Abstract
Mycobacterium tuberculosis is a highly successful human pathogen that evades host innate immunity by interfering with macrophage functions. In addition to avoiding macrophage microbicidal activities, M. tuberculosis triggers secretion of proinflammatory cytokines and chemokines in macrophages. The levels of proinflammatory cytokines induced by clinical M. tuberculosis isolates are thought to play an important role in determining tuberculosis disease progression and severity, but the mechanisms by which M. tuberculosis modulates the magnitude of inflammatory responses remain unclear. Here we show that M. tuberculosis restricts robust macrophage activation and dampens proinflammatory responses through the cell envelope-associated serine hydrolase Hip1 (hydrolase important for pathogenesis 1). By transcriptionally profiling macrophages infected with either wild-type or hip1 mutant bacteria, we found that the hip1 mutant induced earlier and significantly higher levels of several proinflammatory cytokines and chemokines. We show that increased activation of Toll-like receptor 2 (TLR2)- and MyD88-dependent signaling pathways mediates the enhanced cytokine secretion induced by the hip1 mutant. Thus, Hip1 restricts the onset and magnitude of proinflammatory cytokines by limiting TLR2-dependent activation. We also show that Hip1 dampens TLR2-independent activation of the inflammasome and limits secretion of interleukin-18 (IL-18). Dampening of TLR2 signaling does not require viable M. tuberculosis or phagocytosis but does require Hip1 catalytic activity. We propose that M. tuberculosis restricts proinflammatory responses by masking cell surface interactions between TLR2 agonists on M. tuberculosis and TLR2 on macrophages. This strategy may allow M. tuberculosis to evade early detection by host immunity, delay the onset of adaptive immune responses, and accelerate disease progression.
INTRODUCTION
Mycobacterium tuberculosis, the causative agent of tuberculosis (TB), is a highly successful human pathogen that has evolved multiple strategies for manipulating host innate immunity (10, 53). Macrophages are critical for the innate immune response to pathogens because of their potent antimicrobial functions, and they play a central role in shaping adaptive immune responses (4, 12, 36). However, M. tuberculosis can evade macrophage functions and actively grow within their hostile intracellular environment (14). M. tuberculosis inhibits phagosome maturation and acidification, interferes with responses to gamma interferon (IFN-γ), resists antimicrobial agents that damage the bacterial cell envelope, and counters toxic reactive oxygen (ROI) and nitrogen (RNI) intermediates (10, 20, 52). Evading these innate immune defenses allows M. tuberculosis to replicate within the host and escape early immune detection. Manipulation of early innate immune events by pathogens also impacts the initiation of adaptive immunity through induction of proinflammatory cytokines and chemokines which influence the disease outcome (4, 6, 51, 54). Delineating how M. tuberculosis modulates proinflammatory responses is therefore key to understanding the role of innate immune responses in M. tuberculosis pathogenesis.
Upon recognition of pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs), macrophages secrete a number of proinflammatory cytokines, such as interleukin-12 (IL-12), IL-1β, IL-6, and tumor necrosis factor alpha (TNF-α) (24, 60). Many of these responses are downstream of signaling through Toll-like receptors (TLRs), and M. tuberculosis harbors several PAMPs, for example, lipoproteins, phosphatidylinositol mannoside (PIM), and CpG DNA, recognized by TLR2, TLR4, and TLR9, respectively, on macrophages and dendritic cells in vitro (1, 2, 4, 5, 26, 35, 63). While mice lacking individual TLRs are not dramatically susceptible to acute infection with M. tuberculosis, mice lacking myeloid differentiation factor 88 (MyD88), an adaptor protein recruited to the cytoplasmic domain of several TLRs, are highly susceptible to M. tuberculosis infection (1, 2, 9, 11, 17, 21, 55, 58). Moreover, mice deficient in IL-1R1, the receptor for IL-1α and IL-1β, which also utilizes MyD88 as an adaptor molecule, are also highly susceptible to M. tuberculosis infection (16, 33). These studies show that while MyD88 is critical for host control of tuberculosis, the precise role of TLRs in controlling M. tuberculosis infection is less clear. PAMPs are also recognized by cytosolic NOD-like receptors (NLRs) in macrophages, which assemble into an inflammasome complex, resulting in activation of caspase-1 (31). Activation of the inflammasome is required for the secretion of cytokines such as IL-1β and IL-18. M. tuberculosis activates the NLRP3 inflammasome, which consists of the NLRP3 scaffold, the ASC adaptor, and caspase-1 (23, 25, 34, 37, 39).
Although several PAMPs purified from M. tuberculosis can stimulate proinflammatory responses through TLR signaling, several studies suggest that M. tuberculosis also inhibits macrophage activation and cytokine induction via cell envelope-associated and secreted mycobacterial factors (3, 15, 18, 19, 38, 41, 42, 45, 47, 61). Modulation of proinflammatory responses is highly relevant to M. tuberculosis pathogenesis, as clinical isolates of M. tuberculosis have been reported to vary in the ability to induce proinflammatory responses in macrophages, with more virulent strains of M. tuberculosis inducing lower levels of proinflammatory cytokines than their less virulent counterparts (28, 30, 40, 44, 47, 59). These studies suggest that limiting proinflammatory responses may lead to rapid progression to disease and provide a selective advantage to M. tuberculosis (44). However, the host pathways that lead to lowered or enhanced inflammatory responses are not clear. In this study, we show that M. tuberculosis dampens TLR2-dependent proinflammatory responses and that the serine hydrolase Hip1 restricts the onset and magnitude of proinflammatory responses induced by M. tuberculosis by limiting TLR2 activation in macrophages.
Rv2224c, which we have named Hip1 (hydrolase important for pathogenesis 1), is a cell envelope-associated serine hydrolase that we and others have previously shown to be critical for the virulence of M. tuberculosis and for its survival in macrophages (29, 49, 50, 65). The hip1 mutant grows poorly in macrophages and is more susceptible to acidic environments and many cell envelope-directed stresses, i.e., lysozyme, SDS, and lipophilic antibiotics (49, 50, 64, 65). Hip1 and its M. smegmatis ortholog have been implicated in maintaining M. tuberculosis cell envelope integrity (13, 50). Additionally, mice infected with the hip1 mutant survive significantly longer than C57BL/6 or RAG2−/− mice infected with wild-type (wt) M. tuberculosis strains and have severely reduced granulomas in their lungs, despite only modest decreases in lung bacterial burdens (29, 50, 65). The delayed progression to disease and the mild immunopathology in hip1 mutant-infected mice in the face of large numbers of mutant bacteria suggested that Hip1 may also modulate innate immune responses.
To investigate whether Hip1 modulates innate immune responses, we transcriptionally profiled macrophages infected with either a wt or hip1 mutant strain of M. tuberculosis and found that hip1 mutant-infected macrophages rapidly induced dramatically higher levels of proinflammatory cytokines and chemokines. Defining the host signaling pathways modulated by Hip1 therefore provides a unique opportunity to understand how M. tuberculosis dampens proinflammatory responses. Here we show that the enhanced cytokine production induced by the absence of Hip1 is dependent on TLR2 and MyD88 signaling and does not require live bacteria. Our studies suggest that M. tuberculosis dampens inflammatory responses by limiting cell surface interactions between TLR2 agonists on M. tuberculosis and TLR2 on macrophages. This strategy may allow M. tuberculosis to evade early detection by host immunity and may promote disease progression.
MATERIALS AND METHODS
Ethics statement.
All animal experiments were approved by the institutional animal care and use committees at the Emory University School of Medicine and the University of Tennessee Health Science Center (UTHSC). Animal experiments were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (37a), under the assurance of compliance number A3435-01.
Bacterial strains and media.
