Abstract
The xCELLigence real time cell analyzer Cardio system offers a new system for real-time cell analysis that measures impedance-based signals in a label-free noninvasive manner. The aim of this study was to test whether impedance readings are a useful tool to detect compound effects on beating frequency (beats per minute, bpm) and arrhythmias of human induced pluripotent stem cell- and a mouse embryonic stem cell-derived cardiomyocyte line (hiPSC-CM and mESC-CM, respectively). Baseline values for control wells were 45±3 and 179±6 bpm, respectively (n=6). Correspondingly, isoproterenol increased beating frequency by 77% and 71%, whereas carbachol decreased frequency by 11% and 100% (stopped in 5/6 mESC-CM wells). E-4031 decreased beating rate and caused arrhythmias in both cell types, however, more pronounced in the human iPSC-CMs. Amlodipine inhibited contractions in both models, and T-type calcium channel block strongly reduced beating rate and eventually stopped beating in mESC-CM but caused a smaller effect in hiPSC-CM. The results of this initial study show that, under the right conditions, the beating frequency of a monolayer of cells can be stably recorded over several days. Additionally, the system detects changes in beating frequency and amplitude caused by added reference compounds. This assay system has the potential to enable medium-throughput screening, but for implementation into routine daily work, extended validation, testing of additional batches of cardiomyocytes, and further assay optimization (e.g., frequency of media exchange, growth matrix, seeding density, age of cells after plating, and temperature control) will be needed.
Introduction
Continuous improvement of preclinical assays and model systems are of utmost importance for pharmaceutical companies that wish to maintain their competitive strength. Attrition rates during the clinical phase of drug testing are high, with drug safety and lack of efficacy being the two leading causes. Only one out of nine compounds that started a clinical trial during the last decade of the 20th century resulted in a registration.1 Beyond that, only three out of ten drugs on the market recover the original investment made in them.1 By improving predictability and translatability of results acquired during the preclinical phase of drug discovery, attrition rate and with it, cost, will very likely be reduced, and the overall efficiency of drug discovery will be improved.
Cardiotoxicity is a field of study that includes testing new compounds for unwanted side effects on the heart. Different models are employed to assess the effect of various compounds and predict their effect in humans. These models range from cellular over-expression systems to complex in vivo models, and have been explained in detail elsewhere.2,3 A similar range of models as used for cardiotoxicity is also employed at earlier stages of drug discovery, for identification and validation of new targets.
Stem cell-derived cardiomyocytes (SC-CM) are a potentially useful tool for in vitro target identification, validation, and compound screening. Compounds that target ion channels, or regulators thereof, are likely to affect action potential (AP) characteristics and, subsequently, electrocardiogram (ECG) parameters and cardiac beating frequency. The cardiac AP differs significantly throughout the heart to facilitate the specialized functions of the different areas.2,4 Nodal cells are distinguished by spontaneous phase 4 depolarization caused by the pacemaker current (also known as funny current If). The electrical impulse then spreads throughout the working myocardium where activation of sodium channels results in an inward sodium current (INa) that depolarizes the membrane (phase 0). This is reflected by the QRS complex on an ECG. Consequently, voltage-gated calcium channels are activated and open, thereby causing an inward calcium current (ICa). Thereafter, phase 1 rapid repolarization occurs as the result of transient outward potassium currents (Ito), which is quickly followed by a plateau phase where the outward movement of potassium and the inward movement of calcium keep the membrane potential steady for a few hundred milliseconds (phase 2). Influx of calcium causes additional release of calcium from the sarcoplasmic reticulum into the cytoplasm, and contraction takes place. Phase 3 repolarization is the result of mainly slow and rapid delayed rectifier potassium currents (IKs and IKr respectively; the latter is sometimes also known as the hERG current since the channel is encoded by the human Ether-à-go-go related gene [hERG]), eventually leading back to the resting phase 4, which is maintained by the inward delayed rectifier current (IK1). The major difference between the AP of mouse and that of humans is a lack of a plateau phase in mice and Ito based repolarization. Studying the beating frequency of spontaneously active CMs can, thus, give valuable information on the effects of a new compound.5 Several previous publications have shown promising results using embryonic SC (ESC)- or induced pluripotent SC (iPSC)-derived CM for safety pharmacology.6–14 Many of the techniques employed, however, such as patch clamp and sharp microelectrode recordings, are technically challenging, invasive, and allow only low throughput.
One of the obstacles between preclinical and clinical testing is species differences that cause unexpected results when new compounds are tested in man for the first time. The opportunity to use cells of human origin and thereby possibly improving the predictive value of assays and reducing the number of animal experiments is attractive. Clearly, SC-CMs cannot replace all preclinical models. Animal models that represent a complete organism and allow for testing systemic effects are still necessary. For improved understanding of species differences and the translatability of results from animal models, we have tested CMs derived from both mouse (m)ESC and human (h)iPSC. This will allow for mechanistic insights into different responses in animal versus man.
The new xCELLigence real time cell analyzer (RTCA) Cardio system (Roche Diagnostics) offers a system for real-time cell analysis measuring impedance-based signals of adherent cells in a label-free noninvasive manner.15 Using a cell-type of choice, this system allows for long-term recording of cellular signals whereby cells can be left undisturbed in the incubator, thus minimizing external input that may influence the results. In this study, we used two assays to test utility and predictability of this new system. Initially, optimization of signal strength and quality was performed. Thereafter, nine reference compounds were tested on both cell lines to determine their effects on beating frequency and amplitude.
Materials and Methods
Cells, Media, and Reagents
In vitro differentiated CMs from hiPSC were bought from Cellular Dynamics (“iCell® cardiomyocytes”; CMC-100-110-001), and mESC-CM from Lonza (“Cor.At™” cardiomyocytes, medium and puromycin kit; XCAC-1010E, Axiogenesis). Both cell types were obtained as frozen 1 mL aliquots containing approximately 5.8 and 1 million cells (iCells and Cor.At cells, respectively). Both cell types tested negative for mycoplasma (MycoAlert detection kit, LT07-418, Lonza). Proprietory media were used as directed by the cell suppliers (iCell Cardiomyocytes Plating Medium, CMM-100-110-001; iCell Cardiomyocytes Maintainance Medium, CMM-100-120-001; XCAM-250E Cor.At medium). Gelatine (from porcine skin; G1890, Sigma), 0.1% in water (w/v), was autoclaved and used for several months when stored at 4°C. Fibronectin stock solution (1 mg/mL in 0.5 M NaCl, 0.05 M Tris, pH 7.5; from bovine plasma) was purchased from Sigma (No. F-1141).
