Abstract
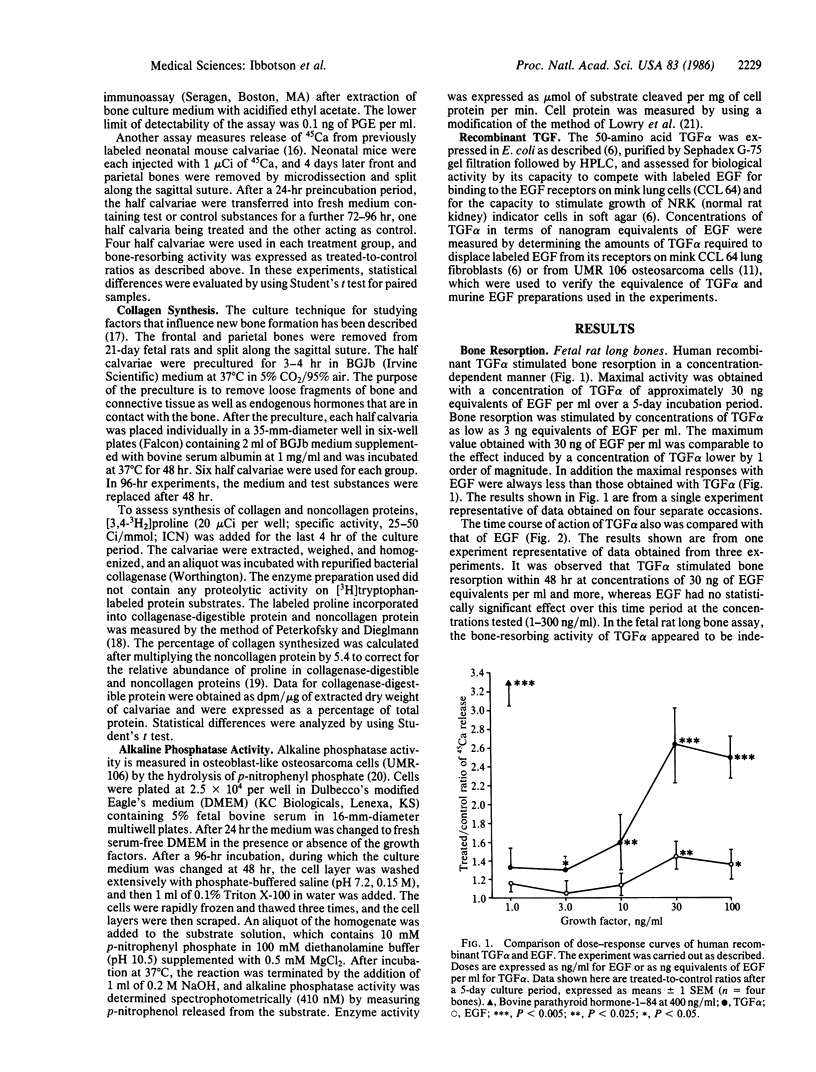
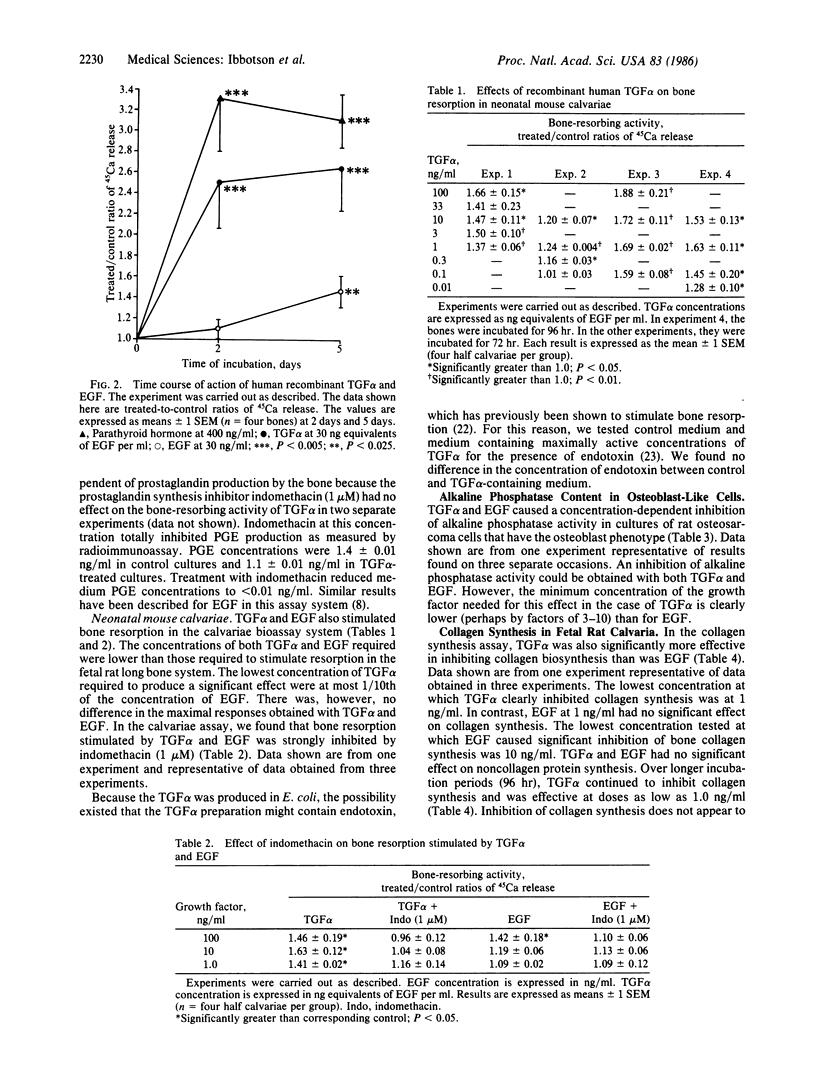
Human recombinant transforming growth factor alpha (TGF alpha), which binds to the epidermal growth factor (EGF) receptor and causes several biological effects similar to those caused by EGF, was compared with murine EGF for its effects on a number of parameters of bone cell metabolism. TGF alpha stimulated bone resorption in two organ culture systems, the fetal rat long bone and neonatal mouse calvarial systems. TGF alpha stimulated bone resorption at concentrations as low as 0.1 ng/ml. TGF alpha effects on bone resorption in mouse calvariae were inhibited by indomethacin, suggesting that, like EGF, its effects were mediated by prostaglandin synthesis. TGF alpha had a different time course of action on bone resorption from that of EGF, causing more rapid release of previously incorporated 45Ca from bone cultures, suggesting that TGF alpha does not function on bone as a simple EGF analogue. TGF alpha also caused effects on osteoblast function resembling those of EGF. It inhibited alkaline phosphatase activity in cultured rat osteosarcoma cells with the osteoblast phenotype and inhibited collagen synthesis in fetal rat calvaria at concentrations of 1.0 ng/ml. The lowest concentration of TGF alpha (expressed as nanogram equivalents of EGF per ml) required to produce a response in all of the systems tested was about 1/10th of that needed for EGF to produce a similar effect. These results indicate that TGF alpha is a potent stimulator of bone resorption and inhibitor of bone formation as assessed by inhibition of collagen synthesis and alkaline phosphatase activity and are consistent with the hypothesis that TGF alpha may be responsible, at least in part, for the bone resorption associated with some tumors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Canalis E. The hormonal and local regulation of bone formation. Endocr Rev. 1983 Winter;4(1):62–77. doi: 10.1210/edrv-4-1-62. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Stoscheck C. M., Preston Y. A., DeLarco J. E. Antibodies to the epidermal growth factor receptor block the biological activities of sarcoma growth factor. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5627–5630. doi: 10.1073/pnas.80.18.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Roberts A. B., Winkler M. E., Chen E. Y., Goeddel D. V. Human transforming growth factor-alpha: precursor structure and expression in E. coli. Cell. 1984 Aug;38(1):287–297. doi: 10.1016/0092-8674(84)90550-6. [DOI] [PubMed] [Google Scholar]
- Dietrich J. W., Canalis E. M., Maina D. M., Raisz L. G. Hormonal control of bone collagen synthesis in vitro: effects of parathyroid hormone and calcitonin. Endocrinology. 1976 Apr;98(4):943–949. doi: 10.1210/endo-98-4-943. [DOI] [PubMed] [Google Scholar]