Mycobacterium tuberculosis H37Rv (wild type), its hip1 mutant strain, the complemented hip1 mutant strain, and the hip1 S228A mutant strain (50, 65) were grown at 37°C in Middlebrook 7H9 broth or 7H10 agar supplemented with 10% oleic acid-albumin-dextrose-catalase (OADC), 0.5% glycerol, and 0.05% Tween 80 (for broth), with the addition of 25 μg/ml kanamycin (Sigma-Aldrich, St. Louis, MO) for the hip1 mutant, 10 μg/ml streptomycin (Sigma-Aldrich, St. Louis, MO) for the complemented hip1 mutant strain, and 50 μg/ml hygromycin (Roche Diagnostics, Indianapolis, IN) for the hip1 S228A mutant. For inactivation of M. tuberculosis strains, bacteria were grown in Middlebrook 7H9 broth until mid-log phase, washed twice with phosphate-buffered saline (PBS), and either heat killed by incubation at 80°C for 2 h or gamma irradiated. Cell pellets were sterilized by gamma irradiation in a JL Shephard Mark I 137Cs irradiator with a dose of 2.4 Mrad (Department of Radiobiology, University of North Carolina at Chapel Hill).
Mice.
All mice were housed under specific-pathogen-free conditions in filter-top cages within the vivarium at the Yerkes National Primate Center, Emory University, and were provided with sterile water and food ad libitum. All knockout mice were backcrossed for 10 generations on a C57BL/6 background. C57BL/6 and IL-1R1−/− mice were purchased from Jackson Laboratory (Bar Harbor, ME). MyD88−/−, TLR2−/−, TLR4−/−, and TLR9 −/− mice were obtained from Bali Pulendran (Emory University, Atlanta, GA). ASC−/− and NLRP3−/− mice were obtained from Vishva Dixit (Genentech, CA) and bred in the UTHSC facility, and bone marrow cells from caspase-1−/− mice were obtained from Denise Monack (Stanford University School of Medicine, CA).
Macrophage infection and cytokine assays.
Murine bone marrow-derived macrophages (BMM) were generated as previously described (49). Briefly, bone marrow cells were grown in Dulbecco's modified Eagle's medium (DMEM)/F-12 medium (Lonza, Walkersville, MD) with 10% fetal bovine serum (FBS; HyClone, Logan, UT), 2 mM glutamine, and 10% L-cell-conditioned medium (LCM) for 7 days of differentiation. Incubations were carried out at 37°C with 5% CO2. Cells were 98% F480+ and 96% CD11b+ by flow cytometry. For infection, macrophages were plated onto 24-well plates (3 × 105 cells per well). Bacteria were filtered through 5-μm filters, resuspended in DMEM/F-12 medium containing 5% LCM, and sonicated twice for 5 s each before addition to adherent monolayers. Each bacterial strain was used for infection (in duplicate or triplicate for each time point) at a multiplicity of infection (MOI) of 10 or as indicated. Infection of macrophages was carried out for 3 h, the medium was replaced with DMEM/F-12 medium containing 200 μg/ml amikacin for 45 min, and the cells were washed 4 times with PBS before the medium was replaced with DMEM/F-12 medium containing 5% LCM. To determine intracellular CFU, one set of macrophages was lysed in PBS containing 0.5% Triton X-100 and plated on 7H10 agar plates containing the appropriate antibiotics. Alternatively, macrophages were infected with heat-killed or irradiated M. tuberculosis at an MOI of 10 or 200 μg, respectively, in DMEM/F-12 medium containing 5% LCM. Cell-free supernatants from macrophage monolayers were isolated at various time points and assayed for cytokines by enzyme-linked immunosorbent assay (ELISA), using duo set kits for IL-1β (BD Biosciences, San Diego, CA), TNF-α and IL-6 (R&D Systems, Minneapolis, MN), and IL-18 (MBL International Corporation, Woburn, MA). Assays were carried out according to the manufacturers' instructions. Uninfected macrophages were used as controls for each experiment. Multiplex ELISAs were carried out using a Luminex multiplex kit and were analyzed using a Bio-Plex 200 system (Millipore, Billerica, MA). For phagosome blocking experiments using cytochalasin D, macrophages were treated with 10 μM cytochalasin D (Sigma-Aldrich, St. Louis, MO) for 1 h before infection. Subsequent steps were the same as those described above.
Microarray analysis.
Macrophages were infected in triplicate with H37Rv or the hip1 mutant as described above. At 24 h postinfection, the supernatant was removed and macrophage RNA was isolated using an RNeasy kit (Qiagen, Valencia, CA). Microarray processing and analysis were carried out by Almac Diagnostics (Durham, NC), using Affymetrix mouse genome 430 2.0 arrays. RNA, cRNA, and fragmented cRNA quality were assessed using a spectrophotometer and bioanalyzer, and samples that passed the quality criteria were used for subsequent processing. Hybridizations were done for 16 h at 45°C in an Affymetrix GeneChip model 640 hybridization oven set to rotate at 60 rpm. Arrays were scanned using a GeneChip model 3000 scanner. The Rosetta error model was applied to the raw data in order to generate processed data. Ratios of gene expression in hip1 mutant-infected macrophages to that in wt-infected macrophages were generated. Genes with a fold change difference in expression of 2-fold or more, with a P value of ≤0.01, were included in the study. Data were normalized using the Z score (number of standard deviations from the mean), and a heat map was generated using Spotfire Inc. The expression level of each gene is represented by the number of standard deviations above (red) or below (green) the average value for that gene across all samples.
Statistical analysis.
The statistical significance of data was analyzed using Student's unpaired t test (GraphPad Prism 5.0). Data are shown as means ± standard deviations (SD) for one representative experiment of three independent experiments.
RESULTS
Proinflammatory responses in M. tuberculosis-infected macrophages are dampened by the serine hydrolase Hip1.
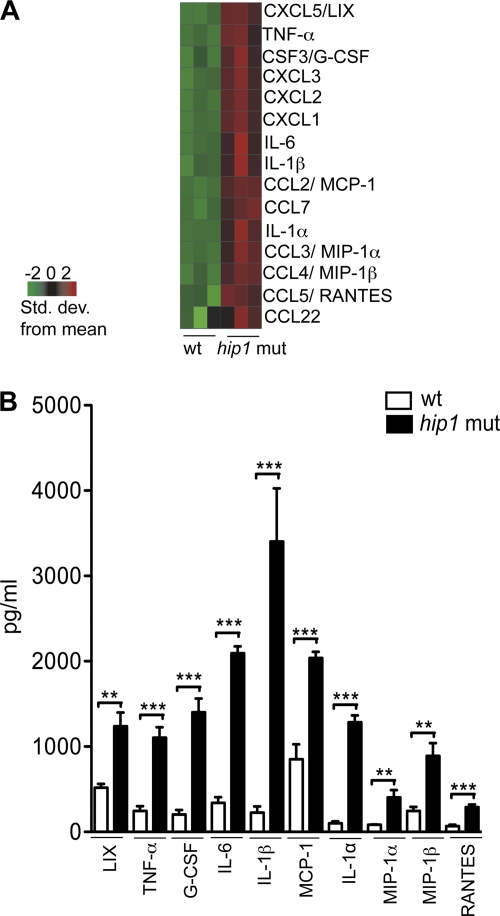
Hip1 is a cell envelope-localized serine hydrolase that is required for M. tuberculosis virulence and disease progression in vivo and promotes pathogen survival in macrophages (29, 49, 50, 65). hip1 mutant strains of M. tuberculosis are more susceptible to antimicrobial agents and cell envelope-directed stresses (50, 64, 65), suggesting that the Hip1 hydrolase modifies the M. tuberculosis cell envelope. Since the M. tuberculosis cell envelope harbors several PAMPs that are recognized by PRRs to elicit innate immune responses in macrophages and dendritic cells, we investigated the hypothesis that Hip1 modulates macrophage responses. We profiled the gene expression of macrophages infected with either a wild-type or hip1 mutant strain of M. tuberculosis at 24 h postinfection, when intracellular bacterial counts are indistinguishable between the two strains (50). We infected BMM from C57BL/6 mice with the wt or hip1 mutant strain at an MOI of 10 and compared their transcriptional profiles 24 and 48 h after infection by using Affymetrix microarrays. The intracellular bacterial counts in infected macrophages at 4 h postinfection were comparable (data not shown). Statistical analyses of the data revealed that the most significant difference between gene expression levels in wt- and hip1 mutant-infected macrophages was a dramatic increase in mRNAs corresponding to several proinflammatory cytokines and chemokines in macrophages infected with the hip1 mutant (Fig. 1A). We confirmed these data by assaying the protein levels of several cytokines and chemokines produced by infected macrophages (Fig. 1B). At 24 h postinfection, cell-free supernatants were harvested from wt- or hip1 mutant-infected macrophages and analyzed by multiplex ELISA. Levels of several proinflammatory cytokines (IL-1α, IL-1β, IL-6, and TNF-α) and chemokines (LIX, granulocyte colony-stimulating factor [G-CSF], monocyte chemoattractant protein 1 [MCP-1], macrophage inflammatory protein 1α [MIP-1α], MIP-1β, and RANTES) were enhanced 2.5- to 14-fold following infection with the hip1 mutant compared to the wt (Fig. 1B). The vigorous induction of proinflammatory responses in macrophages by the hip1 mutant suggests that virulent M. tuberculosis dampens proinflammatory cytokine and chemokine production.
