Abstract
Interleukin-10 (IL-10) modulates inflammatory responses elicited in vitro and in vivo by Borrelia burgdorferi, the Lyme disease spirochete. How IL-10 modulates these inflammatory responses still remains elusive. We hypothesize that IL-10 inhibits effector functions of multiple genes induced by B. burgdorferi in macrophages to control concomitantly elicited inflammation. Because macrophages are essential in the initiation of inflammation, we used mouse J774 macrophages and live B. burgdorferi spirochetes as the model target cell and stimulant, respectively. First, we employed transcriptome profiling to identify genes that were induced by stimulation of cells with live spirochetes and that were perturbed by addition of IL-10 to spirochete cultures. Spirochetes significantly induced upregulation of 347 genes at both the 4-h and 24-h time points. IL-10 inhibited the expression levels, respectively, of 53 and 65 of the 4-h and 24-h genes, and potentiated, respectively, at 4 h and 24 h, 65 and 50 genes. Prominent among the novel identified IL-10-inhibited genes also validated by quantitative real-time PCR (qRT-PCR) were Toll-like receptor 1 (TLR1), TLR2, IRAK3, TRAF1, IRG1, PTGS2, MMP9, IFI44, IFIT1, and CD40. Proteome analysis using a multiplex enzyme-linked immunosorbent assay (ELISA) revealed the IL-10 modulation/and or potentiation of RANTES/CCL5, macrophage inflammatory protein 2 (MIP-2)/CXCL2, IP-10/CXCL10, MIP-1α/CCL3, granulocyte colony-stimulating factor (G-CSF)/CSF3, CXCL1, CXCL5, CCL2, CCL4, IL-6, tumor necrosis factor alpha (TNF-α), IL-1α, IL-1β, gamma interferon (IFN-γ), and IL-9. Similar results were obtained using sonicated spirochetes or lipoprotein as stimulants. Our data show that IL-10 alters effectors induced by B. burgdorferi in macrophages to control concomitantly elicited inflammatory responses. Moreover, for the first time, this study provides global insight into potential mechanisms used by IL-10 to control Lyme disease inflammation.
INTRODUCTION
Lyme disease, the most frequently reported arthropod-borne disease in the United States, results from infection with the spirochete Borrelia burgdorferi. The disease is spread to humans and other mammals through the bite of infected Ixodes ticks (18). Invasion of the mammalian host with spirochetes results in the activation of inflammatory pathways that lead to the release of inflammatory mediators and an influx of inflammatory cells that result in many of the clinical manifestations of Lyme disease (9, 42–44, 79). Manifestations of the disease include, among others, acute or chronic arthritis, carditis, and neuroborreliosis (23, 31, 75, 79, 93). The inflammatory immune response is of crucial importance for the early containment of infection but at the same time has the potential to result in immunopathology. It is thought that inflammation, either induced by the spirochete or by spirochete antigens left in tissues after bacterial demise, plays a major role in disease pathogenesis. The final outcome of infection, therefore, depends on an intricate balance between the pathogen and the host response.
The anti-inflammatory cytokine interleukin-10 (IL-10) plays a pivotal role in limiting the inflammatory response and preventing tissue damage. This is mainly achieved by downregulating the expression of inflammatory mediators as well as inhibiting effector functions of T cells and mononuclear phagocytes (30). In addition to these activities, IL-10 regulates growth and/or differentiation of T and B cells, NK cells, cytotoxic cells, mast cells, granulocytes, dendritic cells (DCs), keratinocytes, and endothelial cells (63). Different preparations of B. burgdorferi spirochetes (live, sonicated, freeze-thawed, and heat inactivated) and lipoproteins induce IL-10 in a variety of cell types. IL-10 production has been detected in joint tissues (24, 61), skin tissues (53) lymph node cells (34), splenocytes (3), glial cells (82), and macrophages (17, 27, 53) in the murine model of Lyme disease. In humans, we have shown that B. burgdorferi induces the production of IL-10 in vitro in mononuclear cells present in peripheral blood (40). Other investigators have shown production of IL-10 in peripheral blood (20, 28, 92), macrophages (94), dendritic cells (92), lymphocytes (49, 73, 76), cerebrospinal fluid (CSF) (20), synovium (98), microglia (19), skin (86), and erythema-migrans skin lesions of B. burgdorferi-infected patients (50).
IL-10 has proved to be a key cytokine in regulating inflammatory responses elicited by B. burgdorferi. We (35) along with others (17, 53, 54, 96) have reported results of experiments conducted in vitro showing that in response to B. burgdorferi or its lipoproteins, IL-10 dampens proinflammatory cytokines such as IL-1β, tumor necrosis factor (TNF), IL-6, IL-12, IL-18, and gamma interferon (IFN-γ) of cells that are involved in innate and adaptive immunity in the murine model. Using human monocytic THP-1 cells, we demonstrated that IL-10 down-modulated the production of IL-1β, TNF, IL-12, and IL-6, as elicited by spirochetal lipoproteins (66). Studies by Lisinski and Furie (56) showed that IL-10 decreases production of CXCL8 in B. burgdorferi-stimulated endothelial cells. The fact that C57BL/6J (17) and C3H (16) mice deficient in IL-10 and infected with B. burgdorferi develop more severe arthritis and harbor fewer spirochetes in joints than wild-type mice suggests an important in vivo role for IL-10 as mediator of anti-inflammatory immune responses induced by B. burgdorferi. The above in vitro and in vivo studies suggest that IL-10 may profoundly inhibit a broad spectrum of inflammatory mediators induced by B. burgdorferi.
Macrophage function has been proposed to be critical for mice to combat B. burgdorferi infection (5). Since monocytes/macrophages are the primary IL-10 responders to pathogen-associated molecular patterns (PAMPS) and since their activation initiates most inflammatory responses (59), we hypothesize that IL-10 inhibits effector functions of multiple genes induced by B. burgdorferi in macrophages to control concomitantly elicited inflammation. The viability of this hypothesis was tested by first using murine whole-genome microarray to identify inflammatory mediators that were induced in cultured J774 mouse macrophages in response to stimulation with live B. burgdorferi spirochetes and that were further perturbed by the addition of IL-10 to these cultures. Next, alternative approaches such as TaqMan quantitative real-time PCR (qRT-PCR) and proteome analysis (cytokine/chemokine multiplex or single enzyme-linked immunosorbent assays [ELISAs]) were used to confirm the microarray data. Findings from the experiments in which live B. burgdorferi spirochetes were used as stimulants were compared to those obtained with macrophages stimulated with sonicated B. burgdorferi or with purified recombinant lipidated outer surface protein A (L-OspA) either via microarray, qRT-PCR, or single and multiplex ELISAs. The results of this study are presented and discussed in the global context of IL-10-mediated control of inflammation in Lyme disease.
MATERIALS AND METHODS
Bacteria and lipoprotein.
B. burgdorferi spirochetes (strain B31, clone 5A19, with the complete plasmid content) were grown in vitro in Barbour-Stoenner-Kelly H (BSK-H) medium as previously described (36, 40). Purified recombinant L-OspA was kindly provided by GlaxoSmithKline Biologicals (Rixensart, Belgium). The L-OspA preparation contained less than 0.25 endotoxin units per mg of protein, as assessed by Limulus amebocyte assay (Associates of Cape Cod, Woods Hole, MA).
Cell stimulation and culture conditions.
The mouse J774 macrophage cell line was obtained from the American Type Culture Collection (Waldorf, MD). We chose this cell line because it has been shown to be phagocytic for B. burgdorferi spirochetes (62). In addition, we have shown employing mouse J774 macrophages (27), human THP-1 monocytic cell line (66), and mouse primary lymph node cells (35) that IL-10 alters the expression levels of several cytokines induced by B. burgdorferi stimuli irrespective of the cells used. Our published work (27) also indicated the ability of IL-10 to diminish live-spirochete-induced cytokines. Thus, to further understand the extent of IL-10 anti-inflammatory effect on live-spirochete-inducible inflammatory mediators, we selected mouse J774 macrophages as our modeled cell line. Cell culture medium for J774 cells consisted of Dulbecco's medium (Gibco Invitrogen, Carlsbad, CA), 10% heat-inactivated fetal bovine serum, 1 mM HEPES (Gibco Invitrogen), 2 mM l-glutamine (Gibco Invitrogen), and 1 μg/ml antibiotic/antimycotic (Gibco Invitrogen). Cells (3 × 106/ml) were cultured in 24-well plates (Costar, Cambridge, MA) and incubated at 37°C in a humidified atmosphere with 5% CO2 for various periods of time depending on the experimental procedure. Macrophages were stimulated with live B. burgdorferi spirochetes at a 10:1 multiplicity of infection (MOI) in the presence or absence of mouse recombinant IL-10 (10 ng/ml). Live spirochetes were incubated with cells in antibiotic-free medium. Some cells were stimulated also with either L-OspA at 1 μg/ml or sonicated B. burgdorferi spirochetes (1 × 107/ml) in the presence or absence of IL-10 (10 ng/ml). Unstimulated cells served as negative controls for all experiments. To study the role of IL-10 alone on constitutive expression of inflammatory mediators, cells incubated with recombinant IL-10 only were included in all real-time PCR and cytokine assays. All cultures were subsequently centrifuged at 400 × g at 4°C for 10 min to collect cell-free supernatants and cell pellets. RNA was extracted from the cell pellets using a Qiagen RNeasy Kit (Qiagen Inc., Valencia, CA), which included a DNase I digestion step. Supernatant and RNA samples were stored at −80°C until they were used.
Mouse whole-genome microarray.
Previous experimental designs routinely used in our laboratory and published previously (27) showed no differences in the IL-10 anti-inflammatory effects on inflammatory mediators induced by B. burgdorferi stimulants in macrophages when (i) stimulants were cocultured simultaneously with IL-10 and (ii) when IL-10 was added to cells first followed by addition of stimulants or vice versa (27); we therefore decided, in the present study, to focus only on stimulants cocultured simultaneously with IL-10. Thus, to fulfill this objective, total RNA obtained from 4-h and 24-h samples of unstimulated cells, live B. burgdorferi spirochetes alone, or live B. burgdorferi spirochetes combined with IL-10 were subjected to mouse whole-genome microarray studies. A quantity of 100 ng of total RNA was used to generate Cy-labeled cDNA samples using a low-RNA input linear amplification kit (LRILAK) (Agilent Technologies, Inc., Foster City, CA). Control samples were labeled with Cy3, whereas experimental samples were labeled with Cy5. Labeled cDNA was hybridized overnight to Agilent's Whole Mouse Genome Oligo microarray printed in a 4 by 44,000 format (Agilent Technologies Inc.), which is comprised of 41,534 60-mer oligonucleotide probes representing over 41,000 mouse genes and transcripts at once. Hybridization was performed in a SciGene 4000 HybOven (SciGene Corp., Sunnyvale, CA) at 65°C for 18 h in a rotary chamber at 10 rpm. The slides were then washed using the manufacturer's protocol (Agilent) and scanned in a dual-confocal continuous microarray scanner (GenePix 4000B; Molecular Devices, Sunnyvale, CA), using GenePix Pro, version 6.1, as the image acquisition and extraction software. The microarray data are based on experiments conducted twice with similar RNA samples, and each data set was scanned twice. In addition, we used RNA samples obtained at 24 h from unstimulated cells and from sonicated B. burgdorferi spirochetes (1 × 107/ml) alone or combined with IL-10 as stimulants for microarray experiments.
The resulting microarray text data were imported into Spotfire DecisionSite for Functional Genomics (Spotfire Inc., Somerville, MA), filtered, and subjected to statistical analysis. Genes whose expression changed by at least 2-fold or more for upregulated genes and −2-fold or less for downregulated genes (with a corrected one-way analysis of variance, P < 0.05) compared with unstimulated cells were considered to be differentially expressed in a statistically significant manner. We then arbitrarily used a cutoff ratio (number of live spirochetes/number of live spirochetes with IL-10) of ≥1.2 to determine genes considered to be inhibited by IL-10. To determine genes that were potentiated by IL-10, we used a corresponding cutoff ratio of ≤0.83, the equivalent of the 1.2 cutoff ratio.
Quantitative real-time PCR.
