Abstract
Blastocystis, one of the most common parasites colonizing the human intestine, is an extracellular, noninvasive, luminal protozoan with controversial pathogenesis. Blastocystis infections can be asymptomatic or cause intestinal symptoms of vomiting, diarrhea, and abdominal pain. Although chronic infections are frequently reported, Blastocystis infections have also been reported to be self-limiting in immunocompetent patients. Characterizing the host innate response to Blastocystis would lead to a better understanding of the parasite's pathogenesis. Intestinal epithelial cells produce nitric oxide (NO), primarily on the apical side, in order to target luminal pathogens. In this study, we show that NO production by intestinal cells may be a host defense mechanism against Blastocystis. Two clinically relevant isolates of Blastocystis, ST-7 (B) and ST-4 (WR-1), were found to be susceptible to a range of NO donors. ST-7 (B), a metronidazole-resistant isolate, was found to be more sensitive to nitrosative stress. Using the Caco-2 model of human intestinal epithelium, Blastocystis ST-7 (B) but not ST-4 (WR-1) exhibited dose-dependent inhibition of Caco-2 NO production, and this was associated with downregulation of inducible nitric oxide synthase (iNOS). Despite its higher susceptibility to NO, Blastocystis ST-7 (B) may have evolved unique strategies to evade this potential host defense by depressing host NO production. This is the first study to highlight a strain-to-strain variation in the ability of Blastocystis to evade the host antiparasitic NO response.
INTRODUCTION
Blastocystis is an extracellular and noninvasive unicellular enteric parasite with zoonotic potential (28, 41, 42). It is among the most common parasites found in the human intestine, with prevalence ranging between 10% of the population in developed countries and 50% in developing countries (42). It is a species complex belonging to the Stramenopile group. According to a recent classification, it is divided into 11 subtypes, 9 of which are known to infect humans. Other common hosts are chickens, rats, pigs, reptiles, and insects (41). Despite recent advances in our understanding of the parasite's classification and pathobiology, several questions concerning the parasite's biology remain unanswered. The parasite's pathogenicity is also controversial owing to asymptomatic carriage, coinfection with other pathogens, and nonresponsiveness to conventional chemotherapeutic agents.
Blastocystis infections or blastocystosis present with common intestinal symptoms, such as abdominal pain, vomiting, and bloating, as well as mucous and watery diarrhea (42). Dermatological disorders (15, 45) and irritable bowel syndrome (39) are also frequently associated with Blastocystis infections. It is transmitted through the feco-oral route, and metronidazole is the treatment of choice for Blastocystis infections. The parasite has a high prevalence in immunocompromised individuals (17, 29, 43). In immunocompetent individuals, Blastocystis infections are often asymptomatic (7, 39) or self-limiting (39), suggesting effective host defense mechanisms against the parasite. Chronic cases, in the absence of obvious immunodeficiencies, are common (39). Because virulence factors of Blastocystis are largely unknown, it is not clear what key interactions between the parasite and host determine the variability in infection chronicity and clinical symptoms.
Although reports of mucosal inflammatory changes in the human gut are inconclusive (46), Blastocystis infections do induce a host immune response (1, 14, 32). Recent studies have shown that the parasite causes NF-κB-mediated upregulation of the proinflammatory cytokine interleukin-8 (IL-8) (32) in human T-84 colonic epithelium. Upregulation of IL-8 and granulocyte-macrophage colony-stimulating factor (GM-CSF) has also been reported in parasite-infected HT-29 colonic cells (22). Rats infected with Blastocystis also exhibited goblet cell hyperplasia and significant upregulation of proinflammatory cytokines gamma interferon (IFN-γ) and IL-12 (14). Among the potential host antiparasitic defense mechanisms, antibodies against Blastocystis have been suggested to play an important role (36). In mice infected with Blastocystis, IgA was the predominant antibody isotype in intestinal secretions (36). In symptomatic human Blastocystis infections, an increase in secretory as well as humoral IgA response was also observed (23). Asymptomatic carriers showed no difference in secretory IgA response (23), suggesting that the parasite might have inhibited the host's anti-Blastocystis immune response to successfully colonize the human gut. Evidence of the parasite cysteine proteases cleaving human secretory IgAs (31) and the higher cysteine protease activity in Blastocystis isolates recovered from symptomatic patients (26) provide further evidence that certain strains of the parasite are capable of modulating host immune response to their advantage.