Fig. 1.
Enhanced proinflammatory responses in hip1 mutant-infected macrophages. C57BL/6 BMM were infected with the wt or hip1 mutant strain at an MOI of 10. At 24 h postinfection, cell-free supernatants were harvested for cytokine assays and macrophage RNA was extracted for gene expression analysis using Affymetrix microarrays. (A) Heat map depicting microarray data corresponding to the expression of several proinflammatory cytokine and chemokine transcripts in wt- versus hip1 mutant-infected macrophages. The expression level of each gene (in rows) is represented by the number of standard deviations above (red) or below (green) the average value for that gene across all samples (in columns). (B) Proinflammatory cytokine and chemokine levels were measured in the supernatants of wt- or hip1 mutant-infected macrophages by multiplex ELISA at 24 h postinfection. Results are representative of 2 independent experiments. Values are presented as means plus SD for triplicate assays. **, P < 0.01; ***, P < 0.001.
M. tuberculosis limits the magnitude of macrophage proinflammatory cytokines through Hip1.
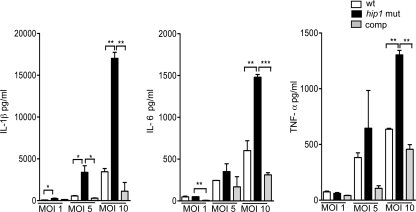
To further investigate the basis for the increased magnitude of proinflammatory responses induced by the hip1 mutant, we infected macrophages with bacteria at different MOIs. We observed increased production of representative proinflammatory cytokines (IL-1β, IL-6, and TNF-α) with escalating MOIs of both wt and hip1 mutant strains (Fig. 2), with an MOI of 10 showing the most significant differences between wt- and hip1 mutant-induced responses. To confirm that disruption of hip1 leads to the hyperinflammatory phenotype of the mutant, we ectopically expressed intact Hip1 protein in the hip1 mutant strain. The high levels of key proinflammatory cytokines (IL-1β, IL-6, and TNF-α) induced by the hip1 mutant were restored to wild-type levels upon infection with the complemented strain (Fig. 2). Taken together, these results indicate that Hip1 limits the magnitude of macrophage proinflammatory responses.
Fig. 2.
Dose-dependent induction of proinflammatory cytokines and complementation of the hip1 mutant phenotype. C57BL/6 BMM were infected with the wt, hip1 mutant, or complemented hip1 mutant (comp) strain at MOIs of 1, 5, and 10. Supernatants were collected at 24 h postinfection and were assayed for IL-1β, IL-6, and TNF-α by ELISA. Values are presented as means plus SD, and the data are representative of 3 independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Hip1 delays induction of proinflammatory cytokines.
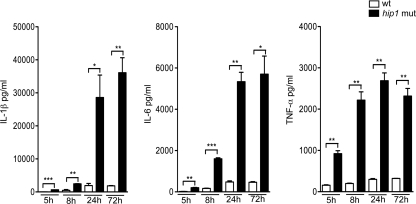
To understand how Hip1 limits robust macrophage responses, we studied the kinetics of IL-1β, IL-6, and TNF-α induction in macrophages infected with the M. tuberculosis wt or hip1 mutant strain at 5, 8, 24, and 72 h postinfection. We detected significantly enhanced cytokine levels in hip1 mutant-infected macrophages as early as 5 h postinfection (Fig. 3), and this trend continued at 8, 24, and 72 h postinfection, with substantial increases in IL-1β, IL-6, and TNF-α levels. The increased induction of these cytokines by the hip1 mutant preceded any detectable cytotoxicity, as measured by lactate dehydrogenase (LDH) release, and was therefore not due to increased cell death (data not shown). Thus, disruption of hip1 leads to faster and higher levels of proinflammatory responses, indicating that wt M. tuberculosis delays the induction of innate immune responses. The rapid onset and increased magnitude of cytokine responses elicited by the hip1 mutant implicate signal transduction pathways downstream of macrophage PRRs. In the next section, we investigate how Hip1 dampens proinflammatory cytokine responses by defining the signaling pathways elicited by wt and hip1 mutant strains of M. tuberculosis.
Fig. 3.
Rapid induction of proinflammatory cytokines in the absence of Hip1. C57BL/6 BMM were infected with the wt or hip1 mutant strain at an MOI of 10. Supernatants were collected at 5, 8, 24, and 72 h postinfection and were assayed for IL-1β, IL-6, and TNF-α by ELISA. Values are presented as means plus SD, and the data are representative of 3 independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