TaqMan PCR was performed to validate the expression level of randomly selected genes from multiple pathways identified by microarray to be upregulated by live B. burgdorferi spirochetes and downregulated in macrophages when IL-10 was added to live B. burgdorferi cultures. qRT-PCR was carried out using a TaqMan RNA-to-CT one-step kit (where CT is threshold cycle) in combination with TaqMan gene expression assays on an ABI Prism HT7900 Sequence Detection System (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. FAM (6-carboxyfluorescein)-labeled TaqMan gene expression assays (Applied Biosystems) were used to measure the transcription level of the genes: Ifi44 (Mm00505670_m1; interferon-induced protein 44), Ifit1 (Mm0051515_m1; interferon-induced protein with tetratricopeptide repeats 1), Tlr2 (Mm00442346_m1; Toll-like receptor 2), Tlr1 (Mm00446095_m1; Toll-like receptor 1), Irg1 (Mm01224529_m1; immunoresponsive gene 1), Ptgs2 (Mm01307329_m1; prostaglandin-endoperoxide synthase 2 [COX2]), CD40 (Mm00441891_m1; CD40 antigen), MMP9 (Mm00442991_m1; matrix metallopeptidase 9), Traf1 (Mm00493827_m1; (TNF receptor-associated factor 1), and Irak3 (Mm00518541_m1; interleukin-1 receptor-associated kinase 3). The relative changes in gene expression levels were calculated using the equation 2−ΔΔCT (58), where all values were normalized with respect to the housekeeping gene Gapdh (glyceraldehyde-3-phosphate dehydrogenase; 4352932E) mRNA levels. Amplification using 50 ng of RNA was performed in duplicate and with a total volume of 20 μl. Each real-time PCR assay was performed two times, and the results are expressed as the means ± standard deviations (SDs).
Measurement of inflammatory mediators.
Concentrations of inflammatory mediators were quantified in cell-free supernatants of macrophage cultures using a Milliplex 32-Plex mouse cytokine detection system (Millipore Corporation, Billerica, MA) according to the manufacturer's instructions. Each sample was assayed in duplicate, and cytokine standards and quality controls supplied by the manufacturer were run on each plate. The multiplex assay was performed two times using cell-free culture supernatants from different experiments. Data were acquired on a Luminex 100 system and analyzed using Bio-Plex Manager software, version 4.1 (Bio-Rad Laboratories, Hercules, CA). Multiplex test kits were validated using high-sensitivity ELISA Opti-EIA sets (BD-Pharmingen, San Jose, CA) or Duo sets (R&D Systems, Minneapolis, MN) where the latter were available. In all cases tested, comparable results were observed using the Luminex-based multiplex assays and individual ELISA kits (data not shown).
Statistical analysis.
A corrected one-way analysis of variance was used to analyze the microarray data using Spotfire software (Spotfire DecisionSite for Functional Genomics, Spotfire Inc.). Cytokine multiplex and qRT-PCR data were analyzed using a two-tailed unpaired Student's t test. P < 0.05 was considered significant.
RESULTS
Live B. burgdorferi spirochetes induce the upregulation of multiple gene transcripts in macrophages.
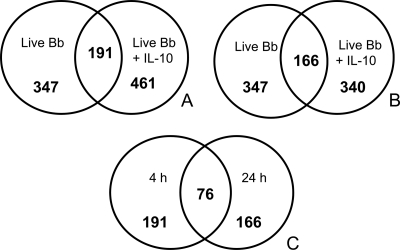
Microarray was employed to first identify genes that are upregulated in macrophages after stimulation with live B. burgdorferi spirochetes either alone or combined with IL-10. RNA samples were obtained from unstimulated and stimulated cells at 4 and 24 h poststimulation and subjected to gene expression analyses. Live spirochetes upregulated a total of 347 (fold change of >2.0) macrophage gene transcripts at both the 4-h and 24-h time points, whereas live spirochetes combined with IL-10 induced upregulation of 461 and 340 genes at 4 h and 24 h, respectively (Fig. 1A and B), suggesting the potentiation of these genes by IL-10. Live spirochetes induced 156 (4 h) and 181 (24 h) exclusive gene transcripts while live spirochetes combined with IL-10 elicited 270 (4 h) and 174 (24 h) exclusive genes (see Tables S3 to S6 in the supplemental material). The numbers of overlapping candidate genes identified at 4 h (191 genes) and 24 h (166 genes) between both stimulants are depicted in Fig. 1A and B and listed in Tables 1 to 4) (see also Tables S1 and S2 in the supplemental material). Of the 191 and 166 overlapping genes, 76 of them were common to the 4-h and 24-h time points (Fig. 1C and Tables 1 to 4; see also Tables S1 and S2). We also observed that live spirochetes alone or with added IL-10 downregulated a total of 723 and 698 gene transcripts at 4 h and 24 h, respectively. There were 238 (33% of the total genes) and 312 (45% of the total genes) common genes represented, respectively, at 4 h and 24 h between both stimulants (see Tables S7 and S8). However, the majority of these genes were poorly characterized with regard to immunological functions, and therefore they were not further evaluated in this study.
Fig. 1.
Distinct gene expression profiles obtained from mouse J774 macrophages stimulated with live B. burgdorferi (Bb) spirochetes alone or in combination with IL-10 at 4 h (A) and 24 h (B) and common genes between 4 h and 24 h (C) poststimulation. Macrophages (3 × 106/ml) were incubated with live B. burgdorferi spirochetes at an MOI of 10 in the presence or absence of 10 ng/ml of mouse recombinant IL-10. The control culture consisted of cells incubated with medium alone (unstimulated). Microarray analysis was conducted on the cDNA produced from RNA extracted from macrophages at 4 h and 24 h poststimulation. Data are reported as fold change induction relative to the values obtained from unstimulated cells. Genes whose expression changed by at least 2-fold or more for upregulated genes and −2-fold or less for downregulated genes (with a corrected one-way analysis of variance, P < 0.05) compared with unstimulated cells were considered to be differentially expressed in a statistically significant manner. The mean fold induction of the expression of each sample was expressed as a ratio of intensities of stimulated to unstimulated cells in two parallel experiments.
Table 1.
Selected upregulated gene transcripts in macrophages 4 h after exposure to live B. burgdorferi which were downregulated in the presence of added exogenous IL-10a
| Functional group and description | Gene no. | Annotation | Fold change in expression by culture condition(s) |
Fold change ratio (live Bb culture/live Bb + IL-10 culture) | |
|---|---|---|---|---|---|
| Live Bbb | Live Bb + IL-10 | ||||
| Cytokines and Chemokines | |||||
| Colony stimulating factor 1 (macrophage) | NM_007778 | Csf1 | 8.14 | 3.13 | 2.6 |
| Chemokine (C-X-C motif) ligand 2 | NM_009140 | Cxcl2 | 98.28 | 38.22 | 2.57 |
| Chemokine (C-X-C motif) ligand 10 | NM_021274 | Cxcl10 | 6.53 | 3.75 | 1.74 |
| Chemokine (C-C motif) ligand 2 | NM_011333 | Ccl2 | 4.6 | 2.75 | 1.67 |
| Chemokine (C-C motif) ligand 5 | NM_013653 | Ccl5 | 4.31 | 3.11 | 1.39 |
| Interleukin 6 | NM_031168 | IL-6 | 6 | 4.54 | 1.32 |
| Tumor necrosis factor | NM_013693 | Tnf | 12.16 | 9.36 | 1.3 |
| Enzymes | |||||
| Cytochrome c oxidase, subunit XVII assembly protein homolog (yeast) | BU555670 | Cox17 | 14.34 | 5.7 | 2.52 |
| Guanylate nucleotide binding protein 2 | NM_010260 | Gbp2 | 5.23 | 3.31 | 1.58 |
| Cytochrome b-245, beta polypeptide | NM_007807 | Cybb | 5.49 | 3.5 | 1.57 |
| Acyl-coenzyme A synthetase long-chain family member 1 | NM_007981 | Acsl1 | 6.45 | 4.52 | 1.43 |
| G-protein coupled receptor | |||||
| G protein-coupled receptor 109A | NM_030701 | Gpr109a | 4.61 | 3.62 | 1.27 |
| Growth factor | |||||
| Jagged 1 | NM_013822 | Jag1 | 5.61 | 2.84 | 1.98 |
| Kinases | |||||
| Polo-like kinase 2 (Drosophila) | NM_152804 | Plk2 | 8.65 | 5.91 | 1.46 |
| Interleukin-1 receptor-associated kinase 2 ligand-dependent nuclear receptor | NM_172161 | Irak2 | 4.36 | 3.06 | 1.42 |
| Nuclear receptor subfamily 4, group A, member 1 | NM_010444 | Nr4a1 | 12.56 | 6.9 | 1.82 |
| Peptidases | |||||
| Mucosa-associated lymphoid tissue lymphoma translocation gene 1 | NM_172833 | Malt1 | 6.83 | 3.1 | 2.21 |
| Complement component 3 | NM_009778 | C3 | 15.32 | 12.12 | 1.26 |
| Phosphatases | |||||
| Protein tyrosine phosphatase, receptor-type, F interacting protein, binding protein 2 | NM_008905 | Ppfibp2 | 3.86 | 2.22 | 1.74 |
| Protein tyrosine phosphatase, receptor type, J | NM_008982 | Ptpri | 4.63 | 2.85 | 1.63 |
| Transcription regulators | |||||
| Human immunodeficiency virus type I enhancer binding protein 3 | AK038070 | Hivep3 | 5.92 | 2.99 | 1.98 |
| Early growth response 2 | NM_010118 | Egr2 | 4.03 | 2.43 | 1.66 |
| V-maf musculoaponeurotic fibrosarcoma oncogene family, protein F (avian) | NM_010755 | Maff | 7.13 | 4.52 | 1.58 |
| Nuclear factor of kappa light chain gene enhancer in B-cells 1, p105 | BC050841 | Nfkb1 | 3.26 | 2.27 | 1.44 |
| Nuclear factor of kappa light chain polypeptide gene enhancer in B-cell inhibitor, epsilon | NM_008690 | Nfkbie | 2.85 | 2.28 | 1.25 |
| Jun-B oncogene | NM_008416 | Junb | 5.35 | 4.43 | 1.21 |
| Transmembrane receptors | |||||
| Macrophage receptor with collagenous structure | NM_010766 | Marco | 29.32 | 9.46 | 3.1 |
| Toll-like receptor 2 | NM_011905 | Tlr2 | 8.65 | 6.55 | 1.32 |
| Poliovirus receptor | NM_027514 | Pvr | 2.82 | 2.27 | 1.24 |
| Macrophage scavenger receptor 1 | NM_031195 | Msr1 | 3.09 | 2.52 | 1.23 |
| Transporters | |||||
| Solute carrier family 11 (proton-coupled divalent metal ion transporters), member 2 | AK148276 | Slc11a2 | 7.73 | 4.82 | 1.61 |
| Syntaxin 11 | AK017897 | Stx11 | 3.91 | 3.26 | 1.2 |
| Other | |||||
| Unknown | NM_173363 | Eif5 | 8.91 | 3.5 | 2.54 |
| Cytokine-inducible SH2-containing protein | NM_009895 | Cish | 4.93 | 2.49 | 1.98 |
| TNF receptor-associated factor 1 | NM_009421 | Traf1 | 10.75 | 6.17 | 1.74 |
| Septin 11 | AK028475 | 3.46 | 2.17 | 1.59 | |
| Intercellular adhesion molecule | BC008626 | Icam1 | 10.21 | 6.63 | 1.54 |
| CD83 antigen | NM_009856 | Cd83 | 3.84 | 2.56 | 1.5 |
| Rho guanine nucleotide exchange factor (GEF) 3 | NM_027871 | Arhgef3 | 6.15 | 4.12 | 1.49 |
| ADP-ribosylation factor-like 5C | NM_207231 | Arl5c | 7.04 | 5.03 | 1.4 |
| CASP8 and FADD-like apoptosis regulator | NM_207653 | Cflar | 3.64 | 2.62 | 1.39 |
| RIKEN cDNA 1190003J15 gene | AK004470 | 1190003J15Rik | 5.94 | 4.27 | 1.39 |
| Zinc finger CCCH type containing 12A | NM_153159 | Zc3h12a | 4.04 | 3.09 | 1.3 |
| Density-regulated protein | NM_026603 | Denr | 2.76 | 2.13 | 1.3 |
| Nucleotide-binding oligomerization domain containing 2 | NM_145857 | Card15 | 3.94 | 3.09 | 1.28 |
| Proline-rich nuclear receptor coactivator 1 | NM_001033225 | Pnrc1 | 3.67 | 2.91 | 1.26 |
| Syndecan 4 | NM_011521 | Sdc4 | 3.82 | 3.07 | 1.24 |
| Unknown | XM_134209 | BC053440 | 2.93 | 2.37 | 1.24 |
| Interferon-related developmental regulator 1 | NM_013562 | Ifrd1 | 2.88 | 2.33 | 1.23 |
| CDC42 effector protein (Rho GTPase binding) 2 | NM_026772 | Cdc42ep2 | 4.7 | 3.84 | 1.22 |
| Growth arrest and DNA-damage-inducible 45 beta | NM_008655 | Gadd45b | 11.09 | 9.07 | 1.22 |
| Deltex 4 homolog (Drosophila) | NM_172442 | Dtx4 | 3.24 | 2.68 | 1.21 |
| Poly (ADP-ribose) polymerase family, member 14 | NM_001039530 | Parp14 | 2.83 | 2.35 | 1.21 |
A corrected one-way analysis of variance was used to analyze the microarray data. Genes whose expression levels were upregulated 2-fold or more (P < 0.05) compared to unstimulated cells were considered to be differentially expressed in a statistically significant manner. The underlined genes are common to both the 4- and 24-h time points.