Nitric oxide (NO) plays multiple functions in the human body, such as maintenance of vascular tone (9), modulation of epithelial barrier function (16), and neurotransmission (38). Generation of nitrosative stress in response to microbes is also an important component of the human cellular defense arsenal (3, 6, 19). The antimicrobial activity of NO has been reported for a wide range of prokaryotic (24) and eukaryotic (20, 30, 33) organisms. A recent study suggested that NO also induces apoptosis-like cell death in Blastocystis (4). It has been suggested that parasites successful in colonizing the mucosa either possess NO-scavenging mechanisms, like Trichomonas (11, 37), or suppress host cell NO production, like Giardia (3) and Entamoeba (6). How Blastocystis thrives in the intestinal lumen despite the toxicity of NO is not known.
A report on anaerobic parasites Trichomonas and Giardia suggested that metronidazole resistance makes the organisms more vulnerable to oxidative stress (5, 34). Metronidazole-resistant (Mzr) Trichomonas strains were found to have inferior oxygen-scavenging capacity (34), even though no apparent changes were observed in ferredoxin or NADH oxidase activity in these strains. No such association between susceptibility to nitrosative stress and Mz resistance in anaerobes has been investigated thus far. Nitrosative stress prevents colonization of host tissues by parasites and bacteria susceptible to nitric oxide (3, 6, 19). A microorganism more susceptible to nitrosative stress will be at a disadvantage in such an environment (34). In a previous study, we identified Mzr and metronidazole-susceptible (Mzs) strains of Blastocystis. We posit that although Mz resistance helps Blastocystis survive antibiotic treatment, it might make Mzr strains more susceptible to nitrosative stress.
NO in intestinal epithelial cells is produced by inducible nitric oxide synthase (iNOS) that catalyzes the conversion of l-arginine to reactive oxygen species of nitrogen. l-Arginine consumption by microbial arginase is considered a survival mechanism of pathogens against host macrophage NO response (3). Arginases of extracellular pathogens such as Helicobacter pylori and Entamoeba were reported to limit macrophage NO production by consuming l-arginine (19) in their microenvironment. Inhibition of iNOS activity under both conditions occurs at a posttranscriptional level and does not involve downregulation of iNOS mRNA. On the other hand, infections of intracellular parasites such as Toxoplasma (35) and Leishmania (25) lead to downregulation of iNOS mRNA expression in microglial cells and macrophages, respectively. Noninvasive, lumen-dwelling, extracellular pathogens like Giardia and Blastocystis seldom come in contact with macrophages. Since these organisms are in close proximity to NO-producing enterocytes, to survive this hostile environment, it is reasonable to suggest that they suppress intestinal epithelial NO production. Giardia is the only organism known to inhibit intestinal epithelial iNOS activity. It does so in the same manner as extracellular pathogens like H. pylori and Entamoeaba do in macrophages, by competing with host cells for l-arginine (3). It would be interesting to investigate the mechanisms Blastocystis has evolved to counter the host antiparasitic NO response.
Clinical (39) as well as in vitro (26) and in vivo (12, 13) studies suggest strain-to-strain variations in Blastocystis pathogenicity as well as antibiotic susceptibility (27). Variations in pathogenicity of the parasite strains are often proposed as a possible explanation for the large number of asymptomatic Blastocystis infections (42). On the other hand, difference in susceptibilities of Blastocystis strains to antiparasitic agents is suspected as the reason for frequent reports of treatment failure in parasite infections (27). A strain-to-strain variation in the ability of Blastocystis to evade host immune response has not been investigated yet. A superior ability of a Blastocystis strain to cope with host defense mechanisms such as enterocyte-generated nitrosative stress might provide it with a survival advantage over other strains in colonizing the human gut.
