Abstract
Staphylococcus aureus is a common cause of prosthetic implant infections, which can become chronic due to the ability of S. aureus to grow as a biofilm. Little is known about adaptive immune responses to these infections in vivo. We hypothesized that S. aureus elicits inflammatory Th1/Th17 responses, associated with biofilm formation, instead of protective Th2/Treg responses. We used an adapted mouse model of biofilm-mediated prosthetic implant infection to determine chronic infection rates, Treg cell frequencies, and local cytokine levels in Th1-biased C57BL/6 and Th2-biased BALB/c mice. All C57BL/6 mice developed chronic S. aureus implant infection at all time points tested. However, over 75% of BALB/c mice spontaneously cleared the infection without adjunctive therapy and demonstrated higher levels of Th2 cytokines and anti-inflammatory Treg cells. When chronic infection rates in mice deficient in the Th2 cytokine interleukin-4 (IL-4) via STAT6 mutation in a BALB/c background were assessed, the mice were unable to clear the S. aureus implant infection. Additionally, BALB/c mice depleted of Treg cells via an anti-CD25 monoclonal antibody (MAb) were also unable to clear the infection. In contrast, the C57BL/6 mice that were susceptible to infection were able to eliminate S. aureus biofilm populations on infected intramedullary pins once the Th1 and Th17 responses were diminished by MAb treatment with anti-IL-12 p40. Together, these results indicate that Th2/Treg responses are mechanisms of protection against chronic S. aureus implant infection, as opposed to Th1/Th17 responses, which may play a role in the development of chronic infection.
INTRODUCTION
Nosocomial infections present a common and costly problem for the U.S. health care system (20). Staphylococcus aureus is a major cause of such infections worldwide, and the prevalence of methicillin-resistant S. aureus (MRSA) strains in hospitals has risen steadily over the years, contributing to increased mortality and hospital costs. S. aureus is the dominant bacterial species involved in cases of indwelling medical device (IMD) infection, whose incidence has increased with a rise in the use of IMDs and the implantation of prosthetic joints (2, 9, 40). In cases of such infections, often the only treatment option is surgical removal of the infected IMD, leading to significant morbidity and mortality (5, 9). The development of a prosthetic implant infection depends on the ability of S. aureus to adhere to host proteins coating implants through cell wall-associated adhesins. Subsequent biofilm formation by S. aureus makes eradication of this bacterium exceptionally difficult due to the increased resistance of biofilm-embedded bacteria to host defenses (15) and antibiotics (34, 36) compared to their planktonic forms.
While in vivo studies are limited, there is a body of evidence that demonstrates the ability of S. aureus to skew the host immune response by influencing cytokine production in vitro. Staphylococcal enterotoxin B (SEB) has been shown to induce the production of interleukin-2 (IL-2) and gamma interferon (IFN-γ) in vitro (3). Staphylococcal enterotoxin A (SEA) induces a Th1 response associated with the production of tumor necrosis factor alpha (TNF-α) and macrophage inflammatory protein 1β (MIP-1β) (13). Similarly, alpha-toxin induces IFN-γ and also increases T-bet binding to DNA, which induces a Th1 response (7). Protein A is also a potent inducer of IFN-γ, TNF-α, and IL-1, all of which are associated with the Th1 response (45). This skewing in turn influences the propensity of S. aureus infection to progress from an acute infection to a biofilm infection that is chronic. During the early stages of an S. aureus infection, host innate immune cells, such as monocytes, produce a number of proinflammatory cytokines, including IL-1, IL-6, IL-12 p70, IL-18, and TNF-α (8, 42). This cytokine milieu drives proinflammatory Th1 and Th17 CD4+ T helper cell responses that can result in substantial damage to host tissues. S. aureus also induces IL-12, IFN-γ, and TNF-α production in CD4+ T cells, which further provokes a Th1 response (25). Unfortunately, Th1 responses may be ineffectual at clearing S. aureus in the low oxygen partial pressure found deep within a biofilm (42). Th1-polarizing factors such as IL-12 and T-bet also serve to downregulate the anti-inflammatory Th2 humoral immune response by blocking IL-4 expression (37). This downregulation may be detrimental to the host during IMD infection, as previous studies have indicated that Th2 responses are effective at clearing biofilm infections during the early stages of biofilm development (43) as well as at clearing subcutaneous infections in BALB/c mice (33). In addition, there is limited evidence suggesting that Th17 responses contribute to chronic infection (4), whereas Treg responses may limit catheter-related infections (19), indicating a role for these T helper cell lineages in other biofilm-mediated infections, such as those associated with prosthetic implants.
Previous studies from our lab utilized a murine model of S. aureus biofilm-mediated prosthetic implant infection that closely mimics key characteristics of similar infections in humans. The resulting infection was chronic, localized, and recalcitrant to treatment with vancomycin, despite its effectiveness at killing planktonic bacteria of the same strain. These studies also demonstrated that S. aureus implant infections in C57BL/6 mice elicited mainly Th1 and Th17 cytokines and downregulated Treg cells during early stages of implant infection (35). Since this inflammatory response failed to clear the infection, augmented Th2 and Treg responses may play a protective role against the development of chronic S. aureus implant infection through their ability to suppress the proinflammatory effects of the Th1 (37) and Th17 (43) responses.
In the present study, we sought to elucidate the mechanisms of protection against S. aureus biofilm-mediated implant infection by using a mouse model of chronic S. aureus implant infection (35) in Th1- and Th2-biased mice (11). After implantation with a pin coated with adherent S. aureus, C57BL/6 mice could not clear the infection, while BALB/c mice did and possessed higher levels of IL-4 and IL-10 cytokines and Treg cell populations than C57BL/6 mice. These results suggested protective roles for the Th2 response and reduced inflammation in staphylococcal clearance. In order to modify the host immune response in C57BL/6 mice, we used a neutralizing monoclonal antibody (MAb) against the inflammatory cytokine IL-6 or IL-12 p40, and this treatment enabled this otherwise susceptible mouse strain to clear the infection. Conversely, reducing the anti-inflammatory Treg cell population with anti-CD25 MAb resulted in the infection-resistant BALB/c mice developing chronic S. aureus biofilm disease. Additionally, STAT6 knockout (KO) mice on a BALB/c background, which are deficient in Th2 responses, were not protected from chronic S. aureus biofilm infection. This study demonstrated not only that the inflammatory immune response is detrimental to the host in both the clearance and prevention of chronic biofilm infection by S. aureus but also that a functional Th2 response is necessary for resolution of the infection.
