Abstract
Helicobacter pylori infection is associated with several autoimmune diseases, in which autoantibody-producing B cells must be activated. Among these B cells, CD5-positive B-1a cells from BALB/c mice were confirmed to secrete autoantibodies when cocultured with purified H. pylori urease in the absence of T cells. To determine the mechanisms for autoantibody production, CD5-positive B-1a cells were sorted from murine spleen cells and stimulated with either purified H. pylori urease or H. pylori coated onto plates (referred to hereafter as plate-coated H. pylori), and autoantibody production was measured by enzyme-linked immunosorbent assay (ELISA). Complete urease was not secreted from H. pylori but was visually expressed over the bacterium-like endotoxin. Urease-positive plated-coated H. pylori stimulated B-1a cells to produce autoantibodies, although urease-deficient isotype-matched H. pylori did not. Autoantibody secretion by B-1a cells was inhibited when bacteria were pretreated with anti-H. pylori urease-specific antibody having neutralizing ability against urease enzymatic activity but not with anti-H. pylori urease-specific antibody without neutralizing capacity. The B-1a cells externally express various Toll-like receptors (TLRs): TLR1, TLR2, TLR4, and TLR6. Among the TLRs, blocking of TLR2 on B-1a cells with a specific monoclonal antibody (MAb), T2.5, inhibited autoantibody secretion when B-1a cells were stimulated with plate-coated H. pylori or H. pylori urease. Moreover, B-1a cells from TLR2-knockout mice did not produce those autoantibodies. The present study provides evidence that functional urease expressed on the surface of H. pylori will directly stimulate B-1a cells via innate TLR2 to produce various autoantibodies and may induce autoimmune disorders.
INTRODUCTION
Helicobacter pylori causes not only a variety of gastroduodenal diseases but also various autoimmune disorders, such as rheumatoid arthritis (RA) (16), idiopathic thrombocytopenic purpura (ITP) (7), and Sjogren's syndrome (SjS) (3); however, the actual underlying relationship between H. pylori infection and the induction of autoimmune diseases remains unknown.
The relationship between pathogen intrusion and the induction of autoimmune disorders has been defined over the last decade; for example, infection of BALB/c mice with either coxsackievirus or murine cytomegalovirus results in the development of myocarditis and the production of autoantibodies to cardiac myosin from 28 days after infection (25). Thus, T cells and autoantibodies specific for the pathogens in the acquired immunity have been thought to be critical for the induction of autoimmune disorders; however, in some cases, infectious viruses, the causative agents, cannot be detected after 14 days of infection and actual evidence of molecular mimicry in the development of myocarditis has not been confirmed (5). This strongly indicates that the nonspecific adjuvant effect (2) derived from pathogens and damaged self-components produced via infection may persistently stimulate innate immune responses to progress chronic autoimmune disorders. These innate immune cells generally do not respond to specific antigenic epitopes on pathogens but do react against pathogen-associated molecular patterns (PAMPs) via pattern recognition receptors, such as Toll-like receptors (TLRs). Thus, autoimmunity accompanied by the production of various autoantibodies is possibly elicited through continual stimulation of innate TLRs by some PAMPs of pathogens causing chronic infection.
In our previous study, we found that purified urease isolated from H. pylori activated murine B cells to produce various autoantibodies, such as immunoglobulin M (IgM)-type rheumatoid factor (RF IgM), anti-single-stranded DNA antibody, and antiphosphatidylcholine (anti-PC) antibody, in a T-cell-independent manner (33). Moreover, as expected, B cells able to be stimulated by H. pylori urease are CD5+ innate B-1a cells that predominantly secrete IgA-, IgM-, and IgG3-type antibodies. In contrast to T-cell-dependent B-2 cells in the acquired arm, T-cell-independent innate B-1a cells mainly localize in the peritoneal and pleural cavities or mucosal compartments so that they may come into direct contact with H. pylori at the gastric mucosa.
In the present study, we found that urease is not actively secreted from H. pylori but, rather, is expressed on the surface of spiral-shaped bacteria and that urease-positive H. pylori-coated plates appear to stimulate purified CD5+ B-1a cells to produce various autoantibodies, although urease-deficient bacteria do not. Moreover, autoantibody secretion by B-1a cells was apparently inhibited when bacterium-coated plates were pretreated with anti-H. pylori urease-specific antibody. In this case, antibodies that could abrogate the enzymatic activity of bacterial urease, such as UB-33-specific antibodies (14), showed an inhibitory ability. Furthermore, blocking of TLR2 on B-1a cells with a specific monoclonal antibody (MAb), T2.5 (19), significantly inhibited the secretion of autoantibodies when stimulated with H. pylori-coated urease-positive plates. This was confirmed by using B-1a cells from TLR2-knockout mice.
These findings indicate that the functional urease expressed on the surface of H. pylori directly stimulates TLR2 on innate B-1a cells in the gastric mucosa to produce various autoantibodies like PAMPs and may induce autoimmune disorders.
MATERIALS AND METHODS
Animals.
Six- to 8-week-old female BALB/c mice and 10-week-old female Japanese white rabbits were purchased from Nisseizai (Tokyo, Japan), and 6-week-old female TLR2-knockout (TLR2−/−) BALB/c mice (29) were purchased from Oriental Bioservice (Kyoto, Japan). These animals were maintained in microisolator cages under pathogen-free conditions and fed autoclaved laboratory chow and water. All animal experiments were performed according to the guidelines of the National Research Council Guide for the Care and Use of Laboratory Animals (22a) and approved by the Review Board of Nippon Medical School.
Bacterial strains and growth conditions.
The bacteria used in the present study were two known wild-type H. pylori strains, Sydney strain 1 (SS-1) (17) and NCTC 11637 (4). To obtain a large amount of bacterial cells, we used the following methods, as described previously (8). In brief, either the SS-1 or NCTC 11637 isolate was cultured on brucella agar (BD Biosciences, San Diego, CA) containing 5% horse serum (Sigma-Aldrich, St. Louis, MO) and 1% β-cyclodextrin (Wako Junyaku, Osaka, Japan) at 37°C under microaerophilic conditions (5% O2, 15% CO2, and 80% N2) with an AnaeroPack sachet (MicroAero; Mitsubishi Gas Chemical, Tokyo, Japan). After a 2-day culture, the colonies were harvested by scraping with a sterile metal spatula, transferred to 50 ml brucella broth (BD Biosciences) containing 5% horse serum and 1% β-cyclodextrin, and further cultured for 24 h at 37°C. Then, 500 μl cell-containing medium was plated on brucella agar for an additional 3 days at 37°C, and the grown bacterial cells were harvested and washed with cold phosphate-buffered saline (PBS) at pH 7.0. The cells were sedimented by centrifugation (10,000 × g for 10 min at 4°C), and the cell pellet was stored at −80°C. A urease-negative mutant of H. pylori, HPP1801, which originated from the NCTC 11637 isolate (32), a kind gift from Tomoko Mizote at Yamaguchi Prefectural University, was cultured on brucella broth containing 5% horse serum, 1% β-cyclodextrin, and 10 μg/ml kanamycin (Wako Junyaku).
Purification of H. pylori urease.