In this study, we evaluated the susceptibility of Mzr ST-7 (B) and Mzs ST-4 (WR-1) isolates of Blastocystis to nitrosative stress. Furthermore, we studied the modulation of epithelial antiparasitic NO production by Blastocystis. Finally, a variation between the abilities of Blastocystis ST-7 (B) and ST-4 (WR-1) to inhibit host NO production was also investigated.
MATERIALS AND METHODS
Culture of Caco-2 colonic epithelial cell line.
All Blastocystis-host interaction experiments were performed using the Caco-2 human colonic cell line (ATCC HTB-37). Caco-2 stock cultures were maintained in T-75 flasks in a humidified incubator with 5% CO2 at 37°C. Cell cultures were grown in Dulbecco's modified Eagle's medium (DMEM) (HyClone) supplemented with 10% heat-inactivated fetal bovine serum (HyClone) and 1% each sodium pyruvate, nonessential amino acids in minimum essential medium (MEM), and antibiotic (Penstrep; Gibco). Culture health was evaluated using the trypan blue exclusion assay, and only cultures with >95% viability were used for the experiments. Cells were trypsinzed with 0.25% trypsin–EDTA. Cell cultures for PCR experiments were grown on standard cell culture 6-well plates (Corning). For Blastocystis-induced nitric oxide inhibition experiments, cells were grown on Millipore Transwell filters with polyethylene terephthalate (PET) membranes of 3-μm pore size and placed in 24-well tissue culture plates. Since epithelial NO production varies with maturity of the cells, it was decided to measure the health and maturation of Caco-2 epithelium, as described previously (3). The transepithelial resistance (TER) of the monolayers was assessed once every 3 days until they reached maturity on day 21 (TER, ∼1,000 Ω cm2) (3). In order to synchronize cells before experiments, all cultures were serum-starved overnight in antibiotic-free and serum-free DMEM. For cytokine stimulation experiments, a cytokine cocktail comprising a combination of 20 ng/ml human IL-1α, 20 ng/ml human tumor necrosis factor alpha (TNF-α), and 50 ng/ml human IFN-γ (Sigma) was added to cultures.
Parasite cultures.
Blastocystis ST-7 (B) and ST-4 (WR-1) cultures were maintained as described previously (26). ST-7 (B) was recovered from a patient admitted to Singapore General Hospital with intestinal symptoms (26, 27). ST-4 (WR-1) was isolated from a healthy rat during an animal survey (2, 26, 27). Both ST-7 and ST-4 represent zoonotic subtypes. They commonly infect humans and are often associated with intestinal symptoms (39). Other than humans, common hosts for ST-7 and ST-4 isolates are birds and rats, respectively (41). For the arginase assay, parasite lysates were prepared as described by the QuantiChrom arginase assay kit (DARG-200) manual (BioAssay Systems, Hayward, CA). In short, parasites were suspended in 10 mM Tris-HCl (pH 7.4) containing 1 μM pepstatin A, 1 μM leupeptin, and 0.4% (wt/vol) Triton X-100. Samples were centrifuged at 20,000 × g at 4°C for 10 min. Lysate supernatant was used for the arginase assay.
Parasite viability assay.