Bb, B. burgdorferi spirochetes.
Table 4.
Selected upregulated gene transcripts in macrophages 24 h after exposure to live Borrelia burgdorferi which were potentiated in the presence of added exogenous IL-10a
| Functional group and description | Gene no. | Annotation | Fold change in expression by culture condition(s) |
Fold change ratio (live Bb culture/live Bb + IL-10 culture) | |
|---|---|---|---|---|---|
| Live Bbb | Live Bb + IL-10 | ||||
| Cytokines | |||||
| Chemokine (C-C motif) ligand 4 | NM_013652 | Ccl4 | 4.52 | 5.44 | 0.83 |
| Chemokine (C-C motif) ligand 2 | NM_011333 | Ccl2 | 13.33 | 16.69 | 0.8 |
| Chemokine (C-C motif) ligand 9 | NM_011338 | Ccl9 | 3.03 | 3.92 | 0.77 |
| Interleukin 1 beta | NM_008361 | Il1b | 52.97 | 83.67 | 0.63 |
| Chemokine (C-C motif) ligand 12 | NM_011331 | Ccl12 | 2.59 | 5.99 | 0.43 |
| Chemokine (C-C motif) ligand 7 | NM_013654 | Ccl7 | 8.24 | 34.01 | 0.24 |
| Enzymes | |||||
| Prolyl 4-hydroxylase, beta polypeptide | NM_011032 | P4hb | 2.7 | 3.23 | 0.83 |
| Ras homolog gene family, member Q | NM_145491 | Rhoq | 2.31 | 2.82 | 0.82 |
| Phosphodiesterase 4B, cAMP specific | NM_019840 | Pde4b | 3.94 | 5.02 | 0.79 |
| Glutaredoxin | NM_053108 | Glrx | 2.95 | 3.98 | 0.74 |
| DNA segment, Chr 1, Brigham and Women's Genetics 1363 expressed | NM_001001566 | D1Bwg1363e | 2.39 | 3.31 | 0.72 |
| Bone marrow stromal cell antigen 1 | NM_009763 | BstI | 2.69 | 6.54 | 0.41 |
| G-protein coupled receptor | |||||
| Formyl peptide receptor 1 | NM_013521 | Fpr1 | 2.84 | 5.15 | 0.55 |
| Peptidases | |||||
| HtrA serine peptidase 1 | NM_019564 | Htra1 | 5.1 | 9.65 | 0.53 |
| Matrix metallopeptidase 13 | NM_008607 | Mmp13 | 2.62 | 12.5 | 0.21 |
| Phosphatases | |||||
| Dual specificity phosphatase 2 | NM_010090 | Dusp2 | 2.4 | 5.85 | 0.41 |
| Protein tyrosine phosphatase, receptor type, J | NM_008982 | Ptprj | 2.11 | 7.22 | 0.29 |
| Transcription regulators | |||||
| Zinc finger protein 36 | NM_011756 | Zfp36 | 2.25 | 3.45 | 0.65 |
| CCR4 carbon catabolite repression 4-like (S. cerevisiae) | NM_009834 | Ccrn4l | 2.95 | 4.66 | 0.63 |
| B-cell leukemia/lymphoma 3 | NM_033601 | Bcl3 | 3.9 | 7.12 | 0.55 |
| FBJ osteosarcoma oncogene | NM_010234 | Fos | 2.53 | 4.77 | 0.53 |
| Transmembrane receptors | |||||
| C-type lectin domain family 4, member a2 | NM_011999 | Clec4a2 | 3.29 | 5.28 | 0.62 |
| Colony stimulating factor 2 receptor, beta, low-affinity (granulocyte-macrophage) | AK154286 | Csf2rb1 | 2.29 | 4.05 | 0.57 |
| Fc receptor, IgG, high affinity I | BC025535 | Fcgr1 | 2.25 | 5.82 | 0.39 |
| Interleukin 4 receptor, alpha | NM_001008700 | Il4ra | 2.91 | 11.12 | 0.26 |
| Transporters | |||||
| Solute carrier family 28 (sodium-coupled nucleoside transporter), member 2 | NM_172980 | Slc28a2 | 3.53 | 5.44 | 0.65 |
| Lipocalin 2 | NM_008491 | Lcn2 | 12.13 | 20.55 | 0.59 |
| Other | |||||
| Nuclear factor of kappa light chain gene enhancer in B-cells inhibitor, alpha | NM_010907 | Nfkbia | 5.76 | 6.9 | 0.83 |
| Mitochondrial ribosomal protein L52 | NM_026851 | Mrpl52 | 3.35 | 4.04 | 0.83 |
| DNA segment, Chr 3, University of California at Los Angeles 1 | NM_030685 | D3Ucla1 | 2.6 | 3.12 | 0.83 |
| Ring finger protein 149 | NM_001033135 | Rnf149 | 3.01 | 3.72 | 0.81 |
| Transmembrane protein 176A | NM_025326 | 0610011I04Rik | 2.75 | 3.4 | 0.81 |
| RIKEN cDNA 1200002N14 gene | NM_027878 | 1200002N14Rik | 2.67 | 3.3 | 0.81 |
| RIKEN cDNA 1190003J15 gene | AK004470/BC051545 | 1190003J15Rik | 93.75 | 123.62 | 0.76 |
| Syndecan 4 | NM_011521 | Sdc4 | 2.23 | 3.02 | 0.74 |
| Serum amyloid A 1 | NM_009117 | Saa1 | 90.62 | 123.61 | 0.73 |
| Interferon induced transmembrane protein 2 | NM_030694 | Ifitm2 | 2.19 | 2.98 | 0.73 |
| Rho guanine nucleotide exchange factor (GEF) 3 | NM_027871 | Arhgef3 | 2.84 | 4 | 0.71 |
| Myristoylated alanine rich protein kinase C substrate | NM_008538 | Marcks | 2.72 | 3.89 | 0.7 |
| FGF receptor activating protein 1 | NM_145583/AK152420 | Frag1 | 2.33 | 3.35 | 0.7 |
| RIKEN cDNA 5730508B09 gene | AK162420/NM_027482 | 5730508B09Rik | 2.71 | 4.07 | 0.67 |
| Ral guanine nucleotide dissociation stimulator, -like 1 | NM_016846 | Rgl1 | 5.28 | 7.92 | 0.67 |
| Leucine rich repeat containing 25 | NM_153074 | Lrrc25 | 2.92 | 4.6 | 0.64 |
| Transmembrane protein 176B | NM_023056 | 1810009M01Rik | 3.26 | 5.18 | 0.63 |
| Unknown | ENSMUST00000094405 | ENSMUST00000094405 | 4.02 | 6.52 | 0.62 |
| Transformed mouse 3T3 cell double minute 4 | NM_008575 | Mdm4 | 3.24 | 5.49 | 0.59 |
| C-type lectin domain family 4, member a3 | AK156040 | Clec4a3 | 2.92 | 6.68 | 0.44 |
| Predicted gene, OTTMUSG00000000971 | BC089618 | BC089618 | 2.92 | 7.86 | 0.37 |
| CD244 natural killer cell receptor 2B4 | NM_018729 | Cd244 | 8.25 | 25.88 | 0.32 |
| C-type lectin domain family 4, member b1 | NM_027218 | Clec4b1 | 3.06 | 9.47 | 0.32 |
A corrected one-way analysis of variance was used to analyze the microarray data. Genes whose expression levels were upregulated at least 2-fold (P < 0.05) compared to unstimulated cells were considered to be differentially expressed in a statistically significant manner. The underlined genes are common to both the 4- and 24-h time points.
Bb, B. burgdorferi spirochetes.
IL-10 alters the expression levels of multiple genes induced by live B. burgdorferi spirochetes in macrophages.
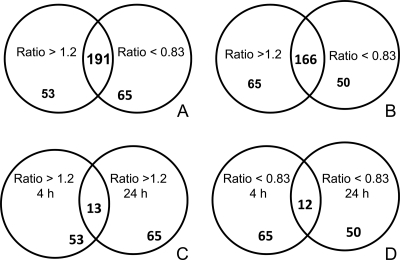
As the focus of our study was to identify genes whose expression was induced by live spirochetes and altered by IL-10, we subjected the 191 (4 h) and 166 (24 h) upregulated overlapping genes to further analyses. Two groups of genes were identified within the overlapping gene groups as being induced by live spirochetes but altered by IL-10. These included (i) genes that were induced by live spirochetes and whose expression levels were downregulated by IL-10 and (ii) genes that were induced by live spirochetes and whose expression levels were potentiated by IL-10 (Fig. 2A and B and Tables 1 to 4; see also Tables S1 and S2). IL-10 downregulated the expression levels of 53 and 65 live spirochete-induced genes with a corresponding ratio of >1.2 at 4 h and 24 h, respectively (Tables 1 and 3). Of these 53 and 65 genes whose expression was inhibited by IL-10, 13 of them overlapped at the 4-h and 24-h time points (Fig. 2C and Tables 1 and 3; see also Tables S1 and S2 in the supplemental material). Many of the IL-10-downregulated genes are of known biological functions, encoding protein and membrane transport, receptor signaling, metabolism, cell adhesion, phosphorylation, development, cell cycle, immune responses, signal transduction, proliferation and apoptosis, translation and transcription, and cytokines/chemokines, among many others. Some of the genes on this list that are well characterized and worthy of mention are as follows: chemokine (C-X-C motif) ligand genes Cxcl2, Cxcl3 and Cxcl10, immunoresponsive gene Irg1; genes that encode monocyte-derived chemokines, Ccl3 and Ccl5 (67); proinflammatory cytokine Tnf and Il-6 (66, 85, 86); Toll-like receptors, Tlr1 and Tlr2 (1, 2, 14, 47, 97); and Ptgs2, which codes for prostaglandins-endoperoxide synthase 2, COX2 (1). Other genes downregulated by IL-10 include the following: IFN response genes such as interferon regulatory factor, Irf7; activating transcription factor 3, Atf3; interferon-activated gene 202B, Ifi202b; interleukin receptor-associated protein kinase 2 and kinase 3, Irak2 and Irak3; TNF receptor-associated factor 1 and factor 2, Traf1 and Traf2; Fas ligand (TNF superfamily, member 6), Fas; tumor necrosis factor alpha-induced protein 3, Tnfaip3; intracellular adhesion molecule 1, Icam1; matrix metallopeptidases, Mmp9 (37, 38, 85), complement 3, C3; and Cd40 and Cd47 transcripts (77).
Fig. 2.
IL-10 alters the gene expression pattern induced by live B. burgdorferi spirochetes in macrophages. Diagrams of the 191 (4 h) and 166 (24 h) overlapping genes (Fig. 1) that were induced by live spirochetes and whose expression levels were inhibited by IL-10 (ratio of ≥1.2) and genes that were induced by live spirochetes and whose expression levels were potentiated by IL-10 (ratio of ≤0.83) are shown for the 4-h (A) and 24-h (B) time points. The common IL-10-inhibited genes between the 4-h and 24-h (C) and the common IL-10-potentiated genes between the 4-h and 24-h (D) time points are also represented.
Table 3.