- Gowen M., Meikle M. C., Reynolds J. J. Stimulation of bone resorption in vitro by a non-prostanoid factor released by human monocytes in culture. Biochim Biophys Acta. 1983 Jun 2;762(3):471–474. doi: 10.1016/0167-4889(83)90014-9. [DOI] [PubMed] [Google Scholar]
- Gregorou M., Rees A. R. Properties of a monoclonal antibody to epidermal growth factor receptor with implications for the mechanism of action of EGF. EMBO J. 1984 May;3(5):929–937. doi: 10.1002/j.1460-2075.1984.tb01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann E., Raisz L. G., Miller W. A. Endotoxin: stimulation of bone resorption in tissue culture. Science. 1970 May 15;168(3933):862–864. doi: 10.1126/science.168.3933.862. [DOI] [PubMed] [Google Scholar]
- Ibbotson K. J., D'Souza S. M., Ng K. W., Osborne C. K., Niall M., Martin T. J., Mundy G. R. Tumor-derived growth factor increases bone resorption in a tumor associated with humoral hypercalcemia of malignancy. Science. 1983 Sep 23;221(4617):1292–1294. doi: 10.1126/science.6577602. [DOI] [PubMed] [Google Scholar]
- Ibbotson K. J., D'Souza S. M., Smith D. D., Carpenter G., Mundy G. R. EGF receptor antiserum inhibits bone resorbing activity produced by a rat Leydig cell tumor associated with the humoral hypercalcemia of malignancy. Endocrinology. 1985 Jan;116(1):469–471. doi: 10.1210/endo-116-1-469. [DOI] [PubMed] [Google Scholar]
- Ibbotson K. J., Twardzik D. R., D'Souza S. M., Hargreaves W. R., Todaro G. J., Mundy G. R. Stimulation of bone resorption in vitro by synthetic transforming growth factor-alpha. Science. 1985 May 24;228(4702):1007–1009. doi: 10.1126/science.3859011. [DOI] [PubMed] [Google Scholar]
- Kumegawa M., Hiramatsu M., Hatakeyama K., Yajima T., Kodama H., Osaki T., Kurisu K. Effects of epidermal growth factor on osteoblastic cells in vitro. Calcif Tissue Int. 1983 Jul;35(4-5):542–548. doi: 10.1007/BF02405091. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levin J., Tomasulo P. A., Oser R. S. Detection of endotoxin in human blood and demonstration of an inhibitor. J Lab Clin Med. 1970 Jun;75(6):903–911. [PubMed] [Google Scholar]
- Marquardt H., Hunkapiller M. W., Hood L. E., Todaro G. J. Rat transforming growth factor type 1: structure and relation to epidermal growth factor. Science. 1984 Mar 9;223(4640):1079–1082. doi: 10.1126/science.6320373. [DOI] [PubMed] [Google Scholar]