H. pylori urease was purified biochemically as described previously (33). Briefly, the stored cell pellet containing about 1 g H. pylori cells was thawed and vortexed with sterile distilled water. The cells were removed from the mixture by centrifugation, and the supernatant was filtered with a 0.22-μm-pore-size filter (Millipore, Billerica, MA) to obtain water extract. Then, the column containing Cellufine sulfate (Millipore) was equilibrated with PE65 buffer (20 mM phosphate buffer [PB] and 1 mM EDTA at pH 6.5). About 6.5 ml of prepared water extract was then applied to the column and eluted with the PE65 buffer. Urease-containing fractions were harvested by measuring enzyme activity, adjusted to pH 5.5, and adsorbed to the second-step column that had been preequilibrated with an another buffer, termed PO55 (20 mM PB at pH 5.5), for washing. Gel-bound urease was also eluted using PO74 buffer (20 mM PB and 0.15 M NaCl at pH 7.4). Each eluted fraction was analyzed for its enzyme activity quantitatively, and the positive fractions were collected in a single tube. For the collected sample, it was confirmed whether it contained H. pylori urease by Western blot analysis, as described below. The purity of the eluted urease was examined by silver staining using a silver staining kit (Wako Junyaku), and the purified urease protein concentration was estimated using a Micro BCA protein assay reagent kit (Pierce Co., Inc., Rockford, IL).
Western blotting for H. pylori urease.
Samples were loaded onto a sodium dodecyl sulfate (SDS)-polyacrylamide gel for electrophoresis and then transferred to a nitrocellulose-polyvinylidene difluoride membrane (Atto, Tokyo, Japan) using blotting buffer (2 M Tris [pH 8.0], 1.43% glycine, 5% methanol). The blots were blocked overnight at 4°C with Block Ace (Dainihon Seiyaku, Osaka, Japan). The membrane was incubated with anti-whole H. pylori urease-specific antibody from immune rabbit serum and two murine H. pylori urease-specific MAbs, termed L2 for the large subunit (UreB) (22) and S2 for the small subunit (UreA) (15). The blots were washed five times with PBS containing 0.05% Tween 20 (T-PBS) and incubated with biotinylated goat anti-rabbit immunoglobulin (Ig) and anti-mouse Ig (Vector Laboratories, Burlingame, CA) at 1:200 in PBS for 2 h at room temperature (RT). After being washed three times, the blots were incubated with streptavidin-alkaline phosphatase (Nichirei, Tokyo, Japan) diluted 1:5 in PBS for 30 min at RT. Then, the blots were detected with a ProtoBlot nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate color development system (Promega Corporation, Madison, WI).
Purification of H. pylori lipopolysaccharide (LPS).
Cells were lysed in Tris HCl (pH 6.8) containing 2% SDS at 90°C for 10 min. Protein content was measured using the BCA protein assay reagent kit. Samples of cell lysates were adjusted to equal protein contents (1 mg/ml) and then proteolyzed in reaction mixtures containing Tris HCl, SDS, and proteinase K at 60°C for 2 h. The proteolyzed samples were extracted with hot phenol, mixed with equal volumes of water and phenol, and incubated at 70°C with repeated vortexing for 20 min. After centrifugation at 12,000 × g for 15 min at 10°C, the aqueous phases were collected. The phenolic phases were reextracted with 1 volume of H2O at 70°C for 10 min, and the centrifugation was repeated. The combined aqueous extracts were adjusted to 0.5 M NaCl, precipitated with 10 volumes of ethanol in the refrigerator overnight, and then centrifuged at 20,000 × g for 20 min at 10°C and air dried.
Electrophoretic analyses of H. pylori LPS.
Samples were applied to a 15% polyacrylamide separating gel containing 3.2 M urea and a 5% stacking gel. After electrophoresis at 25 mA for 90 min, the gel was either fixed for silver staining (30) or transferred to a polyvinylidene difluoride membrane using blotting buffer (20). Primary antibody, monoclonal anti-Lewis x (Lex; clone P12 [21]; Signet, Dedham, MA) or anti-Lewis y (Ley; 1:100; clone F3 [21]; Signet), and secondary antibody (biotinylated goat anti-mouse IgM; 1:1,000; BD Bioscience) were diluted in PBS containing 0.5% bovine serum albumin (BSA) (13). Then, streptavidin peroxidase (Nichirei) was diluted 1:5 in PBS. The blots were detected with a tetramethylbenzidine (TMB) development system (Vector Laboratories, Burlingame, CA).
Preparation of anti-H. pylori urease-specific antibodies.
Anti-H. pylori urease-specific antibodies were obtained from rabbits immunized with either purified urease or synthetic peptides purchased from Takara Bio (Tokyo, Japan). To induce the neutralizing antibody that abrogates the enzymatic activity of H. pylori urease, a 19-mer peptide, UB-33 (amino acids 321 to 339 [CHHLDKSIKEDVQFADSRI]), from a large subunit of H. pylori urease (UreB) (14) was administered intramuscularly into rabbits with the same volume of complete Freund adjuvant. At 2 and 8 weeks after immunization, rabbits were boosted with UB33 peptide mixed with the same volume of incomplete Freund adjuvant. Antibody-containing serum was collected 3 weeks after the last immunization and purified using a protein A column (GE Healthcare, Uppsala, Sweden).
Detection of H. pylori urease in growth medium.
A 100-μl aliquot of the known H. pylori urease-specific MAb L2 (5 μg/ml) (14) was added to 96-well flat-bottom plates (Thermo Fisher Scientific, Roskilde, Denmark), and the mixture was incubated overnight at 4°C. After incubation, antibody-coated plates were blocked with PBS containing 1% BSA for 3 h at RT and washed twice with T-PBS. A 50-μl aliquot of sample was added, and 50 μl diluted anti-whole urease rabbit serum (1:200) was added. After washing with T-PBS, 50 μl diluted peroxidase-conjugated goat antirabbit antibody (Rockland, Gilbertsville, PA) was added, the mixture was incubated with 50 μl TMB-soluble reagent (Scy-Tek Laboratories, Logan, UT) for 20 min, and 50 μl TMB stopping solution (Scy-Tek Laboratories) was added. The color changes were quantitated by measurement of the absorbance at 450 nm with a microplate reader (Bio-Rad, Hercules, CA).
Immunohistological staining for H. pylori urease and LPS.
Cultured H. pylori was plated on a micro-slide glass and fixed with 4% paraformaldehyde (PFA)-PBS (pH 7.2) for 30 min at RT. In the light microscopic view, after washing with PBS, blocking solution (PBS containing 1% BSA and 0.3% normal goat serum) was added to the micro-slide glass and incubated for 1 h. After washing with PBS, anti-urease-specific rabbit serum was added and the mixture was incubated for an additional 1.5 h. Then, biotinylated goat antirabbit antibody (Dako, Glostrup, Denmark) was added for 1 h and the mixture was incubated with streptavidin complex (ABC complex kit; Vector Laboratories) for 20 min. After washing with PBS, diaminobenzidine (DAB; Vector Laboratories) was added and the reaction was stopped with distilled water.
As for H. pylori LPS staining, either monoclonal anti-Lewis x (clone P12) or anti-Lewis y (clone F3) (1:40) was added and the mixture was incubated at 37°C for 2 h. After washing with PBS, secondary antibody (biotinylated goat anti-mouse IgM, 1:200) was added, and the mixture was incubated for an additional 1 h and then further incubated with streptavidin complex (ABC complex kit; Vector Laboratories) for 30 min. After washing with PBS, DAB (Vector Laboratories) was added and the reaction was stopped with distilled water. In electron microscopic analysis, fixed H. pylori cells were embedded in a 1% agarose gel, which was then cut into thin sections (1 to 2 mm3) and stained with anti-urease-specific rabbit serum. The stained block was fixed with 2.5% glutaraldehyde-0.1 M PB (pH 7.3) and 1% osmium tetroxide-0.1 M PB. Then, the sample was dehydrated with a series of graded ethanol and polymerized with Epon 812 resin (TAAB Laboratories, Berks, United Kingdom). Thin sections were made using an ultramicrotome (Leica, Solms, Germany), and the sections were stained with lead citrate and observed with an electron microscope (JEOL-1010; Nihon Denshi, Tokyo, Japan).