M. tuberculosis dampens proinflammatory responses through MyD88- and TLR2-dependent signaling pathways.
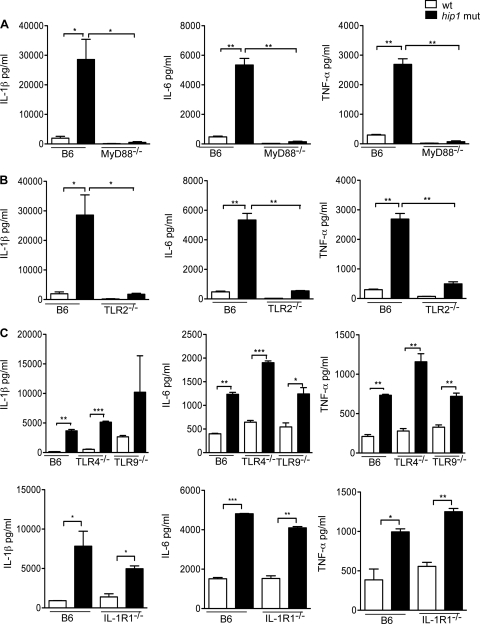
The enhanced levels of proinflammatory cytokines secreted by hip1 mutant-infected macrophages could result from increased signaling through pathways that are known to be downstream of M. tuberculosis infection or could be due to the involvement of additional pathways that wt M. tuberculosis does not normally engage. To delineate the signaling pathways utilized by the hip1 mutant strain, we first analyzed the production of IL-1β, IL-6, and TNF-α in macrophages derived from mice deficient in MyD88, a cytosolic protein which functions as an adaptor protein for signal transduction through several members of the TLR family. We infected resting BMM from MyD88−/− mice with the wt or hip1 mutant strain and assayed for TNF-α, IL-1β, and IL-6 in the supernatants. We found that the production of these cytokines was severely reduced in MyD88−/− macrophages infected with either wt or hip1 mutant bacteria (Fig. 4A), indicating that the enhanced levels of proinflammatory cytokines induced in the absence of Hip1 are generated through MyD88-dependent pathways. To determine which upstream receptors are responsible for this phenotype, we infected macrophages derived from mice deficient in TLR2, TLR4, or TLR9 with the wt or hip1 mutant strain of M. tuberculosis. These TLRs utilize MyD88 as an adaptor and sense PAMPs derived from M. tuberculosis. In macrophages derived from TLR2−/− mice, production of TNF-α, IL-1β, and IL-6 was severely reduced after infection with wt M. tuberculosis as well as with the hip1 mutant (Fig. 4B), indicating that TLR2 signaling mediates the enhanced proinflammatory cytokine responses. In contrast, TLR4 and TLR9 do not contribute to this phenotype, as induction of all three cytokines by either the wt or hip1 mutant strain was not affected in macrophages derived from TLR4−/− or TLR9−/− mice (Fig. 4C). In addition, signaling through IL-1R1, which also utilizes MyD88 as an adaptor, is not required, as the high levels of IL-1β, IL-6, and TNF-α induced by the hip1 mutant in C57BL/6 macrophages were maintained in IL-1R1−/− macrophages (Fig. 4C). The amounts of these cytokines were either at or below the limit of detection in all uninfected macrophages (data not shown). These results show that increased signaling through TLR2 and MyD88 leads to high levels of proinflammatory cytokines by hip1 mutant-infected macrophages and suggest that Hip1 functions by limiting the magnitude of TLR2-dependent innate immune responses. Dampening innate immune responses by restricting TLR2 activation thus appears to be an important strategy employed by M. tuberculosis to evade optimal detection by host innate immunity.
Fig. 4.
Enhanced induction of proinflammatory cytokines by the hip1 mutant requires TLR2 and MyD88 signaling. BMM from C57BL/6 and MyD88−/− mice (A), TLR2−/− mice (B), and TLR4−/−, TLR9−/−, and IL-1R1−/− mice (C) were infected with the wt or hip1 mutant strain at an MOI of 10. Supernatants were collected at 24 h postinfection and assayed for IL-1β, IL-6, and TNF-α by ELISA. Values are presented as means plus SD, and the data are representative of 3 independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Hip1 modulates activation of the NLRP3 inflammasome.
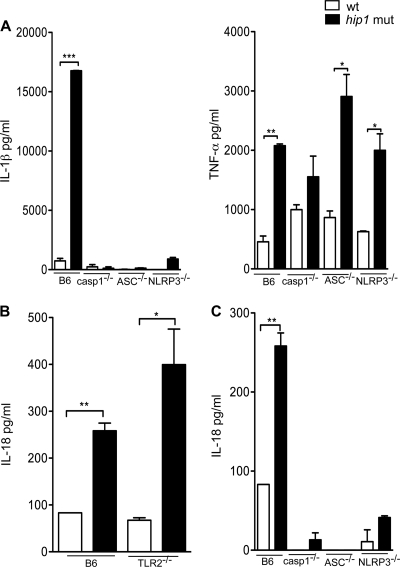
Secretion of IL-1β depends on recognition of PAMPs by both TLRs and cytosolic NLRs and on activation of the inflammasome complex. In response to TLR ligation by microbial ligands, IL-1β is synthesized as inactive pro-IL-1β in the macrophage cytosol; conversion to its biologically active form and its secretion require proteolytic cleavage of pro-IL-1β into mature IL-1β by caspase-1, which in turn is activated by the inflammasome (7, 31). To determine whether the potent IL-1β production in the hip1 mutant was dependent on components of the inflammasome, we infected macrophages from mice deficient in ASC, caspase-1, or NLRP3, each of which has been shown to be required for IL-1β secretion in M. tuberculosis-infected macrophages (25, 34, 37). IL-1β secretion induced by wt or hip1 mutant M. tuberculosis was severely reduced in the absence of each of these proteins (Fig. 5A), while TNF-α production was unperturbed. The amounts of these cytokines were either at or below the limit of detection in all uninfected macrophages (data not shown). Taken together with the data presented in Fig. 4B, these results show that both TLR2 and the NLRP3 inflammasome complex are required for enhanced secretion of IL-1β.
Fig. 5.
IL-1β and IL-18 secretion is dependent on components of the inflammasome complex. (A) BMM from C57BL/6, caspase-1−/−, ASC−/−, and NLRP3−/− mice were infected with the wt or hip1 mutant strain at an MOI of 10. Supernatants collected at 24 h postinfection were assayed for IL-1β and TNF-α by ELISA. BMM from C57BL/6 or TLR2−/− mice (B) or from C57BL/6, caspase-1−/−, ASC−/−, and NLRP3−/− mice (C) were also infected with the wt or hip1 mutant strain at an MOI of 10. Supernatants collected at 24 h postinfection were assayed for IL-18 by ELISA. Values are presented as means plus SD, and the data are representative of 3 independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We also observed 3-fold higher levels of IL-18 in supernatants of hip1 mutant-infected macrophages than in those of wt-infected macrophages (Fig. 5B). Like IL-1β secretion, IL-18 secretion is dependent on activation of the inflammasome complex. However, unlike IL-1β, which is transcriptionally regulated through sensing of PAMPs by TLRs, IL-18 production in response to many bacterial pathogens does not depend on TLR signaling. Pro-IL-18 is expressed constitutively and requires processing by caspase-1 for its maturation and extracellular secretion. To determine whether induction of IL-18 by M. tuberculosis is dependent on TLR2, we assayed the IL-18 levels in TLR2−/− macrophages. The absence of TLR2 did not compromise IL-18 production in either wt- or hip1 mutant-infected macrophages (Fig. 5B). Similar results were observed in MyD88−/− macrophages (data not shown). These data indicate that TLR2 signaling does not regulate induction of IL-18 by either wt or hip1 mutant strains of M. tuberculosis. Enhanced IL-18 secretion induced by hip1 mutant-infected macrophages was entirely dependent on components of the inflammasome complex, i.e., ASC, caspase-1, and NLRP3 proteins (Fig. 5C). Since assaying IL-18 gives a direct readout of caspase-1 and inflammasome activation, these results show that in addition to dampening TLR2-dependent signaling, Hip1 modulates TLR2-independent pathways, i.e., it decreases the magnitude of inflammasome activation.
Heat-killed or gamma-irradiated M. tuberculosis retains the capacity to dampen TLR2-dependent cytokines and requires Hip1 catalytic activity.
