Abstract
Oral epithelial cells discriminate between the yeast and hyphal forms of Candida albicans via the mitogen-activated protein kinase (MAPK) signaling pathway. This occurs through phosphorylation of the MAPK phosphatase MKP1 and activation of the c-Fos transcription factor by the hyphal form. Given that fungal cell wall polysaccharides are critical in host recognition and immune activation in myeloid cells, we sought to determine whether β-glucan and N- or O-glycosylation was important in activating the MAPK/MKP1/c-Fos hypha-mediated response mechanism and proinflammatory cytokines in oral epithelial cells. Using a series of β-glucan and N- and O-mannan mutants, we found that N-mannosylation (via Δoch1 and Δpmr1 mutants) and O-mannosylation (via Δpmt1 and Δmnt1 Δmnt2 mutants), but not phosphomannan (via a Δmnn4 mutant) or β-1,2 mannosylation (via Δbmt1 to Δbmt6 mutants), were required for MKP1/c-Fos activation, proinflammatory cytokine production, and cell damage induction. However, the N- and O-mannan mutants showed reduced adhesion or lack of initial hypha formation at 2 h, resulting in little MKP1/c-Fos activation, or restricted hypha formation/pseudohyphal formation at 24 h, resulting in minimal proinflammatory cytokine production and cell damage. Further, the α-1,6-mannose backbone of the N-linked outer chain (corresponding to a Δmnn9 mutant) may be required for epithelial adhesion, while the α-1,2-mannose component of phospholipomannan (corresponding to a Δmit1 mutant) may contribute to epithelial cell damage. β-Glucan appeared to play no role in adhesion, epithelial activation, or cell damage. In summary, N- and O-mannosylation defects affect the ability of C. albicans to induce proinflammatory cytokines and damage in oral epithelial cells, but this may be due to indirect effects on fungal pathogenicity rather than mannose residues being direct activators of the MAPK/MKP1/c-Fos hypha-mediated immune response.
INTRODUCTION
Candida albicans is a commensal fungus and a constituent of the normal mucosal microbiota in humans. Under suitable predisposing conditions, Candida is able to cause a variety of mucosal diseases with significant morbidity (36) and potentially fatal disseminated infections in immunocompromised individuals and patients on immunosuppressive regimens (37). C. albicans is regarded as the most pathogenic Candida species, probably due in part to its ability to form hyphae under different environmental conditions (8) and its superior ability to induce damage and effector responses in different cell types (10–12, 19, 27, 34, 39, 43).
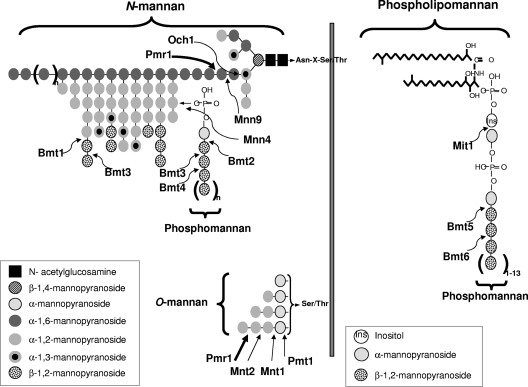
The fungus mediates many of these effects through direct interaction of its cell wall with host cells. The C. albicans cell wall consists of three key polysaccharides: chitin, β-glucans and N-/O-mannans) (16, 18, 26). Chitin (β-1,4-linked homopolymer of N-acetylglucosamine) forms the rigid, inner layer of the cell wall and is covalently attached to β-1,3-glucan, which also contributes to rigidity and is itself attached to branched β-1,6-glucan. Biosynthesis of this β-glucan structure requires the activity of three gene products, those of GSC1 (FKS1), GSL1, and GSL2 (25), together with the small GTPase encoded by RHO1 (17). Cell wall proteins, which are either associated with the wall or covalently attached to the cell wall through a glycosylphosphatidylinositol (GPI) anchor, are glycosylated through N- or O-linkages and are termed mannoproteins. N-Glycosylation is complexed with the outer chains of N-mannans and consists of an α-1,6-mannose backbone to which α-1,2- and α-1,3-linked mannose side chain residues are attached, and requires the activity of multiple genes, including OCH1, PMR1, MNN9, MNN4, MNN1, and MNN2 (families) and BMT1 to BMT4 (BMT1-BMT4) (2, 6, 9, 23, 41). Another major constituent of the cell wall is phospholipomannan (PLM), and glycosylation of PLM requires the gene activity of MIT1, BMT5, and BMT6 (6, 23, 24). O-Linked mannans consist of two to six linear chains of α-1,2-linked mannose residues and require the gene activity of PMR1, PMT genes, MNT1, and MNT2 (1, 29, 42). The locations for the activities of these N- and O-mannan gene products are depicted in Fig. 1 and Table 1, and full details of their function have been reviewed elsewhere (26).
Fig. 1.
Schematic diagram representing the locations of N- and O-mannosylation gene activity on cell wall glycosylation. For N-mannosylation, the first α-1,6-mannose residue is added to the triantennary core N-mannan structure by the α-1,6-mannosyltransferase Och1, followed by a second α-1,6-mannose via Mnn9. This creates an α-1,6-mannose backbone onto which α-1,2- and α-1,3-linked mannose residues are subsequently attached by a series of other Mnn protein family members to form the N-linked outer chain (not shown). Phosphomannan is attached to this outer chain via a phosphodiester bond, which requires Mnn4. β-1,2-Oligomannoses are also added onto the N-linked outer chain via Bmt1 and Bmt3 and onto phosphomannan via Bmt2, Bmt3, and Bmt4. Another major constituent of the cell wall is phospholipomannan, which has α-1,2-mannose attached via Mit1 followed by multiple additions of β-1,2-oligomannoses via Bmt5 and Bmt6. For O-mannosylation, addition of the initial α-mannan is performed by Pmt1, and the remaining α-1,2-mannoses are added by Mnt1 and Mnt2. Pmr1 is not a glycosyltransferase and does not add any mannans directly but exerts its effects on both N-mannosylation and O-mannosylation, as it is a Golgi P-type Ca2+/Mn2+ ATPase that floods the Golgi apparatus with manganese ions, which represent an essential cofactor for the Mnt, Mnn, Och1, Bmt, and Pmt enzymes. The thick arrows represent where truncations in N-mannosylation and O-mannosylation occur.
Table 1.