Cell Impedance Measurements
Recordings of cellular impedance were performed with a prototype “xCELLigence RTCA Cardio” instrument (Roche Diagnostics). This system consists of 96-well sensor plates “E-Plate Cardio 96” (where the measurements on the cultured beating CMs take place), the “RTCA Cardio Analyzer” (for measurement of the electrical signals), and a computer for storage, analysis, and display of the data. The sensor plate is electrically connected to the “RTCA Cardio Station” by a clamp mechanism, and both are placed inside a cell incubator during the measurements. Before each experiment, background impedance signals were recorded of the coated, growth medium-filled, and temperature-equilibrated sensor plate (see Table 1 for a protocol summary). At preprogrammed intervals during cell culture and after compound addition, impedance values for all 96 wells in parallel were measured and recorded at a rate of 12.9 milliseconds per measurement. Raw data are displayed by the instrument as “cell index (CI)” values, which are calculated from changes of the impedance signal. CI (at 10 kHz)=(sample impedance−background impedance)/15.
Table 1.
Protocol for Screening of Compounds for Effects on Beating of Induced Pluripotent Stem Cell- or Mouse Embryonic Stem Cell-Derived Cardiomyocytes Using the xCELLigence System
| Step | Time | Description | Parameter | Value |
|---|---|---|---|---|
| 1 | Day 1 | (a) Coating of sensor plate wells | 0.1% gelatin in water | 100 μL/well |
| 2 | (b) Read impedance for medium only | Growth medium | 140 μL/well | |
| 3 | (c) Thaw cells, count and seed | Viable cells | 70k/well (140 μL) | |
| 4 | (d) Start recording data | Sweeps per hour | As desired | |
| 5 | Day 2 | Medium refresh (for Cor. At culture only) | Growth medium containing puromycin | Remove to 30 μL, add to 150 μL |
| 6 | Day 3 | Partial medium change (2×) | iCell CM: maintainance medium; Cor. At: medium without puromycin | Remove to 30 μL, add to 150 μL |
| 7–10 | Day 5–11 | Partial medium refresh; every other day | Same medium as in step 6 | Remove to 30 μL, add to 150 μL |
| 11 | Day 12– | (a) Partial medium refresh | Same medium as in step 6 | Remove to 30 μL, add to 150 μL |
| 12 | (b) Check baseline | Beating frequency | >30 bpm for iCells; >150 bpm for Cor.At | |
| 13 | (c) Increase sweep rate | Sweeps per time unit | As desired; e.g., 1/min | |
| 14 | (d) Reduce volume to 100 μL | |||
| 15 | (e) Add compound dilutions (10×) in prewarmed medium | Compound dilution | 1/10 of final |
1. Incubate 1.5 h in cell incubator; plates can be stored for up to 6 days in the fridge.
2. Ideally, the same sensor plate should be used as in the experiment (though not absolutely necessary).
3. Allow for initial attachment to the sensor plate by letting the plate sit in the hood for 30 min before transfering to Cardio Station.
4. For example, sweeps of 40 s for iCells (20 s for Cor.At).
5. Use automated pipettor, sterile tips, defined position from bottom, slow pipetting speed; not necessary for iCells.
6. As in step 5, but for both iCells and Cor.At cells.
7–10. As in step 5, but for both iCells and Cor.At cells.
11. As in step 5, but for both iCells and Cor.At cells.
12. Only proceed if baseline frequency and pattern (irregularity parameter; standard deviation of frequency approximately 10% of average) are ok; otherwise, continue culture until baseline check is ok.
14. Use automated pipettor, sterile tips, defined height position, slow pipetting speed.
15. Use automated pipettor, sterile tips, defined height position, slow pipetting speed.
bpm, beats per minute; CM, cardiomyocytes.
Cell Plating and Culture
Before seeding with CMs, the 96-well sensor plates (“E-plates Cardio 96,” Roche Diagnostics) were coated with either 0.1% gelatine or 10 μg/mL fibronectin (for initial experiments only).
Proprietary media were used according to the protocols provided by the cell suppliers. Briefly, cells were thawed, diluted in media, and centrifuged (200×g, 4 min, room temperature). After removing the medium, the cells were resuspended in fresh medium and counted. For consistency and ease of counting, we used an automated cell counter (Cedex Cell counter, Roche Diagnostics). However, it should be noted that manual counts (hemocytometer) gave up to 50% lower counts, most likely due to noncounting of small cells. Before seeding into the sensor plates, the cell suspensions were diluted with medium to 0.5 million cells/mL, and 140 μL (70,000 cells) were plated per well. The seeded sensor plates were left covered for 30 min at room temperature to allow initial attachment before transfer to the cell incubator. To avoid edge effects during the approximately 20 days of cell culture, the outermost rows and columns of the sensor plates were not used for plating cells but instead filled with medium. The sensor plate was mounted on the RTCA Cardio Station inside a regular cell incubator (6% CO2, 37°C) for recording of signals.
Medium Exchange and Compound Additions
To minimize disturbance of the cell layer and cooling down of the cell samples, a 96-channel pipettor (“Cybi™ SELMA” from CyBio) was used for careful and fast media exchanges and compound additions.
At regular time intervals (2 days), the medium was refreshed by partial removal of medium in the wells down to 30 μL (at a predetermined pipetting height by the calibrated SELMA pipettor), followed by addition of 120 μL of fresh warm medium. Pipetting speed was 20 μL/s, no mixing. At the switchover of plating media to maintainance media on day 3 (see step 6 in Table 1), this procedure was repeated once more for more complete medium change. Complete removal of the medium was avoided, as it often lead to irregular beating after fresh medium addition. On the day of the experiment, the medium was partially exchanged (120/150 μL) to fresh medium, because depleted media often lead to irregular beating patterns. Two compounds dilutions (10×) for two final concentrations (see Table 2) in media were prepared, adjusted for equal dimethyl sulfoxide (DMSO) concentrations, and pre-equilibrated to incubator temperature. The additions of compounds to CMs (both cell types) were performed on the same day by using the same batches of compounds. Immediately before compound addition (1/10 of final volume), the liquid level in the sensor plate was adjusted to 100 μL (SELMA pipettor). Data were recorded for baseline values, and after first and second compound addition. To minimize temperature changes, the compound addition procedure was performed within approximately 3 min. Since fresh medium can influence the beating frequency, blank control wells were included that only received additions of medium and DMSO.