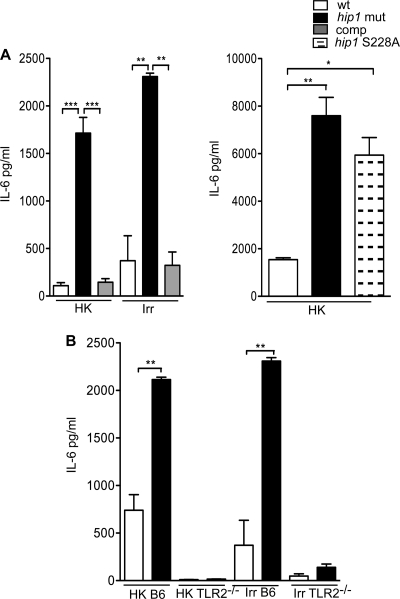
The rapid induction of TLR2-dependent proinflammatory cytokines by the hip1 mutant suggests that PAMPs present in its cell envelope mediate this response. We reasoned that stronger innate immune responses induced by the hip1 mutant might be a result of alterations in surface-localized and/or extracellular TLR2 ligands. To address whether the viability of M. tuberculosis is necessary for the hyperinflammatory phenotype of the hip1 mutant, we tested the ability of live or dead bacteria of the wt and hip1 mutant strains to induce IL-6. Live M. tuberculosis is required for inhibition of phagosome maturation, but other immune evasion strategies, for example, TLR2-dependent inhibition of major histocompatibility complex (MHC) class II expression, can be mediated by dead mycobacteria (15, 61). To determine whether live, replicating M. tuberculosis is required for augmented cytokine responses, we infected macrophages with the inactivated (heat-killed or gamma-irradiated) wt or hip1 mutant strain and measured IL-6 levels. We showed that the inactivated (by either heat killing or gamma irradiation) hip1 mutant induced 6- to 16-fold more IL-6 than inactivated wt M. tuberculosis (Fig. 6A). Therefore, bacterial viability is not necessary for eliciting enhanced levels of IL-6 in macrophages. Wild-type levels of IL-6 were restored upon infection with the hip1-complemented strain. However, expression of a catalytically inactive form of Hip1 (S228A mutant) failed to restore IL-6 levels (Fig. 6A), suggesting that Hip1 activity is necessary for dampening proinflammatory responses. Furthermore, TLR2 activation is required, since IL-6 production in response to inactivated wt and hip1 mutant strains was substantially decreased in TLR2−/− macrophages (Fig. 6B). Since heat-killed or gamma-irradiated bacteria were sufficient for inducing IL-6 in a TLR2-dependent manner, surface-localized TLR2 ligands rather than secreted factors are likely to be involved in dampening innate immune responses. Indeed, we observed that short-term culture filtrates of wt and hip1 mutant strains induced comparable levels of IL-6 (data not shown).
Fig. 6.
Bacterial viability is not required for Hip1-mediated restriction of TLR2-dependent cytokines. (A) C57BL/6 BMM were infected at an MOI of 10 with the heat-killed (HK) or irradiated (Irr) wt, hip1 mutant, or complemented hip1 (comp) strain or with the hip1 mutant complemented with catalytically inactive Hip1 (hip1 S228A). Supernatants collected at 24 h postinfection were assayed for IL-6 by ELISA. (B) C57BL/6 and TLR2−/− BMM were infected with the heat-killed (HK) wt, hip1 mutant, or complemented hip1 mutant (comp) strain at an MOI of 10. Supernatants collected at 24 h postinfection were assayed for IL-6 by ELISA. Values are presented as means plus SD, and the data are representative of 3 independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Limiting TLR2 engagement does not require phagocytosis of M. tuberculosis.
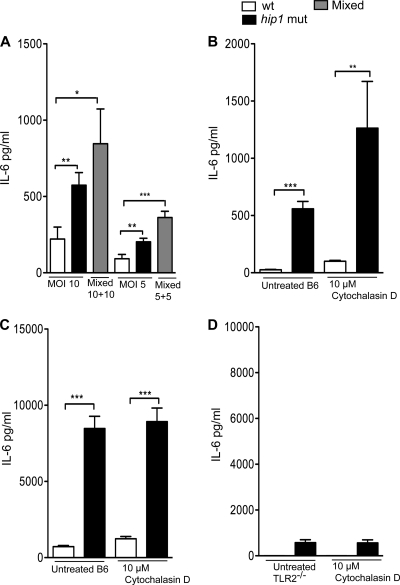
We reasoned that Hip1 might dampen TLR2 activation either through active suppression, i.e., by inhibiting components of the TLR2 signaling pathway, or by limiting activation of TLR2 by promoting the masking of TLR2 ligands in the M. tuberculosis cell envelope. To distinguish between these two possibilities, we combined wt and hip1 mutant strains in mixed infections and compared these to single infections with either strain. If Hip1 exerted an inhibitory effect on TLR2 signaling, we would expect the phenotype of wt M. tuberculosis to dominate in mixed infections. However, if TLR2 ligands were already revealed on the cell surface of the hip1 mutant, we would expect the mutant to retain its ability to elicit enhanced cytokine responses, even in the presence of the wt. Macrophages were exposed to the heat-killed wt and hip1 mutant strains, either singly or in combination (1:1 ratio), at an MOI of 5 or 10. Supernatants were assayed for IL-6 production at 24 h postexposure. The cytokine response elicited by mixed cultures was additive, showing that the wt strains did not suppress the ability of the hip1 mutant to elicit higher levels of IL-6 (Fig. 7A). Rather, heightened cytokine responses were likely driven by increased engagement of TLR2 ligands by the hip1 mutant. These data suggest that Hip1 dampens TLR2 signaling in macrophages by limiting recognition of M. tuberculosis TLR2 ligands.
Fig. 7.
hip1 mutant-induced proinflammatory response is dominant over wt response and is independent of phagocytosis. (A) C57BL/6 BMM were infected with heat-killed (HK) cultures of the wt or the hip1 mutant at an MOI of 10 or 5 or with mixed cultures of the wt and the hip1 mutant at a 1:1 ratio (MOIs of 10 and 10 or 5 and 5). Supernatants collected at 24 h postinfection were assayed for IL-6 by ELISA. (B) C57BL/6 BMM were left untreated or treated with 10 μM cytochalasin D for 1 h and then infected with the heat-killed wt or hip1 mutant strain, and supernatants collected at 6 h postinfection were assayed for IL-6 by ELISA. C57BL/6 (C) or TLR2−/− (D) BMM were left untreated or treated with 10 μM cytochalasin D for 1 h and then infected with the live wt or hip1 mutant strain, and supernatants collected at 24 h postinfection were assayed for IL-6 by ELISA. Values are presented as means plus SD, and the data are representative of 3 independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We next examined the basis for limited TLR2 recognition. Increased activation of TLR2 signaling by the hip1 mutant could occur because of an altered repertoire of PAMPs that are recognized by TLR2 on macrophages. In wt M. tuberculosis, these TLR2 ligands may be masked by the cell envelope architecture. Unmasking of these TLR2 ligands in the absence of Hip1 could lead to cell surface interactions between M. tuberculosis and macrophages upon contact. Alternatively, phagocytosis of the bacteria and damage from antimicrobial agents may be required for release of TLR2 ligands and subsequent sensing by TLR2 on macrophage phagosomes. To distinguish between these possibilities, we either left macrophages untreated prior to infection or pretreated macrophages with cytochalasin D, which inhibits phagocytosis and blocks uptake of M. tuberculosis by macrophages (8). Exposure of cytochalasin D-treated macrophages to the inactivated wt or hip1 mutant strain for 6 h did not diminish the enhanced IL-6 production, indicating that phagocytosis is not necessary for induction of these responses (Fig. 7B). Similarly, cytochalasin D did not prevent the induction of IL-6 by live M. tuberculosis in C57BL/6 mice (Fig. 7C). These results show that cell surface interactions are sufficient for enhanced induction of IL-6 by the hip1 mutant. In addition, IL-6 production was severely reduced in TLR2-deficient macrophages (Fig. 7D), indicating that interaction of M. tuberculosis cell surface-localized TLR agonists with TLR2 on the macrophage cell surface is sufficient for initiating proinflammatory responses. These data suggest that Hip1 dampens TLR2 activation by limiting recognition of TLR2 ligands on the M. tuberculosis cell surface.