Understanding the mechanisms behind the development of chronic S. aureus implant infection in mice will further our understanding of how host responses to chronic S. aureus implant infections affect the development and clearance of in vivo biofilm infections. Also, this understanding will provide insight into how the host response may be manipulated by therapeutic agents to improve the chances of successfully preventing and treating these infections clinically.
MATERIALS AND METHODS
Mice.
Inbred C57BL/6 and BALB/c mice (6 to 8 weeks old) were purchased from Jackson Laboratories (Bar Harbor, ME). STAT6 KO mice on a BALB/c background were bred in-house. Mice were maintained under microisolator conditions in the animal facilities at the University of Maryland School of Medicine and the University of Maryland Dental School (Baltimore, MD), in accordance with protocols reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Maryland School of Medicine (Baltimore, MD).
Bacterial strain and prep of implants.
The strain of S. aureus used in these experiments, designated MRSA-M2, is a clinical isolate obtained from an osteomyelitis patient undergoing treatment at the University of Texas Medical Branch (Galveston, TX) and has been used in a number of biofilm infection models and molecular studies (6, 24, 35). This strain is an ST30, spa type T019, and agr III strain. Autoclaved 0.25-mm insect pins (Fine Science Tools, Foster City, CA) were incubated for 2 h in 10 ml of an overnight culture of S. aureus that was diluted 1:100 in sterile Trypticase soy broth, with sufficient time and continuous flow of bacterial culture medium to mimic physiological conditions that favor biofilm formation (10, 35). The inoculating dose of S. aureus was determined to be 3.0 × 105 CFU/pin (standard deviation [SD] = 5 × 104 CFU/pin).
Surgical implantation.
Six to 10 mice per experimental group received tibial implants. Mice were anesthetized via intraperitoneal (i.p.) injection of 100 mg ketamine/kg of body weight (Ketaset-Fort Dodge Laboratories, Inc., Fort Dodge, IA) and 10 mg/kg xylazine (Rugby Laboratories, Inc., Rockville Center, NY). The left leg of each mouse was cleansed with povidone iodine and rinsed with 70% ethanol before surgical implantation of an S. aureus-coated pin according to previously described methods (26, 35). Mice did not undergo any additional treatments after surgery until sacrifice, except for mice receiving immunomodulatory antibodies (described below). All animal experiments were performed in accordance with protocols reviewed and approved by the IACUC at the University of Maryland School of Medicine (Baltimore, MD).
Bone cultures.
At 7, 14, 21, 28, and 49 days postimplantation, infected and uninfected BALB/c and C57BL/6 mice were euthanized. Left tibiae were removed, cut into small pieces, and placed in 300 μl of sterile 0.85% saline per 100 μg of bone. As previously described, bones were homogenized using a Polytron PT 1200 handheld homogenizer (Kinematica, Bohemia, NY) at 25,000 rpm, and serial 10-fold dilutions of bone homogenates were plated on sheep's blood agar plates to enumerate the number of viable S. aureus cells per g bone (16, 35). At 21 days postimplantation, infected BALB/c mice and STAT6 KO mice were euthanized, and left tibiae were processed and plated as described above.
PNA-FISH.
At day 21 postimplantation, pins were carefully removed from the tibiae of infected and uninfected mice to prevent perturbation of biofilm mass. Pins were placed in Eppendorf tubes and fixed in 2% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) before peptide nucleic acid-fluorescence in situ hybridization (PNA-FISH) with a fluorescein isothiocyanate (FITC)-labeled universal bacterial probe and a rhodamine-labeled universal eukaryotic cell probe, specific for bacterial and eukaryotic rRNAs, respectively, per the manufacturer's instructions (AdvanDx, Woburn, MA). Each pin was then examined with a Zeiss LSM 510 confocal laser scanning microscope (Carl Zeiss, Thornwood, NY) for both green and red fluorescence, using a FITC/Texas Red dual-band filter and a 63× objective.
Cytokine production assay.
To compare levels of various Th1, Th2, and Th17 cytokines at the implant site, implanted tibiae were harvested from C57BL/6 and BALB/c mice at day 7 postimplantation and stored at −70°C. Samples were homogenized on ice in sterile PBS containing an EDTA-free protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN). Tissue homogenates were centrifuged for 15 min at 14,000 × g at 4°C, and supernatants were analyzed by Lisa Hester at the Cytokine Core Laboratory at the University of Maryland School of Medicine (Baltimore, MD), using quantitative sandwich enzyme-linked immunosorbent assay (ELISA). Cytokines tested included murine IL-1β, IL-2, IL-4, IL-6, IL-10, IFN-γ, and TNF-α.
Treg cell frequency.
To compare Treg cell frequencies between C57BL/6 and BALB/c mice, 1 × 106 draining lymph node (LN) cells were aliquoted into fluorescence-activated cell sorter (FACS) tubes (Becton Dickinson, Bedford, MA), and a Treg FACS staining kit (eBioscience, San Diego, CA) was used to determine the frequency of Treg cells in draining LN cells. Cells were surface stained with FITC-labeled anti-mouse CD4 and phycoerythrin (PE)-labeled anti-mouse CD25 MAbs and stained intracellularly with PE-Cy5-labeled anti-mouse Foxp3 MAb in accordance with the manufacturer's protocol. Results are expressed as percentages of Foxp3+ CD25+ CD4+ T cells in C57BL/6 versus BALB/c mice. Cells were analyzed using an LSR II flow cytometer (BD Biosciences, San Jose, CA).
In vivo Treg cell neutralization.
To determine if Treg cells play a role in bacterial clearance, BALB/c mice were injected i.p. with 1.0 mg of the anti-CD25 MAb PC61.5.3 (BioXCell, West Lebanon, NH) to neutralize natural Treg cells in vivo. Control mice received i.p. injections of rat IgG1. MAbs were injected 2 h before surgical implantation of an S. aureus-coated pin into the left tibia, as previous described. Mice received subsequent i.p. injections of 1.0 mg MAb at days 7 and 14 postimplantation to maintain reduced Treg cell frequencies for the duration of the experiment. Mice were euthanized at day 21 postimplantation, tibiae were harvested and processed, and serial dilutions of bone homogenates were plated on blood agar plates to enumerate CFU/g bone as previously described.
Histology and immunohistochemistry.
Tibiae from infected and uninfected control C57BL/6 and infected BALB/c mice were evaluated by histology and immunohistochemistry in order to observe differences in histology and in infiltrating neutrophils. Tibiae were removed at 7 days postimplantation from each mouse strain and were fixed in formalin. Tibiae were then decalcified, and paraffin-embedded sections were stained with hematoxylin and eosin (H&E). Additional paraffin-embedded sections were immunostained using the anti-Ly-6g MAb Gr-1 (eBioscience, San Diego, CA) to detect neutrophil infiltration into the implant site. A peroxidase-conjugated secondary Ab and colorimetric substrate were used to visualize labeled cells. All tissue sections were counterstained with hematoxylin. Each section was examined using an Omega 4000 light microscope (Alpha and Omega Microscopes, Gaithersburg, MD) with a 10× objective. All tissue processing and staining were performed by Elizabeth Smith at the University of Maryland, Baltimore, Center for Vascular and Inflammatory Disease Histology Core.