Selected upregulated gene transcripts in macrophages 24 h after exposure to live Borrelia burgdorferi which were downregulated in the presence of added exogenous IL-10a
| Functional group and description | Gene no. | Annotation | Fold change in expression by culture condition(s) |
Fold change ratio (live Bb culture/live Bb + IL-10 culture) | |
|---|---|---|---|---|---|
| Live Bbb | Live Bb + IL-10 | ||||
| Cytokines | |||||
| Chemokine (C-X-C motif) ligand 2 | NM_009140 | Cxcl2 | 37.17 | 3.31 | 11.23 |
| Chemokine (C-C motif) ligand 5 | BC033508 | Ccl5 | 13.51 | 5.54 | 2.44 |
| Tumor necrosis factor | NM_013693 | Tnf | 6.77 | 3.39 | 2.0 |
| Enzymes | |||||
| Diacylglycerol O-acyltransferase 2 | NM_026384 | Dgat2 | 6.96 | 2.68 | 2.6 |
| Ceruloplasmin | NM_007752 | Cp | 21.69 | 9.26 | 2.34 |
| Guanylate nucleotide binding protein 3 | NM_018734 | Gbp4 | 6.89 | 3.62 | 1.9 |
| Cytochrome b-245, beta polypeptide | NM_007807 | Cybb | 8.66 | 5.44 | 1.59 |
| 2′-5′ Oligoadenylate synthetase 1A | NM_145211 | Oas1a | 3.7 | 2.56 | 1.45 |
| Prostaglandin-endoperoxide synthase 2 | NM_011198 | Ptgs2 | 7.51 | 5.47 | 1.37 |
| Carbonic anhydrase 13 | NM_024495 | Car13 | 5.17 | 3.95 | 1.31 |
| DEXH (Asp-Glu-X-His) box polypeptide 58 | NM_030150 | D11Lgp2e | 4.3 | 3.35 | 1.28 |
| Baculoviral IAP repeat-containing 3 | NM_007464 | Birc3 | 4.81 | 3.79 | 1.27 |
| Superoxide dismutase 2, mitochondrial | NM_013671 | Sod2 | 10.41 | 8.17 | 1.27 |
| 2′-5′ Oligoadenylate synthetase 2 | NM_145227 | Oas2 | 3.23 | 2.6 | 1.24 |
| Crystallin, mu | NM_016669 | Crym | 3.01 | 2.46 | 1.22 |
| Cytochrome b5 type B | NM_025558 | Cyb5b | 2.68 | 2.23 | 1.2 |
| G-protein coupled receptors | |||||
| EGF-like module containing, mucin-like, hormone receptor-like sequence 1 | NM_010130 | Emr1 | 5.86 | 4.1 | 1.43 |
| G protein-coupled receptor 84 | NM_030720 | Gpr84 | 5.09 | 4.15 | 1.22 |
| Kinases | |||||
| Interleukin-1 receptor-associated kinase 3 | NM_028679 | Irak3 | 9.68 | 4.27 | 2.27 |
| Spleen tyrosine kinase | NM_011518 | Syk | 3.13 | 2.31 | 1.35 |
| Ligand-dependent nuclear receptor | |||||
| Nuclear receptor subfamily 4, group A, member 1 | NM_010444 | Nr4a1 | 3.98 | 3.08 | 1.29 |
| Peptidases | |||||
| Ubiquitin specific peptidase 18 | NM_011909 | Usp18 | 9.44 | 4.28 | 2.2 |
| Matrix metallopeptidase 9 | NM_013599 | Mmp9 | 6.38 | 4.1 | 1.55 |
| Complement component 3 | NM_009778 | C3 | 28.86 | 22.15 | 1.3 |
| Phosphatase | |||||
| Protein tyrosine phosphatase, receptor-type, F interacting protein, binding protein 2 | NM_008905 | Ppfibp2 | 5.3 | 2.25 | 2.36 |
| Transcription regulators | |||||
| Interferon regulatory factor 7 | NM_016850 | Irf7 | 7.44 | 3.47 | 2.15 |
| Nuclear antigen Sp100 | BC069183 | Sp100 | 4.51 | 2.59 | 1.74 |
| Elongation factor RNA polymerase II 2 | BC006925 | Ell2 | 5.26 | 3.54 | 1.48 |
| Transmembrane receptors | |||||
| Toll-like receptor 1 | NM_030682 | Tlr1 | 8.63 | 4.89 | 1.77 |
| Toll-like receptor 2 | NM_011905 | Tlr2 | 5.17 | 3.08 | 1.68 |
| Fas (TNF receptor superfamily member 6) | NM_007987 | Fas | 9.25 | 7.1 | 1.3 |
| Macrophage receptor with collagenous structure | NM_010766 | Marco | 67.71 | 52.45 | 1.29 |
| CD40 antigen | NM_170701 | Cd40 | 3.34 | 2.77 | 1.2 |
| Transporters | |||||
| Solute carrier family 11 (proton-coupled divalent metal ion transporters), member 2 | AK148276 | Slc11a2 | 4.99 | 2.63 | 1.9 |
| Solute carrier family 31, member 2 | NM_025286 | Slc31a2 | 5.94 | 3.73 | 1.59 |
| Fatty acid binding protein 4, adipocyte | NM_024406 | Fabp4 | 3.96 | 2.94 | 1.35 |
| Serum amyloid A 3 | NM_011315 | Saa3 | 39.27 | 30.33 | 1.29 |
| Other | |||||
| Immunoresponsive gene 1 | L38281/AK1521 | Irg1 | 196.02 | 24.55 | 7.99 |
| TNF receptor-associated factor 1 | NM_009421 | Traf1 | 23.29 | 5 | 4.66 |
| Bone marrow stromal cell antigen 2 | NM_198095 | Bst2 | 6.82 | 3.04 | 2.24 |
| ISG15 ubiquitin-like modifier | NM_015783 | Isg15 | 7.85 | 3.52 | 2.23 |
| C-type lectin domain family 4, member e | NM_019948 | Clec4e | 4.79 | 2.23 | 2.15 |
| Tumor necrosis factor, alpha-induced protein 3 | NM_009397 | Tnfaip3 | 6.79 | 3.47 | 1.95 |
| ADP-ribosylation factor-like 5C | NM_207231 | Arl5c | 4.58 | 2.43 | 1.88 |
| Argininosuccinate synthetase 1 | NM_007494/M3 | Ass1 | 5.25 | 2.82 | 1.86 |
| Interferon-activated gene 202B | NM_011940 | Ifi202b | 3.93 | 2.41 | 1.63 |
| Unknown | ENSMUST00000073378 | ENSMUST00000073378 | 4.22 | 2.87 | 1.47 |
| RAS p21 protein activator 4 | NM_133914 | Rasa4 | 3.03 | 2.06 | 1.47 |
| EH-domain containing 1 | NM_010119 | Ehd1 | 4.2 | 2.89 | 1.45 |
| Unknown | NM_022431 | Ms4a11 | 7.42 | 5.19 | 1.43 |
| Unknown | XM_484397 | Dgkh | 4.75 | 3.33 | 1.42 |
| Wingless-related MMTV integration site 6 | NM_009526 | Wnt6 | 2.97 | 2.12 | 1.4 |
| Zinc finger CCCH type containing 12C | XM_146893 | Zc3h12c | 5.6 | 4.03 | 1.39 |
| Membrane-spanning 4-domains, subfamily A, member 6B | NM_027209 | Ms4a6b | 8.13 | 5.95 | 1.37 |
| Immediate early response 3 | NM_133662 | Ier3 | 3.85 | 2.99 | 1.29 |
| CD47 antigen (Rh-related antigen, integrin-associated signal transducer) | NM_010581 | Cd47 | 2.66 | 2.11 | 1.26 |
| Lymphocyte cytosolic protein 2 | NM_010696 | Lcp2 | 3.1 | 2.46 | 1.26 |
| Membrane-spanning 4-domains, subfamily A, member 6C | NM_028595 | Ms4a6c | 7.91 | 6.33 | 1.25 |
| Secretory leukocyte peptidase inhibitor | NM_011414 | Slpi | 14.21 | 11.82 | 1.2 |
| Coiled-coil domain containing 50 | NM_026202 | Ccdc50 | 3.21 | 2.37 | 1.36 |
| SH3-domain binding protein 5 (BTK-associated) | NM_011894 | Sh3bp5 | 2.99 | 2.24 | 1.34 |
| Ras association (RalGDS/AF-6) domain family 4 | NM_178045 | Rassf4 | 3.28 | 2.46 | 1.33 |
| RAB32, member RAS oncogene family | NM_026405 | Rab32 | 2.83 | 2.14 | 1.32 |
| CDC42 effector protein (Rho GTPase binding) 2 | NM_026772 | Cdc42ep2 | 2.83 | 2.15 | 1.32 |
| Membrane-spanning 4-domains, subfamily A, member 6D | NM_026835 | Ms4a6d | 8.57 | 6.65 | 1.29 |
A corrected one-way analysis of variance was used to analyze the microarray data. Genes whose expression levels were upregulated at least 2-fold (P < 0.05) compared to unstimulated cells were considered to be differentially expressed in a statistically significant manner. The underlined genes are common to both 4- and 24-h time points.
Bb, B. burgdorferi spirochetes.
The second group of overlapping genes induced by live spirochetes that were potentiated by IL-10 are shown in Fig. 2D. There were 65 and 50 live-spirochete-induced gene transcripts at 4 h and 24 h that were enhanced by IL-10, as determined by a reduced ratio of <0.83 (Tables 2 and 4). There were 12 IL-10-potentiated genes common to the 4-h and 24-h time points, of which 11 were functionally recognizable (Bcl3, Ccl4, Saa1, Ccl9, Rnf149, Il-1B, Rgl1, Mmp13, Pde4b, Mrp, and Ccrn4) (Fig. 2D; Tables 2 and 4). Interestingly, some genes, such as Ptgs2, Cd40, Tnfaip3, and Saa3, which were enhanced by IL-10 at 4 h subsequently were downregulated by IL-10 at 24 h, suggesting early and late regulation by IL-10. Overall, many of the live-spirochete-induced genes altered (inhibited or potentiated) by IL-10 were similarly perturbed when sonicated B. burgdorferi spirochetes were used as the stimulant in microarray studies (data not shown).
Table 2.