The optimized Blastocystis viability assay, as described previously (27), was used to measure NO toxicity. Fifty percent inhibitory concentrations (IC50s) of nitric oxide donors sodium nitroprusside (SNP), S-nitroso-N-acetyl-penicillamine (SNAP), S-nitrosoglutathione (GSNO), and sodium nitrite (NaNO2) were calculated for the Mzr and Mzs isolates ST-7 (B) and ST-4 (WR-1), respectively. Stock solutions of all NO donor compounds were prepared in dimethyl sulfoxide (DMSO), diluted in prereduced Blastocystis medium, and transferred to 96-well plates. A total of 0.5 × 106 cells/well were incubated for 24 h with dilutions of NO donor agents ranging between 0 and 100 μg/ml. The final DMSO concentration was kept constant at 0.5% in each well, while the total volume per well was kept constant at 200 μl. After 24 h of exposure, resazurin solution (Sigma) was added to each well at a final concentration of 10% (vol/vol). Three hours after incubation, fluorescence readings of resazurin were taken at 550-nm excitation and 570-nm emission wavelengths using a TECAN Infinite M200 reader.
Confocal microscopy (annexin-FITC-PI staining).
Confocal micrographs of the parasites were taken in order to confirm NO cytotoxicity against Blastocystis. ST-7 was treated for 24 h with a 10-μg/ml concentration of SNAP. After drug exposure, the parasites were washed and resuspended in annexin V binding buffer (BioVision). Fluorescein isothiocyanate (FITC)-labeled annexin V and propidium iodide (PI) (BioVision) were then added to the cell suspension. Confocal imaging of cell suspensions was done using an Olympus Fluoview FV1000 (Japan) equipped with a dual filter set for fluorescein isothiocyanate (FITC) and rhodamine. Images were captured using Olympus Fluoview version 1.6b.
Determination of nitrite/nitrate in culture supernatants.
For estimation of reactive NO species produced by Caco-2 after 24 h, culture supernatants were collected from apical compartments of the Transwell setup. To ensure that any changes in NO production by Caco-2 are not due to induction of host cell death by Blastocystis, before testing NO concentrations, the postincubation viability of all Caco-2 cultures was confirmed using the trypan blue assay, and only supernatants of inserts with >95% viable cells were used. 2,3-Diaminonaphthalene (DAN) reacts with NO2 to give a fluorescent product, naphthotriazole. To determine levels of the stable NO end products nitrite and nitrate, nitrate was first reduced to nitrite by incubating the samples for 60 min at room temperature with 0.05 U/ml nitrate reductase in the presence of β-NADH and flavin adenine dinucleotide (FAD). 2,3-DAN was then incubated with the sample for 15 min at room temperature. After the incubation, the fluorescent product of the reaction between DAN and nitrite, naphthotriazole, was measured by an enzyme-linked immunosorbent assay (ELISA) reader at excitation and emission wavelengths of 360 and 415 nm, respectively.
Real-time PCR.
Total RNA was extracted from Caco-2 cultures using the RNeasy minikit (Qiagen, Chatsworth, CA), following the manufacturer's instructions. Only cultures with >95% viability were used for RNA extraction to ensure that Blastocystis-induced changes in Caco-2 iNOS mRNA levels are not due to enterocyte cell death. iNOS gene expression was quantified by real-time reverse transcription-PCR (RT-PCR) using the SYBR green PCR master mix system (Qiagen, Chatsworth, CA) on a Stratagene MX3000P PCR thermocycler (Stratagene, La Jolla, CA). cDNA samples (2 μl for a total volume of 20 μl per reaction) analyzed for the gene of interest were normalized to the reference gene coding for β-actin. The level of expression of iNOS in each sample was then calculated by the threshold cycle (CT) method as 2−ΔΔCT. All experiments were repeated at least three times. Real-time RT-PCR was performed at 95°C for 15 min, followed by 40 cycles of 15 s of denaturation at 94°C, 30 s of annealing at 55°C, and 30 s of elongation at 72°C. The following iNOS primers were used: forward primer 5′-GGC CCC ACA CCC CAC CAG AC-3′ and reverse primer 5′-GCC AGG CCC GAT GAG GAT G-3′.
Arginase assay.