Immunomodulation.
To determine if neutralization of Th17 and/or Th1 responses increased rates of bacterial clearance, C57BL/6 mice were injected i.p. with 1.0 mg of the anti-IL-6 MAb MP5-20F3 or the anti-IL-12 p40 MAb C17.8 (BioXCell, West Lebanon, NH) to neutralize the effects of these cytokines on the production of Th17 or Th1/Th17 responses in vivo, respectively. Control mice received i.p. injections of either rat IgG1 or rat IgG2a isotype control Ab or no additional treatment. MAbs were injected 2 h before surgical implantation of an S. aureus-coated pin into the left tibia, as previous described. Mice received subsequent i.p. injections of 1.0 mg MAb at days 7 and 14 postimplantation to maintain the neutralization of IL-6 and IL-12 for the duration of the experiment. Mice were euthanized at day 21 postimplantation, tibiae were harvested and processed, and serial dilutions of bone homogenates were plated on blood agar plates to enumerate CFU/g bone as previously described.
Statistical analysis.
Means and SD were calculated and analyzed using Student's t test, with P values of <0.05 denoting statistical significance. Experiments determining the percentages of mice still infected at various time points postimplantation were analyzed using Fisher's exact test, with P values of <0.05 denoting statistical significance.
RESULTS
Chronic S. aureus implant infection is less prevalent in BALB/c mice than in C57BL/6 mice.
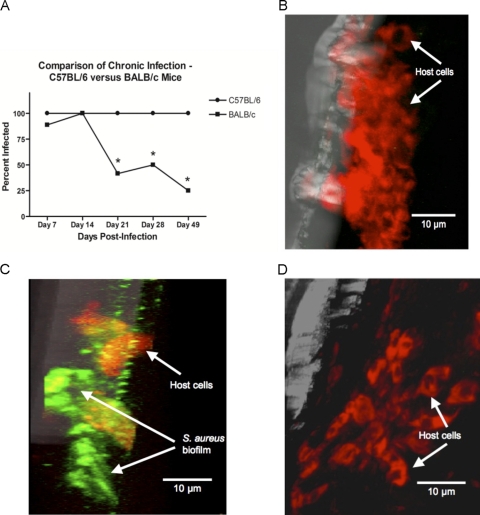
Tibiae from Th2-biased BALB/c and Th1-biased C57BL/6 mice receiving implants of S. aureus-coated pins were harvested and processed at days 7, 14, 21, 28, and 49 postinfection. Viable S. aureus was cultured from homogenized bone tissue as previously described. One hundred percent of C57BL/6 mice were still infected at all postinfection time points tested. Initially, at days 7 and 14 postimplantation, there was no significant difference in the percentages of infected BALB/c and C57BL/6 mice (Fig. 1A). However, the percentage of BALB/c mice infected at day 21 postinfection dropped significantly, to 41.67%. By day 49, there was an even more dramatic decrease in infection levels, with viable S. aureus cultured from only 25% of BALB/c tibiae (Fig. 1A). Biofilm formation was not evident on implanted pins removed from uninfected C57BL/6 mice receiving sterile control pins (Fig. 1B), as determined by confocal laser scanning microscopy. Interestingly, biofilm formation was also not evident on implanted pins removed from any of the infected BALB/c mice examined (Fig. 1D). This was in sharp contrast to implanted pins removed from infected C57BL/6 mice, which clearly demonstrated S. aureus biofilm formation on the pin surface (Fig. 1C).
Fig. 1.
Comparison of chronic, biofilm-mediated infection between C57BL/6 and BALB/c mice. (A) Percentages of C57BL/6 versus BALB/c mice still infected 7, 14, 21, 28, and 49 days after receiving an S. aureus-coated pin. The host immune response was unable to clear the infection in any C57BL/6 mice, as 100% of the mice were still infected at all time points. In contrast, 41.67%, 50.00%, and 25.00% of BALB/c mice were still infected at days 21, 28, and 49, respectively. Confocal laser scanning microscopic images are shown for pins removed at day 21 postimplantation from C57BL/6 mice receiving either sterile pins (B) or S. aureus-coated pins (C) or from BALB/c mice receiving S. aureus-coated pins (D). The pins were labeled using a FITC-labeled universal bacterial probe and a rhodamine-labeled universal eukaryotic cell probe, specific for bacterial and eukaryotic rRNAs, respectively. In contrast to the case for C57BL/6 mice, biofilm formation was not evident on the pins removed from infected BALB/c mice. Experiments were performed in triplicate (n = 5 to 8 mice per group). *, P < 0.05 for BALB/c compared to C57BL/6 mice (by Fisher's exact test).
S. aureus implant infection results in increased Th2 cytokines in BALB/c but not C57BL/6 mice.
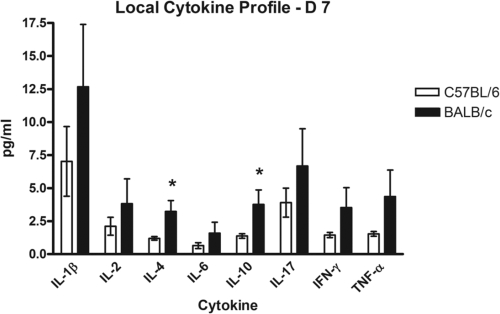
Cytokine levels were compared between infected BALB/c and C57BL/6 mice in order to determine the character of the immune response that may be responsible for the differential susceptibility to S. aureus biofilm infection observed. At day 7 postimplantation, tibiae were removed from infected BALB/c and C57BL/6 mice and then homogenized, and supernatants of bone homogenates were analyzed for cytokine production by quantitative sandwich ELISA. The results indicate that both mouse strains produced at least some level of all of the Th1, Th2, and Th17 cytokines tested, including IL-1β, IL-2, IL-4, IL-6, IL-10, IFN-γ, and TNF-α. However, the levels of the major Th2 cytokines, IL-4 and IL-10, were significantly higher in infected BALB/c mice than in C57BL/6 mice (Fig. 2), indicating a more robust Th2 response in the BALB/c mouse strain. These data support the hypothesis that a host Th2 response plays a protective role against the development of chronic S. aureus implant infection.
Fig. 2.