Table 2.
Reference Compounds and Final Assay Concentrations
| Compound | Target | After first addition (μM) | After second addition (μM) |
|---|---|---|---|
| Amlodipine | L-type calcium channel blocker | 0.09 | 0.36 |
| Carbachol | Cholinergic agonist | 2.78 | 10.84 |
| DMSO | Vehicle | 0.03% | 0.11% |
| E-4031 | hERG channel blocker | 0.09 | 0.36 |
| Isoproterenol | β-adrenergic agonist | 0.08 | 0.33 |
| Mibefradil | T-type calcium channel blocker | 0.09 | 0.36 |
| Ref1 | T-type calcium channel blocker | 0.83 | 3.25 |
| Ref2 | T-type calcium channel blocker | 0.67 | 2.60 |
| Ref3 | T-type calcium channel blocker | 0.67 | 2.60 |
| Zatebradine | Pacemaker channel blocker | 1.11 | 4.34 |
DMSO, dimethyl sulfoxide; hERG, human Ether-à-go-go related gene.
Reference compounds used were either bought from Sigma-Aldrich or obtained from Compound Management at AstraZeneca.
Data Analysis and Statistics
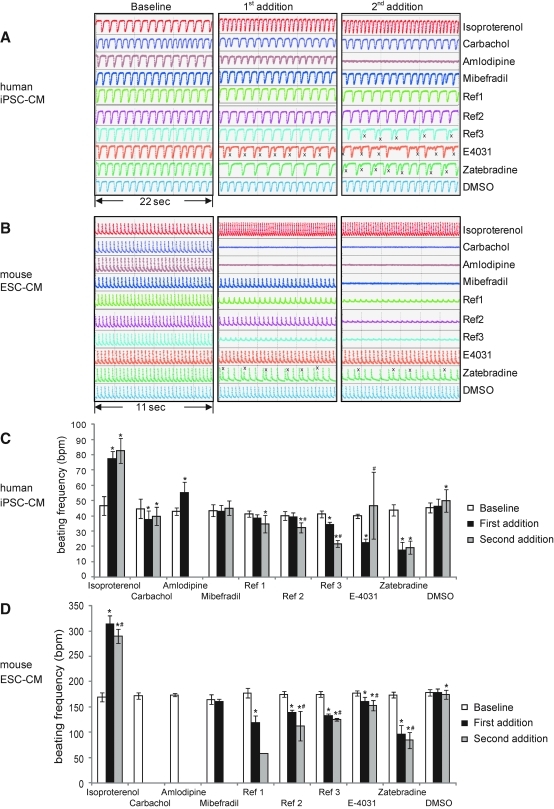
The recorded data were analyzed with the RTCA Cardio Software 1.0.0.1009. Threshold level 10 was used to suppress noise for improved peak recognition; for display of raw data in beat curves (Fig. 1), threshold level 0 was used. Data recordings (“sweeps”; 40 s) with amplitude values <0.02 or beating pattern irregularity values >20% were excluded or marked as irregular, respectively. The beating frequency was manually determined for wells where the beating was irregular, as the prototype instrument software could not distinguish between main regular contractions and “extra” beats (see the effect of E-4031 on hiPSC-CM in Fig. 1 for example). Extra beats generally had an amplitude <80% of the main peaks, and were excluded during the frequency count.
Fig. 1.
(A, B) Raw data traces showing control (DMSO) beating (changes in cell index) and the effect of all nine compounds tested on both hiPSC-CM and mESC-CM. Plating density 70k/well and gelatin coating for both cell types. Note the irregular beating detected using Ref3, E-4031, and Zatebradine. x denotes extra, smaller, beats that were excluded when the beating rate was calculated. The results are quantified for hiPSC-CM in (C) and for mESC-CM in (D). The numerical data presented are means±standard deviation (4≤n≤6,) except for Ref1 and Ref3 for mESC-CM at second addition where n=1 and 2, respectively). * denotes significantly different from control, and # denotes significantly different from first compound addition.
All values are presented as mean±standard deviation. One-way analysis of variance with Bonferroni post hoc test was used to test statistical significance, and p<0.05 was considered as significant. Throughout the text, * denotes significantly different from control, and # denotes significantly different from first compound addition.
Results
Formation of Beating Cell Layer in Sensor Plates
To establish conditions for compound screening, we initially determined the conditions that resulted in reproducible and rhythmic beating of the seeded CMs. The likely parameters affecting the beating and/or its recording were seeding density, surface coating, time, medium exchange, and temperature.
Seeding Density, Surface Coating, and Medium Exchange
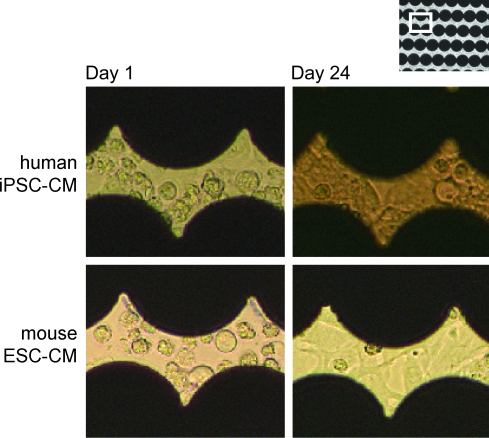
Human iPSC-CM (“iCells”) and mouse ESC-CM (“Cor.At”) were seeded in separate sensor plates at densities ranging from 5.6k/well to 200k/well (hiPSC-CM) and 11k/well to 400k/well (mESC-CM) in wells coated with either fibronectin, gelatin, or in uncoated wells. Viabilities of the cells before plating ranged from 63% to 80% for the iCells and from 74% to 84% for Cor.At cells (in different experiments). The plating densities were based on the total viable cell count (as determined by the Cedex reader). The main cell population for both cell types (in the size range of 9.1 to 17 μm) constituted 40%–50% of the viable cell count, the remainder were mostly either smaller (3.6 to 9.1 μm) or, usually to a lesser degree, larger particles. All these cell populations did not stain with trypan blue dye, thus indicating viable cells. The sensor plates were then kept in the cell incubator for approximately 3 weeks, with partial media changes every 2 days, and impedance signals were recorded. Figure 2 shows the phase contrast appearance of the seeded cells in the sensor plate at day 1 and 24 (both cell types) when seeded on gelatin at 72k/well (iCells) or 52k/well (Cor.At cells). At day 1, the CMs were not yet contracting and showed clear cell borders. During continued cell culture, many of the cells interconnected, forming a contracting “cell sheet.”