The QuantiChrom arginase assay kit/DARG-200 (BioAssay Systems) was used to measure parasite arginase activity, following the manufacturer's instructions. In short, 40 μl of parasite lysate (2, 4, 6, or 8 × 107 cells) was coincubated with 10 μl of 5× l-arginine substrate buffer in a standard clear-bottom 96-well plate (Corning) for 2 h at 37°C. Forty microliters of sample without substrate buffer (optical density of sample blank control [ODsample]), 50 μl H2O (optical density of standard background [ODbackground]), or 50 μl 1 mM urea standard (ODstandard) were added in separate wells as controls. To stop the reaction and to calculate the final urea concentrations, 200 μl of urea reagent was added to all wells and 10 μl of 5× l-arginine substrate buffer was used as a sample blank control. The plate was tapped gently and incubated for 60 min at room temperature. After incubation, optical density was measured at 430 nm (OD430). Arginase activity (units/liter) of the sample was calculated as1 arginase unit = ODsample − ODblank/ODstandard − ODwater × [urea standard] × 50 × 103/(40 × t), where (i) ODsample, ODblank, ODstandard, and ODwater are the optical density values of the sample, sample blank, standard, and water, respectively, (ii) [urea standard] = 1 mM, t is reaction time (120 min), and 50 and 40 are reaction volumes.
One unit of arginase converts 1 μmol of l-arginine to ornithine and urea per minute at pH 9.5 and 37°C.
Statistical analysis.
Student's t test was used to determine the statistical significance of the data illustrated in Fig. 2, 4, and 5b, while the significance of data represented in Fig. 3 and 5a as well as Table 1 was estimated using the analysis of variance (ANOVA) test.
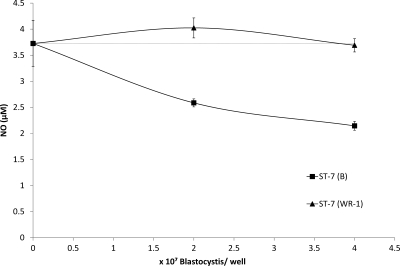
Fig. 2.
Graph representing NO production by Blastocystis-cocultured Caco-2 intestinal epithelium. Blastocystis ST-4 (WR-1) or ST-7 (B) cells were incubated with 21-day-old mature and polarized Caco-2 intestinal epithelial monolayers on a Transwell setup. Twenty-four hours post-coculture, samples of media from apical compartments were analyzed for nitrate and nitrite concentrations. Coculture with 2 × 107 and 4 × 107 cells of ST-7 (B) significantly reduced apical NO secretion by intestinal epithelium (P < 0.01). ST-4 (WR-1) did not alter the baseline NO concentration significantly. Viability of monolayers remained >95% during cocultures. Error bars represent the standard errors of 6 samples, taken from 2 independent experiments (each in triplicate).
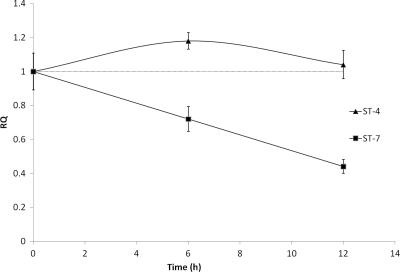
Fig. 4.
Graph representing downregulation of epithelial iNOS expression by Blastocystis ST-7 (B) coculture. Caco-2 iNOS mRNA levels were quantified by real-time PCR. As expected, a significant time-dependent reduction in iNOS mRNA levels was observed in Caco-2 epithelium after 6 and 12 h of coculture with ST-7 (B) (P < 0.01), while ST-4 (WR-1)-cocultured Caco-2 monolayers remained unaffected. These results are consistent with our earlier findings that Blastocystis ST-7 (B) inhibits antiparasitic NO production by intestinal epithelium. Error bars represent standard errors (n = 3). RQ, relative quantity of mRNA.