Purification and sorting of B-1a cell subset.
After red blood cells had been depleted with ammonium chloride, the remaining splenic lymphocytes or peritoneal cavity-derived cells from mice were incubated with anti-Thy1.2 MAb (Serotec Ltd., Oxford, United Kingdom), followed by the addition of rabbit complement (Cederane, Ontario, Canada) at 37°C for 1 h to deplete T lymphocytes as described previously (28). Live cells were harvested and confirmed to be B cells of >90% purity by flow cytometry (BD Bioscience) using fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse B220 MAb (RA3-6B2; PharMingen, San Diego, CA). Purified B cells were incubated in 200 μl RPMI 1640-based culture medium (CM) containing 10% heat-inactivated fetal calf serum (FCS), 20 mM HEPES (Gibco BRL, Grand Island, NY), 10 mM 2-mercaptoethanol (Sigma-Aldrich), 100 U/ml penicillin, 0.1 mg/ml streptomycin, and 10 μg/ml gentamicin at 37°C in a 5% CO2 atmosphere on 96-well U-bottom plates (BD Falcon, Franklin Lakes, NJ). To obtain a B-1a (B220+/CD5+) or B-2 (B220+/CD5−) cell subset, purified splenic B cells were further sorted using a FACSAria-II cell sorter (BD Bioscience) and FITC-conjugated rat anti-mouse B220 MAb and phycoerythrin (PE)-conjugated anti-mouse CD5 MAb (53-7.3; PharMingen). Sorted cells were confirmed to be either B-1a or B-2 cells of >90% purity. In some experiments, we used peritoneal cavity-derived B-1a cells (perC B-1a cells), obtained by flushing the peritoneal cavity with RPMI 1640 supplemented with 10% FCS and 1 mM EDTA. perC B-1a cells were isolated using a two-step process. First, macrophages were depleted with two successive pannings of adherent cells for 2 h at 37°C in 5% CO2, followed by depletion of T cells with antibodies to the pan-T-cell marker Thy1.2 with Dynabeads according to the manufacturer's instructions (Dynal Biotech, Oslo, Norway). The purified cells were 92% B220+ CD5+ B-1a cells. However, we could always obtain fewer perC B-1a cells than sorted splenic B-1a cells from a mouse. Thus, we used sorted splenic B-1a cells for further experiments.
Enzyme-linked immunosorbent assay (ELISA).
Purified B cells, B-1a cells, or B-2 cells either were added to H. pylori coated onto plates (referred to hereafter as plate-coated H. pylori) or cultured with 10 μg/ml purified H. pylori urease or PBS for 7 days in vitro, and the culture supernatants were harvested and analyzed. All the experiments were performed in triplicate.
Detection of IgG3.
A 50-μl aliquot of affinity-purified rabbit anti-mouse IgG3 (10 μg/ml in PBS; Rockland) was added and the mixture was incubated at 4°C. After overnight incubation, the antigen-coated plates were blocked with BSA in PBS, and then 50 μl cultured supernatant was plated for an additional 60 min. After washing with T-PBS, 50 μl diluted biotinylated goat anti-mouse Ig (1:5,000; Amersham Bioscience) was added for 60 min, followed by horseradish peroxidase-avidin D (1:2,000; Vector Laboratories). After washing with T-PBS, 50 μl TMB staining solution was added, the mixture was incubated for 20 min, and a 100-μl aliquot of TMB stopping solution was added. The changes were quantitated by measurement of the absorbance at 450 nm with a microplate reader. On the basis of the mouse reference serum for standard IgG3 (Bethyl Laboratories, Inc., Montgomery, TX), we determined the concentration of serum IgG3.
Detection of ssDNA.
Stock solution containing calf thymus DNA (type I; 1 mg/ml in H2O; Sigma-Aldrich) was boiled for 10 min in a 1/10 volume of 1 N NaOH. The boiled solution was immediately put on ice for 10 min and diluted to 3 μg/ml with cold borate-buffered saline. A 100-μl aliquot of prepared single-stranded DNA (ssDNA) was added and blocked with 0.5% BSA and 0.04% Tween 20 in borate-buffered saline (BBT). A 50-μl aliquot of diluted (1:10) culture supernatant was incubated overnight at 4°C, a 50-μl aliquot of diluted biotinylated goat anti-mouse Ig (1:5,000) was added, and bound Ig was detected with a similar procedure. On the basis of the mouse reference serum for standard anti-ssDNA MAb (clone F7-26; Millipore), we determined the concentration of anti-ssDNA.
Detection of PC.
A 100-μl aliquot of l-α-phosphatidylcholine from frozen egg yolk (type XIII-E; 50 μg/ml in ethanol; Sigma-Aldrich) was added to the plates, and the plates were incubated overnight at 4°C. After blocking, a 50-μl aliquot of the culture supernatant was plated, followed by biotinylated goat anti-mouse Ig, and bound Ig was measured with a similar procedure.
Detection of RF IgM.
RF IgM was detected with an RF IgM (mouse) ELISA kit (Shibayagi, Gunma, Japan). According to the manufacturer's procedure, IgM type RF was measured.
Coating of culture plates with H. pylori.
The grown H. pylori cells were harvested and sedimented by centrifugation (3,000 × g for 10 min). The cell pellet was adjusted to a McFarland index 2 standard with PBS. For fixation, the same volume of 2% PFA solution was added to the pellet, and the mixture was incubated for 10 min at 10°C (9). Then, a 100-μl aliquot of PFA-fixed H. pylori was further incubated for 40 min at 60°C for plate coating.
Blocking of urease on the surface of H. pylori with specific rabbit antibodies.
After coating 96-well round-bottom plates with H. pylori, 50 μg diluted (1:200) antibodies for H. pylori urease (anti-whole urease antibody, anti-UB-33 antibody) was added to each well, the plates were incubated at 37°C for 90 min, and the cells were washed three times with CM.
Effect of antibodies on H. pylori urease activity.
To investigate the inhibitory effect of antibodies on the enzymatic activity of H. pylori urease, the urease (0.2 μg in 50 μl of 20 mM PB, pH 6.8) was mixed with purified antibody in 96-well plates. The mixture was incubated for 90 min. After incubation, 100 μl of reacted solution was mixed with 100 μl of 100 mM Sorensen's PB (pH 6.8) containing 100 mM urea (Wako) and 0.005% phenol red (Wako). Using a microreader, the time course of the color development was monitored by measurement of the absorbance at 540 nm at 60-min intervals for 7 h.
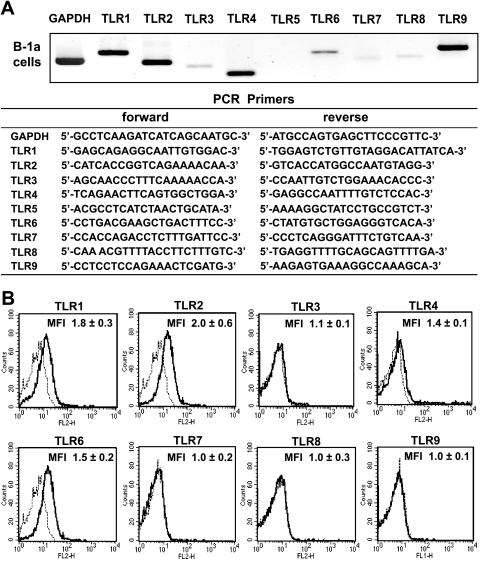
Detection of TLRs by reverse transcription-PCR analysis.