Fig. 2.
Phase-contrast micrographs (20×objective) of hiPSC-CM and mESC-CM plated on the sensor plates. Black areas are the microelectrodes in the well bottom. At day 1, cells are round and isolated from other cells. At day 24, they have coupled to surrounding cells and formed a monolayer on the plate. hiPSC-CM, human induced pluripotent stem cell-derived cardiomyocytes; mESC-CM, mouse embryonic stem cell-derived cardiomyocytes. Color images available online at www.liebertonline.com/adt
Time Needed Before Initiating Experiments
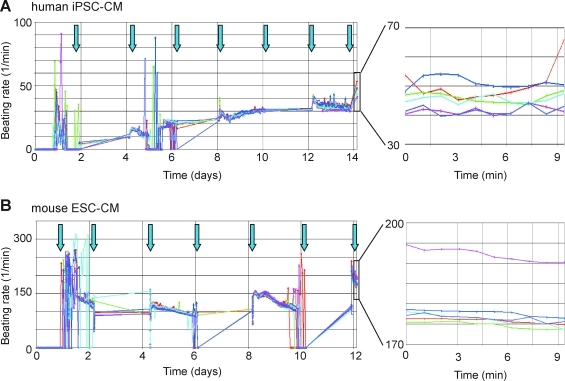
First regular beats for hiPSC-CM appeared after approximately 4 days on all three surfaces, but the beating frequency was still rather low (approximately 10 beats per minute [bpm]). The most regular patterns were seen after about 9–11 days in the concentration range of 26k/well–72k/well. The other conditions often gave irregular or no beating signal at all, presumably because of either absence of contractions or lack of synchronization. Regular beating continued for the duration of the experiment (11 more days). Therefore, we chose a seeding density of 70,000 cells (total viable cell count by Cedex Counter) per well (gelatin-coated) of either of the two cell types for subsequent experiments. When sensor plates were prepared that way, the frequency of beating increased during culture, reaching plateau-like phases at day 9 of about 30 bpm for hiPSC-CM (Fig. 3A) and about 140–150 bpm for mESC-CM (Fig. 3B). Media exchange before compound addition at day 13 (for mESC-CM) and day 15 (for hiPSC-CM) caused a further increase in frequency to approximately 180 and 45 bpm, respectively (see Table 3). Although the frequency pattern for hiPSC-CM beats remained fairly constant in-between media changes, it only remained steady (with a slight downward trend) for mESC-CM during the first 24 h after each medium refreshment; thereafter the beat became irregular, with some wells showing no beating at all. However, at the next medium refresh, all wells regained a steady beating, but again only for about 24 h (repeated pattern at each medium refresh). This indicated medium exhaustion and suggested that medium (in particular for mESC-CM) should be exchanged even more frequently (e.g., every 24 h) to keep the cells in a more steady condition. Therefore, to avoid instabilities of beating due to medium exhaustion, the medium was always refreshed on the day of compound testing. The optimal time for testing of compounds appeared to be after about 12–14 days in culture (either cell type)—at about that time, a clear and regular rhythmic beating pattern was established that also had reached a plateau phase of stable beating pattern (58/60 and 60/60 wells for hi-PS and mESC-CM, respectively).
Fig. 3.
Beating rate recorded from hiPSC-CM in (A) and mESC-CM in (B) over the time period up to testing of compounds (day 15 and 13, respectively) for the DMSO control wells (n=6). Plating density was 70k/well, and gelatin was used for coating. Arrows indicate time points of media exchanges. Note that the rate stayed fairly constant after media exchanges for hiPSC-CM, but decreased slowly for mESC-CM during 1 day and became irregular up to the next media refresh, thus suggesting that more frequent media exchanges for mESC-CM may be required. During cell culture, the data were not continuously recorded; the graphs show, therefore, simple line connections for those time intervals (see days 2–4, 6–8, and 10–12 in A and B). DMSO, dimethyl sulfoxide.
Table 3.
Effect of Nine Reference Compounds on Beating Frequency in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes and Mouse Embryonic Stem Cell-Derived Cardiomyocytes
| |
Human iPSC-CM |
Mouse ESC-CM |
||||
|---|---|---|---|---|---|---|
| Compound | Baseline | First addition | Second addition | Baseline | First addition | Second addition |
| DMSO (baseline) | 45±3 | 46±5 | 50±7* (+11%) |
179±6 | 178±8 | 175±9* (−2%) |
| Amlodipine | 43±2 | 55±7* (+30%) |
6 weak/abs.‡ | 174±3 | 6 weak/abs.‡ | 6 weak/abs.‡ |
| Carbachol | 45±7 | 37±6* (−18%) |
40±6* (−11%) |
172±7 | 6 weak/abs.‡ | 6 weak/abs.‡ |
| E-4031 | 40±1 | 22±3* (−45%) five irregular |
47±22#& 2 weak/abs.‡ two irregular |
177±5 | 161±8* (−9%) two irregular |
153±10*# (−15%) |
| Isoproterenol | 47±6 | 77±5* (+64%) |
83±8* (+77%) |
170±9 | 315±16* (+85%) |
290±14*# (+71%) |
| Mibefradil | 43±4 | 43±4 | 45±5 | 165±9 | 160±6 2 weak/abs.‡ |
6 weak/abs.‡ |
| Ref1 | 41±2 | 38±2 | 35±6* (−15%) two irregular |
178±9 | 118±14* (−34%) 1 weak/abs.‡ two irregular |
58 (n=1) 5 weak/abs.‡ |
| Ref2 | 40±3 | 39±3 | 32±3*# (−20%) one irregular |
175±6 | 139±6* (−21%) |
113±29*# (−35%) 2 weak/abs.‡ one irregular |
| Ref3 | 41±2 one irregular‡ |
34±2* (−17%) one irregular‡ |
22±2*# (−46%) 2 weak/abs.‡ three irregular |
175±6 | 133±4* (−24%) |
125±3*# (−29%) 4 weak/abs.‡ |
| Zatebradine | 44±4 | 18±5* (−59%) four irregular |
19±4* (−57%) six irregular |
174±6 | 96±18* (−45%) five irregular |
84±16*# (−52%) five irregular |
Significantly different from control.