C. albicans strains utilized in this study
| Strain | Function | Reference/source |
|---|---|---|
| C. albicans SC5314 | Wild-type strain | 7 |
| C. albicans CA14(CIp10) (Ura3+) | Parent of N- and O-mutants | 30 |
| C. albicans BWP17 (Arg4+ His1+ Ura3+) | Parent of Δbmt mutants | 35 |
| C. albicans Δoch1(CIp10) (Ura+) | Och1 is an α-1,6-mannosyltransferase; attaches first α-1,6-mannose residue to triantennary core N-mannan structure | 2 |
| C. albicans Δoch1(CIp10-OCH1) (Ura+) | Revertant strain incorporating one copy of OCH1 | 2 |
| C. albicans Δpmr1(CIp10) (Ura+) | Pmr1 is a Golgi P-type Ca2+/Mn2+ ATPase that floods the Golgi apparatus with manganese ions; essential cofactor for the Mnt, Mnn, Och1, and Bmt enzymes; the Δpmr1 strain is defective in both N-mannosylation and O-mannosylation | 1 |
| C. albicans Δpmr1(CIp10-PMR1) (Ura+) | Revertant strain incorporating one copy of PMR1 | 1 |
| C. albicans Δpmt1(CIp10) (Ura+) | Pmt1 attaches the initial α-mannan to proteins | 42 |
| C. albicans Δmnt1 Δmnt2(CIp10) (Ura+) | Mnt1 and Mnt2 attach the second and third α-1,2-mannans to proteins | 29 |
| C. albicans Δmnt1 Δmnt2(CIp10-MNT1) (Ura+) | Revertant strain incorporating one copy of MNT1 | 29 |
| C. albicans Δmnn4(CIp10) (Ura+) | Mnn4 attaches phosphomannan to the N-linked outer chain via a phosphodiester bond | 9 |
| C. albicans Δmnn9(CIp10) (Ura+) | Mnn9 attaches the second α-1,6-mannose to the triantennary core N-mannan structure after Och1 | 41 |
| C. albicans Δmit1(CIp10) (Ura+) | Mit1 attaches first α-mannan to phospholipomannan | 24 |
| C. albicans Δbmt1(CIp10) (Ura+) | Bmt1 attaches β-1,2-linked mannans onto the N-linked outer chain | 23 |
| C. albicans Δbmt2(CIp10) (Ura+) | Bmt2 attaches β-1,2-linked mannans onto phosphomannan in N-linked outer chain | 23 |
| C. albicans Δbmt3(CIp10) (Ura+) | Bmt3 attaches β-1,2-linked mannans onto the N-linked outer chain and phosphomannan in N-linked outer chain | 23 |
| C. albicans Δbmt4(CIp10) (Ura+) | Bmt4 attaches β-1,2-linked mannans onto phosphomannan in N-linked outer chain | 23 |
| C. albicans Δbmt5(CIp10) (Ura+) | Bmt5 attaches β-1,2-linked mannans onto phosphomannan in phospholipomannan | Provided by D. Poulain |
| C. albicans Δbmt6(CIp10) (Ura+) | Bmt6 attaches β-1,2-linked mannans onto phosphomannan in phospholipomannan | Provided by D. Poulain |
| C. albicans Δgsc1/GSC1 (heterozygote) (Ura−) | Approximate 20% reduction in β-1,6-glucan and 31% reduction in β-1,3-glucan | 25 |
| C. albicans Δgsl1 (Ura−) | Approximate 18% reduction in β-1,6-glucan and 12% reduction in β-1,3 glucan | 25 |
| C. albicans Δgsl2 (Ura−) | Approximate 27% reduction in β-1,6-glucan, but β-1,3-glucan is unaffected | 25 |
Both β-glucan and N-/O-mannan are known to play multiple roles in host interactions and are required for adhesion, immune stimulation and virulence in systemic models of C. albicans infection (15, 29, 33, 42). Recognition of these fungal agonists by myeloid/lymphoid cells (macrophages, monocytes, dendritic cells, and neutrophils) is mediated by pattern recognition receptors (PRRs), including dectin-1 (β-1,3-glucan) (3), Toll-like receptor 2 (TLR2) (phospholipomannan) (14), TLR4 (O-mannan) (32), mannose receptor (N-mannan) (32), and galectin-3 (β-1,2-mannan) (13). Recently, other C. albicans cell wall glycosylated moieties have also been identified as being targets of myeloid cell PRRs, including high-mannose structures (dectin-2 and DC-SIGN [dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin]) (5, 21) and α-mannans (dectin-2) (21, 38). Although polysaccharide components of the C. albicans cell wall are strong activators of myeloid/lymphoid cells (33), it is currently unclear whether they can also activate epithelial immune responses. Given that the epithelial cell is the first cell that encounters C. albicans during mucosal infections, it is important to determine whether C. albicans cell wall β-glucan and N-/O-mannan are also major activating factors of epithelial cells.
Recently, we reported that oral epithelial cells discriminate between C. albicans yeasts and hyphae via a biphasic mitogen-activated protein kinase (MAPK) response (27). Activation of the second MAPK phase, constituting phosphorylation of the MAPK phosphatase MKP1 and induction of the c-Fos transcription factor, correlated directly with hypha formation and was required for full activation of the epithelial cells, resulting in the production of proinflammatory cytokines. In that study, we also demonstrated that purified C. albicans β-glucan and N- and O-mannan did not directly activate the MAPK/MKP1/c-Fos mechanism or proinflammatory cytokines. However, our approach had major limitations, as the fungal agonist preparations were isolated only from C. albicans yeast cells (which do not activate the MAPK/MKP1/c-Fos mechanism) and devoid of protein components. Therefore, these purified polysaccharide components may differ greatly from their steric presentation in the complex structure of a viable cell wall. We hypothesized that epithelial activation via MAPK/MKP1/c-Fos may require hypha-specific modifications in β-glucan composition or specific N- or O-glycosylation of hyphal proteins. Thus, in this study we utilized a series of β-glucan, N- and O-mannan mutants to determine their role (in the context of a viable fungal cell) in mediating activation of the MAPK/MKP1/c-Fos mechanism, proinflammatory cytokines and epithelial damage.
MATERIALS AND METHODS
Cell lines, reagents, and Candida strains.
Experiments were carried out using the TR146 buccal epithelial carcinoma cell line. Monolayer cultures were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS). DMEM-10% FBS was removed, and cells were rinsed with Hanks' balanced salt solution (HBSS) and then incubated with serum-free DMEM the day before (∼16 h) the experiment. Antibodies to phospho-MKP1 and c-Fos were purchased from Cell Signaling Technologies (New England BioLabs, United Kingdom). Mouse monoclonal antibody to human α-actin was purchased from Millipore, and goat anti-mouse and anti-rabbit horseradish peroxidase (HRP)-conjugated antibodies were purchased from Jackson Immunologicals Ltd. (Stratech Scientific, United Kingdom). The fungal strains used are listed in Table 1 and were grown in YPD medium (1% yeast extract, 2% peptone, 2% dextrose) overnight at 30°C to stationary phase prior to experimentation. All growth and experimental conditions with Δgsc1, Δgsl1, and Δgsl2 strains were performed in the presence of 50 μg/ml uridine, as these were originally constructed as Ura− strains.
Candida infection of epithelium and morphological analysis.