Selected upregulated gene transcripts in macrophages 4 h after exposure to live B. burgdorferi which were potentiated in the presence of added exogenous IL-10a
| Functional group and description | Gene no. | Annotation | Fold change in expression by culture condition(s) |
Fold change ratio (live Bb culture/live Bb + IL-10 culture) | |
|---|---|---|---|---|---|
| Live Bbb | Live Bb + IL-10 | ||||
| Cytokines | |||||
| Interleukin 1 beta | NM_008361 | Il1b | 146.37 | 180.93 | 0.81 |
| Interleukin 10 | NM_010548 | Il10 | 3.81 | 4.74 | 0.8 |
| Chemokine (C-C motif) ligand 9 | NM_011338 | Ccl9 | 4.18 | 5.62 | 0.74 |
| Chemokine (C-C motif) ligand 4 | NM_013652 | Ccl4 | 11.32 | 16.23 | 0.7 |
| Interleukin 1 receptor antagonist | NM_031167 | Il1rn | 3.91 | 6.79 | 0.57 |
| Enzymes | |||||
| RAB12, member RAS oncogene family | NM_024448 | Rab12 | 2.26 | 2.75 | 0.82 |
| Superoxide dismutase 2, mitochondrial | NM_013671 | Sod2 | 6.41 | 8.33 | 0.77 |
| Diacylglycerol O-acyltransferase 2 | NM_026384 | Dgat2 | 3.15 | 4.47 | 0.71 |
| CTAGE family, member 5 | AK164018 | Mgea6 | 2.08 | 3.05 | 0.68 |
| Carbonic anhydrase 13 | NM_024495 | Car13 | 5.5 | 8.82 | 0.62 |
| UDP-glucose ceramide glucosyltransferase | NM_011673 | Ugcg | 3.37 | 5.5 | 0.61 |
| Sphingomyelin synthase 1 | NM_144792 | Tmem23 | 3.91 | 6.49 | 0.6 |
| Prostaglandin-endoperoxide synthase 2 | NM_011198 | Ptgs2 | 15.95 | 27.45 | 0.58 |
| G-protein coupled receptor | |||||
| Adenosine A2b receptor | NM_007413 | Adora2b | 2.98 | 6.85 | 0.44 |
| Ion channel | |||||
| Mucolipin 2 | NM_026656 | Mcoln2 | 2.96 | 4.05 | 0.73 |
| Kinases | |||||
| Proviral integration site 3 | NM_145478 | Pim3 | 2.85 | 3.6 | 0.79 |
| Phosphoinositide-3-kinase, regulatory subunit 5, p101 | NM_177320 | Pik3r5 | 3.1 | 4.03 | 0.77 |
| Inhibitor of κB kinase epsilon | NM_019777 | Ikbke | 2.93 | 3.95 | 0.74 |
| Proviral integration site 1 | NM_008842 | Pim1 | 2.18 | 2.99 | 0.73 |
| Mitogen-activated protein kinase kinase kinase kinase 4 | AK020498 | 9430080K19Rik | 2.4 | 3.49 | 0.69 |
| Hemopoietic cell kinase | NM_010407 | Hck | 2.6 | 4.19 | 0.62 |
| Unknown | NM_010884 | Ndrg1 | 2.68 | 5.9 | 0.46 |
| Peptidases | |||||
| Caspase 1 | NM_009807 | Casp1 | 2.58 | 3.48 | 0.74 |
| Matrix metallopeptidase 13 | NM_008607 | Mmp13 | 8.11 | 11.74 | 0.69 |
| A disintegrin-like and metallopeptidase (reprolysin type) with thrombospondin type 1 motif, 1 | NM_009621 | Adamts1 | 2.73 | 6.27 | 0.44 |
| Phosphatase | |||||
| Dual specificity phosphatase 1 | NM_013642 | Dusp1 | 3.51 | 5.71 | 0.61 |
| Transcription regulators | |||||
| B-cell leukemia/lymphoma 3 | NM_033601 | Bcl3 | 4.52 | 5.47 | 0.83 |
| E2F transcription factor 5 | X86925 | E2f5 | 2.54 | 3.49 | 0.73 |
| Hypoxia inducible factor 1, alpha subunit | NM_010431 | Hif1a | 3.44 | 4.78 | 0.72 |
| Kruppel-like factor 7 (ubiquitous) | NM_033563 | Klf7 | 2.22 | 3.09 | 0.72 |
| Activating transcription factor 3 | NM_007498 | Atf3 | 6.87 | 9.65 | 0.71 |
| Microphthalmia-associated transcription factor | NM_008601 | Mitf | 2.86 | 4.4 | 0.65 |
| Nuclear factor, interleukin 3, regulated | NM_017373 | Nfil3 | 2.81 | 4.78 | 0.59 |
| MAX dimerization protein 1 | AK137548 | Mxd1 | 2.99 | 5.53 | 0.54 |
| Signal transducer and activator of transcription 3 | NM_213659 | Stat3 | 2.91 | 5.89 | 0.49 |
| CCR4 carbon catabolite repression 4-like (S. cerevisiae) | NM_009834 | Ccrn4l | 4.74 | 10.29 | 0.46 |
| Transmembrane receptors | |||||
| CD40 antigen | NM_170701 | Cd40 | 3.26 | 4.18 | 0.78 |
| Fc receptor, IgG, low-affinity IIb | NM_010187 | Fcgr2b | 7.83 | 15.52 | 0.5 |
| Fc receptor, IgG, low-affinity III | NM_010188 | Fcgr3 | 3.3 | 6.63 | 0.5 |
| Tumor necrosis factor receptor superfamily, member 9 | NM_011612 | Tnfrsf9 | 3.09 | 5.42 | 0.57 |
| Transporters | |||||
| Solute carrier family 16 (monocarboxylic acid transporters), member 1 | NM_009196 | Slc16a1 | 2.22 | 3.01 | 0.74 |
| Synaptotagmin X | NM_018803 | Syt10 | 2.47 | 3.89 | 0.64 |
| Serum amyloid A 1 | NM_009117 | Saa1 | 20.57 | 64.77 | 0.32 |
| Serum amyloid A 3 | NM_011315 | Saa3 | 34 | 66.07 | 0.51 |
| Other | |||||
| TNFAIP3 interacting protein 3 | NM_001001495 | TNIP3 | 4.19 | 14.90 | 0.28 |
| Tumor necrosis factor, alpha-induced protein 3 | NM_009397 | Tnfaip3 | 19.96 | 58.62 | 0.34 |
| SAM domain, SH3 domain and nuclear localization signals, 1 | NM_023380 | Samsn1 | 3.04 | 6.97 | 0.44 |
| RIKEN cDNA 4933426M11 gene | NM_178682 | 4933426M11Rik | 2.45 | 2.97 | 0.82 |
| Unknown | AK031731 | Nfe2l2 | 2.21 | 2.71 | 0.82 |
| Zinc finger, AN1-type domain 5 | NM_009551 | Zfand5 | 2.15 | 2.67 | 0.81 |
| Phosphodiesterase 4B, cAMP specific | NM_019840/AK171700 | Pde4b | 6.83 | 9.07 | 0.75 |
| RIKEN cDNA 1810022K09 gene | BC045157 | 1810022K09Rik | 2.64 | 3.65 | 0.72 |
| Unknown | AT_ssM_RR_3 | AT_ssM_RR_3 | 2.46 | 3.47 | 0.71 |
| RIKEN cDNA 1810029B16 gene | NM_025465 | 1810029B16Rik | 3.35 | 4.75 | 0.71 |
| Mesoderm development candidate 1 | NM_030705 | Mesdc1 | 2.15 | 3.09 | 0.7 |
| Pleckstrin | NM_019549 | Plek | 2.64 | 3.81 | 0.69 |
| Ral guanine nucleotide dissociation stimulator, -like 1 | NM_016846 | Rgl1 | 3.09 | 4.59 | 0.67 |
| Immediate-early response 3 | NM_133662 | Ier3 | 3.72 | 5.71 | 0.65 |
| CDNA sequence BC031781 | NM_145943 | BC031781 | 2.43 | 3.82 | 0.64 |
| Ring finger protein 149 | NM_001033135 | Rnf149 | 2.68 | 4.3 | 0.62 |
| RIKEN cDNA E130014J05 gene | NM_001040400 | E130014J05Rik | 2.33 | 3.75 | 0.62 |
| RIKEN cDNA 5730508B09 gene | AK162420/NM_027482 | 5730508B09Rik | 4.07 | 9.32 | 0.44 |
| Unknown | AK035396 | 1200016E24Rik | 3.71 | 8.91 | 0.42 |
| Mitochondrial ribosomal protein L52 | AK081551 | Mrpl52 | 3.20 | 6.20 | 0.52 |
| Activity regulated cytoskeletal-associated protein | NM_018790 | Arc | 3.83 | 6.67 | 0.57 |
A corrected one-way analysis of variance was used to analyze the microarray data. Genes whose expression levels were upregulated by at least 2-fold or more (P < 0.05) compared to unstimulated cells were considered to be differentially expressed in a statistically significant manner. The underlined genes are common to both the 4- and 24-h time points.
Bb, B. burgdorferi spirochetes.
We also observed several genes to be induced by live spirochetes that were not detected in the live spirochete cultures to which IL-10 was added at either the 4-h or 24-h time points, which may suggest the complete downregulation of these genes by IL-10. A snapshot of these genes include cytokines (Il-20), chemokines (Cxcl4), signaling (Nos2), apoptosis (Casp1), and multiple interferon-induced protein transcripts (Ifit1 [Ifi204, Ifi27 and Ifi44] (see Tables S3 to S5 in the supplemental material). Many of these genes represent newly identified genes inducible by live spirochetes that are unperturbed or downregulated by IL-10.
Validation of B. burgdorferi-inducible genes that are downregulated by IL-10 by real-time PCR.
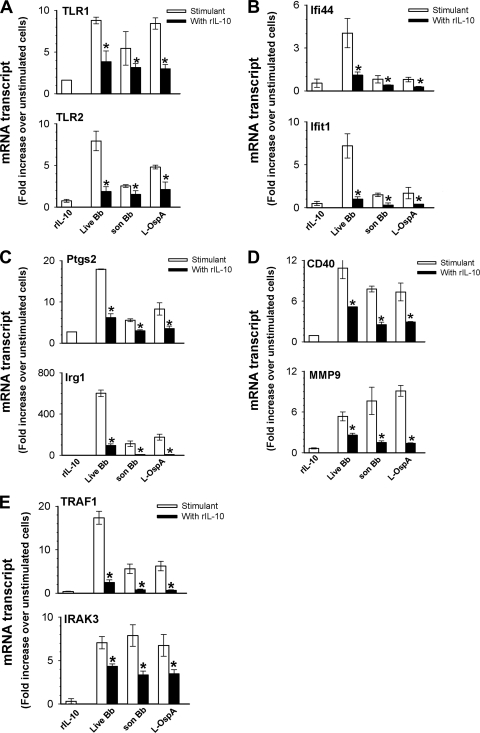
The reliability of the gene array analysis was validated by randomly selecting 10 of the spirochete-induced, IL-10-inhibited noncytokine and nonchemokine genes (as these were assessed at the protein level) and subjecting them to TaqMan qRT-PCR. For this study, we used RNA samples collected at 24 h from cells stimulated with live spirochetes and also from cells stimulated with sonicated B. burgdorferi or with the lipoprotein outer surface protein A (L-OspA) in the presence and absence of IL-10. To study the effect of IL-10 on the constitutive expression of inflammatory mediators in J774 cells, RNA collected from cells exposed to IL-10 alone was used in all real-time PCR studies. As shown in Fig. 3 A to E all selected genes (Ifit1, Ifi44, Tlr1, Tlr2, Irg1, Ptgs2, Mmp9, Cd40, Traf1, and Irak3) were significantly (P < 0.05) downregulated by IL-10 in response to all stimulants. Worthy of note was the ability of IL-10 by itself to alter the marginal constitutive mRNA expression levels of Ifit1, Ifi44, Tlr1, Tlr2, Ptgs2, Mmp9, Cd40, and Irak3 genes, indicating its inherent capacity to modulate selective genes in J774 cells. Live spirochetes overall significantly (P < 0.05 to 0.0001) induced levels of expression of all genes higher than those induced by sonicated spirochetes and L-OspA.
Fig. 3.
Confirmation of selected genes by qRT-PCR. Macrophages (3 × 106/ml) were incubated with live B. burgdorferi (Bb) spirochetes at an MOI of 10, sonicated (son) spirochetes (1 × 107/ml), or L-OspA (1 μg/ml) in the presence (black bars) or absence (stimulant; white bars) of 10 ng/ml of mouse recombinant IL-10 (rIL-10). Controls consisted of cells incubated with rIL-10 or medium alone (unstimulated cells). RNA samples were collected after 24 h of incubation, and gene transcripts were quantified by TaqMan qRT-PCR. All values were normalized with respect to the housekeeping gene Gapdh mRNA levels. Results are presented as fold increase over control (the level in unstimulated cells). Asterisks indicate significant differences from cells incubated with stimulants alone (P < 0.05). P values were calculated by an unpaired Student's t test. Results are representative of one of two experiments. Each bar represents the mean ± SD from samples run in duplicates.
IL-10 regulates the protein expression levels of cytokines and chemokines in macrophages stimulated by live B. burgdorferi spirochetes.
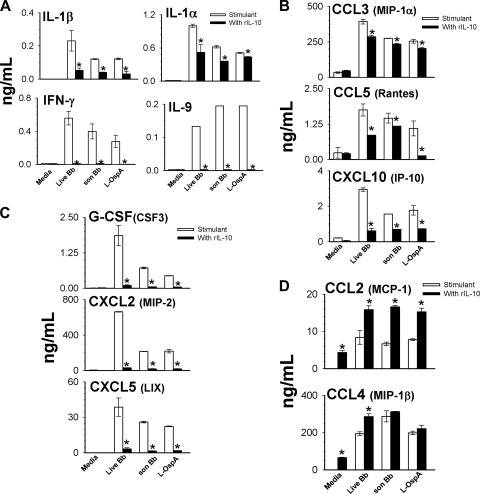
Cytokine/chemokine multiplex assays were performed to validate at the protein level live-spirochete-induced cytokine and chemokine mRNA gene transcripts that were altered by IL-10. These experiments were performed using 24-h culture supernatants from live and sonicated spirochetes or L-OspA-stimulated cells in the presence and absence of IL-10. As the microarray data showed the potentiating effect of IL-10 on select cytokine/chemokine genes, cultured supernatants collected from cells stimulated with IL-10 alone were also analyzed to assess whether or not IL-10 similarly potentiates selected genes at the protein level. By using this multiplex approach, 18 cytokines/chemokines were observed to be significantly induced by the three stimulants compared to unstimulated or IL-10-modulated cells. The production levels of the prototypic IL-6 and TNF cytokines were significantly (P < 0.05 to 0.008) downregulated by IL-10 in macrophages in response to live spirochetes (data not shown). IL-1β elicited by live spirochetes was markedly downregulated by IL-10 (Fig. 4A) although its mRNA transcript was enhanced by IL-10 (Tables 1 and 2), suggesting that IL-10 may regulate its expression at the translational and not transcriptional level. Similar significant (P < 0.05) results were obtained when cells were stimulated with sonicated spirochetes or with L-OspA (Fig. 4A), as already reported by us (27) and others (17). IL-10 also significantly (P < 0.03 to 0.01) inhibited the production of IL-1α, IFN-γ, and IL-9 in macrophages as induced by all stimulants (Fig. 4A) even though their gene transcripts were not observed by microarray. Of significance was the enhanced production of IL-1β, IL-1α, and IFN-γ by live spirochetes compared to that of sonicated spirochetes and L-OspA.
Fig. 4.
IL-10-mediated regulation of cytokine (A) and chemokine (B to D) production by macrophages exposed in vitro to live or sonicated B. burgdorferi spirochetes or with L-OspA. Macrophages (3 × 106/ml) were stimulated as described in the legend of Fig. 3. Cell-free supernatants were harvested from cultures at 24 h, and protein determinations were made by multiplex ELISA. The lower limit of detection of the multiplex ELISA was 3.2 pg/ml. Cytokine and chemokine production levels are shown in ng/ml. Asterisks indicate significant differences from cells incubated with stimulants alone (P < 0.05 to P < 0.0000001). P values were calculated by use of an unpaired Student's t test. Each bar represents the mean ± SD of duplicate cultures.