Significantly different from first addition.
Beating frequency was increased, possibly due to triggered activity.
Excluded data; n=6 otherwise.
ESC-CM, embryonic stem cell-derived cardiomyocytes; iPSC-CM, induced pluripotent stem cell-derived cardiomyocytes; weak/abs., weak or absent signal; amplitude <0.02 cell index units.
Influence of Temperature
CMs quickly reduced beating rate when handled at room temperature, for example, during compound addition (approximately 50% reduction; data not shown). It then took approximately 15 min for the control cells to regain the original beating rates. Therefore, any time outside the incubator was kept to a maximum of 3 min, and the compound effects were analyzed after the plates had been back in the incubator for 15–20 min.
Effect of Reference Compounds
Nine reference compounds (Table 2) were used to assess the effects on beating frequency in both cell types and evaluate the ability of the system to detect frequency and beating pattern changes.
Human iPSC-CM in freshly changed medium had a steady baseline beating frequency of 45±3 bpm (n=6; day 15). Frequency was unchanged (46±5 bpm) during first compound addition, but increased slightly (50±7* bpm) during the second. The opposite pattern was seen for mouse ESC-CM, where the beating frequency at baseline was 179±6 bpm (n=6; day 13), at first addition 178±8 bpm, and slightly decreased to 175±9* bpm at second addition. The change in beating frequency at second addition was taken into account during statistical analysis. The amplitudes (differences of CI units) of the six DMSO control wells during baseline were twice as high for the mESC-CM as compared with the hiPSC-CMs: 0.128±0.008 and 0.064±0.006, respectively (data not shown). The frequency data have been summarized in Table 3 and Figure 1.
Autonomic Nervous System
At first, responses to adrenergic (isoproterenol) and cholinergic (carbachol) agonists were tested in both cell types. As expected, adrenergic stimulation strongly increased beating rate (+64%* and +77%* at first, respectively, second addition in human iPSC-CM). For mouse ESC-CM, the corresponding values were +85%* (first addition) and +71%* (second addition). Cholinergic stimulation slowed beating (−18%* and −11%* at first and second addition, respectively, in human iPSC-CM). Mouse ESC-CM reacted stronger to carbachol than human iPSC-CM did, with cessation of contraction already at first compound addition. One of the six replicate wells showed a slow beating pattern at later time points (data not shown). Notably, when mouse ESC-CMs had been plated at a lower density (18k/well) on fibronectin-coated surfaces in an earlier experiment, carbachol at the same concentrations did not disrupt the beating, only reduced the frequency of beating (data not shown). Whether this difference was caused by the different substrates or other factors is currently unclear, but it should be further investigated during the extended validation that is still required to assure a screening assay which is robust enough for routine medium throughput screening (see also in discussion).
L-Type Calcium Current
Blocking the L-type calcium current (ICa,L) using amlodipine caused cessation of beating in human cells at second compound addition and in mouse cells already at first addition. In human cells, an increase in beating frequency (43±2 to 55±7* bpm) and a decrease of beating amplitude was seen at first addition. Altogether, this suggests that the concentrations used were too high and, therefore, became toxic immediately.
T-Type Calcium Current
Effect of blocking T-type calcium (ICa,T) was tested by using four different compounds. Mibefradil did not show any effect at the concentrations used in human iPSC-CM, whereas contractions in mouse ESC-CM were inhibited in 2/6 wells at the first addition and in all six wells at the second addition.
The higher sensitivity of mouse ESC-CM to ICa,T block was confirmed by using three additional compounds (Ref1–Ref3), all of which decreased beating rate and amplitudes (to varying extents) at first addition and caused irregular beating patterns and/or ceased contractions in a majority of the wells at second addition. In human iPSC-CM, only Ref3 showed an effect at first addition and irregularity of beating in one replicate well. At second addition, Ref1 and Ref3 caused irregular beating in several of the six replicate wells, whereas Ref2 did so in only in one replicate well.
hERG Current
The hERG current (IKr) plays an important role during repolarization of the AP. Block of the IKr channel is a frequent side effect of many compounds, and may lead to prolonged QT interval and development of the lethal ventricular arrhythmia Torsade de Pointes. Using E-4031 as a specific hERG channel blocker, both assays responded by decreased beating rate (as a result of prolonged repolarization time) and irregular beating (a sign of arrhythmias) already at first compound addition.
Human iPSC-CM changed from 40±1 to 22±3* bpm with 5/6 wells showing irregularity at first addition. At second addition, 4/6 wells showed irregular beating, two of them with amplitude <0.02 CI units. In the two remaining wells, the beating frequency had increased, a possible sign of triggered activity.
In mouse ESC-CM, the beating rate was reduced from 177±5 bpm at baseline to 161±8* bpm at first addition and 153±10*# bpm at second addition. Two of six wells were irregular at first addition, but the irregularity disappeared at second addition (regular beating pattern is shown in Fig. 1B).
Pacemaker Current
The pacemaker current (If) plays a key role in the spontaneous beating of nodal cells. The spontaneous beating of both cell types used in this study suggests functional If channels. Zatebradine reduced beating rate in both models as anticipated.
In human iPSC-CM, the beating rate of 44±4 bpm at baseline decreased to 18±5* bpm at first addition, with 4/6 wells showing irregular beating, to 19±4* bpm at second addition with 6/6 being irregular. For mouse ESC-CM, the baseline rate of 174±6 bpm decreased to 96±18* bpm at first addition, with 5/6 wells showing irregular beating, and to 84±16*# bpm at second addition, with 5/6 being irregular. The irregular beating detected may be due to the slow beating frequency in combination with an additional hERG block at higher drug concentrations. In addition to If channels, Zatebradine also blocks hERG with an IC50 of 10 μM at 20°C in HEK-hERG cells (in house data).
Discussion
Spontaneously beating CMs, derived through in vitro differentiation of iPSC and ESC, are a promising cellular model for testing the effects of compounds on beating rhythm and for cardiotoxicity. The aim of this study was to test whether impedance measurement, as performed with the xCELLigence RTCA Cardio system, is suitable to detect effects of compounds on the beating frequency and pattern of hiPSC- and mESC-CMs and to correlate the effects to known compound effects in vivo.