Total RNA was extracted from 3 × 105 cells of each cell preparation using a commercial RNeasy kit (Qiagen, Hilden, Germany). Transcripts of TLRs as well as the housekeeping gene glyceraldehyde-3-phosphate (GAPDH) were amplified by PCR. The primer sets used to perform the PCR analysis are shown in Fig. 6A. After 35 cycles of PCR, the PCR products were resolved by electrophoresis in agarose gels and visualized by ethidium bromide staining using a UV light source.
Fig. 6.
TLR expression on murine B-1 cells. (A) TLR expression in B-1a cells was analyzed by reverse transcription-PCR. B-1a cells strongly expressed TLR1, TLR2, TLR4, TLR6, and TLR9 and weakly expressed TLR3, TLR7, and TLR8. (B) Surface expression of TLRs on purified B-1a cells was also analyzed by flow cytometry. B-1a cells expressed TLR1, TLR2, TLR4, and TLR6 on their surface. MFI, maximum fluorescence intensity.
Flow cytometric analysis of TLRs on B-1a cells.
Purified B-1a cells were stained with the following anti-mouse TLR-specific rat antibodies and PE-conjugated goat anti-rat IgG (clone 405406; BioLegend, San Diego, CA) and analyzed by flow cytometry: anti-mouse TLR1 antibody (clone TR23; eBioscience, San Diego, CA), anti-mouse TLR2 antibody (clone 6C2; eBioscience) (23), anti-mouse TLR3 antibody (clone T3.7C3; eBioscience), anti-mouse TLR4/MD-2 antibody (clone MTS510; eBioscience), and anti-mouse TLR6 antibody (clone 41861; R&D Systems Inc., Minneapolis, MN). B-1a cells were also stained with either PE-conjugated anti-mouse TLR7 antibody (polyclonal; Imgenex, San Diego, CA), PE-conjugated anti-mouse TLR8 antibody (clone 44C143; Imgenex), or FITC-conjugated anti-mouse TLR9 antibody (clone M9.D6; eBioscience).
Blocking of cell surface TLRs on B-1a cells.
Purified murine B-1a cells were incubated with anti-mouse TLR1 antibody, anti-mouse TLR2 (clone T2.5; eBioscience) (19), anti-mouse TLR4/MD-2 antibody, anti-mouse TLR6 antibody, or anti-mouse IgG1 isotype control (clone 11711; R&D Systems) (final concentration, 1 μg/106 cells) for 30 min at 37°C. After incubation, cells were washed three times with CM and added to round-bottom 96-well plates.
Statistical analysis.
The results were analyzed using Student's t test, and the results are presented as means ± standard deviations (SDs). Differences at P values of <0.05 were considered significant.
RESULTS
Secretion of anti-ssDNA-specific autoantibody by purified murine B-1a cells stimulated with purified H. pylori urease.
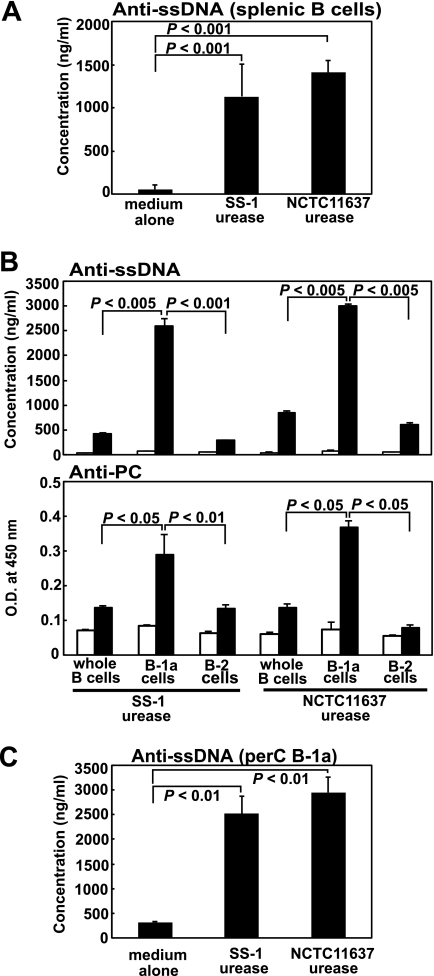
We have reported previously that murine B cells, in particular, CD5+ B-1a cells, secreted various autoantibodies, such as IgM-type RF, anti-ssDNA-specific antibody, and anti-PC-specific antibody, when stimulated with purified H. pylori urease in vitro (33). Here, we confirmed the production of anti-ssDNA-specific autoantibody by enriched murine splenic B cells stimulated with purified H. pylori urease from either isolate SS-1 or isolate NCTC 11637 (Fig. 1A). We observed similar results in autoantibody production against ssDNA and PC when sorted splenic CD5+ B-1a cells but not CD5−− B-2 cells were stimulated with purified H. pylori urease (Fig. 1B). These findings indicate that among B cells, murine B-1a cells are the actual targets for H. pylori urease. We have seen similar results by using murine B-1a cells obtained from the peritoneal cavity (perC B-1a) (Fig. 1C). Thus, there seems to be no difference between splenic B-1a cells and perC B-1a cells in the response against purified H. pylori urease to produce autoantibodies. However, we could always obtain far fewer perC B-1a cells than sorted splenic B-1a cells from a mouse. Thus, we used sorted splenic B-1a cells for further experiments.
Fig. 1.
Autoantibody secretion from purified murine splenic B cells as well as their sorted B-1 or B-2 cells stimulated with purified H. pylori urease. (A) Purified B cells (1 × 106) were cultured with 10 μg/ml purified H. pylori urease from either the SS-1 or NCTC 11637 isolate for 7 days, and the supernatants were harvested to measure their anti-ssDNA autoantibody production by ELISA. Data are means ± SDs (n = 6). (B) Either 2 × 105 purified B cells or the same number of their sorted B-1a or B-2 cells were cultured with 10 μg/ml purified H. pylori urease (filled columns) or PBS (open columns) for 7 days in CM, and the supernatants were harvested to measure either their anti-ssDNA (top) or anti-PC (bottom) autoantibody production. Data are means ± SDs (n = 6). O.D., optical density. (C) Purified peritoneal cavity-derived B-1a (perC B-1a) cells (1 × 105) were cultured with 10 μg/ml purified H. pylori urease for 7 days, and the supernatants were harvested to measure their anti-ssDNA autoantibody production. Statistical significance was determined by the Student t test, and the values are given in each graph.
Detection and localization of H. pylori urease.
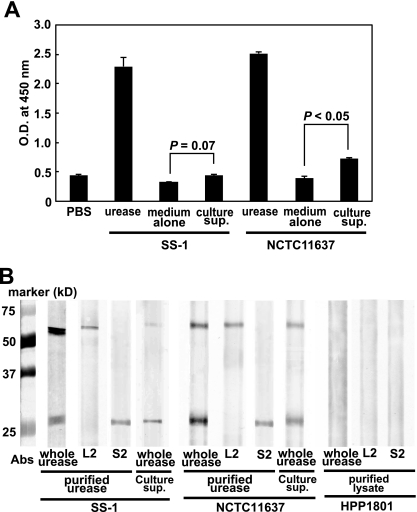
To confirm whether urease is actually secreted from H. pylori in the growth state, we examined the bacterial culture supernatant of H. pylori. H. pylori urease was slightly detected in the culture supernatant of NCTC 11637 isolates with a sensitive ELISA system using highly purified H. pylori urease-specific MAb (8, 14) (Fig. 2A). We tried to confirm the results by Western blotting by using whole urease-specific antibody from immune rabbit sera and two H. pylori urease-specific MAbs, L2 for the large subunit (UreB) and S2 for the small subunit (UreA), as described in Materials and Methods. As shown in Fig. 2B, similar to the purified H. pylori urease, we could detect both the large subunit (UreB) and the small subunit (UreA) in the culture supernatants of both SS-1 and NCTC 11637 by Western blotting. Here, we have also confirmed that HPP1801, a urease-negative isogenic mutant from NCTC 11637, did not express urease (Fig. 2B). These findings indicate that, although the amount was very small, we could detect complete H. pylori urease composed of both the large (UreB) and small (UreA) subunits in the culture supernatants of both SS-1 and NCTC 11637.