The chemokines, RANTES/CCL5, IP-10/CXCL10, and MIP-2/CXCL2, were all significantly downregulated by IL-10 in macrophages in response to all stimulants (Fig. 4B and C). MIP-1α/CCL3, whose gene transcript level was moderately downregulated by IL-10 (ratio of 1.15) was moderately but significantly (P < 0.01) downregulated at the protein level (Fig. 4B). The production levels of the chemokines granulocyte colony-stimulating factor (G-CSF)/CSF3 and LIX/CXCL5 were markedly decreased (P < 0.02 to 0.007) by IL-10 in stimulated macrophages, as shown in Fig. 2C. These highly expressed chemokines were not detected or did not reach a level of significance in the live or sonicated spirochete microarray results. The reason for this discrepancy is not clear. The selective upregulation of MCP-1/CCL2 and MIP-1β/CCL4 by IL-10 in live-spirochete cultures was also confirmed at the protein level (Fig. 4D). IL-10 by itself also significantly (P < 0.05 to 0.004) upregulated both of these chemokines compared to unstimulated cells (Fig. 4D), indicating the independent ability of IL-10 to stimulate their production. CCL2 levels were similarly upregulated (P < 0.001 to 0.009) in macrophages stimulated with sonicated spirochetes and L-OspA in the presence of IL-10. CCL4 enhancement by IL-10 reached a level of significance only when live B. burgdorferi spirochetes were used as stimulant but not in the presence of either sonicated spirochetes or L-OspA (Fig. 4D). IL-5, which induces proliferation and activation of cells, was enhanced by IL-10 in the presence of each and all of the stimulants that were used (data not shown). Live spirochetes induced levels of G-CSF, CXCL2, CXCL5, CCL3, CCL5, and CXCL10 in macrophages higher than those induced by sonicated spirochetes and L-OspA, suggesting differences in stimulation of these mediators by live organisms and their lipoproteins or lysates.
DISCUSSION
Lyme disease is thought to ensue largely as a consequence of both acute and chronic inflammatory responses, induced by either the spirochete or spirochetal antigens left in tissues after bacterial death. Excessive, unchecked inflammation of any origin can be deleterious to the host, and, consequently, several regulatory mechanisms have evolved to control its magnitude and duration. One such pivotal regulator of inflammation is the anti-inflammatory cytokine IL-10. This cytokine is produced in response to stimulation by B. burgdorferi in different cells and tissue types, as reported in mice (17, 24, 27, 53, 61), rhesus macaques (40), and human patients (50, 94). Moreover, IL-10 has been shown to control Lyme disease inflammation in vitro (17, 35, 53, 54, 96) and in vivo (16, 17). In this paper, we focused on understanding the mechanism(s) by which IL-10 controls B. burgdorferi-induced inflammatory responses in macrophages. Our results show the following: (i) IL-10 simultaneously altered numerous effector genes induced by live spirochetes in macrophages, and many of these genes encoded mediators from multiple inflammatory pathways; (ii) IL-10-mediated alteration of effector genes correlated with its ability to affect the expression of spirochete-induced macrophage inflammatory mediators both at the mRNA and/or protein levels.
B. burgdorferi triggers the production of inflammatory mediators in macrophages via recognition of both Toll-like receptors (TLRs) and non-TLRs (8, 12, 22, 71, 78, 95, 97). In the present study, gene array analysis identified candidate genes from multiple pathways induced by live spirochetes that were significantly inhibited by IL-10. Classification of these genes showed many of them encoding mediators from several inflammatory pathways. Notable among the B. burgdorferi-induced genes subjected to IL-10-mediated suppression were those encoding the TLR pathway. Although several members of the TLR family contribute to the host inflammatory response to B. burgdorferi (74, 90), our study revealed that IL-10 specifically inhibits the B. burgdorferi transcriptional activation of TLR1 and TLR2, along with several of their downstream signaling components, such as interleukin-1 receptor-associated kinase 2 (IRAK2), IRAK3, TRAF1, and TNFAIP3. Binding of IRAKs to the downstream molecule TRAF6 leads to activation of NF-κB, an important regulator of cellular events (13) and mitogen-activated protein kinase (MAPK) kinase signaling pathways (52). Even though all IRAKs bind to TRAF6, only IRAK2 and IRAK3, both of which were inhibited by IL-10, are known to activate NF-κB signaling in cells (52). Interestingly, in the absence of added B. burgdorferi stimuli, the marginal constitutive expression of TLR1, TLR2, and IRAK3 were inhibited by IL-10, suggesting the inherent capacity of IL-10 to modulate these select genes. The magnitude of the anti-inflammatory effect of IL-10 on these genes, as induced by B. burgdorferi stimuli, varied and may indicate a limitation on its inhibitory capacity for them. Alternatively, it may be that an IL-10 concentration greater than 10 ng/ml is necessary to control their heightened expression levels. Combined, these findings additionally suggest that the pathway IL-10 uses to inhibit constitutive expression of select genes is similar to that used when these genes are induced by B. burgdorferi. To our knowledge, our study provides the first documentation of the IL-10 hijacking of the TLR pathway at multiple levels to regulate inflammation in Lyme disease. The inhibition by IL-10 of key genes in the TLR pathway suggests its multifaceted approach to control B. burgdorferi-induced inflammatory responses in macrophages.
Many of the IL-10-regulated mediators identified in the present study have been recognized as components of B. burgdorferi-induced inflammation in cells or tissues (9, 15–17, 45, 65, 67, 78, 80, 85, 90). However, only the prototypic TNF, IL-6, IL-12, and IL-1β cytokines have been previously shown to be downregulated by IL-10 in vitro in mouse or human macrophages (17, 65, 66, 84). Paradoxically, in the present study, IL-1β was inhibited by IL-10 at the protein and not the transcriptional level, contrasting with our previous observation where IL-1β gene transcript was significantly diminished by IL-10 in mouse macrophages stimulated with freeze-thawed B. burgdorferi (JD1 strain) or with L-OspA (27). This discrepancy may be due to differences in spirochete strains or preparation and or/techniques used. Alternatively, it may be that live spirochetes do not stimulate IL-1β via a pathway by which IL-10 inhibits its transcriptional expression level. This might possibly explain why adenoviral delivery of IL-10 to B. burgdorferi-infected C3H mice failed to alter the transcriptional expression of IL-1β in infected joints (16). B. burgdorferi infection of C3H IL-10 knockout mice also resulted in reduced pathogen load and decreased in vivo expression of IL-1β mRNA gene transcripts. Indeed, as demonstrated in the present study (see Table S5 in the supplemental material) and by other investigators (71), B. burgdorferi can activate caspase-1 to induce IL-1β in mouse macrophages, indicating a caspase-1-dependent pathway for production of this cytokine. Studies by Liu and colleagues (57) provide further evidence to suggest that IL-1β also may be induced by B. burgdorferi in mouse macrophages via caspase-1-dependent and -independent pathways, which adds complexity to the regulation of this cytokine.
Our present findings have relevance to our recent observations that silencing of the Tlr1 and Tlr2 genes by RNA interference (RNAi) in human monocytes stimulated with live spirochetes diminished production of inflammatory mediators elicited by the spirochetes (26). In agreement with our results, there are also those of studies where immune cell activation by live spirochetes or lipoprotein has generally been ascribed to TLR1/TLR2-mediated inflammatory responses (2, 14, 26, 55, 69, 70, 86, 87, 97). Studies have also shown that the mechanism(s) of IL-10-mediated inhibition of lipopolysaccharide (LPS)-induced proinflammatory gene expression involves inhibition of NF-κB or P38 MAPK pathways, as well as destabilization of RNA message (29, 51). We have now provided a global perspective of novel mediators that are regulated by IL-10 in addition to other previously reported prototypical mediators (15, 17, 27). To our knowledge, this is the first documentation of IL-10-mediated inhibition of effectors of the TLR pathway, thus providing some insight into the role of this cytokine in the control of Lyme disease inflammation.
We also confirmed by TaqMan analysis the significant inhibition by IL-10 of the inflammatory mediators IRG1, MMP9, and PTGS2 as induced by live spirochetes, as well as by spirochetal sonicate and L-OspA. IRG1 is worthy of mention because of the robust expression induced in macrophages by live B. burgdorferi spirochetes and the subsequent marked (6-fold) inhibitory effect of IL-10. Other pathogens known to induce the IRG1 gene are Mycobacterium tuberculosis (89) and Mycobacterium paratuberculosis (6) in addition to LPS (48). The significance of the upregulation of IRG1 is unclear at this time, given that it has not been functionally characterized in macrophages. Its upregulation in B. burgdorferi-stimulated macrophages is probably worthy of further investigation. MMP9, a granulocyte-secreted type IV collagenase (88), has been shown previously to be induced by B. burgdorferi in both human and murine monocytes in a TLR2-dependent manner (37, 38). MMP9 is also upregulated in erythema migrans lesions of Lyme disease patients (99, 100) and in joints of Lyme disease-susceptible mice (7). Recent studies by Heilpern et al. (46) using MMP9 knockout mice infected with B. burgdorferi revealed reduced arthritis in these animals, suggesting that MMP9 plays a role in the genesis of this form of Lyme disease. In the present study, B. burgdorferi-induced MMP9 was downregulated 2-fold in the presence of IL-10, using TaqMan assays, indicating the ability of this cytokine to target select genes that contribute to the overall B. burgdorferi-induced inflammation. The Ptgs2 gene also known as COX2 (1) was downregulated 3-fold in spirochete cultures with added IL-10. COX2 has been reported to be expressed in murine B cells (10), microglia (81), peripheral blood mononuclear cells (PBMCs) (74), and joints (4) after exposure to B. burgdorferi. The expression of COX2 in joints of B. burgdorferi-infected mice has been associated with the initiation of arthritis (4) although a recent study showed that COX2 is also essential for resolution of the inflammatory arthritis induced by B. burgdorferi (11). Overall, our data show the collective inhibition of these inflammatory mediators by IL-10 in B. burgdorferi-stimulated cultures, thus revealing an aspect of IL-10 regulatory function in Lyme disease not previously investigated.
TLR-stimulated macrophages induce effectors of the adaptive immune system, such as CD40, CD80, and CD86, to drive T-cell activation and proliferation, (60) as well as IFN-α/IFN-β-inducible genes. Our study showed the transcriptional activation by B. burgdorferi of the Cd40 gene and many interferon-inducible genes, namely, Irf7, Ifi202b, Ifit1, Ifi44, Ifi27, Oas1, and oas2, all of which were inhibited by IL-10. Studies by Qin et al. (77) have indicated that IL-10 inhibits LPS-induced CD40 gene expression through the inhibition of LPS-induced IFN-β gene expression and induction of suppressor of cytokine signaling 3 (SOCS3). SOCS3 was also shown to be significantly upregulated in cultures stimulated with live spirochetes either alone or when combined with IL-10 at both 4 and 24 h in the present study (see Tables S1 and S2 in the supplemental material). A SOCS3 synergistic effect by live spirochetes combined with IL-10 was also seen using TaqMan analysis (data not shown) as previously reported by us (27). We have shown that enhanced SOCS expression correlated with the IL-10-mediated downregulation of inflammatory mediators in macrophages (27). Consistent with a role for SOCS in B. burgdorferi-induced inflammation was the recent observation that CD14 recognition by B. burgdorferi triggers p38-dependent SOCS and that reduced SOCS expression in cells resulted in greater expression of cytokines through diminished regulation of the TLR2 pathway (83).
The observed B. burgdorferi IFN-inducible genes in the present study are consistent with previous reports by other investigators (24, 61, 74, 83, 85). Salazar and coworkers (85) have shown that live spirochetes induced transcription of several type I interferon-associated genes in human PBMCs, such as Irf7, Ifit1, Ifi44, Oas1, and Oas2 seen here. The Irf7 (83) as well as the Oas1, Ifiti, Ifi44, and Oas2 (74) genes are also induced by live spirochetes in human immune cells. Other studies have shown marked upregulation of similar IFN-responsive genes in the joints of Lyme arthritis-resistant mice (24) and in mouse bone marrow-derived macrophages stimulated with live spirochetes (61). These findings indicate that the role of type I IFN in arthritis development after a B. burgdorferi infection is independent of TLR2, suggesting an alternative pathway for induction of IFN-responsive genes, as also demonstrated employing PBMCs and purified human monocytes (85). Cervantes et al. (21) recently demonstrated a TLR8-mediated induction of IFN-β by live B. burgdorferi spirochetes in human monocytes. In contrast, spirochete recognition of TLR7 and TLR9 was necessary for the expression of IFN genes in human plasmacytoid DCs (74). Whether the IFN-inducible genes elicited by B. burgdorferi in macrophages that were downregulated by IL-10 in this study are TLR dependent or independent remains to be investigated.