The results of this study are encouraging and show that, under the right conditions, the beating frequency of a monolayer of cells can be stably recorded over several days. Additionally, the system is able to detect even small changes in beating frequency and amplitude as a result of added reference compounds. Further, irregular beating or complete disappearance of beating on compound addition can indicate arrhythmias and cardiotoxicity. The exact nature and the mechanisms behind these observations need to be studied, though, in greater detail. Previous reports have used CI changes to study cytotoxicity and determine cell viability.15–17 For investigation of compounds with unknown effects, it will be crucial to determine whether inhibition of contraction is due to ion channel blocking activities or due to toxicity.
Current Models
Models currently used during the preclinical phase of drug discovery and safety pharmacology range from simple over-expression systems (often in HEK293 cells) to complex in vivo systems.2 Stem cell-derived CMs are under evaluation as a tool either as single cells or as more complex two- or three-dimensional systems.
At the single cell level, conventional patch clamp recordings allow for studying ion channel kinetics and current densities.11,18,19 Additionally, APs can be recorded from single cells using patch clamp, or from multicellular stem cell clusters using sharp microelectrodes. By recording APs, the effect of a compound at several ion channels simultaneously can be studied.10,12–14,20,21 Both the patch clamp and the sharp microelectrode technique give high-quality results but are labor intense, require a high degree of technical skill, and yield low throughput. An alternative technique to study APs that is less invasive, requires less skill, and yields higher throughput than the traditional techniques is optical recording using a fast voltage-sensitive dye (di-4-ANEPPS).22 Analysis gives information on AP duration and changes therein, but the information is less detailed compared with the techniques just mentioned.
Multi-electrode arrays are used to study the electrical activity of SC-CM clusters by recording a field potential.23 This technique gives information on the duration of the contraction and, to some detail, the depolarizing Na+ and Ca2+ currents plus repolarizing K+ currents. You can, however, not distinguish between the currents with the same precision as when recording APs. Nevertheless, it is possible to study compound effects and possibly also pick up arrhythmic responses.6
Studying contractility is another way of assessing cardiac toxicity. By means of sarcomere movement or by cell edge detection using specialized software, contractility of a CM can be determined in a noninvasive manner, and effects of compounds can be detected.
Compared with these existing models, the xCELLigence RTCA Cardio does not provide detailed electrophysiological readouts and mechanistic insight on the single cell level or on cell clusters. However, it offers a relevant physiological readout of CM contraction on inter-connected CMs in a thin layer (functional syncytium) at higher throughput, both of which are of great interest for screening applications. Moreover, this system is currently the only one that can measure in vitro synchronized CM beating for an extended period of time at a remarkable steady performance, thus representing a viable model for the study of chronotropy and ultimately arrhythmia, once optimized.
The xCELLigence RTCA Cardio System
The xCELLigence RTCA Cardio system is an impedance reader capable of reading signals at high sampling rates corresponding to the contraction movements of CMs plated in sensor plates. The CMs cover most of the interdigitated electrode surfaces of the sensor plate wells when seeded at a density of 70,000 cells/well. Contractions of the CMs cause redistribution of cellular components near the electrode surface and/or changes in cell adhesion, thereby modulating current flow between the electrodes in the wells, resulting in a recordable signal. To be able to detect a signal, it is further necessary that the cells in the well beat in synchrony; otherwise the individual signals, if they are not in phase, would cancel each other out, as the signal at any time is the sum of all the signals on the electrode surfaces in one well. We show that these CMs indeed form a “functional syncytium” (interconnected cells allowing electrical signal transfer) that thereby enables synchronization and measurement of contractions. The current system is limited to measuring signals from one sensor plate (96 recording channels) at a time; however, several plates can be prepared in parallel and only connected to the measurement device at the actual measurement time. In that way, increased throughput can be achieved; however, automation for the change of the sensor plates would be desired. Additionally, automation for reproducible and frequent media exchanges is crucial to achieve uniform rhythmic baseline beating patterns, as it is difficult to reproducibly perform with manual pipettes or suction devices. Importantly, temperature control during compound addition could minimize the time needed for cells in control wells to regain baseline beating frequencies. Although the software readily reports frequency and amplitude data (at a time point chosen, or in a time interval), the noise reduction settings have to be carefully chosen to deal also with “noisy” signal curves of small amplitudes. Curves representing irregular beating patterns were difficult to analyze directly by the prototype software, as they could not automatically determine the main beating frequency in a complex beating pattern consisting of a main frequency overlaid with a beating pattern of usually smaller amplitudes. Although there are a number of additional beat descriptor readouts available via the software (e.g., rising time and slope, falling time and slope, etc.), we used only a few others (e.g., “beating pattern irregularity”). For the others, we found it difficult to obtain robust values for the weaker signal patterns.
The Stem Cell Assay
Improvements in generating pure (near 100%) SC-CM cultures have been made by utilizing selection based on alpha myosin heavy chain-driven antibiotic resistance of genetically engineered human iPS and mouse ES cells. Although pure by the criterium of antibiotic resistance, these cells are heterogenous mixtures representing CMs showing ventricular, atrial, and nodal electrophysiological characteristics. To obtain robust and consistent responses from those cell samples, it will be important to use CM batches with the same sub-type composition. Using the commercially available, subtype-heterogenous, SC-CM lines purified by antibiotic resistance (“iCells” and “Cor.At” cells), we tested their sensitivity to nine reference compounds and the accuracy of their response in comparison to in vivo models. Most compounds caused comparable results in both stem cell types, though the mouse cells tended to respond stronger at lower concentrations compared with the human cells; whereas the human iPSC-CM reacted to E-4031 with a more clearly detectable arrhythmia (Fig. 1A). The latter is in line with the fact that the major repolarizing current in mice is Ito and not IKr as in human CM, and underlines that important species differences exist.4 As the data in Table 3 show, cells in replicate wells often did not respond equally with changes of beating patterns. For example, only two out of six wells of mESC-CM showed beating irregularity after the first compound addition. Moreover, the irregularities even disappeared at the higher compound concentration (second addition). Therefore, to get a representative picture about the responses to compounds, it is important to test several replicates (n>3), and in ranges of compound concentrations.