C. albicans strains were inoculated onto TR146 monolayer cultures at a multiplicity of infection (MOI) of 10 (10 C. albicans cells per epithelial cell) for signaling work or an MOI of 0.01 for cytokine analysis and cell damage assays. MOIs for assessing signaling and cytokine responses from epithelial cells were previously optimized (27). Monolayers were incubated at 37°C in 5% CO2 for 2 h or 24 h as previously described (27). Noninfected controls contained phosphate-buffered saline (PBS) alone. For morphological analysis, monolayers were fixed in 10% buffered formalin and examined by differential interference contrast (DIC) microscopy at ×400 for the 2-h time point and ×200 for the 24-h time point.
Western blotting.
TR146 oral epithelial cells were lysed using a modified radioimmunoprecipitation assay (RIPA) lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS) containing protease (Sigma-Aldrich, United Kingdom) and phosphatase inhibitors (Perbio, United Kingdom), left on ice for 30 min, and then centrifuged for 10 min in a refrigerated microcentrifuge. Supernatants were assayed for total protein by using the bicinchoninic acid (BCA) protein quantitation kit (Perbio, United Kingdom). A 20-μg sample of protein was separated on 12% NuPAGE Bis:Tris minigels (Invitrogen, United Kingdom) before transfer to polyvinylidene difluoride (PVDF) membranes (GE Healthcare). After probing with primary (1:1,000 dilution) and secondary (1:10,000 dilution) antibodies, membranes were developed using Immobilon chemiluminescent substrate (Millipore, United Kingdom) and exposed to ECL film (GE Healthcare, United Kingdom). α-Actin was used a loading control.
Cytokine determination.
Cytokine levels (interleukin 1α [IL-1α], IL-6, granulocyte colony-stimulating factor [G-CSF], and granulocyte-macrophage colony-stimulating factor [GM-CSF]) in cell culture supernatants were determined at 24 h using Fluorokine MAP cytokine multiplex kits (R&D Systems), coupled with the Luminex 100 machine according to the manufacturer's protocol. The trimmed median value was used to derive the standard curve and calculate sample concentrations.
Cell damage assay.
Epithelial cell damage was determined at 24 h by measuring lactate dehydrogenase (LDH) activity in the culture supernatant as described previously (27, 31, 40). This was performed using the Cytox 96 nonradioactive cytotoxicity assay kit (Promega) according to the manufacturer's protocol and using a recombinant porcine LDH (Sigma-Aldrich) to generate a standard curve. Sample values were then extrapolated from this curve.
Adherence assay.
TR146 oral epithelial cells were grown to confluence in a six-well tissue culture plate and serum starved overnight, and 100 C. albicans yeast cells in 100 μl of PBS were added to each well containing 1 ml serum-free DMEM. Following incubation for 90 min at 37°C in 5% CO2, the nonadhered yeast cells were removed by aspiration. Each well was washed twice (1 ml PBS), overlaid with molten Sabouraud's dextrose agar (SDA) at 45°C, and incubated at 30°C for 24 h for colony development. The total number of fungal cells added (100%) was determined as CFU in control plates. Aliquots of 100 μl of C. albicans yeast cells (from the same yeast suspension used for the adherence assays) were plated in SDA and incubated at 30°C for 24 h for colony development. The numbers of CFU were determined for both experimental and control plates, and adherence was expressed as the percentage of total cells that adhered in the experimental plates.
Statistics.
Cytokine, damage, and adhesion data were analyzed by using a two-tailed t test. In all cases, P values of <0.05 were taken to be significant.
RESULTS
Activation of the epithelial MAPK/MKP1/c-Fos signaling response.
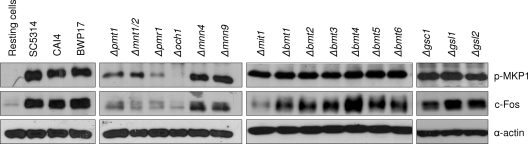
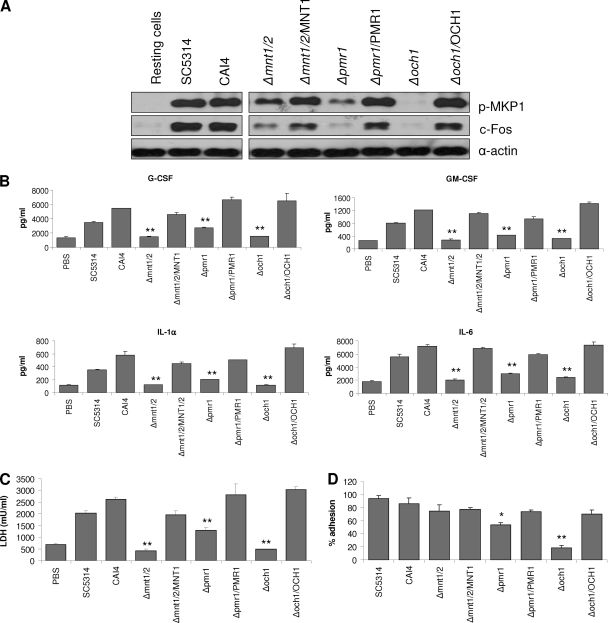
In oral epithelial cells, induction of MKP1 phosphorylation and c-Fos is mediated by the hyphal form of C. albicans and can be assessed at 2 h postinfection (27). To determine the role of β-glucan and protein glycosylation in the activation of epithelial cells, we screened a panel of β-glucan-, N- and O-mannan-, and β-1,2-mannan-deficient mutants (Table 1) and assessed for MKP1 and c-Fos phosphorylation. Western blot analysis of TR146 oral epithelial cells demonstrated that only the N- and O-mannosylation mutants possessed defects in both MKP1 and c-Fos activation, with Δpmr1 and Δoch1 N-mutants exhibiting a more significant defect than Δpmt1 or Δmnt1 Δmnt2 O-mutants (Fig. 2). Both the Δmnn9 and Δmnn4 mutants induced MKP1 phosphorylation and c-Fos at levels similar to those of the parent/wild-type strains, suggesting little role for α-1,6-mannan residues and phosphomannan in MKP1 or c-Fos activation. Interestingly, the Δmit1 mutant was able to induce MKP1 phosphorylation but possessed a reduced ability to induce c-Fos, suggesting a possible role for PLM mannosylation in epithelial cell activation. The β-glucan mutants (Δgsc1/GSC1, Δgsl1/Δgsl1, and Δgsl2/Δgsl2) induced MKP1 phosphorylation and c-Fos to a level similar to that of the parent/wild type, indicating no role for β-glucan in activation of the MAPK-based discriminatory response. (Note that GSC1 appears essential for C. albicans, so a Δgsc1/Δgsc1 null mutant is unavailable [25].) The data suggest that certain features of N- and O-glycosylation of proteins may be required for activation of oral epithelial cells via the MAPK/MKP1/c-Fos pathway.
Fig. 2.
Activation of MAPK signaling by different C. albicans cell wall mutants. Different C. albicans cell wall mutants, the parent strains (CAI4 and BWP17), and wild-type strain (SC5314) were added to TR146 oral epithelial cells under standard culture conditions for 2 h. Total protein was isolated, and phosphorylation of MKP1 and induction of c-Fos were assessed. Bands are shown relative to an α-actin loading control. A fungal/epithelial cell MOI of 10:1 was used. Data are representative of three independent experiments.