Fig. 2.
Detection of H. pylori urease in the bacterial culture supernatant by sensitive ELISA using H. pylori urease-specific MAb. (A) Urease-positive H. pylori strains (isolates SS-1 and NCTC 11637) were cultured in brucella broth containing 5% horse serum for 2 days, and cell-free culture supernatant (culture sup.) was collected carefully and further sedimented by centrifugation (3,000 rpm for 5 min at 4°C) to remove debris. Compared with positive controls containing purified H. pylori urease (100 μg/ml), H. pylori urease was only slightly detected in the bacterial culture supernatant. Results are expressed as means ± SDs (n = 5). Statistical significance was determined by the Student t test, and the values are given in the graph. (B) We then tried to confirm the results by Western blotting by using whole urease-specific antibody from immune rabbit sera and two H. pylori urease-specific MAbs, L2 for the large subunit (UreB) and S2 for the small subunit (UreA), as described in Materials and Methods. We could detect both the large subunit (UreB) and the small subunit (UreA) in the culture supernatants of both SS-1 and NCTC 11637 compared with purified urease obtained from the bacteria. We have confirmed that HPP1801, a urease-negative mutant from the NCTC 11637 isolate, did not express urease in its lysates.
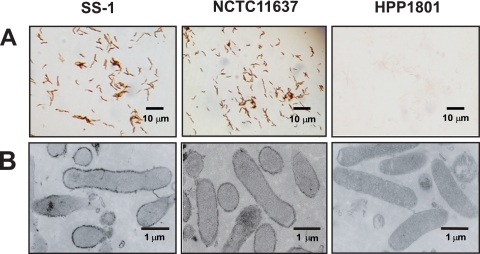
Then, we examined whether H. pylori urease is expressed on/in the bacteria by immunohistochemical staining using anti-H. pylori urease-specific antibody. Here, we took advantage of a urease-negative H. pylori isolate (HPP1801) as a negative control. We found the expression of H. pylori urease on the surface of bacteria rather than inside by both light microscopy (Fig. 3A) and electron microscopy (Fig. 3B). Similar to the previous observation (24), these findings indicate that H. pylori expresses urease on its surface and secretes only small amounts of urease. In general, endotoxins, such as LPSs and peptide-glycans (PGs), are components expressed on various pathogens, while exotoxins, such as enterotoxins and verotoxin, are released from the pathogens. The former endotoxins may stimulate immunocompetent cells through TLRs. Thus, we thought that H. pylori urease on the bacteria would stimulate innate cells through TLRs.
Fig. 3.
Detection of H. pylori urease on/in the bacteria by immunohistological staining using murine H. pylori urease-specific antibody. H. pylori urease-positive isolates (SS-1 and NCTC 11637) and an H. pylori urease-negative isolate (HPP1801) were used for staining. Immunohistological analysis to detect the localization of H. pylori urease was carried out using light microscopy (magnification, ×800) (A) and electron microscopy (magnification, ×15,000) (B).
Stimulation of murine B-1a cells by H. pylori urease expressed on the bacterial surface.
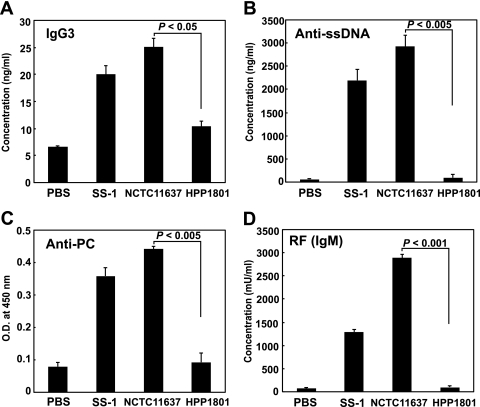
We then asked whether the H. pylori urease expressed on the surface of the bacterium would directly stimulate murine B-1a cells. To keep the expression of urease on the surface, H. pylori was fixed with 2% PFA, as indicated in Materials and Methods. We coated a 96-well round-bottom culture plate with the fixed H. pylori cells and added purified murine B-1a cells to examine the amount of autoantibodies secreted in the culture supernatant. To eliminate the possibility that B-1a cells were stimulated by components of H. pylori other than urease, such as LPS, we cultured B-1a cells with PFA-fixed urease-negative H. pylori (strain HPP1801). Consequently, the secretion of various autoantibodies (Fig. 4A to D), such as IgG3 (A), anti-ssDNA (B), anti-PC (C), and RF (D), was detected when B-1a cells were cocultured with the urease-positive H. pylori isolate but not with the urease-negative H. pylori isolate. It should be noted that the isotypes of the anti-ssDNA and anti-PC autoantibodies secreted by B-1a cells were IgA or IgM (data not shown). These findings strongly suggest that murine B-1a cells can be stimulated to secrete autoantibodies by H. pylori urease expressed on the bacterial surface.
Fig. 4.
Secretion of autoantibodies from purified B-1a cells stimulated with fixed plate-coated H. pylori. Purified murine B-1a cells (2 × 105) were cultured with either urease-positive H. pylori (SS-1 and NCTC 11637) or urease-negative H. pylori (HPP1801) fixed by 2% PFA for 10 min and cultured for an additional 7 days, and the supernatants were harvested to measure the production of autoantibodies by ELISA. Results are expressed as means ± SDs (n = 5). Statistical significance was determined by the Student t test, and the P values are given in each graph.
Effect of anti-H. pylori urease-specific antibodies on autoantibody secretion by B-1a cells stimulated with bacterium-coated plates.
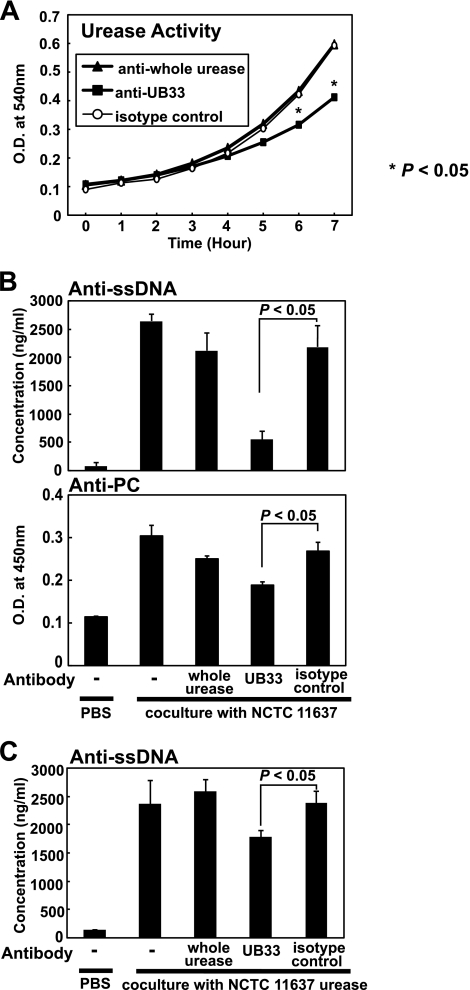
Next, we examined the effects of various H. pylori urease-specific antibodies on autoantibody secretion by B-1a cells. As we have reported previously (14), there are two distinct types of H. pylori urease-specific antibodies: one can neutralize urease enzymatic activity, and the other cannot, despite its binding capacity. We have identified an epitope for neutralizing antibody, termed the UB-33 peptide, within the large subunit (UreB) of H. pylori urease and demonstrated that rabbit immune sera specific for that epitope neutralized urease enzymatic activity (14), although sera from rabbits immunized with purified whole H. pylori urease did not.