Both E-4031 and the If blocker Zatebradine block hERG and can cause arrhythmic events through delayed repolarization and formation of early afterdepolarizations (Zatebradine blocks hERG with an IC50 of 10 μM in HEK-hERG cells, in house data).24–27 Both compounds caused irregular beating and additional small extra beats in the two assays tested here (see Fig. 1), which we have classified as arrhythmias. Other compounds resulted in undetectable signals at various concentrations in the two models. No detectable signal implies that contractions had ceased or were too weak to be detected, thereby indicating toxicity. However, dedicated cytotoxicity tests are required to distinguish between compounds that merely stop the contractions of the CMs, without affecting viability, and those which are indeed cytotoxic as well.
Adding either an agonist (isoproterenol) or an antagonist (carbachol) of the autonomic nervous system showed the expected increase respective decrease in beating rate in both cell models. This is in line with what is seen in in vivo mouse models.28,29
Blocking the L-type Ca2+ channel using Amlodipine stopped contractions in both models. This agrees with previous reports using other ICa,L blockers in stem cells.30 Amlodipine is a very potent dihydropyridine Ca2+ antagonist. It is highly vasoselective and neither significantly depresses heart rate nor produces significant negative inotropic effects in adult animals or humans.31 The cessation of contraction after addition of amlodipine was most likely due to the high concentrations used in the present study. In contrast, blocking T-type Ca2+ current only slightly decreased the beating rate in human iPSC-CM, whereas a major decrease in combination with arrhythmias and toxicity were detected in the mouse ESC-CM. These data correspond well to what is previously published for Mibefradil32 and to our in-house data for Ref1 in a mouse model.* The arrhythmias and toxicity seen in the mouse cell assay indicate that the mouse cardiac cells are more sensitive to T-type Ca2+ channel blockade than human cells, which is in agreement with the findings by Tanaka et al., who demonstrated that the contribution of T-type Ca2+ current to cardiac pacing is much greater in the mouse than in larger species.33
Future Directions
Using the current assay conditions (as described in Materials and Methods section), a reproducible beating pattern can be achieved. However, a systematic exploration of well coating matrices and optimal media exchange conditions may increase the time window (currently approximately 1 week) and overall robustness of the assay further. Also, in view of the cost of the CMs, the lower limits of seeding density need to be determined if more throughput is desired. For such a study, a careful characterization of the seeded cells (e.g., in terms of size range of counted cells) will be important to be able to compare experimental conditions.
We also noticed that the mESC-CM assay setup (70k/well, gelatine coating, proprietary medium) was particularly sensitive to medium exhaustion, which became apparent by irregular or ceased beating before media refreshes. Therefore, fresh medium was crucial before compound testing to obtain stable baseline beats.
The assay system has the potential to enable medium-throughput screening of compounds for effects on cardiac beating patterns: SC-CMs are available in screening scale quantities, and the data acquisition rate is sufficiently high to allow for the recording of even the fast beating pattern of mouse ESC-CMs in the 96-well format. However, to fulfill this potential, we see it necessary that several further improvements are achieved: (i) extended validation involving a larger range of reference compounds with known in vivo effects, (ii) testing of additional batches of CMs to assess consistency of CM quality, and (iii) further technical improvements as just outlined. Continuous effort should also be made to further improve maturity of the cells for better translatability to the adult situation. Similarly, generation of CM representing ventricular, atrial, and nodal subtypes could open new areas of screening for arrhythmia and cardiotoxicity.
Abbreviations
- AP
action potential
- bpm
beats per minute
- CM
cardiomyocytes
- CI
cell index
- DMSO
dimethyl sulfoxide
- ECG
electrocardiogram
- ESC
embryonic stem cell
- hERG
human Ether-à-go-go related gene
- hiPSC-CM
human induced pluripotent stem cell-derived cardiomyocytes
- ICa,L
calcium current, L-type
- ICa,T
calcium current, T-type
- If
pacemaker (funny) current
- IKr
rapid delayed rectifier potassium current (also known as hERG)
- mESC-CM
mouse embryonic stem cell-derived cardiomyocytes
- RTCA
real time cell analyzer
- SC
stem cell
- SC-CM
stem cell-derived cardiomyocytes.
Footnotes
Admyre T: Validation of the conscious mouse model for studying heart rate reduction by T-type calcium channel inhibition [unpublished]. AstraZeneca R&D, Mölndal, Sweden, 2010.
Acknowledgments
This work was supported by ZonMW-NWO (grant no. 114000102 TAB vV).
The authors are grateful for the help and useful comments from Dr. Markus Schmitz, Dr. Udo Eichenlaub, and Dr. Manfred Watzele from Roche Diagnostics (Penzberg, Germany) during alpha tests of the xCELLigence RTCA Cardio instrument and Dr. Blake Anson for providing iCell CMs in the “early access program” from Cellular Dynamics.
Author Disclosure Statement
No competing financial interests exist for any of the authors.