Activation of epithelial cytokines and cell damage.
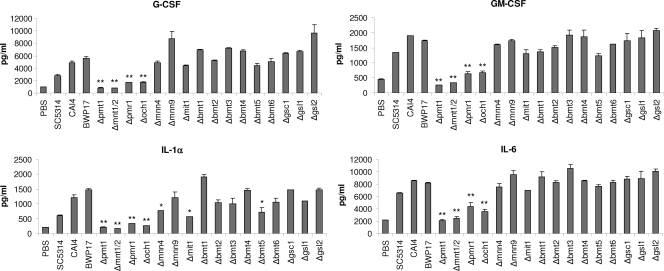
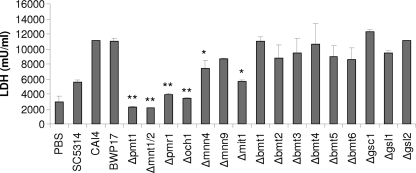
C. albicans-induced MKP1 phosphorylation and c-Fos DNA binding in oral epithelial cells correlates with cytokine production (27). As N-glycosylation and O-glycosylation appeared to be required for MKP1 phosphorylation and c-Fos induction (Fig. 2), we hypothesized that cell wall glycosylation would also contribute to epithelial cytokine production. Analysis of cell culture supernatants of C. albicans-infected TR146 oral epithelial cells demonstrated that both N-glycosylation (Δpmr1 and Δoch1) and O-glycosylation (Δpmt1 and Δmnt1 Δmnt2) mutants were defective in cytokine induction, with near abolishment of G-CSF, GM-CSF, and IL-6 production when using Δpmt1 or Δmnt1 Δmnt2 O-mannan mutants (Fig. 3). All other glycosylation mutants stimulated G-CSF, GM-CSF, and IL-6 at levels similar to those of the parent/wild-type strains. The lack of cytokine production correlated well with a lack of cell damage induction as determined by a significant decrease in the release of lactate dehydrogenase (LDH) into the medium with Δpmr1, Δoch1, Δpmt1, and Δmnt1 Δmnt2 mutants (Fig. 4). Interestingly, the Δmnn4 and Δmit1 mutants also had a reduced ability to damage epithelial cells. The correlation between damage and cytokine release was further supported by the significant reduction in the release of the damage-associated cytokine IL-1α when infecting epithelial cells with Δpmr1, Δoch1, Δpmt1, Δmnt1 Δmnt2, Δmnn4, and Δmit1 strains (Fig. 3). The Δbmt5 mutant also induced a reduced amount of IL-1α, but we are unclear as to the biological meaning of these data, as no other obvious phenotype was evident.
Fig. 3.
Cytokine production by different C. albicans cell wall mutants. Different C. albicans cell wall mutants, the parent strains (CAI4 and BWP17), and a wild-type strain (SC5314) were added to TR146 oral epithelial cells under standard culture conditions for 24 h. Cell culture medium was collected and assessed for cytokine proteins by multiplex microbead assay (Luminex). A fungal/epithelial cell MOI of 0.01 was used. Data represent mean values ± standard errors of the means (SEM) and are representative of at least two independent experiments. *, P < 0.05; **, P < 0.01 (compared with the respective parent strain).
Fig. 4.
Induction of cell damage by different C. albicans cell wall mutants. Different C. albicans cell wall mutants, the parent strains (CAI4 and BWP17), and wild-type strain (SC5314) were added to TR146 oral epithelial cells under standard culture conditions for 24 h. Cell culture medium was collected and assessed for lactate dehydrogenase (LDH) release as a measure of epithelial cell damage. A fungal/epithelial cell MOI of 0.01 was used. Data represent mean values ± SEM and are representative of two independent experiments. *, P < 0.05; **, P < 0.01 (compared with the respective parent strain).
Hypha formation and adherence.
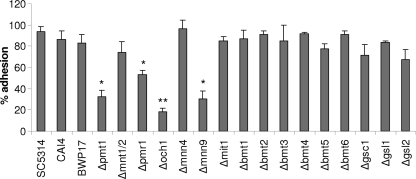
Epithelial MKP1/c-Fos activation, cytokine induction, and cell damage correlate directly with C. albicans hypha formation via a process that is contact dependent (27). Given that N-glycosylation and O-glycosylation of proteins appear to be required for epithelial activation, we assessed whether the respective mutants had any defects in epithelial adherence or hypha formation. Both N-glycosylation and O-glycosylation were required for epithelial adherence, but differing data were obtained for the two respective mutants that represented N-glycosylation and O-glycosylation events (Fig. 5). Of the N-mutants, the Δoch1 mutant adhered significantly less than the Δpmr1 mutant, and of the O-mutants, the Δpmt1 mutant adhered significantly less than the Δmnt1 Δmnt2 mutant. The Δmnt1 Δmnt2 mutant adhered to epithelial cells equally as well as the parent/wild-type strains. Notably, the Δmnn9 mutant also possessed defects in epithelial adherence. All other mutants adhered as well as the parent/wild-type strains.
Fig. 5.
Adhesion of C. albicans cell wall mutants to oral epithelial cells. Yeast cells (100 CFU) of C. albicans cell wall mutants, the parent strains (CAI4 and BWP17), and wild-type strain (SC5314) were added to TR146 oral epithelial cells for 90 min. After extensive washing, molten (45°C) Sabouraud's dextrose agar was added and incubated at 37°C for 24 h for colony development of adhered yeasts. Results are expressed as the percent adhered yeast cells. Data represent mean values ± SEM and are representative of two independent experiments. *, P < 0.05; **, P < 0.01 (compared with the respective parent strain).
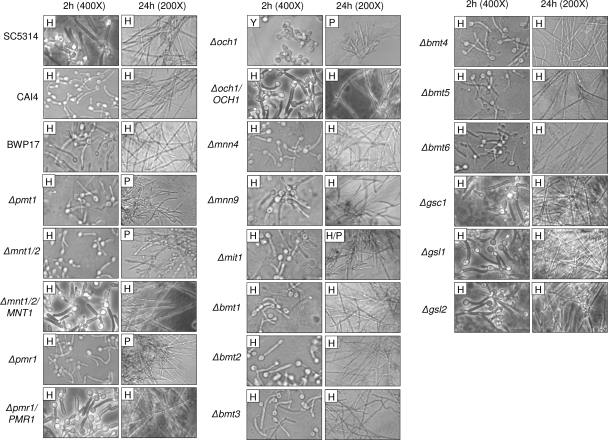
The adherence phenotypes were then compared with the abilities of these mutants to form germ tubes at 2 h (in relation to MKP1 phosphorylation and c-Fos induction) and hyphae at 24 h (in relation to cytokine production and cell damage) (Fig. 6). At 2 h, only the Δoch1 mutant was unable to form germ tubes. While Δpmt1, Δmnt1 Δmnt2, and Δpmr1 mutants appeared to form normal germ tubes at 2 h, these were generally shorter than parent/wild-type germ tubes. All other mutants formed normal germ tubes at 2 h. However, at 24 h, all four N- and O-mutants (Δoch1, Δpmt1, Δmnt1 Δmnt2, and Δpmr1) were unable to form true hyphae and grew as pseudohyphae in our epithelial culture system, and they exhibited significant clumping and aggregate formation. The Δmit1 mutant also exhibited clumping and aggregate formation, but to a lesser extent than the N- and O-mutants, and appeared to grow as a mixture of hyphae and pseudohyphae. All other mutants formed normal hyphae at 24 h.