The influx of inflammatory cells in pathogen-induced diseases can be either beneficial or detrimental to the host. An interesting observation made in our study was the ability of IL-10 to selectively potentiate B. burgdorferi-induced expression levels of well-characterized CC chemokines, including CCL2 and CCL4, which attract monocytes, and CCL7 and CCL9, which attract T cells. This potentiating effect of IL-10 was due to its ability to distinctly upregulate the mRNA transcripts of these chemokines. Indeed, we previously observed, using PCR array, that IL-10 alone induced the mRNA gene transcripts of the above mentioned chemokines along with several others (CCL11, CCL12, CCL17, and CCL24) and their putative receptors (CCR1, CCR2, CCR6, and CCR9) in mouse J774 macrophages (our unpublished observations). Some of these chemokines have been previously recognized as part of the B. burgdorferi-induced inflammatory milieu. These include CCL2 (39, 44, 67, 80, 91, 100), CCL4 (67), and CCL9 (72). The anti-inflammatory effects of IL-10, manifested by this cytokine's ability to control the influx of inflammatory cells in tissues and, ultimately, their level of production of inflammatory mediators in Lyme disease, are very intriguing. This ability of IL-10 to repress the expression of a large fraction of live-spirochete-induced genes and potentiate others suggests its selective regulation of genes to control inflammation in Lyme disease. How IL-10 concomitantly enhances and represses B. burgdorferi-inducible inflammatory mediators is not known. However, a recent report that inappropriate proinflammatory responses can be selectively controlled through epigenetic modifications to individual promoters while leaving other responses intact (32) suggests a phenomenon that is worth exploring and that may help explain the IL-10 selective anti-inflammatory effect in Lyme disease.
Finally, our finding of the enhanced amplification of the transcription and secretion of inflammatory mediators as elicited by live spirochetes compared to the effects of L-OspA and sonicated spirochetes in J774 macrophages is worthy of mention since studies have demonstrated that in vitro monocyte/macrophage models may not be as representative of the true phagocytic capacity as primary cells (64). Although we did not perform phagocytosis studies here, the mouse J774 macrophages are known to be phagocytic for several bacterial pathogens (33, 41, 68) including B. burgdorferi, where degraded spirochetes were observed in intracellular compartments (62). Our findings of heightened responses to live spirochetes corroborated those of other investigators who have noted that live spirochetes induce greater responses in innate immune cells than those of lipoproteins or lysates (25, 64, 74, 85, 90). As suggested, this enhancing effect may be attributed to phagocytosis and degradation of live spirochetes in phagolysosomes, which ultimately leads to synergistic amplification of multiple signaling pathways and enhancement of inflammatory mediators compared to those generated by a single agonist (64, 85). Thus, in all likelihood the greater inflammatory responses elicited by live spirochetes in the present study may have resulted from phagocytosis and degradation of live spirochetes in macrophages.
In conclusion, we have found that IL-10 inhibits B. burgdorferi-induced effectors that participate in several pathways and, especially, the TLR pathway. Consequently, multiple inflammatory mediators underwent changes when exposed to IL-10. A number of these mediators are newly identified IL-10-regulated genes potentially in the context of Lyme disease. Our study provides a more global understanding of potential mechanisms used by IL-10 to control Lyme disease inflammation. Functional studies are now necessary to identify specific mediators of IL-10 anti-inflammatory activities. This may allow the development of specific immunotherapeutic approaches for both early- and late-stage Lyme disease.
Supplementary Material
ACKNOWLEDGMENTS
The project described was supported by grant R21AI073356 from the National Institute of Allergy and Infectious Diseases, grant RR00164 from the National Center for Research Resources, National Institutes of Health, and grant HRD-0734232 from NSF-CREST.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
Published ahead of print on 26 September 2011.
REFERENCES
- 1. Alexopoulou L., et al. 2002. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat. Med. 8:878–884 [DOI] [PubMed] [Google Scholar]
- 2. Aliprantis A. O., et al. 1999. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science 285:736–739 [DOI] [PubMed] [Google Scholar]
- 3. Anguita J., et al. 1997. B7-1 and B7-2 monoclonal antibodies modulate the severity of murine Lyme arthritis. Infect. Immun. 65:3037–3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anguita J., et al. 2002. Cyclooxygenase 2 activity modulates the severity of murine Lyme arthritis. FEMS Immunol. Med. Microbiol. 34:187–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barthold S. W., de Souza M. S., Janotka J. L., Smith A. L., Persing D. H. 1993. Chronic Lyme borreliosis in the laboratory mouse. Am. J. Pathol. 143:959–971 [PMC free article] [PubMed] [Google Scholar]
- 6. Basler T., Jeckstadt S., Valentin-Weigand P., Goethe R. 2006. Mycobacterium paratuberculosis, Mycobacterium smegmatis, and lipopolysaccharide induce different transcriptional and post-transcriptional regulation of the IRG1 gene in murine macrophages. J. Leukoc. Biol. 79:628–638 [DOI] [PubMed] [Google Scholar]
- 7. Behera A. K., Hildebrand E., Scagliotti J., Steere A. C., Hu L. T. 2005. Induction of host matrix metalloproteinases by Borrelia burgdorferi differs in human and murine Lyme arthritis. Infect. Immun. 73:126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berende A., Oosting M., Kullberg B. J., Netea M. G., Joosten L. A. 2010. Activation of innate host defense mechanisms by Borrelia. Eur. Cytokine Netw. 21:7–18 [DOI] [PubMed] [Google Scholar]
- 9. Bernardino A. L., Kaushal D., Philipp M. T. 2009. The antibiotics doxycycline and minocycline inhibit the inflammatory responses to the Lyme disease spirochete Borrelia burgdorferi. J. Infect. Dis. 199:1379–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blaho V. A., Buczynski M. W., Dennis E. A., Brown C. R. 2009. Cyclooxygenase-1 orchestrates germinal center formation and antibody class-switch via regulation of IL-17. J. Immunol. 183:5644–5653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blaho V. A., Mitchell W. J., Brown C. R. 2008. Arthritis develops but fails to resolve during inhibition of cyclooxygenase 2 in a murine model of Lyme disease. Arthritis Rheum. 58:1485–1495 [DOI] [PubMed] [Google Scholar]
- 12. Bolz D. D., et al. 2004. MyD88 plays a unique role in host defense but not arthritis development in Lyme disease. J. Immunol. 173:2003–2010 [DOI] [PubMed] [Google Scholar]
- 13. Bourteele S., et al. 2007. Alteration of NF-κB activity leads to mitochondrial apoptosis after infection with pathological prion protein. Cell. Microbiol. 9:2202–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brightbill H. D., et al. 1999. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science 285:732–736 [DOI] [PubMed] [Google Scholar]
- 15. Brown C. R., Blaho V. A., Loiacono C. M. 2003. Susceptibility to experimental Lyme arthritis correlates with KC and monocyte chemoattractant protein-1 production in joints and requires neutrophil recruitment via CXCR2. J. Immunol. 171:893–901 [DOI] [PubMed] [Google Scholar]
- 16. Brown C. R., et al. 2008. Adenoviral delivery of interleukin-10 fails to attenuate experimental Lyme disease. Infect. Immun. 76:5500–5507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brown J. P., Zachary J. F., Teuscher C., Weis J. J., Wooten R. M. 1999. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect. Immun. 67:5142–5150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burgdorfer W., et al. 1982. Lyme disease—a tick-borne spirochetosis? Science 216:1317–1319 [DOI] [PubMed] [Google Scholar]
- 19. Cassiani-Ingoni R., et al. 2006. Borrelia burgdorferi Induces TLR1 and TLR2 in human microglia and peripheral blood monocytes but differentially regulates HLA-class II expression. J. Neuropathol. Exp. Neurol. 65:540–548 [DOI] [PubMed] [Google Scholar]
- 20. Cepok S., et al. 2003. The immune response at onset and during recovery from Borrelia burgdorferi meningoradiculitis. Arch. Neurol. 60:849–855 [DOI] [PubMed] [Google Scholar]
- 21. Cervantes J. L., et al. 2011. Phagosomal signaling by Borrelia burgdorferi in human monocytes involves Toll-like receptor (TLR) 2 and TLR8 cooperativity and TLR8-mediated induction of IFN-β. Proc. Natl. Acad. Sci. U. S. A. 108:3683–3688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coleman J. L., Benach J. L. 2003. The urokinase receptor can be induced by Borrelia burgdorferi through receptors of the innate immune system. Infect. Immun. 71:5556–5564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coyle P. K. 1993. Lyme disease, p. 179–183In Manning S.(ed.), Pathogenesis of Lyme disease. Mosby-Year Book, Inc., St Louis, MO [Google Scholar]
- 24. Crandall H., et al. 2006. Gene expression profiling reveals unique pathways associated with differential severity of Lyme arthritis. J. Immunol. 177:7930–7942 [DOI] [PubMed] [Google Scholar]
- 25. Cruz A. R., et al. 2008. Phagocytosis of Borrelia burgdorferi, the Lyme disease spirochete, potentiates innate immune activation and induces apoptosis in human monocytes. Infect. Immun. 76:56–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dennis V. A., et al. 2009. Live Borrelia burgdorferi spirochetes elicit inflammatory mediators from human monocytes via the Toll-like receptor signaling pathway. Infect. Immun. 77:1238–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dennis V. A., Jefferson A., Singh S. R., Ganapamo F., Philipp M. T. 2006. Interleukin-10 anti-inflammatory response to Borrelia burgdorferi, the agent of Lyme disease: a possible role for suppressors of cytokine signaling 1 and 3. Infect. Immun. 74:5780–5789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diterich I., Harter L., Hassler D., Wendel A., Hartung T. 2001. Modulation of cytokine release in ex vivo-stimulated blood from borreliosis patients. Infect. Immun. 69:687–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dokter W. H., Koopmans S. B., Vellenga E. 1996. Effects of IL-10 and IL-4 on LPS-induced transcription factors (AP-1, NF-IL6 and NF-κB) which are involved in IL-6 regulation. Leukemia 10:1308–1316 [PubMed] [Google Scholar]
- 30. Donnelly R. P., Dickensheets H., Finbloom D. S. 1999. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J. Interferon Cytokine Res. 19:563–573 [DOI] [PubMed] [Google Scholar]
- 31. England J. D., Bohm R. P., Jr, Roberts E. D., Philipp M. T. 1997. Mononeuropathy multiplex in rhesus monkeys with chronic Lyme disease. Ann. Neurol. 41:375–384 [DOI] [PubMed] [Google Scholar]
- 32. Foster S. L., Medzhitov R. 2009. Gene-specific control of the TLR-induced inflammatory response. Clin. Immunol. 130:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fukuto H. S., Svetlanov A., Palmer L. E., Karzai A. W., Bliska J. B. 2010. Global gene expression profiling of Yersinia pestis replicating inside macrophages reveals the roles of a putative stress-induced operon in regulating type III secretion and intracellular cell division. Infect. Immun. 78:3700–3715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ganapamo F., Dennis V. A., Philipp M. T. 2001. CD19(+) cells produce IFN-gamma in mice infected with Borrelia burgdorferi. Eur. J. Immunol. 31:3460–3468 [DOI] [PubMed] [Google Scholar]
- 35. Ganapamo F., Dennis V. A., Philipp M. T. 2003. Differential acquired immune responsiveness to bacterial lipoproteins in Lyme disease-resistant and -susceptible mouse strains. Eur. J. Immunol. 33:1934–1940 [DOI] [PubMed] [Google Scholar]
- 36. Gautam A., Hathaway M., McClain N., Ramesh G., Ramamoorthy R. 2008. Analysis of the determinants of bba64 (P35) gene expression in Borrelia burgdorferi using a gfp reporter. Microbiology 154:275–285 [DOI] [PubMed] [Google Scholar]
- 37. Gebbia J. A., Coleman J. L., Benach J. L. 2001. Borrelia spirochetes upregulate release and activation of matrix metalloproteinase gelatinase B (MMP-9) and collagenase 1 (MMP-1) in human cells. Infect. Immun. 69:456–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gebbia J. A., Coleman J. L., Benach J. L. 2004. Selective induction of matrix metalloproteinases by Borrelia burgdorferi via Toll-like receptor 2 in monocytes. J. Infect. Dis. 189:113–119 [DOI] [PubMed] [Google Scholar]