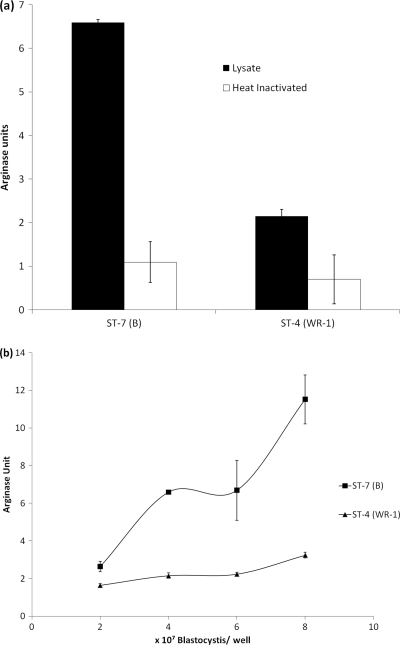
Fig. 5.
Arginase activity of Blastocystis ST-4 (WR-1) and ST-7 (B). (a) Lysate of 6 × 107 Blastocystis ST-7 (B) cells showed significantly higher arginase activity than ST-4 (WR-1) cells (P < 0.01). Five minutes of incubation of the parasite lysates at 95°C completely inhibited arginase activity. (b) Cell-density-dependent increase in arginine degradation by parasite lysate was observed in ST-7 (B), while arginase activity of ST-4 (WR-1) did not change much between lysates of 2 × 107 and 8 × 107 parasites. These findings suggest that higher arginine degradation by Blastocystis ST-7 (B) might be responsible for epithelial iNOS inhibition by Blastocystis ST-7 (B). Each point represents a mean of six samples collected from two independent experiments (each in triplicate). Error bars represent standard errors.
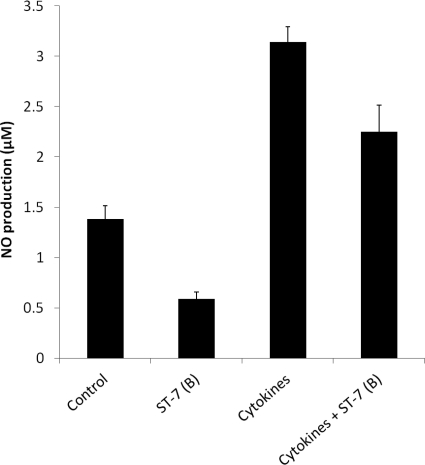
Fig. 3.
Graph representing NO production by Caco-2 intestinal epithelium. Twenty-four hours post-coculture, Blastocystis ST-7 (B) significantly inhibited antiparasitic epithelial NO production (P < 0.01). Stimulation of Caco-2 epithelium with a proinflammatory cytokine cocktail (IL-1α, IFN-γ, and TNF-α) from the basolateral side significantly increased NO production by Caco-2 epithelium. Blastocystis ST-7 (B) coculture reduced cytokine-stimulated NO upregulation by >50%. Error bars represent the standard errors of 6 samples, taken from two independent experiments (each in triplicate).
Table 1.
IC50s of anti-Blastocystis activity of NO donors
| NO donora | IC50 for: |
|||
|---|---|---|---|---|
| ST-7 (B) |
ST-4 (WR-1) |
|||
| μg/ml | μM | μg/ml | μM | |
| SNP | 2.07 ± 0.43 | 6.947474 | 2.03 ± 0.68 | 6.813224 |
| SNAP | 5.86 ± 1.83 | 26.61217 | 17.2 ± 7.37 | 78.11081 |
| GSNO | 36.66 ± 3.78 | 109.0098 | 83.33 ± 2.49 | 247.7847 |
| NaNO2 | 54 ± 16.16 | 782.6087 | >100b | |
SNP, sodium nitroprusside; SNAP, S-nitro-acetyl-penicillamine; GSNO, S-nitrosoglutathione.
One hundred micrograms per milliliter is the highest concentration of the compound tested in our study.
RESULTS
Blastocystis ST-4 (WR-1) and ST-7 (B) exhibit variation in susceptibility to nitrosative stress.