Fig. 6.
Morphologies of different C. albicans cell wall mutants. Different C. albicans cell wall mutants, the parent strains (CAI4 and BWP17), and wild-type strain (SC5314) were added to TR146 oral epithelial cells for 2 or 24 h and formalin fixed. Morphology was assessed by DIC microscopy at ×400 (2 h) or ×200 (24 h). H, hyphae; P, pseudohyphae; Y, yeast.
Complementation of epithelial activation with reintegrated N- and O-glycosylation strains.
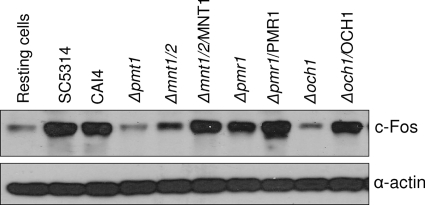
Given the defects in MKP1/c-Fos activation, cytokine release, damage induction, adhesion, and morphology of the N- and O-glycosylation mutants, we utilized C. albicans reintegrated strains in which a single copy of each respective gene (Δmnt1 Δmnt2/MNT1, Δpmr1/PMR1, and Δoch1/OCH1) was reintegrated into the genome to determine whether these phenotype defects could be complemented. We did not have access to the Δpmt1/PMT1 reintegrated strain, so this strain was not tested. In each case the reintegrated strains were able to induce MKP1 phosphorylation, c-Fos, cytokine production, and epithelial damage and restore adhesion and, importantly, hyphal growth (Fig. 6 and Fig. 7), demonstrating that the observed phenotypes were associated with general defects in N-glycosylation and O-glycosylation. Finally, since hypha formation is key to epithelial cell activation via the c-Fos transcription factor (27), we determined whether the reintegrated strains, which form hyphae, could maintain c-Fos activation at 24 h in comparison with the respective mutant strains, which are unable to form hyphae at 24 h. Figure 8 shows that the reintegrated strains activated c-Fos at 24 h at levels similar to those of the parent/wild-type strains, whereas the mutants strains either were unable to (Δpmt1 and Δoch1 mutants) or possessed a significantly reduce ability to (Δmnt1 Δmnt2 and Δpmr1 mutants) activate c-Fos at 24 h.
Fig. 7.
Infection of oral epithelial cells with C. albicans N- and O-glycosylation mutants and reintegrant strains. (A) Immunoblot of phosphorylated MKP1 and c-Fos induction after 2 h infection of TR146 cells with Δmnt1 Δmnt2, Δmnt1 Δmnt2/MNT1, Δpmr1, Δpmr1/PMR1, Δoch1, Δoch1/OCH1, parent (CAI4), and wild-type (SC5314) strains. Production of cytokines (B) and induction of cell damage assessed by LDH release (C) after 24 h infection with these strains. A MOI of 10 was used for panel A, and an MOI of 0.01 was used for panels B and C. (D) Percent adhesion to TR146 cells by these strains after 90 min. Data are representative of three (A) or two (B to D) independent experiments (±SEM). *, P < 0.05; **, P < 0.01 [compared with CAI4(CIp10) parent strain].
Fig. 8.
Late activation of c-Fos by different C. albicans N- and O-glycosylation mutants and reintegrant strains. Different C. albicans N- and O-glycosylation mutants, their reintegrated strains, the parent strain (CAI4), and a wild-type strain (SC5314) were added to TR146 oral epithelial cells under standard culture conditions for 24 h. Total protein was isolated and induction of c-Fos assessed. Bands are shown relative to an α-actin loading control. A fungal/epithelial cell MOI of 0.01 was used. Data are representative of two independent experiments.
DISCUSSION
This study demonstrates that protein glycosylation, but not β-glucan, affects the ability of C. albicans to modulate activation of innate immune responses from oral epithelial cells. Utilizing a series of mutant strains, we found that N-mannosylation and O-mannosylation of proteins, as represented by the Δpmr1, Δoch1, Δpmt1, and Δmnt1 Δmnt2 mutants, affects MKP1/c-Fos activation, proinflammatory cytokines, and cell damage in oral epithelial cells. However, these phenotypes correlated with the inability of these glycosylation mutants to become a filament, maintain hyphae, and/or adhere to epithelial cells. For example, the lack of epithelial activation by the Δoch1 mutant is most probably due to defects in hypha formation (and thus lack of expression of certain hypha-associated proteins) and adherence rather than a direct contribution of the OCH1 gene product to epithelial activation. In contrast, while the Δpmr1 mutant was also defective in epithelial adhesion, it was capable of forming germ tubes, albeit they were shorter than parent/wild-type germ tubes. This ability to form germ tubes explains why the Δpmr1 mutant, unlike the Δoch1 mutant, was able to induce MKP1 phosphorylation and c-Fos at 2 h (albeit weaker than parent/wild-type strains). It should be noted that although the Δpmr1 mutant has truncated N-mannan, it is less truncated than that of the Δoch1 mutant and thus possesses more of the α-1,6-mannan backbone than Δoch1. Together with the fact that the Δpmr1 mutant can form germ tubes and phosphorylate MKP1, this may indicate that N-mannosylation of hyphal proteins is required for MKP1 phosphorylation. However, at 24 h, both Δoch1 and Δpmr1 mutants were unable to form hyphae and grew as pseudohyphal cultures, which explains why both these mutants were unable to induce proinflammatory cytokine responses or cell damage at this later time point, as this appears to require sustained hypha formation (28).
Like the Δpmr1 mutant, the O-mannosylation mutant Δpmt1 and Δmnt1 Δmnt2 strains also formed shorter germ tubes at 2 h and grew as pseudohyphal cultures at 24 h. This again explains why Δpmt1 and Δmnt1 Δmnt2 mutants were able to induce MKP1 phosphorylation and c-Fos (weakly like the Δpmr1 mutant) at 2 h but not proinflammatory cytokine production or cell damage induction at 24 h. Notably, despite similar morphologies at 2 h, both Δpmt1 and Δmnt1 Δmnt2 mutants were able to induce MKP1 phosphorylation to a greater extent than Δpmr1 (but weaker than parent/wild-type strains). Since the Δpmr1 mutant has defects in N-mannan in addition to O-mannan, this supports the conclusion above that N-mannosylation of hyphal proteins may be required for MKP1 phosphorylation. However, since epithelial activation is dependent upon both hypha formation and fungal burdens (i.e., threshold levels of activation need to be reached) (27), it is possible that the Δpmr1 mutant may not express sufficient levels of the hypha-associated proteins required to strongly phosphorylate MKP1 compared with the Δpmt1 or Δmnt1 Δmnt2 mutant, resulting in the lower activation levels.