- 39. Gergel E. I., Furie M. B. 2004. Populations of human T lymphocytes that traverse the vascular endothelium stimulated by Borrelia burgdorferi are enriched with cells that secrete gamma interferon. Infect. Immun. 72:1530–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Giambartolomei G. H., Dennis V. A., Philipp M. T. 1998. Borrelia burgdorferi stimulates the production of interleukin-10 in peripheral blood mononuclear cells from uninfected humans and rhesus monkeys. Infect. Immun. 66:2691–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Giri P. K., Kruh N. A., Dobos K. M., Schorey J. S. 2010. Proteomic analysis identifies highly antigenic proteins in exosomes from M. tuberculosis-infected and culture filtrate protein-treated macrophages. Proteomics 10:3190–3202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Glickstein L., et al. 2003. Inflammatory cytokine production predominates in early Lyme disease in patients with erythema migrans. Infect. Immun. 71:6051–6053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grygorczuk S., et al. 2004. Concentrations of macrophage inflammatory proteins MIP-1α and MIP-1β and interleukin 8 (IL-8) in Lyme borreliosis. Infection 32:350–355 [DOI] [PubMed] [Google Scholar]
- 44. Guerau-de-Arellano M., Alroy J., Huber B. T. 2005. β2 Integrins control the severity of murine Lyme carditis. Infect. Immun. 73:3242–3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hamilton J. A. 2008. Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 8:533–544 [DOI] [PubMed] [Google Scholar]
- 46. Heilpern A. J., et al. 2009. Matrix metalloproteinase 9 plays a key role in lyme arthritis but not in dissemination of Borrelia burgdorferi. Infect. Immun. 77:2643–2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hirschfeld M., et al. 1999. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by Toll-like receptor 2. J. Immunol. 163:2382–2386 [PubMed] [Google Scholar]
- 48. Hoshino K., Kaisho T., Iwabe T., Takeuchi O., Akira S. 2002. Differential involvement of IFN-beta in Toll-like receptor-stimulated dendritic cell activation. Int. Immunol. 14:1225–1231 [DOI] [PubMed] [Google Scholar]
- 49. Jarefors S., et al. 2006. Lyme borreliosis reinfection: might it be explained by a gender difference in immune response? Immunology 118:224–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jones K. L., et al. 2008. Higher mRNA levels of chemokines and cytokines associated with macrophage activation in erythema migrans skin lesions in patients from the United States than in patients from Austria with Lyme borreliosis. Clin. Infect. Dis. 46:85–92 [DOI] [PubMed] [Google Scholar]
- 51. Kishore R., Tebo J. M., Kolosov M., Hamilton T. A. 1999. Cutting edge: clustered AU-rich elements are the target of IL-10-mediated mRNA destabilization in mouse macrophages. J. Immunol. 162:2457–2461 [PubMed] [Google Scholar]
- 52. Kobayashi K., et al. 2002. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 110:191–202 [DOI] [PubMed] [Google Scholar]
- 53. Lazarus J. J., Kay M. A., McCarter A. L., Wooten R. M. 2008. Viable Borrelia burgdorferi enhances interleukin-10 production and suppresses activation of murine macrophages. Infect. Immun. 76:1153–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lazarus J. J., Meadows M. J., Lintner R. E., Wooten R. M. 2006. IL-10 deficiency promotes increased Borrelia burgdorferi clearance predominantly through enhanced innate immune responses. J. Immunol. 177:7076–7085 [DOI] [PubMed] [Google Scholar]
- 55. Lien E., et al. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419–33425 [DOI] [PubMed] [Google Scholar]
- 56. Lisinski T. J., Furie M. B. 2002. Interleukin-10 inhibits proinflammatory activation of endothelium in response to Borrelia burgdorferi or lipopolysaccharide but not interleukin-1β or tumor necrosis factor alpha. J. Leukoc. Biol. 72:503–511 [PubMed] [Google Scholar]
- 57. Liu N., Belperron A. A., Booth C. J., Bockenstedt L. K. 2009. The caspase 1 inflammasome is not required for control of murine Lyme borreliosis. Infect. Immun. 77:3320–3327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 59. Medzhitov R., Janeway C. A., Jr 2002. Decoding the patterns of self and nonself by the innate immune system. Science 296:298–300 [DOI] [PubMed] [Google Scholar]
- 60. Medzhitov R., Janeway C. A., Jr 1998. Innate immune recognition and control of adaptive immune responses. Semin. Immunol. 10:351–353 [DOI] [PubMed] [Google Scholar]
- 61. Miller J. C., Ma Y., Crandall H., Wang X., Weis J. J. 2008. Gene expression profiling provides insights into the pathways involved in inflammatory arthritis development: murine model of Lyme disease. Exp. Mol. Pathol. 85:20–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Montgomery R. R., Malawista S. E. 1996. Entry of Borrelia burgdorferi into macrophages is end-on and leads to degradation in lysosomes. Infect. Immun. 64:2867–2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Moore K. W., de Waal Malefyt R., Coffman R. L., O'Garra A. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683–765 [DOI] [PubMed] [Google Scholar]
- 64. Moore M. W., et al. 2007. Phagocytosis of Borrelia burgdorferi and Treponema pallidum potentiates innate immune activation and induces gamma interferon production. Infect. Immun. 75:2046–2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mullegger R. R., et al. 2000. Differential expression of cytokine mRNA in skin specimens from patients with erythema migrans or acrodermatitis chronica atrophicans. J. Invest. Dermatol. 115:1115–1123 [DOI] [PubMed] [Google Scholar]
- 66. Murthy P. K., Dennis V. A., Lasater B. L., Philipp M. T. 2000. Interleukin-10 modulates proinflammatory cytokines in the human monocytic cell line THP-1 stimulated with Borrelia burgdorferi lipoproteins. Infect. Immun. 68:6663–6669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Myers T. A., Kaushal D., Philipp M. T. 2009. Microglia are mediators of Borrelia burgdorferi-induced apoptosis in SH-SY5Y neuronal cells. PLoS Pathog. 5:e1000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Niemann G. S., et al. 2011. Discovery of novel secreted virulence factors from Salmonella enterica serovar Typhimurium by proteomic analysis of culture supernatants. Infect. Immun. 79:33–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Norgard M. V., et al. 1996. Activation of human monocytic cells by Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides proceeds via a pathway distinct from that of lipopolysaccharide but involves the transcriptional activator NF-κB. Infect. Immun. 64:3845–3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Norgard M. V., Riley B. S., Richardson J. A., Radolf J. D. 1995. Dermal inflammation elicited by synthetic analogs of Treponema pallidum and Borrelia burgdorferi lipoproteins. Infect. Immun. 63:1507–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Oosting M., et al. 2010. Recognition of Borrelia burgdorferi by NOD2 is central for the induction of an inflammatory reaction. J. Infect. Dis. 201:1849–1858 [DOI] [PubMed] [Google Scholar]
- 72. Pashenkov M., et al. 2002. Recruitment of dendritic cells to the cerebrospinal fluid in bacterial neuroinfections. J. Neuroimmunol. 122:106–116 [DOI] [PubMed] [Google Scholar]
- 73. Perticarari S., et al. 2003. Lymphocyte apoptosis co-cultured with Borrelia burgdorferi. Microb. Pathog. 35:139–145 [DOI] [PubMed] [Google Scholar]
- 74. Petzke M. M., Brooks A., Krupna M. A., Mordue D., Schwartz I. 2009. Recognition of Borrelia burgdorferi, the Lyme disease spirochete, by TLR7 and TLR9 induces a type I IFN response by human immune cells. J. Immunol. 183:5279–5292 [DOI] [PubMed] [Google Scholar]
- 75. Philipp M. T., Johnson B. J. 1994. Animal models of Lyme disease: pathogenesis and immunoprophylaxis. Trends Microbiol. 2:431–437 [DOI] [PubMed] [Google Scholar]
- 76. Pohl-Koppe A., Balashov K. E., Steere A. C., Logigian E. L., Hafler D. A. 1998. Identification of a T cell subset capable of both IFN-γ and IL-10 secretion in patients with chronic Borrelia burgdorferi infection. J. Immunol. 160:1804–1810 [PubMed] [Google Scholar]
- 77. Qin H., et al. 2006. IL-10 inhibits lipopolysaccharide-induced CD40 gene expression through induction of suppressor of cytokine signaling-3. J. Immunol. 177:7761–7771 [DOI] [PubMed] [Google Scholar]
- 78. Radolf J. D., et al. 1995. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytes/macrophages. J. Immunol. 154:2866–2877 [PubMed] [Google Scholar]
- 79. Ramesh G., et al. 2008. Interaction of the Lyme disease spirochete Borrelia burgdorferi with brain parenchyma elicits inflammatory mediators from glial cells as well as glial and neuronal apoptosis. Am. J. Pathol. 173:1415–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ramesh G., et al. 2009. Possible role of glial cells in the onset and progression of Lyme neuroborreliosis. J. Neuroinflammation 6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rasley A., Marriott I., Halberstadt C. R., Bost K. L., Anguita J. 2004. Substance P augments Borrelia burgdorferi-induced prostaglandin E2 production by murine microglia. J. Immunol. 172:5707–5713 [DOI] [PubMed] [Google Scholar]
- 82. Rasley A., Tranguch S. L., Rati D. M., Marriott I. 2006. Murine glia express the immunosuppressive cytokine, interleukin-10, following exposure to Borrelia burgdorferi or Neisseria meningitidis. Glia 53:583–592 [DOI] [PubMed] [Google Scholar]
- 83. Sahay B., et al. 2009. CD14 signaling restrains chronic inflammation through induction of p38-MAPK/SOCS-dependent tolerance. PLoS Pathog. 5:e1000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Salazar J. C., et al. 2003. Coevolution of an aminoacyl-tRNA synthetase with its tRNA substrates. Proc. Natl. Acad. Sci. U. S. A. 100:13863–13868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Salazar J. C., et al. 2009. Activation of human monocytes by live Borrelia burgdorferi generates TLR2-dependent and -independent responses which include induction of IFN-beta. PLoS Pathog. 5:e1000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Salazar J. C., et al. 2005. Lipoprotein-dependent and -independent immune responses to spirochetal infection. Clin. Diagn. Lab. Immunol. 12:949–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sellati T. J., et al. 1999. Activation of human monocytic cells by Borrelia burgdorferi and Treponema pallidum is facilitated by CD14 and correlates with surface exposure of spirochetal lipoproteins. J. Immunol. 163:2049–2056 [PubMed] [Google Scholar]
- 88. Senior R. M., et al. 1991. Human 92- and 72-kilodalton type IV collagenases are elastases. J. Biol. Chem. 266:7870–7875 [PubMed] [Google Scholar]
- 89. Shi S., et al. 2005. Expression of many immunologically important genes in Mycobacterium tuberculosis-infected macrophages is independent of both TLR2 and TLR4 but dependent on IFN-α/β receptor and STAT1. J. Immunol. 175:3318–3328 [DOI] [PubMed] [Google Scholar]
- 90. Shin O. S., et al. 2008. Distinct roles for MyD88 and Toll-like receptors 2, 5, and 9 in phagocytosis of Borrelia burgdorferi and cytokine induction. Infect. Immun. 76:2341–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Singh S. K., Morbach H., Nanki T., Girschick H. J. 2005. Differential expression of chemokines in synovial cells exposed to different Borrelia burgdorferi isolates. Clin. Exp. Rheumatol. 23:311–322 [PubMed] [Google Scholar]
- 92. Sjowall J., et al. 2005. Innate immune responses in Lyme borreliosis: enhanced tumour necrosis factor-alpha and interleukin-12 in asymptomatic individuals in response to live spirochetes. Clin. Exp. Immunol. 141:89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Steere A. C. 2001. Lyme disease. N. Engl. J. Med. 345:115–125 [DOI] [PubMed] [Google Scholar]
- 94. Strle K., et al. 2009. Borrelia burgdorferi stimulates macrophages to secrete higher levels of cytokines and chemokines than Borrelia afzelii or Borrelia garinii. J. Infect. Dis. 200:1936–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Talkington J., Nickell S. P. 2001. Role of Fc gamma receptors in triggering host cell activation and cytokine release by Borrelia burgdorferi. Infect. Immun. 69:413–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wang G., Petzke M. M., Iyer R., Wu H., Schwartz I. 2008. Pattern of proinflammatory cytokine induction in RAW264.7 mouse macrophages is identical for virulent and attenuated Borrelia burgdorferi. J. Immunol. 180:8306–8315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wooten R. M., et al. 2002. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 168:348–355 [DOI] [PubMed] [Google Scholar]
- 98. Yin Z., et al. 1997. T cell cytokine pattern in the joints of patients with Lyme arthritis and its regulation by cytokines and anticytokines. Arthritis Rheum. 40:69–79 [DOI] [PubMed] [Google Scholar]
- 99. Zhao Z., et al. 2003. Selective up-regulation of matrix metalloproteinase-9 expression in human erythema migrans skin lesions of acute Lyme disease. J. Infect. Dis. 188:1098–1104 [DOI] [PubMed] [Google Scholar]
- 100. Zhao Z., McCloud B., Fleming R., Klempner M. S. 2007. Borrelia burgdorferi-induced monocyte chemoattractant protein-1 production in vivo and in vitro. Biochem. Biophys. Res. Commun. 358:528–533 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.