DISCUSSION
Our studies describe a novel strategy utilized by M. tuberculosis to dampen proinflammatory responses and show a critical role for the serine hydrolase Hip1 in mediating this effect. We showed that disruption of hip1 leads to earlier and significantly higher levels of key proinflammatory cytokines and chemokines (Fig. 1 and 3). While these findings differ from what has been reported previously (50), the enhanced induction of cytokines in response to infection reported here was characterized extensively in multiple experiments and reflects the true phenotype of the hip1 mutant. The modest reduction in cytokine levels induced by the hip1 mutant in the earlier study was likely due to the lower MOIs used and to differences related to preparation of the inocula. Using macrophages derived from a panel of knockout mice, we showed that the enhanced production of key proinflammatory cytokines by hip1 mutant-infected macrophages requires MyD88- and TLR2-dependent signaling. Increased transcription of cytokines such as IL-1β, TNF-α, and IL-6, downstream of TLR2 recognition, leads to increased protein levels in macrophage supernatants (Fig. 1 and 4). Residual levels of these cytokines observed in TLR2−/− and MyD88−/− macrophages (Fig. 4 and 6) suggest that TLR2/MyD88-independent pathways also contribute to proinflammatory responses in M. tuberculosis-infected macrophages, but these responses are independent of TLR4, TLR9, or IL-1R1 signaling (Fig. 4). Overall, we concluded that M. tuberculosis normally limits the magnitude of TLR2 activation and thereby prevents robust activation of macrophage proinflammatory responses. Interestingly, increased cytokine production in response to infection with the hip1 mutant does not appear to be a generalized phenomenon, since the chemokine IP-10 is stimulated to the same extent by wt and hip1 mutant strains (R. Madan-Lala and J. Rengarajan, unpublished data). Several groups, including ours, have shown that in human and murine cells, IP-10 induction in response to TLR agonists or M. tuberculosis infection occurs through the MyD88-independent IRF3 pathway and that TLR2-mediated signaling is unable to induce this chemokine (22, 46, 56, 66).
Our studies suggest that restricting TLR2 activation contributes to M. tuberculosis pathogenicity, while augmentation of early TLR2 activation, as observed with the hip1 mutant, is associated with reduced virulence (50). Indeed, several studies have suggested that virulent strains of M. tuberculosis are associated with decreased inflammatory responses (28, 40, 44, 47, 59, 67). Clinical isolates corresponding to evolutionarily modern lineages of M. tuberculosis have lowered proinflammatory responses compared to more ancient lineages (44). Thus, it is possible that restricting the magnitude of inflammatory responses may provide a selective advantage to the pathogen. The attenuated virulence exhibited by the hip1 mutant in vivo suggests that increased production of TLR2-dependent proinflammatory cytokines may be beneficial to the host. It is tempting to speculate that dampening the induction of innate cytokines by limiting TLR2 activation enables M. tuberculosis to influence adaptive immunity and accelerate the progression of TB disease. It will be interesting to test whether hip1 expression is altered in different M. tuberculosis lineages, thereby contributing to the various abilities of these strains to induce inflammatory responses.
Many immune evasion mechanisms require replication of live M. tuberculosis organisms within macrophages, but dead mycobacteria are sufficient for other mechanisms. For example, inhibition of MHC class II expression, which is also dependent on TLR2 signaling, does not require M. tuberculosis viability (15, 61). Interestingly, we showed that M. tuberculosis replication is not necessary for the enhanced TLR2-dependent proinflammatory phenotype, as both heat-killed and gamma-irradiated hip1 mutant strains retained the ability to induce higher levels of IL-6 (Fig. 6). This induction requires Hip1 hydrolase activity, as a catalytically inactive Hip1 protein failed to restore wt levels of IL-6 in the mutant (Fig. 6A). To elucidate the mechanism for dampened proinflammatory responses, we next examined whether Hip1 functions by suppressing components of the TLR2 signal transduction pathway. For example, the secreted protein ESAT-6 has been shown to inhibit TLR2 signaling and NF-κB activation by preventing interaction between MyD88 and IRAK (42). We carried out mixed infection experiments using 1:1 ratios of wt and hip1 mutant strains and demonstrated that Hip1 does not suppress the TLR2 signal transduction pathway, since the wt strain was unable to prevent the hip1 mutant from inducing high levels of IL-6 (Fig. 7A). Rather, these data are consistent with increased recognition of TLR2 ligands by the mutant strain, leading to enhanced production of TLR2-dependent cytokines. Cell surface interactions between TLR2 ligands on the hip1 mutant and TLR2 on macrophages are sufficient to induce rapid and robust cytokine responses, since we showed that phagocytosis is not required for cytokine induction (Fig. 7B and C). These data also imply that damage to the hip1 mutant cell envelope in macrophage phagosomes and subsequent release of PAMPs are not required for engaging the TLR2-MyD88 pathway. Instead, we propose that in wt M. tuberculosis, Hip1 hydrolase activity masks TLR agonists in the cell envelope that could potentially be recognized by TLR2. This limits TLR2 activation and dampens proinflammatory responses.
Although M. tuberculosis possesses several potential TLR2 ligands, our data suggest that there may be limited recognition of TLR2 agonists in the context of infection with the whole organism. Indeed, despite the ability of several purified lipoproteins (e.g., LpqH, LprG, and LprA) and lipids to act as potent TLR2 agonists in vitro, TLR2-deficient mice are not especially susceptible to acute M. tuberculosis infection (9, 18, 19, 43, 48, 58). However, TLR2/TLR9 doubly deficient mice are more susceptible to infection, and MyD88-deficient mice succumb rapidly to infection (2, 17, 21, 55). These studies suggest redundancy in TLR usage in vivo but may also reflect limited TLR2 engagement during the innate phase of infection. Based on our data, we propose that many M. tuberculosis TLR2 agonists in the cell envelope may not interact with TLR2 on macrophages. They may be inaccessible to macrophages due to Hip1-dependent modifications of the cell envelope that mask these PAMPs, or processing of TLR2 ligands by Hip1 hydrolase activity may prevent recognition of TLR2 agonists by macrophages. In the absence of Hip1, altered accessibility or changes in the diversity of TLR2 ligands could lead to increased recognition by TLR2 and to enhanced proinflammatory responses.
While TLR agonists on the cell surface of M. tuberculosis are likely to be sufficient for inducing IL-6, the secretion of inflammasome-dependent IL-1β and IL-18 requires sensing of cytosolic PAMPs by NLRs in the macrophage cytoplasm (31). While M. tuberculosis has been shown to induce inflammasome activation (23, 25, 34, 37), stronger activation of the NLRP3 inflammasome by the hip1 mutant raises the interesting question of how NLRP3 senses M. tuberculosis. While the ligands for NLRP3 in M. tuberculosis have not been identified, a wide array of molecules, such as muramyl dipeptide, bacterial RNA, uric acid crystals, ATP, alum, and asbestos, have been implicated in activating the NLRP3 inflammasome, although the molecular basis for activation is not understood (31, 62). It is likely that NLR ligands are able to access the macrophage cytosol. Possible mechanisms for the cytosolic localization and recognition of M. tuberculosis NLR ligands include the rupture of phagosomes due to the particulate nature of M. tuberculosis antigens and the release of PAMPs into the cytosol, as well as entry of M. tuberculosis components into the macrophage cytoplasm (27, 57, 62). This may be mediated in part by ESAT-6, which is implicated in mediating membrane damage and has been reported to be required for activation of caspase-1 and the NLRP3 inflammasome (37). However, ESAT-6 secretion is comparable in both wt and mutant strains (Madan-Lala and Rengarajan, unpublished data). We speculate that the higher susceptibility to cell envelope-directed stresses (50, 65) of the hip1 mutant may facilitate release of mycobacterial cell wall components that can activate NLRP3 into the macrophage cytosol, resulting in increased IL-1β and IL-18 secretion. Interestingly, disruption of a putative zinc metalloprotease, Zmp1, also results in stronger caspase-1 activation and more IL-1β secretion than those in wt M. tuberculosis, suggesting that similar mechanisms may be involved (32). Overall, these data show that in addition to limiting TLR2 engagement, restricting the magnitude of inflammasome activation is an important mechanism employed by M. tuberculosis for dampening proinflammatory responses and avoiding immune detection.