It is interesting that despite Δpmt1 and Δmnt1 Δmnt2 mutants exhibiting significant differences in epithelial adherence (the Δpmt1 mutant exhibited poor adherence and Δmnt1 Δmnt2 mutant adherence was similar to that of the wild type), neither mutant induced proinflammatory cytokine responses or cell damage at 24 h. This implies that the lack of proinflammatory cytokine production and cell damage by the Δmnt1 Δmnt2 and Δpmt1 mutants was probably due to the inability of these mutants to maintain hypha formation rather than their ability to adhere to epithelial cells. It should be noted that the epithelial adherence phenotype of the Δmnt1 Δmnt2 mutant (it adhered equally well as the wild type) is in contrast to the results of previous studies showing deficiency in adhesion to oral and vaginal epithelial cells (4, 29). However, this is likely explained by differences in using primary versus carcinoma epithelial cells, the type of adhesion assay, and cell culture conditions used between the studies. Thus, the role of MNT1 and MNT2 in adhesion in vivo is still unknown. Together, the data suggest that although N-mannosylation of hyphal proteins may be important for epithelial cell activation, MKP1 phosphorylation, c-Fos activation, proinflammatory cytokine production, and cell damage require maintenance of hypha formation and are probably independent of glycosylation. Since all parent/wild-type phenotypes were restored when a single copy of each gene (OCH1, PMR1, and MNT1) was reintegrated, this suggests that N-glycosylation and O-glycosylation are important for general pathogenicity of C. albicans at mucosal surfaces. We note that some of the N-glycosylation mutants deep within the core of the glycosylation, such as the Δoch1 mutant, may induce alterations in the cell wall proteome. While these proteome changes could affect the repertoire of peptides presented by major histocompatibility complex (MHC) in antigen-presenting cells, any changes in proteome are unlikely to affect carbohydrate-PRR recognition mechanisms. Furthermore, most of the mnt and mnn mutants that have been studied induce only subtle changes in the C. albicans transcriptome (C. A. Munro and N. A. R. Gow, unpublished). Hence, alterations in the immune recognition of the glycosylation mutants used are likely to be due to changes in the carbohydrate repertoire of the fungus and not the proteome.
Above, N-mannosylation is discussed in context of the Δpmr1 and Δoch1 mutants. However, four additional modifications to N-mannosylation, as represented by Δmnn9, Δmnn4, Δmit1, and Δbmt1 to Δbmt6 mutants, were also assessed. The Δmnn9 mutant was able to form normal germ tubes at 2 h and maintain hypha formation at 24 h and was thus able to induce MKP1 phosphorylation, c-Fos, proinflammatory cytokines, and cell damage. However, despite being able to activate epithelial cells, the Δmnn9 mutant exhibited poor adherence. This suggests that the α-1,6-mannose backbone of N-mannan may be required for C. albicans adherence but not for epithelial activation. (Note that sufficient fungal hyphal burdens were still present to permit epithelial activation despite poor adherence.) This is supported by the data for the Δoch1 and Δpmr1 mutants, which also lack the α-1,6-mannose backbone and have significantly reduced adhesion compared with wild-type/parent cells.
Like the Δmnn9 mutant, the Δmnn4 mutant was able to form normal germ tubes at 2 h and maintain hypha formation at 24 h and also induced MKP1 phosphorylation, c-Fos, and proinflammatory cytokines (G-CSF, GM-CSF, and IL-6). Interestingly, however, despite normal adherence, the Δmnn4 mutant exhibited reduced epithelial damage, which correlated with reduced secretion of the damage-associated cytokine IL-1α. Previously, it was shown that phagocytosis by macrophages was significantly reduced for mutants deficient in phosphomannan biosynthesis (22). Therefore, phosphomannan may be required postadhesion for efficient uptake (induced endocytosis) of C. albicans by oral epithelial cells, after which cell damage is induced from within the cell by hypha formation. In summary, although not directly involved in activating epithelial cells via the MKP1/c-Fos pathway, phosphomannan may contribute to induction of epithelial cell damage via a process that is independent of hypha formation and MKP1/c-Fos activation. This supports the conclusions from our previous study in which we proposed that the MAPK/MKP1/c-Fos activation pathway was partly separated from the cell damage pathway (27).
There appears to be no role or a limited role for β-1,2-mannosylation (of the N-linked outer chain, phosphomannan [PLM]) in epithelial adherence and activation, as the Δbmt1 to Δbmt6 mutants adhered to epithelial cells equally as well as the parent/wild type and exhibited no defects in MKP1/c-Fos activation, cytokine production, or cell damage. However, it is likely that there is redundancy within the BMT gene family, allowing for compensatory mechanisms between individual Δbmt mutants. Data interpretation may be further complicated by the fact that some Δbmt mutants are missing β-mannan epitopes on certain molecules but not on others, e.g., the Δbmt2 mutant, like the Δmnn4 mutant, does not express phosphomannan on the N-mannan outer chain but still expresses phosphomannan on PLM (Fig. 1). Nevertheless, for reasons that are unclear at present, the Δbmt5 mutant induced reduced secretion of IL-1α despite inducing normal cell damage.
Although no role for PLM β-1,2-mannosylation in epithelial activation is evident, there may be a potential role for PLM α-mannosylation in epithelial activation, as the Δmit1 mutant was a poor inducer of c-Fos at 2 h despite normal germ tube formation. Interestingly, the Δmit1 mutant was able to phosphorylate MKP1 at 2 h, providing further support that c-Fos induction and MKP1 phosphorylation are not directly linked (27). However, given that GM-CSF, G-CSF, and IL-6 were all induced by the Δmit1 mutant at 24 h owing to its predominantly hyphal growth phenotype, MKP1 phosphorylation was not surprising, as this phosphatase acts as a negative feedback loop for MAPK activation important in cytokine induction. Furthermore, since the NF-κB pathway also controls cytokine production in oral epithelial cells (27), Mit1 (and potentially PLM) potentially induces cytokine responses via NF-κB activation as previously indicated (20). Notably, like the Δmnn4 mutant, the Δmit1 mutant also induced reduced levels of epithelial damage, which again correlated with reduced secretion of the damage-associated cytokine IL-1α. This leads to the interesting proposition that Mit1 (and potentially PLM) may contribute to induction of epithelial damage via a process that is independent of hypha formation. However, it is important to note that the Δmit1 mutant is not only impaired in PLM α-mannosylation and β-mannosylation but also in the biosynthesis of other glycosphingolipids (24). Thus, the phenotypes we observed with the Δmit1 mutant may be due to defects in glycosphingolipids rather than defects in PLM α-mannosylation. (Note that PLM β-mannosylation does not appear important, since the Δbmt5 and Δbmt6 mutants do not exhibit the same phenotypes as Δmit1.) In summary, Mit1 (and potentially PLM or glycosphingolipids) may be involved in both activating epithelial cells via the MAPK/c-Fos pathway and induction of cell damage, although the precise connection between c-Fos activation and cell damage is unclear.