Local cytokine profiles at implant sites. Tibiae were removed from C57BL/6 and BALB/c mice receiving S. aureus-coated pins. Supernatants from bone homogenates were analyzed for cytokine production at day 7 postimplantation, as described in Materials and Methods. Significant upregulation of IL-4 and IL-10 in BALB/c mice indicates a predominantly Th2-type response in this mouse strain, suggesting a role in protection from chronic implant infection. Experiments were performed in triplicate (n = 5 to 8 mice per group). *, P < 0.05 compared to controls (by Student's t test). Bars represents standard errors of the means (SEM).
S. aureus implant infection results in chronic infection in STAT6 KO BALB/c mice but not in wt BALB/c mice.
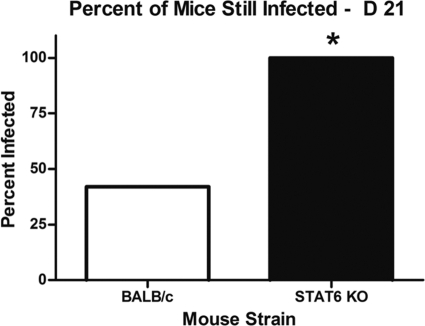
Previous studies from our lab demonstrated that BALB/c mice are less susceptible to the development of chronic S. aureus implant infection than C57BL/6 mice. In order to determine if this difference in disease outcome is due to the production of an effective Th2 response, STAT6 KO mice, which lack a key component of the signaling pathway required to mount a Th2 response, and wild-type (wt) BALB/c mice were implanted with S. aureus-coated pins. Tibiae from both wt and KO mice were harvested and processed on day 21 postimplantation, and viable S. aureus was cultured from homogenized bone tissue. The results indicate that STAT6 KO mice had lost the ability to clear an S. aureus implant infection before chronic disease developed, as viable S. aureus could be cultured from the tibiae of 100% of these mice at day 21 postimplantation, in contrast to only 42.1% of wt BALB/c mice (Fig. 3). These data further support our hypothesis that a Th2 response is protective against chronic S. aureus implant infection. Although 100% of the STAT6 KO mice were still infected at day 21, this mutant mouse strain had similar CFU counts in the tibiae at day 21 to those of wt BALB/c mice and 100-fold lower CFU counts than those of C57BL/6 mice, although these numbers were not statistically significant (data not shown). This may indicate a possible compensatory mechanism in STAT6 KO mice, such as higher Treg cell levels, or may suggest that the robust inflammatory Th1 and Th17 responses observed in C57BL/6 mice may further exacerbate the severity of infection.
Fig. 3.
Comparison of chronic, biofilm-mediated infection between BALB/c and STAT6 KO mice. Tibiae from BALB/c and STAT6 KO mice receiving S. aureus-coated implants were removed at day 21 postimplantation, and serial dilutions of bone homogenates were plated on blood agar plates. The host immune response was unable to effectively clear the infection in STAT6 KO mice, among which 100% were still infected at day 21 postimplantation. In contrast, only 42.1% of BALB/c mice were still infected at day 21. Experiments were performed in duplicate (n = 6 to 10 mice per group). *, P < 0.05 compared to controls (by Fisher's exact test).
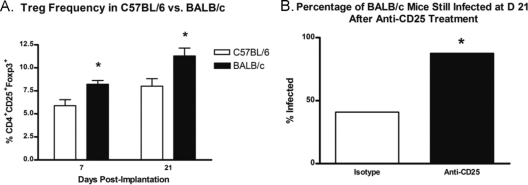
Treg cells protect BALB/c mice from developing chronic infection after implantation of an S. aureus-coated pin.
The role that Treg cells play in protecting BALB/c mice from the development of chronic implant infection was evaluated by determining the relative population level of this T cell subset in each mouse strain. Briefly, draining LN from infected C57BL/6 and BALB/c mice were harvested at 7 and 21 days postinfection, and single-cell suspensions of LN cells were prepared. LN cells were FACS stained for surface CD4 and CD25 and for intracellular Foxp3. The results indicate that the percentage of CD4+ CD25+ T cells expressing Foxp3 was significantly lower at both 7 and 21 days postimplantation in C57BL/6 mice than in BALB/c mice (Fig. 4A). To further evaluate the protective role of Treg cells in BALB/c mice, tibiae from infected BALB/c mice receiving i.p. injections of anti-CD25 MAb to neutralize Treg cells in vivo or receiving an isotype control were harvested and processed at day 21 postinfection. Viable S. aureus was cultured from homogenized bone tissue as previously described. There was a significant difference between BALB/c mice receiving anti-CD25 MAb and mice receiving the isotype control. When mice were injected with rat IgG1, approximately 41% of BALB/c mice were still infected at day 21 postimplantation, consistent with data from previous studies on untreated BALB/c mice. In contrast, the percentage of BALB/c mice still infected at day 21 postimplantation after treatment with anti-CD25 MAb increased to 87.5% (Fig. 4B).
Fig. 4.
Effect of Treg cells on development of chronic infection in C57BL/6 versus BALB/c mice. (A) Draining lymph nodes were removed from infected C57BL/6 and BALB/c mice at days 7 and 21 postimplantation. Single-cell suspensions were stained intracellularly for Foxp3 as described in Materials and Methods. The Treg cell frequency, expressed as the percentage of Foxp3 expression in CD4+ lymphocytes, was significantly lower for infected C57BL/6 mice than for BALB/c mice at both 7 and 21 days postimplantation. (B) BALB/c mice were treated by i.p. injection of the anti-CD25 MAb PC61.5.3 or an isotype control before implantation of an S. aureus-coated pin and at days 7 and 14 postimplantation, as described in Materials and Methods. Treatment with anti-CD25 reversed the protection against chronic implant infection normally observed in BALB/c mice. A single experiment was performed (n = 8 to 13 mice per group). *, P < 0.05 compared to controls (by Student's t test or Fisher's exact test). Bars represent SD.
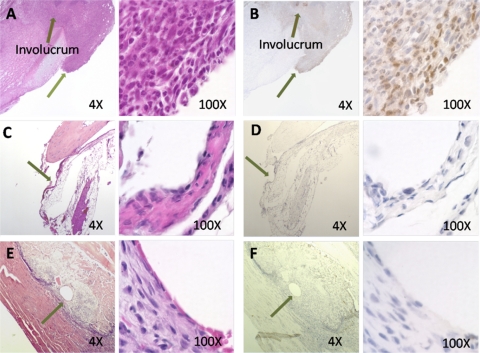
Implantation of an S. aureus-coated pin leads to increased neutrophil infiltration at the implant site in C57BL/6 but not BALB/c mice at day 7 postimplantation.
