOI] [PubMed] [Google Scholar]
- 24. Małunowicz EM, Ginalska-Malinowska M, Romer TE, Ruszczyñska-Wolska A, Dura M. 1995. Heterogeneity of urinary steroid profiles in children with adrenocortical tumors. Horm Res 44:182–188 [DOI] [PubMed] [Google Scholar]
- 25. Shackleton CH, Marcos J. 2011. GC/MS steroid profiling: diagnosis of disorders affecting steroid synthesis and metabolism. In: Gross M, Caprioli R. eds. The encyclopedia of mass spectrometry. Vol 8 Amsterdam: Elsevier; 789–813 [Google Scholar]
- 26. Krone N, Hughes BA, Lavery GG, Stewart PM, Arlt W, Shackleton CH. 2010. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS). J Steroid Biochem Mol Biol 121:496–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kohonen T. 1997. Self-organizing maps. Berlin: Springer [Google Scholar]
- 28. Schneider P, Biehl M, Hammer B. 2009. Adaptive relevance matrices in learning vector quantization. Neural Comput 21:3532–3561 [DOI] [PubMed] [Google Scholar]
- 29. McCullagh P, Nelder JA. 1989. Generalized linear models. London: Chapman and Hall [Google Scholar]
- 30. Ilias I, Sahdev A, Reznek RH, Grossman AB, Pacak K. 2007. The optimal imaging of adrenal tumours: a comparison of different methods. Endocr Relat Cancer 14:587–599 [DOI] [PubMed] [Google Scholar]
- 31. Groussin L, Bonardel G, Silvéra S, Tissier F, Coste J, Abiven G, Libé R, Bienvenu M, Alberini JL, Salenave S, Bouchard P, Bertherat J, Dousset B, Legmann P, Richard B, Foehrenbach H, Bertagna X, Tenenbaum F. 2009. 18F-Fluorodeoxyglucose positron emission tomography for the diagnosis of adrenocortical tumors: a prospective study in 77 operated patients. J Clin Endocrinol Metab 94:1713–1722 [DOI] [PubMed] [Google Scholar]
- 32. Gratz S, Kemke B, Kaiser W, Heinis J, Behr TM, Höffken H. 2010. Incidental non-secreting adrenal masses in cancer patients: intra-individual comparison of 18F-fluorodeoxyglucose positron emission tomography/computed tomography with computed tomography and shift magnetic resonance imaging. J Int Med Res 38:633–644 [DOI] [PubMed] [Google Scholar]
- 33. Blake MA, Cronin CG, Boland GW. 2010. Adrenal imaging. AJR Am J Roentgenol 194:1450–1460 [DOI] [PubMed] [Google Scholar]
- 34. Korobkin M, Brodeur FJ, Francis IR, Quint LE, Dunnick NR, Londy F. 1998. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. AJR Am J Roentgenol 170:747–752 [DOI] [PubMed] [Google Scholar]
- 35. Soldin SJ, Soldin OP. 2009. Steroid hormone analysis by tandem mass spectrometry. Clin Chem 55:1061–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Janzen N, Sander S, Terhardt M, Peter M, Sander J. 2008. Fast and direct quantification of adrenal steroids by tandem mass spectrometry in serum and dried blood spots. J Chromatogr B Analyt Technol Biomed Life Sci 861:117–122 [DOI] [PubMed] [Google Scholar]
- 37. Rossi C, Calton L, Hammond G, Brown HA, Wallace AM, Sacchetta P, Morris M. 2010. Serum steroid profiling for congenital adrenal hyperplasia using liquid chromatography-tandem mass spectrometry. Clin Chim Acta 411:222–228 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.