We found no defects in epithelial cell responses to any of the three β-glucan mutants. All three mutants were unaffected in hypha formation or epithelial adherence/activation, suggesting that β-glucan recognition plays little or no role in mediating epithelial-fungal interactions at mucosal surfaces. However, given that the majority of the cell β-glucan remains in these mutants (Table 1), this may still provide a potential role for β-glucan in activating epithelial cells. Our conclusions, though, are supported by the lack of spleen tyrosine kinase (SYK; the kinase activated during dectin-1/β-glucan interactions) phosphorylation in epithelial cells after stimulation with viable C. albicans and the inability of β-glucan particles to activate the MKP1/c-Fos pathway or stimulate cytokine release (27). Furthermore, although the cell wall structure and composition of these three mutants are altered (25), the expression of the hyphal moiety(ies) that activates the epithelial MAPK/c-Fos discriminatory mechanism is unaffected and remains present. Together with the data from our previous study (27), this work provides further evidence that, unlike myeloid cells, dectin-1 is unimportant in epithelial cell anti-Candida responses.
We conclude that N-mannosylation and O-mannosylation of C. albicans proteins are important for epithelial-fungal interaction. However, this interaction appears to be complex, and it is difficult to disassociate the observed phenotypes (defects in MKP1/c-Fos activation, cytokine production, and cell damage) from hypha formation. Previously, we demonstrated that purified C. albicans N- and O-mannans did not activate MKP1/c-Fos, proinflammatory cytokines, or cell damage in oral epithelial cells (27). However, those fungal preparations were isolated from C. albicans yeast cells, which do not activate epithelial cells, and were devoid of protein components. Taking into account data from our previous and current studies, there are a number of possible explanations for the data. The first possible explanation is that N-glycosylation and O-glycosylation of a viable C. albicans cell have properties different from those of purified N- and O-mannans and these properties are required for epithelial activation. However, this is unlikely, given that viable C. albicans yeast cells, which also possess glycosylated proteins, are unable to activate epithelial cells (27). The second possible explanation is that N-glycosylation and O-glycosylation of hyphal proteins are different from N-glycosylation and O-glycosylation of yeast cells and are able to activate epithelial cells directly. To test this, N- and O-mannans from C. albicans hyphae would need to be isolated; however, thus far, this has been complicated by numerous technical issues, and these experiments cannot yet be undertaken (David Williams, personal communication). The third possible explanation is that activation of epithelial cells by N- and O-mannans is secondary to the main activation event, which is driven by specific hyphal proteins, or that glycosylation of these specific hyphal proteins may be required for full epithelial activation. If this is correct, the identities of these hyphal proteins need to be determined. The third option is our current hypothesis and is the most likely explanation for the data, and it would suggest a more indirect role for N-glycosylation and O-glycosylation in epithelial activation. Whichever of these hypotheses is correct, however, it is clear that recognition of C. albicans by epithelial cells does not follow the same route of recognition of carbohydrate moieties through TLRs and dectin-1 as myeloid cells and indicates that epithelial cells differ from myeloid cells fundamentally in how they interact with this fungus.
We conclude that N-glycosylation and O-glycosylation (but not β-glucan composition) affect the ability of C. albicans to activate epithelial cells, but this probably reflects the fundamental importance of glycosylation in C. albicans biology (fitness, pathogenicity, and hypha formation) rather than glycosylation being a direct activator of the MAPK/c-Fos-mediated response that discriminates between yeast and hyphae.
ACKNOWLEDGMENTS
We thank Toshiyuki Mio for supplying Δgsc1, Δgsl1, and Δgsl2 mutants, Joachim Ernst for the Δpmt1 mutant, Susan Southard for the Δmnn9 mutant, and Stephen Challacombe for helpful discussions.
This work was supported by the NIDCR (grant DE017514 to J.R.N.). The construction of glycosylation mutants in the Gow lab was supported by the European Commission (ALLFUN) and the Wellcome Trust (080088). We also acknowledge financial support from the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy's and St. Thomas' NHS Foundation Trust in partnership with King's College London. D.L.M. is supported by a Wellcome Trust Value In People (VIP) award, C. Murciano by a FEMS Advanced Fellowship, and A.I. by a King's College London Overseas Research Studentship.
Footnotes
Published ahead of print on 19 September 2011.
REFERENCES
- 1. Bates S., et al. 2005. Candida albicans Pmr1p, a secretory pathway P-type Ca2+/Mn2+-ATPase, is required for glycosylation and virulence. J. Biol. Chem. 280:23408–23415 [DOI] [PubMed] [Google Scholar]
- 2. Bates S., et al. 2006. Outer chain N-glycans are required for cell wall integrity and virulence of Candida albicans. J. Biol. Chem. 281:90–98 [DOI] [PubMed] [Google Scholar]
- 3. Brown G. D. 2006. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 6:33–43 [DOI] [PubMed] [Google Scholar]
- 4. Buurman E. T., et al. 1998. Molecular analysis of CaMnt1p, a mannosyl transferase important for adhesion and virulence of Candida albicans. Proc. Natl. Acad. Sci. U. S. A. 95:7670–7675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cambi A., et al. 2003. The C-type lectin DC-SIGN (CD209) is an antigen-uptake receptor for Candida albicans on dendritic cells. Eur. J. Immunol. 33:532–538 [DOI] [PubMed] [Google Scholar]
- 6. Fradin C., et al. 2008. Beta-1,2 oligomannose adhesin epitopes are widely distributed over the different families of Candida albicans cell wall mannoproteins and are associated through both N- and O-glycosylation processes. Infect. Immun. 76:4509–4517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gillum A. M., Tsay E. Y., Kirsch D. R. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 198:179–182 [DOI] [PubMed] [Google Scholar]
- 8. Gow N. A. R., Brown A. J. P., Odds F. C. 2002. Fungal morphogenesis and host invasion. Curr. Opin. Microbiol. 5:366–371 [DOI] [PubMed] [Google Scholar]
- 9. Hobson R. P., et al. 2004. Loss of cell wall mannosylphosphate in Candida albicans does not influence macrophage recognition. J. Biol. Chem. 279:39628–39635 [DOI] [PubMed] [Google Scholar]