Because downregulation of Treg cells results in increased infection rates, it was hypothesized that Treg cells may promote a delay in neutrophil infiltration into areas of localized staphylococcal infection. In order to observe the neutrophil invasion early in infection in the two mouse strains, paraffin-embedded tibia sections from infected C57BL/6 mice and infected BALB/c mice were stained with H&E and compared to tibial sections from uninfected C57BL/6 mice. A separate set of sections were immunostained with an anti-Ly-6g MAb to visualize neutrophil infiltration, a key indicator of inflammation and an active Th17 response. The results demonstrate the infiltration of large numbers of neutrophils into the implant site in C57BL/6 mice, as indicated by the large number of polymorphonuclear leukocytes (PMNs) observed in H&E-stained images (Fig. 5A) and also visible in immunohistochemistry images as dark brown cells (Fig. 5B). A large area of involucrum formation is also clearly observable and is a result of bone remodelling resulting from chronic infection and inflammation at the implant site. In contrast, results from BALB/c mice indicate a lack of both neutrophil infiltration and involucrum formation at the implant site (Fig. 5C and D), which instead resembles the implant site in C57BL/6 mice receiving sterile implants (Fig. 5E and F). Naked eye observation of tibiae from infected BALB/c mice also suggested decreased neutrophil infiltration and inflammation, as indicated by a lack of involucrum formation and pus, in contrast to the case for tibiae from infected C57BL/6 mice (data not shown).
Fig. 5.
Local neutrophil infiltration at implant site. Tibiae were removed from C57BL/6 and BALB/c mice receiving S. aureus-coated pins at 7 days postimplantation. Paraffin-embedded sections were stained with hematoxylin and eosin or immunostained with anti-Ly-6g MAb and peroxidase-labeled secondary Ab and were examined using a light microscope. Areas of pin insertion are noted by arrows. Neutrophil infiltration (seen as a brown stain) and involucrum formation are evident at the implant site for C57BL/6 mice (A and B) but not BALB/c mice (C and D), for which the implant site appears similar to that in uninfected control mice (E and F). H&E staining of day 7 tibiae was performed in triplicate. Anti-Ly-6g immunostaining was performed in a single experiment.
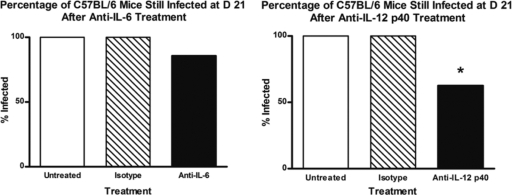
Treatment of C57BL/6 mice with anti-IL-12 p40 but not anti-IL-6 MAb results in decreased levels of chronic infection.
To determine the roles of proinflammatory Th1 and Th17 responses in exacerbation of chronic biofilm infection and the potential to control chronic infection by curbing these responses, C57BL/6 mice were treated presurgically and for the duration of the experiment with either anti-IL-6 or anti-IL-12 p40 MAb to neutralize Th17 or both Th1 and Th17 responses, respectively. Treatment with these cytokine-neutralizing MAbs in vivo also allowed the simultaneous evaluation of these products as potential immunomodulatory agents. Control mice were treated with either rat IgG1 or rat IgG2a as isotype controls. Viable S. aureus was cultured from homogenized bone tissue, as previously described, at day 21 postinfection. With anti-IL-6 treatment, 85.7% of mice were still infected by day 21, in contrast to either untreated mice or mice treated with an isotype control, where 100% of mice were still infected, although this difference was not significant (Fig. 6, left panel). In contrast, treatment of mice with anti-IL-12 p40 MAb resulted in a significant reduction in the percentage of C57BL/6 mice still infected at day 21 compared to untreated mice, from 100% for untreated mice to 62.5% for mice treated with anti-IL-12 MAb (Fig. 6, right panel). Untreated mice and mice treated with an isotype control in both experiments showed comparable levels (100%) of infection.
Fig. 6.
Effects of anti-IL-6 and anti-IL-12 p40 treatment on development of chronic infection in C57BL/6 mice. (Left) C57BL/6 mice were treated by i.p. injection of the anti-IL-6 MAb MP5-20F3 or an isotype control or were left untreated before implantation of an S. aureus-coated pin and at days 7 and 14 postimplantation, as described in Materials and Methods. Treatment with MP5-20F3 did not significantly decrease the percentage of C57BL/6 mice that developed chronic implant infection. (Right) A separate set of C57BL/6 mice were treated by i.p. injection of the anti-IL-12 MAb C17.8 or an isotype control or were left untreated before implantation of an S. aureus-coated pin and at days 7 and 14 postimplantation, as described in Materials and Methods. Treatment with C17.8 resulted in a significant decrease in the percentage of C57BL/6 mice that developed chronic implant infection compared to untreated mice. Mice from untreated and isotype-treated mice had the same rate of infection (100%) (n = 2 to 13 mice per group). *, P < 0.05 compared to controls (by Fisher's exact test).
DISCUSSION
Biofilm-mediated S. aureus infection is a costly and traumatic complication for patients receiving indwelling medical devices (2, 40). Growth in the biofilm state allows S. aureus to successfully evade the host immune response and to establish a chronic infection that is recalcitrant to clearance (12). In the present study, significantly smaller numbers of Th2-biased BALB/c mice were shown to progress from acute to chronic S. aureus infection, as indicated by the 75% infection clearance rate of these mice, whereas none of the Th1-biased C57BL/6 mice were able to accomplish microbial eradication. BALB/c mice were shown to produce a more robust Th2 cytokine response, a higher frequency of Treg cells, and less neutrophil infiltration following S. aureus implant infection than C57BL/6 mice. When we disrupted the Th2 pathway or reduced Treg cell numbers, BALB/c mice were no longer able to clear this chronic infection. In contrast, C57BL/6 mice showed improved bacterial clearance after treatment with anti-IL-12 p40 MAb, which neutralizes both Th1 and Th17 inflammatory responses in vivo.
Initially, at days 7 and 14 postimplantation, the percentage of infected BALB/c mice did not differ from the percentage of infected C57BL/6 mice (Fig. 1A). However, the percentage of infected BALB/c mice dropped significantly at days 21 and 49 postinfection (Fig. 1A). In addition, biofilm formation was not evident on implanted pins removed from infected BALB/c mice (Fig. 1D), as determined by confocal laser scanning microscopy. This was in contrast to implanted pins removed from infected C57BL/6 mice, which demonstrated S. aureus biofilm formation on the pin surface (Fig. 1C).