- Massague J., Czech M. P., Iwata K., DeLarco J. E., Todaro G. J. Affinity labeling of a transforming growth factor receptor that does not interact with epidermal growth factor. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6822–6826. doi: 10.1073/pnas.79.22.6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy G. R., Ibbotson K. J., D'Souza S. M., Simpson E. L., Jacobs J. W., Martin T. J. The hypercalcemia of cancer. Clinical implications and pathogenic mechanisms. N Engl J Med. 1984 Jun 28;310(26):1718–1727. doi: 10.1056/NEJM198406283102607. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B. The effect of ascorbic acid on collagen polypeptide synthesis and proline hydroxylation during the growth of cultured fibroblasts. Arch Biochem Biophys. 1972 Sep;152(1):318–328. doi: 10.1016/0003-9861(72)90221-4. [DOI] [PubMed] [Google Scholar]
- RAISZ L. G. BONE RESORPTION IN TISSUE CULTURE. FACTORS INFLUENCING THE RESPONSE TO PARATHYROID HORMONE. J Clin Invest. 1965 Jan;44:103–116. doi: 10.1172/JCI105117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisz L. G., Simmons H. A., Sandberg A. L., Canalis E. Direct stimulation of bone resorption by epidermal growth factor. Endocrinology. 1980 Jul;107(1):270–273. doi: 10.1210/endo-107-1-270. [DOI] [PubMed] [Google Scholar]
- Smith J. M., Sporn M. B., Roberts A. B., Derynck R., Winkler M. E., Gregory H. Human transforming growth factor-alpha causes precocious eyelid opening in newborn mice. Nature. 1985 Jun 6;315(6019):515–516. doi: 10.1038/315515a0. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Todaro G. J. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980 Oct 9;303(15):878–880. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- Stewart A. F., Vignery A., Silverglate A., Ravin N. D., LiVolsi V., Broadus A. E., Baron R. Quantitative bone histomorphometry in humoral hypercalcemia of malignancy: uncoupling of bone cell activity. J Clin Endocrinol Metab. 1982 Aug;55(2):219–227. doi: 10.1210/jcem-55-2-219. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Levine L. Epidermal growth factor stimulates prostaglandin production and bone resorption in cultured mouse calvaria. Biochem Biophys Res Commun. 1978 Dec 14;85(3):966–975. doi: 10.1016/0006-291x(78)90638-1. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Voelkel E. F., Lazzaro M., Singer F. R., Roberts A. B., Derynck R., Winkler M. E., Levine L. Alpha and beta human transforming growth factors stimulate prostaglandin production and bone resorption in cultured mouse calvaria. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4535–4538. doi: 10.1073/pnas.82.13.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Fryling C., De Larco J. E. Transforming growth factors produced by certain human tumor cells: polypeptides that interact with epidermal growth factor receptors. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5258–5262. doi: 10.1073/pnas.77.9.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]