Elucidating the molecular and biochemical basis for the altered repertoires of PAMPs in wt and hip1 mutant strains should shed light on the nature of the TLR agonists involved. We know from electron microscopy that the cell envelope of the hip1 mutant is not noticeably altered compared to that of the wt (Madan-Lala and Rengarajan, unpublished data). Short-term culture filtrates of the wt and the hip1 mutant show differences in protein composition but induce comparable levels of IL-1β, TNF-α, and IL-6 (data not shown). These data suggest that the differential induction of cytokine levels between these strains is not due simply to altered repertoires of secreted factors or to gross changes in morphology. Rather, alterations in the accessibility or type of cell envelope-associated ligands likely influence how M. tuberculosis modulates macrophage activation. Further studies aimed at defining the protein and lipid profiles of wt and hip1 mutant cell envelopes will provide important insights.
In summary, we show that M. tuberculosis Hip1 modulates the onset and magnitude of proinflammatory responses in macrophages. Limiting recognition of TLR2 ligands on the M. tuberculosis cell surface prevents robust TLR2 activation and leads to dampened proinflammatory responses. Inducing weak innate immune responses is likely to be advantageous to the pathogen during infection.
ACKNOWLEDGMENTS
We gratefully acknowledge Bali Pulendran for the kind gift of TLR2−/−, TLR4−/−, TLR9−/−, and MyD88−/− mice and Denise Monack for bone marrow cells from caspase-1−/− mice. We thank Meghan Feltcher and Miriam Braunstein for the irradiated M. tuberculosis strains, Helder I. Nakaya for help with the microarray heat map, and Kalpana Patel for help with the multiplex ELISA. We are grateful to David Weiss for helpful discussions and to Nina Salinger and Maria Georgieva for technical assistance.
This work was supported by funds from National Institutes of Health grants R00TW008043-04 (FIC) and 5R01AI083366-02 (NIAID).
Footnotes
Published ahead of print on 26 September 2011.
REFERENCES
- 1. Abel B., et al. 2002. Toll-like receptor 4 expression is required to control chronic Mycobacterium tuberculosis infection in mice. J. Immunol. 169:3155–3162 [DOI] [PubMed] [Google Scholar]
- 2. Bafica A., et al. 2005. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J. Exp. Med. 202:1715–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Banaiee N., Kincaid E. Z., Buchwald U., Jacobs W. R., Jr., Ernst J. D. 2006. Potent inhibition of macrophage responses to IFN-gamma by live virulent Mycobacterium tuberculosis is independent of mature mycobacterial lipoproteins but dependent on TLR2. J. Immunol. 176:3019–3027 [DOI] [PubMed] [Google Scholar]
- 4. Bhatt K., Salgame P. 2007. Host innate immune response to Mycobacterium tuberculosis. J. Clin. Immunol. 27:347–362 [DOI] [PubMed] [Google Scholar]
- 5. Brightbill H. D., et al. 1999. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science 285:732–736 [DOI] [PubMed] [Google Scholar]
- 6. Cooper A. M. 2009. Cell-mediated immune responses in tuberculosis. Annu. Rev. Immunol. 27:393–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dinarello C. A. 2009. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27:519–550 [DOI] [PubMed] [Google Scholar]
- 8. Ding A., Yu H., Yang J., Shi S., Ehrt S. 2005. Induction of macrophage-derived SLPI by Mycobacterium tuberculosis depends on TLR2 but not MyD88. Immunology 116:381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drennan M. B., et al. 2004. Toll-like receptor 2-deficient mice succumb to Mycobacterium tuberculosis infection. Am. J. Pathol. 164:49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ehrt S., Schnappinger D. 2009. Mycobacterial survival strategies in the phagosome: defence against host stresses. Cell. Microbiol. 11:1170–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feng C. G., et al. 2003. Mice lacking myeloid differentiation factor 88 display profound defects in host resistance and immune responses to Mycobacterium avium infection not exhibited by Toll-like receptor 2 (TLR2)- and TLR4-deficient animals. J. Immunol. 171:4758–4764 [DOI] [PubMed] [Google Scholar]
- 12. Flannagan R. S., Cosio G., Grinstein S. 2009. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat. Rev. Microbiol. 7:355–366 [DOI] [PubMed] [Google Scholar]
- 13. Flores A. R., Parsons L. M., Pavelka M. S., Jr 2005. Characterization of novel Mycobacterium tuberculosis and Mycobacterium smegmatis mutants hypersusceptible to beta-lactam antibiotics. J. Bacteriol. 187:1892–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flynn J. L., Chan J. 2003. Immune evasion by Mycobacterium tuberculosis: living with the enemy. Curr. Opin. Immunol. 15:450–455 [DOI] [PubMed] [Google Scholar]
- 15. Fortune S. M., et al. 2004. Mycobacterium tuberculosis inhibits macrophage responses to IFN-gamma through myeloid differentiation factor 88-dependent and -independent mechanisms. J. Immunol. 172:6272–6280 [DOI] [PubMed] [Google Scholar]
- 16. Fremond C. M., et al. 2007. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J. Immunol. 179:1178–1189 [DOI] [PubMed] [Google Scholar]
- 17. Fremond C. M., et al. 2004. Fatal Mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88. J. Clin. Invest. 114:1790–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gehring A. J., Dobos K. M., Belisle J. T., Harding C. V., Boom W. H. 2004. Mycobacterium tuberculosis LprG (Rv1411c): a novel TLR-2 ligand that inhibits human macrophage class II MHC antigen processing. J. Immunol. 173:2660–2668 [DOI] [PubMed] [Google Scholar]
- 19. Gehring A. J., et al. 2003. The Mycobacterium tuberculosis 19-kilodalton lipoprotein inhibits gamma interferon-regulated HLA-DR and Fc gamma R1 on human macrophages through Toll-like receptor 2. Infect. Immun. 71:4487–4497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harding C. V., Boom W. H. 2010. Regulation of antigen presentation by Mycobacterium tuberculosis: a role for Toll-like receptors. Nat. Rev. Microbiol. 8:296–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holscher C., et al. 2008. Containment of aerogenic Mycobacterium tuberculosis infection in mice does not require MyD88 adaptor function for TLR2, -4 and -9. Eur. J. Immunol. 38:680–694 [DOI] [PubMed] [Google Scholar]
- 22. Kawai T., et al. 2001. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 167:5887–5894 [DOI] [PubMed] [Google Scholar]
- 23. Kleinnijenhuis J., et al. 2009. Transcriptional and inflammasome-mediated pathways for the induction of IL-1beta production by Mycobacterium tuberculosis. Eur. J. Immunol. 39:1914–1922 [DOI] [PubMed] [Google Scholar]
- 24. Kleinnijenhuis J., et al. 2011. Innate immune recognition of Mycobacterium tuberculosis. Clin. Dev. Immunol. 2011:405310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koo I. C., et al. 2008. ESX-1-dependent cytolysis in lysosome secretion and inflammasome activation during mycobacterial infection. Cell. Microbiol. 10:1866–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kumagai Y., Takeuchi O., Akira S. 2008. TLR9 as a key receptor for the recognition of DNA. Adv. Drug Deliv. Rev. 60:795–804 [DOI] [PubMed] [Google Scholar]
- 27. Lewinsohn D. M., et al. 2006. Secreted proteins from Mycobacterium tuberculosis gain access to the cytosolic MHC class-I antigen-processing pathway. J. Immunol. 177:437–442 [DOI] [PubMed] [Google Scholar]
- 28. Lopez B., et al. 2003. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin. Exp. Immunol. 133:30–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lun S., Bishai W. R. 2007. Characterization of a novel cell wall-anchored protein with carboxylesterase activity required for virulence in Mycobacterium tuberculosis. J. Biol. Chem. 282:18348–18356 [DOI] [PubMed] [Google Scholar]