The cytokine profile at the implant site for infected BALB/c mice was compared to that for infected C57BL/6 mice to determine if cytokine production patterns were different between these two strains. At day 7 postimplantation, both C57BL/6 and BALB/c mice showed production of Th1-, Th2-, and Th17-associated cytokines, including IL-1β, IL-2, IL-4, IL-6, IL-10, IFN-γ, and TNF-α (Fig. 2). This was not entirely unexpected; BALB/c and C57BL/6 are both wild-type mouse strains capable of mounting all three types of T helper immune responses, and many types of infections elicit a combination of these responses. While most of these cytokines were produced at statistically similar levels, BALB/c mice produced statistically significantly higher levels of the Th2 cytokines IL-4 and IL-10 than those produced by C57BL/6 mice (Fig. 2), and these cytokines are important in the initiation and maintenance of a Th2 response. IL-4 is the main inducer of the Th2 response and simultaneously inhibits the initiation of a proinflammatory Th1 response (17). IL-10 inhibits both Th1 and Th17 responses through the inhibition of IFN-γ, IL-12, IL-23, and IL-6 (48). These data demonstrate that BALB/c mice that are effective at clearing chronic infections have a Th2-biased response. However, once this Th2 response is diminished, BALB/c mice are no longer able to clear the infection, as demonstrated by our studies utilizing a STAT6 KO mouse strain (17, 41) (Fig. 3).
While a functional Th2 response was required for clearance, a Treg response might also play a role. Treg cells are a key part of the adaptive immune response, regulating potentially detrimental inflammatory immune responses (46). Previous studies from our lab demonstrated that Treg cells are downregulated at day 7 during S. aureus implant infection in C57BL/6 mice. Therefore, this downregulation may enable the inflammatory response to progress unabated and contribute to the development of a chronic infection (35). To determine if Treg cells aid in protection against chronic S. aureus implant infection in BALB/c mice versus C57BL/6 mice, draining LN cells from infected C57BL/6 and BALB/c mice were analyzed for Foxp3 expression by FACS at 7 and 21 days postimplantation. At both 7 and 21 days postimplantation, draining LN from BALB/c mice contained a higher percentage of Foxp3-expressing CD4+ T cells than LN from C57BL/6 mice (Fig. 4A). In order to determine if these cells play a functional role in disease protection, BALB/c mice were treated with the anti-CD25 MAb PC61.5.3 or an isotype control to neutralize Treg cells in vivo before implantation of an S. aureus-coated pin and throughout the duration of the experiment. The results demonstrated that at day 21, BALB/c mice treated with anti-CD25 lost the ability to effectively clear an S. aureus implant infection before progression to chronic disease. Only 41% of infected BALB/c mice in the control group were still infected at day 21 postimplantation, compared to 87% of BALB/c mice after anti-CD25 treatment (Fig. 4B). These data provide evidence that Treg cells play a protective role against the development of chronic S. aureus implant infection. These data further suggest that promoting Treg cell downregulation during the early stages of implant infection may be a strategy employed by S. aureus to thwart the host immune response in order to form a biofilm. It is possible that Treg cells contribute to the prevention of chronic S. aureus biofilm-mediated infection in BALB/c mice by curbing Th1 and Th17 responses, both of which have been implicated in heightened inflammation and chronic infection. Proinflammatory cytokines and lysed neutrophils damage local host tissues and can lead to decreased vascular sufficiency and an increased amount of devitalized bone and tissue available for S. aureus attachment and biofilm development at the infection site (25).
In addition to the deleterious effects of Treg cell downregulation, Th17 activation may also have a role in promoting the mobilization of neutrophils and may play an important role in bridging innate and adaptive immunity (47). Although the Th17 response is important in preventing life-threatening bacterial infections, such as infections of the lung and bacterial sepsis, Th17 responses have limited effectiveness in long-term protection from bacterial infections (31). Neutrophils are limited in the ability to clear biofilm-embedded S. aureus, and tissue damage resulting from proinflammatory cytokines, reactive oxygen species, and lysosomal enzymes that are released upon neutrophil lysis and death can lead to localized vascular insufficiency and a predisposition toward biofilm development and chronic infection (25). Cytokine data from previous studies in our lab indicated the elevated production of Th17 cytokines in C57BL/7 mice receiving S. aureus-coated implants (35). To determine if neutrophil infiltration at the implant site is involved in chronic infection, we performed immunohistochemical analysis of paraffin-embedded tibial sections removed from infected and uninfected C57BL/6 and infected BALB/c mice at day 21 postimplantation. Immunostaining tibial sections with a MAb directed against the neutrophil marker Ly-6g demonstrated a large influx of neutrophils into the implant site in C57BL/6 mice, which also showed a large area of involucrum formation (Fig. 5A and B). This was in contrast to the implant site in BALB/c mice (Fig. 5C and D), which closely resembled that in uninfected control mice (Fig. 5E and F) in terms of low neutrophil numbers and a lack of involucrum formation. These results suggest that long-term neutrophil infiltration and activation are associated with biofilm formation and chronic disease.
Data from this study and previous studies in our lab have suggested that Th1 and Th17 responses are associated with chronic S. aureus implant infection and may play a detrimental role for the host (35). We sought to neutralize these responses in vivo not only to definitively demonstrate that these responses are mechanisms of disease progression and exacerbation but also to demonstrate that targeted neutralization of these responses can potentially be useful therapeutically in the prevention of chronic S. aureus implant infection. To do this, C57BL/6 mice were treated with the anti-IL-6 MAb MP5-20F3, the anti-IL-12 p40 MAb C17.8, or an isotype control Ab. Treatment with anti-IL-6 MAb resulted in a slight decrease in the percentage of C57BL/6 mice still infected at day 21 postimplantation, but neutralization of IL-6 alone did not result in significantly reduced infection rates (Fig. 6, left panel). IL-6 is required for differentiation of the Th17 lineage, so it is possible that even though the Th17 response may play a detrimental role in biofilm infection, targeting this response alone is not enough to curb the incidence of chronic infection. In contrast, C57BL/6 mice treated with an anti-IL-12 p40 MAb that reduces both Th1 and Th17 responses did show a significant reduction in the percentage of mice still infected at day 21 postinfection (Fig. 6, right panel). IL-12 p40 is shared by both IL-12 and IL-23. IL-12 is a polarizing cytokine that induces the Th1 response, and IL-23 is required for the maintenance of Th17 cells (31). Because neutralization of IL-12 p40 resulted in significantly lower infection rates, it is possible that there is a cumulative effect that results from the combination of Th1 and Th17 responses, both of which may be required to exacerbate chronic infection. Th1 and Th17 responses both lead to the release of a myriad of proinflammatory cytokines and to migration and activation of neutrophils (8, 25, 50). Therefore, by suppressing these inflammatory host responses, conditions that promote biofilm formation in the host are limited and S. aureus infection may be less able to develop into a chronic biofilm infection.