References
- 1.Kola I. Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3:711–716. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 2.Jonsson MKB. van Veen TAB. Goumans M-J. Vos MA. Duker G. Sartipy P. Improvement of cardiac efficacy and safety models in drug discovery by the use of stem cell-derived cardiomyocytes. Expert Opin Drug Discov. 2009;4:357–372. doi: 10.1517/17460440902794912. [DOI] [PubMed] [Google Scholar]
- 3.Nattel S. Duker G. Carlsson L. Model systems for the discovery and development of antiarrhythmic drugs. Prog Biophys Mol Biol. 2008;98:328–339. doi: 10.1016/j.pbiomolbio.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Nerbonne JM. Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev. 2005;85:1205–1253. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- 5.Mangoni M. Nargeot J. Genesis and regulation of heart automaticity. Physiol Rev. 2008;88:919–982. doi: 10.1152/physrev.00018.2007. [DOI] [PubMed] [Google Scholar]
- 6.Braam SR. Tertoolen L. van de Stolpe A. Meyer T. Passier R. Mummery CL. Prediction of drug-induced cardiotoxicity using human embryonic stem cell-derived cardiomyocytes. Stem Cell Res. 2010;4:107–116. doi: 10.1016/j.scr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Caspi O. Itzhaki I. Arbel G, et al. In vitro electrophysiological drug testing using human embryonic stem cell derived cardiomyocytes. Stem Cells Dev. 2009;18:161–172. doi: 10.1089/scd.2007.0280. [DOI] [PubMed] [Google Scholar]
- 8.Dick E. Rajamohan D. Ronksley J. Denning C. Evaluating the utility of cardiomyocytes from human pluripotent stem cells for drug screening. Biochem Soc Trans. 2010;38:1037–1045. doi: 10.1042/BST0381037. [DOI] [PubMed] [Google Scholar]
- 9.He JQ. Ma Y. Lee Y. Thomson JA. Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003;93:32–39. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 10.Jonsson MKB. Duker G. Tropp C, et al. Quantified proarrhythmic potential of selected human embryonic stem cell-derived cardiomyocytes. Stem Cell Res. 2010;4:189–200. doi: 10.1016/j.scr.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Moretti A. Bellin M. Welling A, et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med. 2010;363:1397–1409. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- 12.Otsuji T. Minami I. Kurose Y. Yamauchi K. Tada M. Nakatsuji N. Progressive maturation in contracting cardiomyocytes derived from human embryonic stem cells: qualitative effects on electrophysiological responses to drugs. Stem Cell Res. 2010;4:201–213. doi: 10.1016/j.scr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Pekkanen-Mattila M. Chapman H. Kerkela E, et al. Human embryonic stem cell-derived cardiomyocytes: demonstration of a portion of cardiac cells with fairly mature electrical phenotype. Exp Biol Med. 2010;235:522–530. doi: 10.1258/ebm.2010.009345. [DOI] [PubMed] [Google Scholar]
- 14.Peng S. Lacerda AE. Kirsch GE. Brown AM. Bruening-Wright A. The action potential and comparative pharmacology of stem cell-derived human cardiomyocytes. J Pharmacol Toxicol Methods. 2010;61:277–286. doi: 10.1016/j.vascn.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Yu N. Atienza JM. Bernard J, et al. Real-time monitoring of morphological changes in living cells by electronic cell sensor arrays: an approach to study G protein-coupled receptors. Anal Chem. 2005;78:35–43. doi: 10.1021/ac051695v. [DOI] [PubMed] [Google Scholar]
- 16.Ke N. Wang X. Xu X. Abassi Y. The xCELLigence system for real-time and label-free monitoring of cell viability. Methods Mol Biol. 2011;740:33–43. doi: 10.1007/978-1-61779-108-6_6. [DOI] [PubMed] [Google Scholar]
- 17.Urcan E. Haertel U. Styllou M. Hickel R. Scherthan H. Reichl FX. Real-time xCELLigence impedance analysis of the cytotoxicity of dental composite components on human gingival fibroblasts. Dent Mater. 2010;26:51–58. doi: 10.1016/j.dental.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Itzhaki I. Maizels L. Huber I, et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature. 2011;471:225–229. doi: 10.1038/nature09747. [DOI] [PubMed] [Google Scholar]
- 19.Sartiani L. Bettiol E. Stillitano F. Mugelli A. Cerbai E. Jaconi ME. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: a molecular and electrophysiological approach. Stem Cells. 2007;25:1136–1144. doi: 10.1634/stemcells.2006-0466. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka T. Tohyama S. Murata M, et al. In vitro pharmacologic testing using human induced pluripotent stem cell-derived cardiomyocytes. Biochem Biophys Res Comm. 2009;385:497–502. doi: 10.1016/j.bbrc.2009.05.073. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J. Wilson GF. Soerens AG, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardy MEL. Pollard CE. Small BG, et al. Validation of a voltage-sensitive dye (di-4-ANEPPS)-based method for assessing drug-induced delayed repolarisation in Beagle dog left ventricular midmyocardial myocytes. J Pharmacol Toxicol Methods. 2009;60:94–106. doi: 10.1016/j.vascn.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Liang H. Matzkies M. Schunkert H, et al. Human and murine embryonic stem cell-derived cardiomyocytes serve together as a valuable model for drug safety screening. Cell Physiol Biochem. 2010;25:459–466. doi: 10.1159/000303051. [DOI] [PubMed] [Google Scholar]
- 24.Burashnikov A. Antzelevitch C. Acceleration-induced action potential prolongation and early afterdepolarizations. J Cardiovasc Electrophysiol. 1998;9:934–948. doi: 10.1111/j.1540-8167.1998.tb00134.x. [DOI] [PubMed] [Google Scholar]
- 25.Goethals M. Raes A. van Bogaert P. Use-dependent block of the pacemaker current I(f ) in rabbit sinoatrial node cells by zatebradine (UL-FS 49). On the mode of action of sinus node inhibitors. Circulation. 1993;88:2389–2401. doi: 10.1161/01.cir.88.5.2389. [DOI] [PubMed] [Google Scholar]
- 26.Royer A. Demolombe S. El Harchi A, et al. Expression of human ERG K+ channels in the mouse heart exerts anti-arrhythmic activity. Cardiovasc Res. 2005;65:128–137. doi: 10.1016/j.cardiores.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 27.Stieber J. Wieland K. Stöckl G. Ludwig A. Hofmann F. Bradycardic and proarrhythmic properties of sinus node inhibitors. Mol Pharmacol. 2006;69:1328–1337. doi: 10.1124/mol.105.020701. [DOI] [PubMed] [Google Scholar]
- 28.Lignon JM. Bichler Z. Hivert B, et al. Altered heart rate control in transgenic mice carrying the KCNJ6 gene of the human chromosome 21. Physiol Genomics. 2008;33:230–239. doi: 10.1152/physiolgenomics.00143.2007. [DOI] [PubMed] [Google Scholar]
- 29.Shan J. Kushnir A. Betzenhauser M, et al. Phosphorylation of the ryanodine receptor mediates the cardiac fight or flight response in mice. J Clin Invest. 2010;120:4388–4398. doi: 10.1172/JCI32726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viatchenko-Karpinski S. Fleischmann BK. Liu Q, et al. Intracellular Ca2+ oscillations drive spontaneous contractions in cardiomyocytes during early development. Proc Natl Acad Sci U S A. 1999;96:8259–8264. doi: 10.1073/pnas.96.14.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burges R. Amlodipine: a once daily calcium antagonist. J Hum Hypertens. 1991;5:49–50. [PubMed] [Google Scholar]
- 32.Ertel SI. Clozel J-P. Mibefradil (Ro 40–5967): the first selective T-type Ca2+ channel blocker. Expert Opin Investig Drugs. 1997;6:569–582. doi: 10.1517/13543784.6.5.569. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka H. Komikado C. Namekata I, et al. Species difference in the contribution of T-type calicum current to cardiac pacemaking as revealed by R(-)-efonidipine. J Pharmacol Sci. 2008;107:99–102. doi: 10.1254/jphs.sc0070405. [DOI] [PubMed] [Google Scholar]