When strain NCTC 11637 was treated with anti-UB-33-specific antibody, the enzymatic activity of its urease was apparently inhibited, although anti-whole urease-specific antibody did not cause urease inhibition, as seen with the isotype-matched control antibody (Fig. 5A). Also, B-1a cells were less stimulated to secrete autoantibodies with PFA-fixed urease-positive H. pylori when the fixed bacteria were pretreated with an excess of purified UB-33-specific rabbit serum than with purified whole H. pylori urease-specific serum (Fig. 5B). Similarly, B-1a cells were also less stimulated to secrete autoantibodies with purified H. pylori urease pretreated with purified UB-33-specific rabbit serum (Fig. 5C). Moreover, autoantibody production by B-1a cells declined significantly when the enzymatic activity of purified H. pylori urease was reduced by heat treatment (data not shown). These findings suggest that murine B-1a cells were more strongly activated to secrete autoantibodies by H. pylori urease expressed on the bacterial surface that retained enzymatic activity.
Fig. 5.
Effect of H. pylori-urease specific antibodies on the secretion of autoantibodies by purified B-1a cells stimulated with H. pylori urease. We have identified an epitope for neutralizing antibody, termed the UB-33 peptide, within the large subunit (UreB) of H. pylori urease (14). (A) Anti-UB-33-specific rabbit antibody significantly inhibited the enzymatic activity (urea catalysis) of purified urease from the NCTC 11637 isolate (*, P < 0.005), although anti-whole urease-specific antibody did not neutralize the activity, similar to the isotype-matched control antibody. Results are expressed as means ± SDs (n = 5). (B) Anti-UB-33-specific rabbit antibody significantly suppressed autoantibody secretion by B-1a cells stimulated with urease on the surface of H. pylori for 90 min at 37°C. Results are expressed as means ± SDs (n = 5). (C) Anti-UB-33-specific rabbit antibody also significantly suppressed autoantibody secretion by B-1a cells stimulated with purified H. pylori urease for 90 min at 37°C. Results are expressed as means ± SDs (n = 5). Statistical significance was determined by the Student t test, and the values are given in each graph.
H. pylori urease was a ligand for TLR2 on murine B-1a cells.
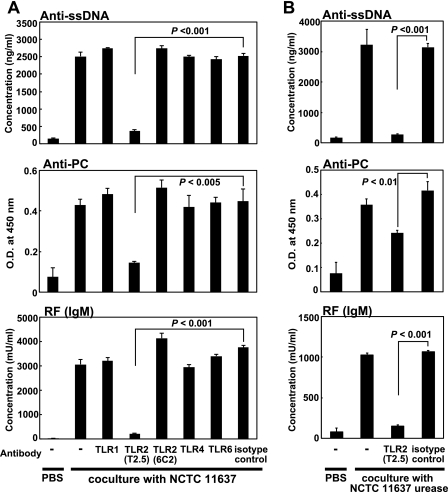
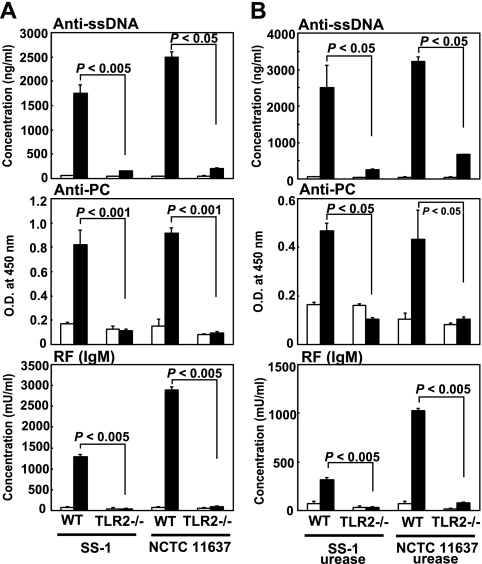
These results indicate that the H. pylori urease retaining enzymatic activity appears to potently stimulate murine B-1a cells to produce various autoantibodies. The fact that B-1a cells were directly activated by urease on the bacterial surface, like endotoxins such as LPSs and PGs, within a few hours suggests that some innate TLRs on B-1a cells may interact with H. pylori urease as an active bacterial functional component. Thus, we analyzed the expression of TLRs on CD5+ B-1a cells and found that the B-1a cells expressed TLR1, TLR2, TLR4, and TLR6 on their surface (Fig. 6A and B). Then, we examined which TLRs might interact with H. pylori urease by blocking each TLR on B-1a cells with each specific antibody. Although TLR1-, TLR4-, and TLR6-specific MAbs did not affect the secretion of autoantibodies by B-1a cells stimulated with H. pylori urease, secretion was significantly inhibited when TLR2-specific MAb (T2.5) (19) was added to the culture (Fig. 7A). Moreover, to prove that H. pylori urease was an actual ligand for TLR2, murine B-1a cells pretreated with anti-TLR2 MAb were stimulated with purified H. pylori urease. Although B-1a cells pretreated with anti-TLR2 MAb (T2.5) did not secrete autoantibodies (Fig. 7B), pretreatment with another TLR2-specific MAb, 6C2 (23), whose binding site was different from that of T2.5, did not inhibit the secretion of autoantibodies. Furthermore, B-1a cells obtained from TLR2-knockout (TLR2−/−) BALB/c mice were not stimulated to secrete autoantibodies by bacterium (strain SS-1 or NCTC 11637)-coated plates (Fig. 8A) or the purified H. pylori urease of strain SS-1 or NCTC 11637 (Fig. 8B).
Fig. 7.
H. pylori urease was a ligand for TLR2 on murine B-1a cells. (A) Purified murine B-1a cells (2 × 105) were incubated for 30 min with either anti-mouse TLR antibodies or an isotype-matched antibody (final concentration, 1 μg/106 cells) and washed with culture medium to remove free antibody. Cells were further cultured with H. pylori (SS-1 and NCTC 11637)-precoated round-bottom 96-well plates for an additional 7 days, and autoantibody secretion in the supernatant was measured. Pretreatment with anti-mouse TLR2 antibody (T2.5) significantly inhibited the secretion of autoantibodies. Results are expressed as means ± SDs (n = 7). (B) Purified murine B-1a cells (2 × 105) were incubated for 30 min with either anti-mouse TLR2 antibody (T2.5) or an isotype-matched antibody and further cultured with 10 μg/ml purified H. pylori urease for 7 days. Pretreatment with the anti-mouse TLR2 antibody (T2.5) again significantly inhibited the secretion of autoantibodies. Results are expressed as means ± SDs (n = 7). Statistical significance was determined by the Student t test, and the values are given in each graph.
Fig. 8.
Murine B-1a cells from TLR2-knockout (TLR2−/−) BALB/c mice did not respond to either H. pylori (SS-1 or NCTC 11637)-coated plates (A) or purified H. pylori (SS-1 or NCTC 11637) urease (B). (A) Two × 105 purified murine B-1a cells from either normal wild-type BALB/c mice (WT) or TLR2−/− mice were cultured in plates precoated with H. pylori (SS-1 or NCTC 11637) (filled columns) or PBS (open columns) for 7 days, and autoantibody secretion in the culture supernatant was measured. Results are expressed as means ± SDs (n = 5). (B) Purified murine B-1a cells (2 × 105) from either normal wild-type BALB/c mice or TLR2−/− BALB/c mice were cultured with 10 μg/ml purified H. pylori urease (filled columns) or PBS (open columns) for 7 days, and autoantibody secretion in the culture supernatant was measured. Results are expressed as means ± SDs (n = 5). Statistical significance was determined by the Student t test, and the values are given in each graph.