These data also indicate that neutralization of IL-12 p40 may be used prophylactically to prevent chronic implant infection in patients before surgical implantation of an indwelling medical device. The administration of intravenous anti-IL-12 p40 MAb has been shown in clinical trials to be generally well tolerated by patients (22), making anti-IL-12 p40 treatment a realistic option for patients in the future.
The deleterious effects of Th1 and Th17 responses in this particular model and their contributions to development of a chronic biofilm infection run in stark contrast to the case in a number of other studies. For example, cell-mediated and innate immune responses have been shown to play vital roles in conferring protection in several nonbiofilm infection models (1, 18, 21, 23, 27, 28, 32). Also, in other studies, neutrophils are the dominant cell type elicited during S. aureus infection and play a key role in protection against nonbiofilm infections (30, 38, 39). Even in an acute superficial biofilm infection model (49), attenuation of the inflammatory response contributes to persistence.
The differences between these studies can be explained by differences in types of infections (biofilm and chronic versus planktonic and acute infections) as well as in the immunological properties in the various compartments of the host where the infection occurs. For those infections within or just below the skin, as well as nonbiofilm septic infections, a robust innate and adaptive inflammatory response seems necessary for clearance. However, in other locations, such as the intramedullary bone infection model in mice (35) and cases of osteomyelitis (29), endocarditis (44), and endophthalmitis (14), a robust response walls the infection off to prevent systemic spread. However, it also forms a nidus of infection that promotes chronic and progressive tissue damage and occasional escape and seeding of other areas. Also, even in cases such as osteomyelitis without an indwelling medical device, early acute inflammation may also produce the devitalized tissue necessary for S. aureus to attach and form biofilms and a chronic infection (29).
Once S. aureus has colonized an implant and chronic infection has developed, the only treatment option available is removal of the infected implant, which is a costly and traumatic procedure. Understanding how host adaptive immune responses contribute to S. aureus biofilm-mediated implant infection may lead to the development of better strategies employed by clinicians to treat and prevent these types of infections. The results of this study not only contribute to a greater understanding of the role of host immunity in the development of biofilm infections but also suggest a new form of adjuvant therapies that may promote effective clearance of S. aureus biofilm infections, either alone or in combination with antimicrobial therapy and vaccination strategies.
ACKNOWLEDGMENTS
We thank Eileen M. Barry and John B. Sacci for their advice and assistance throughout this study, as well as Brian Peters for assistance with PNA-FISH and confocal microscopy, Daniel Powell and Laureanne Lorenzo for assistance with FACS data acquisition, and Steven Bowen for assistance with light microscopy of histological samples. We cordially thank AdvanDx for the donation of various PNA-FISH probes.
This study was funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant R01AI69568), and the National Institute of Dental and Craniofacial Research, National Institutes of Health (grant 1R01DE20939).
Footnotes
Published ahead of print on 26 September 2011.
REFERENCES
- 1. Alexander E. H., Hudson M. C. 2001. Factors influencing the internalization of Staphylococcus aureus and impacts on the course of infections in humans. Appl. Microbiol. Biotechnol. 56:361–366 [DOI] [PubMed] [Google Scholar]
- 2. Arciola C. R., Cervellati M., Pirini V., Gamberini S., Montanaro L. 2001. Staphylococci in orthopaedic surgical wounds. New Microbiol. 24:365–369 [PubMed] [Google Scholar]
- 3. Assenmacher M., et al. 1998. Sequential production of IL-2, IFN-gamma and IL-10 by individual staphylococcal enterotoxin B-activated T helper lymphocytes. Eur. J. Immunol. 28:1534–1543 [DOI] [PubMed] [Google Scholar]
- 4. Aujla S. J., Dubin P. J., Kolls J. K. 2007. Th17 cells and mucosal host defense. Semin. Immunol. 19:377–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barberan J., et al. 2006. Conservative treatment of staphylococcal prosthetic joint infections in elderly patients. Am. J. Med. 119:993.e7–993.e10 [DOI] [PubMed] [Google Scholar]
- 6. Brady R. A., et al. 2006. Identification of Staphylococcus aureus proteins recognized by the mediated immune response to a biofilm infection. Infect. Immun. 74:3415–3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Breuer K., et al. 2005. Alpha-toxin is produced by skin colonizing Staphylococcus aureus and induces a T helper type 1 response in atopic dermatitis. Clin. Exp. Allergy 35:1088–1095 [DOI] [PubMed] [Google Scholar]
- 8. Buzas K., et al. 2004. Different staphylococcal strains elicit different levels of production of T-helper 1-inducing cytokines. Acta Microbiol. Immunol. Hung. 51:371–384 [DOI] [PubMed] [Google Scholar]
- 9. Byren I., et al. 2009. One hundred and twelve infected arthroplasties treated with ‘DAIR’ (debridement, antibiotics and implant retention): antibiotic duration and outcome. J. Antimicrob. Chemother. 63:1264–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cassat J. E., Lee C. Y., Smeltzer M. S. 2007. Investigation of biofilm formation in clinical isolates of Staphylococcus aureus. Methods Mol. Biol. 391:127–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen L., et al. 2005. The involvement of neutrophils in the resistance to Leishmania major infection in susceptible but not in resistant mice. Parasitol. Int. 54:109–118 [DOI] [PubMed] [Google Scholar]
- 12. Costerton J. W., Stewart P. S., Greenberg E. P. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322 [DOI] [PubMed] [Google Scholar]
- 13. Dauwalder O., et al. 2006. Comparative inflammatory properties of staphylococcal superantigenic enterotoxins SEA and SEG: implications for septic shock. J. Leukoc. Biol. 80:753–758 [DOI] [PubMed] [Google Scholar]
- 14. De Kaspar H. M., et al. 2008. Effects of intravitreal corticosteroid in the treatment of Staphylococcus aureus-induced experimental endophthalmitis. Retina 28:326–332 [DOI] [PubMed] [Google Scholar]
- 15. Fedtke I., Gotz F., Peschel A. 2004. Bacterial evasion of innate host defenses—the Staphylococcus aureus lesson. Int. J. Med. Microbiol. 294:189–194 [DOI] [PubMed] [Google Scholar]