- 30. Manca C., et al. 2004. Differential monocyte activation underlies strain-specific Mycobacterium tuberculosis pathogenesis. Infect. Immun. 72:5511–5514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mariathasan S., Monack D. M. 2007. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat. Rev. Immunol. 7:31–40 [DOI] [PubMed] [Google Scholar]
- 32. Master S. S., et al. 2008. Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe 3:224–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mayer-Barber K. D., et al. 2010. Caspase-1 independent IL-1beta production is critical for host resistance to Mycobacterium tuberculosis and does not require TLR signaling in vivo. J. Immunol. 184:3326–3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McElvania Tekippe E., et al. 2010. Granuloma formation and host defense in chronic Mycobacterium tuberculosis infection requires PYCARD/ASC but not NLRP3 or caspase-1. PLoS One 5:e12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Means T. K., et al. 1999. Human Toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J. Immunol. 163:3920–3927 [PubMed] [Google Scholar]
- 36. Medzhitov R., Janeway C., Jr 2000. Innate immune recognition: mechanisms and pathways. Immunol. Rev. 173:89–97 [DOI] [PubMed] [Google Scholar]
- 37. Mishra B. B., et al. 2010. Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell. Microbiol. 12:1046–1063 [DOI] [PubMed] [Google Scholar]
- 37a. National Research Council 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC [Google Scholar]
- 38. Nau G. J., et al. 2002. Human macrophage activation programs induced by bacterial pathogens. Proc. Natl. Acad. Sci. U. S. A. 99:1503–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Netea M. G., et al. 2006. Mycobacterium tuberculosis induces interleukin-32 production through a caspase-1/IL-18/interferon-gamma-dependent mechanism. PLoS Med. 3:e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Newton S. M., et al. 2006. A deletion defining a common Asian lineage of Mycobacterium tuberculosis associates with immune subversion. Proc. Natl. Acad. Sci. U. S. A. 103:15594–15598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pai R. K., et al. 2004. Prolonged Toll-like receptor signaling by Mycobacterium tuberculosis and its 19-kilodalton lipoprotein inhibits gamma interferon-induced regulation of selected genes in macrophages. Infect. Immun. 72:6603–6614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pathak S. K., et al. 2007. Direct extracellular interaction between the early secreted antigen ESAT-6 of Mycobacterium tuberculosis and TLR2 inhibits TLR signaling in macrophages. Nat. Immunol. 8:610–618 [DOI] [PubMed] [Google Scholar]
- 43. Pecora N. D., Gehring A. J., Canaday D. H., Boom W. H., Harding C. V. 2006. Mycobacterium tuberculosis LprA is a lipoprotein agonist of TLR2 that regulates innate immunity and APC function. J. Immunol. 177:422–429 [DOI] [PubMed] [Google Scholar]
- 44. Portevin D., Gagneux S., Comas I., Young D. 2011. Human macrophage responses to clinical isolates from the Mycobacterium tuberculosis complex discriminate between ancient and modern lineages. PLoS Pathog. 7:e1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Quesniaux V., et al. 2004. Toll-like receptor pathways in the immune responses to mycobacteria. Microbes Infect. 6:946–959 [DOI] [PubMed] [Google Scholar]
- 46. Re F., Strominger J. L. 2001. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J. Biol. Chem. 276:37692–37699 [DOI] [PubMed] [Google Scholar]
- 47. Reed M. B., et al. 2004. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature 431:84–87 [DOI] [PubMed] [Google Scholar]
- 48. Reiling N., et al. 2002. Cutting edge: Toll-like receptor (TLR)2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J. Immunol. 169:3480–3484 [DOI] [PubMed] [Google Scholar]
- 49. Rengarajan J., Bloom B. R., Rubin E. J. 2005. Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proc. Natl. Acad. Sci. U. S. A. 102:8327–8332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rengarajan J., et al. 2008. Mycobacterium tuberculosis Rv2224c modulates innate immune responses. Proc. Natl. Acad. Sci. U. S. A. 105:264–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Roy C. R., Mocarski E. S. 2007. Pathogen subversion of cell-intrinsic innate immunity. Nat. Immunol. 8:1179–1187 [DOI] [PubMed] [Google Scholar]
- 52. Russell D. G. 2001. Mycobacterium tuberculosis: here today, and here tomorrow. Nat. Rev. Mol. Cell. Biol. 2:569–577 [DOI] [PubMed] [Google Scholar]
- 53. Russell D. G. 2007. Who puts the tubercle in tuberculosis? Nat. Rev. Microbiol. 5:39–47 [DOI] [PubMed] [Google Scholar]
- 54. Salgame P. 2005. Host innate and Th1 responses and the bacterial factors that control Mycobacterium tuberculosis infection. Curr. Opin. Immunol. 17:374–380 [DOI] [PubMed] [Google Scholar]
- 55. Scanga C. A., et al. 2004. MyD88-deficient mice display a profound loss in resistance to Mycobacterium tuberculosis associated with partially impaired Th1 cytokine and nitric oxide synthase 2 expression. Infect. Immun. 72:2400–2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shi S., et al. 2005. Expression of many immunologically important genes in Mycobacterium tuberculosis-infected macrophages is independent of both TLR2 and TLR4 but dependent on IFN-alphabeta receptor and STAT1. J. Immunol. 175:3318–3328 [DOI] [PubMed] [Google Scholar]
- 57. Stanley S. A., Johndrow J. E., Manzanillo P., Cox J. S. 2007. The type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J. Immunol. 178:3143–3152 [DOI] [PubMed] [Google Scholar]
- 58. Sugawara I., et al. 2003. Mycobacterial infection in TLR2 and TLR6 knockout mice. Microbiol. Immunol. 47:327–336 [DOI] [PubMed] [Google Scholar]
- 59. Tanveer M., Hasan Z., Kanji A., Hussain R., Hasan R. 2009. Reduced TNF-alpha and IFN-gamma responses to Central Asian strain 1 and Beijing isolates of Mycobacterium tuberculosis in comparison with H37Rv strain. Trans. R. Soc. Trop. Med. Hyg. 103:581–587 [DOI] [PubMed] [Google Scholar]
- 60. Taylor P. R., et al. 2005. Macrophage receptors and immune recognition. Annu. Rev. Immunol. 23:901–944 [DOI] [PubMed] [Google Scholar]
- 61. Ting L. M., Kim A. C., Cattamanchi A., Ernst J. D. 1999. Mycobacterium tuberculosis inhibits IFN-gamma transcriptional responses without inhibiting activation of STAT1. J. Immunol. 163:3898–3906 [PubMed] [Google Scholar]
- 62. Tschopp J., Schroder K. 2010. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 10:210–215 [DOI] [PubMed] [Google Scholar]
- 63. Underhill D. M., Ozinsky A., Smith K. D., Aderem A. 1999. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc. Natl. Acad. Sci. U. S. A. 96:14459–14463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vandal O. H., Pierini L. M., Schnappinger D., Nathan C. F., Ehrt S. 2008. A membrane protein preserves intrabacterial pH in intraphagosomal Mycobacterium tuberculosis. Nat. Med. 14:849–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vandal O. H., et al. 2009. Acid-susceptible mutants of Mycobacterium tuberculosis share hypersusceptibility to cell wall and oxidative stress and to the host environment. J. Bacteriol. 191:625–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vogel S., Hirschfeld M. J., Perera P. Y. 2001. Signal integration in lipopolysaccharide (LPS)-stimulated murine macrophages. J. Endotoxin Res. 7:237–241 [PubMed] [Google Scholar]
- 67. Wang C., et al. 2010. Innate immune response to Mycobacterium tuberculosis Beijing and other genotypes. PLoS One 5:e13594. [DOI] [PMC free article] [PubMed] [Google Scholar]