- 10. Jayatilake J., Samaranayake Y., Samaranayake L. 2008. A comparative study of candidal invasion in rabbit tongue mucosal explants and reconstituted human oral epithelium. Mycopathologia 165:373–380 [DOI] [PubMed] [Google Scholar]
- 11. Jayatilake J. A., Samaranayake L. P., Lu Q., Jin L. J. 2007. IL-1alpha, IL-1ra and IL-8 are differentially induced by Candida in experimental oral candidiasis. Oral Dis. 13:426–433 [DOI] [PubMed] [Google Scholar]
- 12. Jayatilake J. A. M. S., Samaranayake Y. H., Cheung L. K., Samaranayake L. P. 2006. Quantitative evaluation of tissue invasion by wild type, hyphal and SAP mutants of Candida albicans, and non-albicans Candida species in reconstituted human oral epithelium. J. Oral Pathol. Med. 35:484–491 [DOI] [PubMed] [Google Scholar]
- 13. Jouault T., et al. 2006. Specific recognition of Candida albicans by macrophages requires galectin-3 to discriminate Saccharomyces cerevisiae and needs association with TLR2 for signaling. J. Immunol. 177:4679–4687 [DOI] [PubMed] [Google Scholar]
- 14. Jouault T., et al. 2003. Candida albicans phospholipomannan is sensed through toll-like receptors. J. Infect. Dis. 188:165–172 [DOI] [PubMed] [Google Scholar]
- 15. Jouault T., et al. 2009. Host responses to a versatile commensal: PAMPs and PRRs interplay leading to tolerance or infection by Candida albicans. Cell. Microbiol. 11:1007–1015 [DOI] [PubMed] [Google Scholar]
- 16. Klis F. M., Mol P., Hellingwerf K., Brul S. 2002. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 26:239–256 [DOI] [PubMed] [Google Scholar]
- 17. Kondoh O., Tachibana Y., Ohya Y., Arisawa M., Watanabe T. 1997. Cloning of the RHO1 gene from Candida albicans and its regulation of beta-1,3-glucan synthesis. J. Bacteriol. 179:7734–7741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lenardon M. D., Munro C. A., Gow N. A. 2010. Chitin synthesis and fungal pathogenesis. Curr. Opin. Microbiol. 13:416–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li L., Dongari-Bagtzoglou A. 2007. Oral epithelium-Candida glabrata interactions in vitro. Oral Microbiol. Immunol. 22:182–187 [DOI] [PubMed] [Google Scholar]
- 20. Li M., Chen Q., Shen Y., Liu W. 2009. Candida albicans phospholipomannan triggers inflammatory responses of human keratinocytes through Toll-like receptor 2. Exp. Dermatol. 18:603–610 [DOI] [PubMed] [Google Scholar]
- 21. McGreal E. P., et al. 2006. The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology 16:422–430 [DOI] [PubMed] [Google Scholar]
- 22. McKenzie C. G., et al. 2010. Contribution of Candida albicans cell wall components to recognition by and escape from murine macrophages. Infect. Immun. 78:1650–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mille C., et al. 2008. Identification of a new family of genes involved in beta-1,2-mannosylation of glycans in Pichia pastoris and Candida albicans. J. Biol. Chem. 283:9724–9736 [DOI] [PubMed] [Google Scholar]
- 24. Mille C., et al. 2004. Inactivation of CaMIT1 inhibits Candida albicans phospholipomannan beta-mannosylation, reduces virulence, and alters cell wall protein beta-mannosylation. J. Biol. Chem. 279:47952–47960 [DOI] [PubMed] [Google Scholar]
- 25. Mio T., et al. 1997. Cloning of the Candida albicans homolog of Saccharomyces cerevisiae GSC1/FKS1 and its involvement in beta-1,3-glucan synthesis. J. Bacteriol. 179:4096–4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mora-Montes H. M., et al. 2009. Protein glycosylation in Candida. Future Microbiol. 4:1167–1183 [DOI] [PubMed] [Google Scholar]
- 27. Moyes D. L., et al. 2010. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe 8:225–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moyes D., et al. 2011. Activation of MAPK/c-Fos induced responses in oral epithelial cells is specific to Candida albicans and Candida dubliniensis hyphae. Med. Microbiol. Immunol. [Epub ahead of print.] doi: 10.1007/s00430-011-0209-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Munro C. A., et al. 2005. Mnt1p and Mnt2p of Candida albicans are partially redundant alpha-1,2-mannosyltransferases that participate in O-linked mannosylation and are required for adhesion and virulence. J. Biol. Chem. 280:1051–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murad A. M., Lee P. R., Broadbent I. D., Barelle C. J., Brown A. J. 2000. CIp10, an efficient and convenient integrating vector for Candida albicans. Yeast 16:325–327 [DOI] [PubMed] [Google Scholar]
- 31. Naglik J. R., et al. 2008. Quantitative expression of the Candida albicans secreted aspartyl proteinase gene family in human oral and vaginal candidiasis. Microbiology 154:3266–3280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Netea M. G., et al. 2006. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J. Clin. Invest. 116:1642–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Netea M. G., Brown G. D., Kullberg B. J., Gow N. A. R. 2008. An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Microbiol. 6:67–78 [DOI] [PubMed] [Google Scholar]
- 34. Netea M. G., et al. 2010. Variable recognition of Candida albicans strains by TLR4 and lectin recognition receptors. Med. Mycol. 48:897–903 [DOI] [PubMed] [Google Scholar]
- 35. Nobile C. J., Mitchell A. P. 2005. Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Curr. Biol. 15:1150–1155 [DOI] [PubMed] [Google Scholar]
- 36. Odds F. C. 1988. Candida and Candidosis. Bailliere Tindall, Philadelphia, PA [Google Scholar]
- 37. Pfaller M. A., Diekema D. J. 2010. Epidemiology of invasive mycoses in North America. Crit. Rev. Microbiol. 36:1–53 [DOI] [PubMed] [Google Scholar]
- 38. Sato K., et al. 2006. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J. Biol. Chem. 281:38854–38866 [DOI] [PubMed] [Google Scholar]
- 39. Schaller M., et al. 2002. Infection of human oral epithelia with Candida species induces cytokine expression correlated to the degree of virulence. J. Invest. Dermatol. 118:652–657 [DOI] [PubMed] [Google Scholar]
- 40. Schaller M., Zakikhany K., Naglik J. R., Weindl G., Hube B. 2006. Models of oral and vaginal candidiasis based on in vitro reconstituted human epithelia. Nat. Protoc. 1:2767–2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Southard S. B., Specht C. A., Mishra C., Chen-Weiner J., Robbins P. W. 1999. Molecular analysis of the Candida albicans homolog of Saccharomyces cerevisiae MNN9, required for glycosylation of cell wall mannoproteins. J. Bacteriol. 181:7439–7448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Timpel C., Strahl-Bolsinger S., Ziegelbauer K., Ernst J. F. 1998. Multiple functions of Pmt1p-mediated protein O-mannosylation in the fungal pathogen Candida albicans. J. Biol. Chem. 273:20837–20846 [DOI] [PubMed] [Google Scholar]
- 43. van de Veerdonk F. L., et al. 2009. Bypassing pathogen-induced inflammasome activation for the regulation of interleukin-1beta production by the fungal pathogen Candida albicans. J. Infect. Dis. 199:1087–1096 [DOI] [PubMed] [Google Scholar]