- 16. Fux C. A., Wilson S., Stoodley P. 2004. Detachment characteristics and oxacillin resistance of Staphylococcus aureus biofilm emboli in an in vitro catheter infection model. J. Bacteriol. 186:4486–4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Glimcher L. H., Murphy K. M. 2000. Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes Dev. 14:1693–1711 [PubMed] [Google Scholar]
- 18. Gomez M. I., Sordelli D. O., Buzzola F. R., Garcia V. E. 2002. Induction of cell-mediated immunity to Staphylococcus aureus in the mouse mammary gland by local immunization with a live attenuated mutant. Infect. Immun. 70:4254–4260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Halabi-Tawil M., et al. 2009. Cutaneous manifestations of immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome. Br. J. Dermatol. 160:645–651 [DOI] [PubMed] [Google Scholar]
- 20. Horan T. C., et al. 1993. Nosocomial infections in surgical patients in the United States, January 1986-June 1992. Infect. Control Hosp. Epidemiol. 14:73–80 [DOI] [PubMed] [Google Scholar]
- 21. Hu D. L., et al. 2003. Vaccination with nontoxic mutant toxic shock syndrome toxin 1 protects against Staphylococcus aureus infection. J. Infect. Dis. 188:743–752 [DOI] [PubMed] [Google Scholar]
- 22. Kauffman C. L., et al. 2004. A phase I study evaluating the safety, pharmacokinetics, and clinical response of a human IL-12 p40 antibody in subjects with plaque psoriasis. J. Invest. Dermatol. 123:1037–1044 [DOI] [PubMed] [Google Scholar]
- 23. Kinoshita M., et al. 2011. Enhancement of neutrophil function by interleukin-18 therapy protects burn-injured mice from methicillin-resistant Staphylococcus aureus. Infect. Immun. 79:2670–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koreen L., et al. 2004. spa typing method for discriminating among Staphylococcus aureus isolates: implications for use of a single marker to detect genetic micro- and macrovariation. J. Clin. Microbiol. 42:792–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leid J. G., Shirtliff M. E., Costerton J. W., Stoodley A. P. 2002. Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect. Immun. 70:6339–6345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li D., et al. 2008. Quantitative mouse model of implant-associated osteomyelitis and the kinetics of microbial growth, osteolysis, and humoral immunity. J. Orthop. Res. 26:96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin L., et al. 2009. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 5:e1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lowy F. D. 2000. Is Staphylococcus aureus an intracellular pathogen? Trends Microbiol. 8:341–343 [DOI] [PubMed] [Google Scholar]
- 29. Mader J. T., Shirtliff M. E. 1999. The rabbit model of bacterial osteomyelitis of the tibia, p. 581–591 In Zak O., Sande M. A.(ed.), Handbook of animal models of infection. Academic Press Ltd., London, England [Google Scholar]
- 30. Mayer-Scholl A., Averhoff P., Zychlinsky A. 2004. How do neutrophils and pathogens interact? Curr. Opin. Microbiol. 7:62–66 [DOI] [PubMed] [Google Scholar]
- 31. McKenzie B. S., Kastelein R. A., Cua D. J. 2006. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 27:17–23 [DOI] [PubMed] [Google Scholar]
- 32. Narita K., et al. 2010. Role of interleukin-17A in cell-mediated protection against Staphylococcus aureus infection in mice immunized with the fibrinogen-binding domain of clumping factor A. Infect. Immun. 78:4234–4242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nippe N., et al. 2011. Subcutaneous infection with S. aureus in mice reveals association of resistance with influx of neutrophils and Th2 response. J. Invest. Dermatol. 131:125–132 [DOI] [PubMed] [Google Scholar]
- 34. Nishimura S., Tsurumoto T., Yonekura A., Adachi K., Shindo H. 2006. Antimicrobial susceptibility of Staphylococcus aureus and Staphylococcus epidermidis biofilms isolated from infected total hip arthroplasty cases. J. Orthop. Sci. 11:46–50 [DOI] [PubMed] [Google Scholar]
- 35. Prabhakara R., Harro J. M., Leid J. G., Harris M., Shirtliff M. E. 2011. Murine immune response to a chronic Staphylococcus aureus biofilm infection. Infect. Immun. 79:1789–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raad I., Darouiche R., Hachem R., Sacilowski M., Bodey G. P. 1995. Antibiotics and prevention of microbial colonization of catheters. Antimicrob. Agents Chemother. 39:2397–2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rao P. S., Pallela V. R., Vassileva-Belnikolovska D., Jungkind D., Thakur M. L. 2000. A receptor-specific peptide for imaging infection and inflammation. Nucl. Med. Commun. 21:1063–1070 [DOI] [PubMed] [Google Scholar]
- 38. Roos D., van Bruggen R., Meischl C. 2003. Oxidative killing of microbes by neutrophils. Microbes Infect. 5:1307–1315 [DOI] [PubMed] [Google Scholar]
- 39. Roos D., Winterbourn C. C. 2002. Immunology. Lethal weapons. Science 296:669–671 [DOI] [PubMed] [Google Scholar]
- 40. Sanderson P. J. 1991. Infection in orthopaedic implants. J. Hosp. Infect. 18(Suppl. A):367–375 [DOI] [PubMed] [Google Scholar]
- 41. Shimoda K., et al. 1996. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature 380:630–633 [DOI] [PubMed] [Google Scholar]
- 42. Shirtliff M. E., Mader J. T., Camper A. K. 2002. Molecular interactions in biofilms. Chem. Biol. 9:859–871 [DOI] [PubMed] [Google Scholar]
- 43. Shkreta L., Talbot B. G., Diarra M. S., Lacasse P. 2004. Immune responses to a DNA/protein vaccination strategy against Staphylococcus aureus induced mastitis in dairy cows. Vaccine 23:114–126 [DOI] [PubMed] [Google Scholar]
- 44. Siaperas P., et al. 2001. Evidence of less severe aortic valve destruction after treatment of experimental staphylococcal endocarditis with vancomycin and dexamethasone. Antimicrob. Agents Chemother. 45:3531–3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sinha P., Ghosh A. K., Das T., Sa G., Ray P. K. 1999. Protein A of Staphylococcus aureus evokes Th1 type response in mice. Immunol. Lett. 67:157–165 [DOI] [PubMed] [Google Scholar]
- 46. Sojka D. K., Huang Y. H., Fowell D. J. 2008. Mechanisms of regulatory T-cell suppression—a diverse arsenal for a moving target. Immunology 124:13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stockinger B., Veldhoen M., Martin B. 2007. Th17 T cells: linking innate and adaptive immunity. Semin. Immunol. 19:353–361 [DOI] [PubMed] [Google Scholar]
- 48. Stumhofer J. S., Silver J., Hunter C. A. 2007. Negative regulation of Th17 responses. Semin. Immunol. 19:394–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thurlow L. R., et al. 2011. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J. Immunol. 186:6585–6596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Veeh R. H., et al. 2003. Detection of Staphylococcus aureus biofilm on tampons and menses components. J. Infect. Dis. 188:519–530 [DOI] [PubMed] [Google Scholar]