These findings collectively suggest that some portion of innate TLR2 on B-1a cells may interact with functional bacterial urease expressed on the surface of H. pylori to secrete various autoantibodies.
Detection and localization of H. pylori LPS.
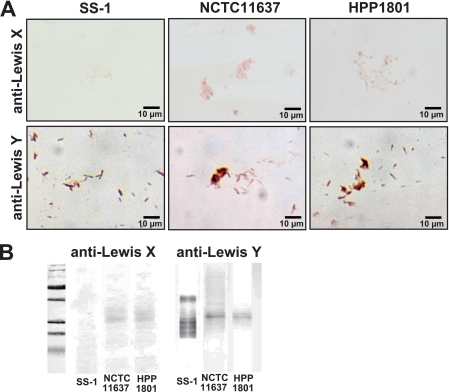
There are some reports showing that H. pylori LPS can stimulate TLR2 rather than classical TLR4 for LPS in other bacteria. Indeed, Smith et al. recently reported (27) that H. pylori LPS detected by anti-Lewis x (Lex) (clone P12) or anti-Lewis y (Ley) (clone F3) murine IgM MAbs (21) stimulated cells to induce a set of chemokines in a TLR2-dependent fashion. Thus, to eliminate the possibility that the H. pylori LPS stimulates B-1a cells to secrete autoantibodies, we tried to identify the Lex and/or Ley antigen on the urease-negative HPP1801 mutant that did not stimulate B-1a cells. As demonstrated in Fig. 9, although the expression of Lex antigen was very weak, apparent Ley antigen expression was seen on the surface of the HPP1801 mutant as well as isolates NCTC 11637 and SS-1 by light microscopy (Fig. 9A). Also, we could detect Ley antigens in the purified LPSs obtained from HPP1801 as well as NCTC 11637 and SS-1 by Western blotting (Fig. 9B). These results clearly indicate that H. pylori LPS will not stimulate B-1a cells to secrete autoantibodies via TLR2.
Fig. 9.
Detection of H. pylori LPS on/in mutant HPP1801 by immunohistological staining and Western blotting. We tried to identify H. pylori LPS (Lex and Ley antigens) in/on the urease-negative HPP1801 mutant that did not stimulate B-1a cells by anti-Lex (clone P12) or anti-Ley (clone F3) murine IgM MAbs. (A) Both Lex and Ley were apparently expressed on the surface of mutant HPP1801 as well as isolates NCTC 11637 and SS-1 by light microscopy. (B) Lex and Ley antigens were seen in the purified LPS obtained from HPP1801 as well as NCTC 11637 and SS-1 by Western blotting.
DISCUSSION
There are two distinct types of murine B cells, B-1 and B-2 cells. Although B-2 cells are conventional T-cell-dependent B cells, in that T cells help B cells to promote their clonal expansion, somatic hypermutation, isotype switch, and differentiation into antibody-secreting cells, B-1 cells, particularly B-1a cells expressing CD5, showing an enriched expression of autoreactive B-cell-antigen receptors, produce several types of natural autoantibodies (11).
In the present study, we found that murine B-1a cells can be stimulated to produce various autoantibodies, such as anti-ssDNA, anti-PC, and RF, by H. pylori urease expressed on the surface of the bacteria. This unique response seems to be mediated through the direct interaction between TLR2 on B-1a cells and urease on the bacteria, indicating that H. pylori urease acts like a bacterial endotoxin, such as PG or LPS, rather than an exotoxin released from the bacteria. Indeed, we have shown here that H. pylori urease was expressed on the bacterial surface and proved that H. pylori (either NCTC 11637 or SS-1 isolate)-coated plates bearing bacterial urease on the surface stimulated B-1a cells to produce autoantibodies, although plates coated with urease-negative H. pylori HPP1801, which originated from NCTC 11637, did not. These findings indicate that bacterial urease expressed on H. pylori but not other products, like LPS or secreted H. pylori-associated toxins, such as cytotoxin-associated gene A (CagA) or vacuolating cytotoxin (VacA), is a ligand for TLR2 recognition by B-1a cells.
Moreover, it should be noted that the enzymatic activity of urease on PFA-coated bacteria was maintained and the magnitude of production of autoantibodies by B-1a cells was markedly inhibited when the enzymatic activity of H. pylori urease was blocked with UB-33-specific neutralizing antibody (14) but not with urease-specific antibody without a neutralizing capacity. Although this is caused by the steric hindrance of binding due to the attached anti-urease-specific antibody, the results reveal that the innate TLR2 on B-1a cells may have a potency to distinguish the functional enzymatic capacity of bacterial ligands, and thus, those B-1a cells can recognize whether the pathogens that have intruded the body express live functional products. We are currently planning to immunize BALB/c mice with the UB-33 peptide plus an appropriate adjuvant to see whether neutralizing antibodies against H. pylori urease can be obtained and the production of various autoantibodies can be manipulated in the presence of H. pylori or its urease in vivo.
To our knowledge, only a few reports have demonstrated that innate TLRs can discriminate between functional and damaged ligands on pathogens; for example, intestinal acyloxyacyl hydrolase, which cleaves acyl chains from the lipid A portion of LPS, downmodulates the proinflammatory response through TLR4-mediated innate immune signaling (6), and in mice lacking this enzyme, acylated LPS persists for longer periods after infection with Gram-negative bacteria and elicits increased B-cell proliferation as well as antibody production (18). In addition, alkaline phosphatase in the brush border of the intestinal mucosa detoxifies LPS by dephosphorylating its lipid A with two phosphate groups coupled to glucosamines into a monophosphoryl lipid A that is a 100-fold less toxic than the unmodified version for TLR4 signaling (26). Such a reduction of TLR4 signaling may prevent intestinal inflammation in response to commensal nontoxic microflora. Also, TLR2 on intraepithelial cells enhanced the enzymatic activity of associated protein kinase C by binding to the appropriate ligands in response to luminal bacteria (1); therefore, as we have shown here, innate TLRs may have the ability to distinguish the functional activity of ligands expressed on pathogens. Evolutionary analysis of TLRs and their functional ligands will determine the actual role of TLRs.
Various types of autoantibodies were produced when TLR2 on B-1a cells was stimulated by urease expressed on H. pylori without T-cell help. In this case, H. pylori urease seems to act as a bacterial endotoxin and TLR2 on B-1a cells recognizes it as a ligand in the production of autoantibodies. Herlands et al. recently suggested a new paradigm of TLR-dependent B-cell initiation of autoimmunity (12). Moreover, two other groups have recently reported similar T-cell-independent activation of autoreactive B cells to produce autoantibodies via B-cell-intrinsic TLR7/9-associated MyD88 signaling, although the actual TLR ligands remain to be elucidated (10, 31). Collectively, as far as we know, our current findings are the first to demonstrate autoantibody production by B cells in a T-cell-independent fashion through the combination of an external TLR with a pathogen-associated ligand.
We have described here that the direct interaction of extracellular TLR2 with H. pylori urease as a ligand expressed on the bacterial surface stimulated B-1a cells to produce various autoantibodies in a T-cell-independent manner. This may be why various autoimmune diseases, such as RA (16) and ITP (7), have been associated with H. pylori infection. The magnitude of these autoimmune disorders can be manipulated using an anti-TLR2 antibody, such as T2.5, or anti-UB-33-specific antibody if autoantibody production is associated with the interaction between TLR2 and H. pylori urease. The findings shown from the current study offer a new notion that H. pylori urease is a bacterial endotoxin that can stimulate innate TLR-bearing B-1a cells to secrete a number of autoantibodies and initiate various autoimmune disorders.
ACKNOWLEDGMENTS
This work was supported in part by grants from the Ministry of Education, Science, Sport, and Culture, from the Ministry of Health and Labor and Welfare, Japan, and from the Japanese Health Sciences Foundation and by the Promotion and Mutual Aid Corporation for Private Schools of Japan.
Footnotes
Published ahead of print on 26 September 2011.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Cario E., Gerken G., Podolsky D. K. 2004. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology 127:224–238 [DOI] [PubMed] [Google Scholar]
- 2. Dempsey P. W., Allison M. E., Akkaraju S., Goodnow C. C., Fearon D. T. 1996. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science 271:348–350 [DOI] [PubMed] [Google Scholar]
- 3. De Vita S., et al. 1996. Widespread clonal B-cell disorder in Sjogren's syndrome predisposing to Helicobacter pylori-related gastric lymphoma. Gastroenterology 110:1969–1974 [DOI] [PubMed] [Google Scholar]
- 4. Drouet E. B., et al. 1991. Characterization of an immunoreactive species-specific 19-kilodalton outer membrane protein from Helicobacter pylori by using a monoclonal antibody. J. Clin. Microbiol. 29:1620–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fairweather D., Kaya Z., Shellam G. R., Lawson C. M., Rose N. R. 2001. From infection to autoimmunity. J. Autoimmun. 16:175–186 [DOI] [PubMed] [Google Scholar]
- 6. Feulner J. A., et al. 2004. Identification of acyloxyacyl hydrolase, a lipopolysaccharide-detoxifying enzyme, in the murine urinary tract. Infect. Immun. 72:3171–3178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Franchini M., Veneri D. 2006. Helicobacter pylori-associated immune thrombocytopenia. Platelets 17:71–77 [DOI] [PubMed] [Google Scholar]
- 8. Futagami S., Takahashi H., Norose Y., Kobayashi M. 1998. Systemic and local immune responses against Helicobacter pylori urease in patients with chronic gastritis: distinct IgA and IgG productive sites. Gut 43:168–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Giannasca K. T., Giannasca P. J., Neutra M. R. 1996. Adherence of Salmonella typhimurium to Caco-2 cells: identification of a glycoconjugate receptor. Infect. Immun. 64:135–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Groom J. R., et al. 2007. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J. Exp. Med. 204:1959–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hardy R. R. 2006. B-1 B cells: development, selection, natural autoantibody and leukemia. Curr. Opin. Immunol. 18:547–555 [DOI] [PubMed] [Google Scholar]
- 12. Herlands R. A., Christensen S. R., Sweet R. A., Hershberg U., Shlomchik M. J. 2008. T cell-independent and Toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity 29:249–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hildebrandt E., McGee D. J. 2009. Helicobacter pylori lipopolysaccharide modification, Lewis antigen expression, and gastric colonization are cholesterol-dependent. BMC Microbiol. 9:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hirota K., et al. 2001. Identification of an antigenic epitope in Helicobacter pylori urease that induces neutralizing antibody production. Infect. Immun. 69:6597–6603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iizumi T., et al. 2005. Augmentation of Helicobacter pylori urease activity by its specific IgG antibody: implications for bacterial colonization enhancement. Biomed. Res. 26:35–42 [DOI] [PubMed] [Google Scholar]
- 16. Ishikawa N., et al. 2002. Helicobacter pylori infection in rheumatoid arthritis: effect of drugs on prevalence and correlation with gastroduodenal lesions. Rheumatology (Oxford) 41:72–77 [DOI] [PubMed] [Google Scholar]
- 17. Lee A., et al. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386–1397 [DOI] [PubMed] [Google Scholar]
- 18. Lu M., et al. 2005. Lipopolysaccharide deacylation by an endogenous lipase controls innate antibody responses to Gram-negative bacteria. Nat. Immunol. 6:989–994 [DOI] [PubMed] [Google Scholar]
- 19. Meng G., et al. 2004. Antagonistic antibody prevents Toll-like receptor 2-driven lethal shock-like syndromes. J. Clin. Invest. 113:1473–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Monteiro M. A., et al. 1998. Simultaneous expression of type 1 and type 2 Lewis blood group antigens by Helicobacter pylori lipopolysaccharides. Molecular mimicry between H. pylori lipopolysaccharides and human gastric epithelial cell surface glycoforms. J. Biol. Chem. 273:11533–11543 [DOI] [PubMed] [Google Scholar]
- 21. Moran A. P., et al. 2002. Phenotypic variation in molecular mimicry between Helicobacter pylori lipopolysaccharides and human gastric epithelial cell surface glycoforms. Acid-induced phase variation in Lewis (x) and Lewis (y) expression by H. pylori lipopolysaccharides. J. Biol. Chem. 277:5785–5795 [DOI] [PubMed] [Google Scholar]
- 22. Nagata K., et al. 1992. Monoclonal antibodies against the native urease of Helicobacter pylori: synergistic inhibition of urease activity by monoclonal antibody combinations. Infect. Immun. 60:4826–4831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22a. National Research Council 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC [Google Scholar]
- 23. Paterson H. M., et al. 2003. Injury primes the innate immune system for enhanced Toll-like receptor reactivity. J. Immunol. 171:1473–1483 [DOI] [PubMed] [Google Scholar]
- 24. Phadnis S. H., et al. 1996. Surface localization of Helicobacter pylori urease and a heat shock protein homolog requires bacterial autolysis. Infect. Immun. 64:905–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rose N. R. 2008. Autoimmunity in coxsackievirus infection. Curr. Top. Microbiol. Immunol. 323:293–314 [DOI] [PubMed] [Google Scholar]
- 26. Schromm A. B., et al. 1998. The charge of endotoxin molecules influences their conformation and IL-6-inducing capacity. J. Immunol. 161:5464–5471 [PubMed] [Google Scholar]
- 27. Smith S. M., et al. 2011. Tribbles 3: a novel regulator of TLR2-mediated signaling in response to Helicobacter pylori lipopolysaccharide. J. Immunol. 186:2462–2471 [DOI] [PubMed] [Google Scholar]
- 28. Takahashi H., Cease K. B., Berzofsky J. A. 1989. Identification of proteases that process distinct epitopes on the same protein. J. Immunol. 142:2221–2229 [PubMed] [Google Scholar]
- 29. Takeuchi O., et al. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443–451 [DOI] [PubMed] [Google Scholar]
- 30. Tsai C. M., Frasch C. E. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115–119 [DOI] [PubMed] [Google Scholar]
- 31. Tsao P. Y., Jiao J., Ji M. Q., Cohen P. L., Eisenberg R. A. 2008. T cell-independent spontaneous loss of tolerance by anti-double-stranded DNA B cells in C57BL/6 mice. J. Immunol. 181:7770–7777 [DOI] [PubMed] [Google Scholar]
- 32. Tsuda M., Karita M., Morshed M. G., Okita K., Nakazawa T. 1994. A urease-negative mutant of Helicobacter pylori constructed by allelic exchange mutagenesis lacks the ability to colonize the nude mouse stomach. Infect. Immun. 62:3586–3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamanishi S., et al. 2006. Implications for induction of autoimmunity via activation of B-1 cells by Helicobacter pylori urease. Infect. Immun. 74:248–256 [DOI] [PMC free article] [PubMed] [Google Scholar]