It was previously reported that nitrosative stress induces apoptosis-like features in Blastocystis (4). A recently optimized resazurin-based drug susceptibility assay for Blastocystis that measures redox activity of the cells was used to assess the cytotoxicity of NO donors against the parasite (27). We are reporting for the first time that Blastocystis ST-4 (WR-1) and ST-7 (B) are susceptible to nitrosative stress (Table 1). With the exception of NaNO2, 24 h of exposure to common NO donors killed both Blastocystis isolates at concentrations known to suppress growth of other parasites (8, 33) (Table 1). Susceptibility to SNAP, GSNO, and NaNO2 varied between the two isolates tested (Table 1). Mzr isolate ST-7 (B) was more susceptible to all of these NO donors than Mzs isolate ST-4 (WR-1) (Table 1). The IC50s of ST-7 (B) to SNAP, GSNO, and NaNO2 were 26.61, 109, and 782.6 μM, respectively (Table 1). The IC50s for SNAP and GSNO against ST-4 WR-1 were 78 and 247 μM, respectively, while NaNO2 had no effect on ST-4 (WR-1) even at the highest concentration tested (100 μg/ml) (Table 1). SNP had a similar effect against both isolates. The IC50s of SNP against ST-7 (B) and ST-4 (WR-1) were calculated to be 2.07 ± 0.43 and 2.03 ± 0.68 μg/ml, respectively (Table 1). Overall, these findings suggest that compared to ST-7 (B), ST-4 (WR-1) is better able to cope with nitrosative stress. The apparent lack of variation in ST-4 (WR-1) and ST-7 (B) susceptibility to and considerably lower IC50 values of SNP (Table 1) might be due to toxicity of cyanide derivatives produced by this compound (44).
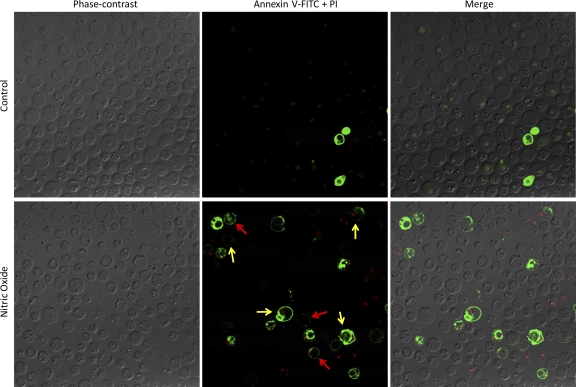
Blastocystis also exhibited typical features of cell death after 3 h of exposure to 10 μg/ml of SNAP (Fig. 1). Annexin V binds to exposed phosphatidylserine (PS) in apoptotic cells. The permeability of cells to PI indicates that necrosis had occurred. After exposure to nitrosative stress, some cells in the Blastocystis culture only stained with annexin-FITC (without PI inclusion), while others exhibited PI inclusion, suggesting that both apoptosis and necrosis had occurred (Fig. 1).
Fig. 1.
Confocal micrographs illustrating cell death features of Blastocystis under nitrosative stress. Blastocystis ST-7 (B) was cultured under normal parasite culture conditions, in the presence of 10 μg/ml of SNAP. Features of necrosis (yellow arrows) and programmed cell death (red arrows) were observed as early as 3 h of incubation with an NO donor. Necrotic cells incorporated both PI and annexin V-FITC stain. Blastocystis undergoing programmed cell death bind annexin V-FITC alone. Very few dying cells were observed in SNAP-free cultures of the parasite. The micrographs are representative of 6 pictures taken in two separate experiments (each in triplicate).
Coculture of intestinal epithelial cells by Blastocystis ST-7 (B) inhibited apical NO release.
Blastocystis is a noninvasive luminal parasite. Under physiological conditions, only the apical side of the polarized intestinal epithelium should come in contact with Blastocystis. Apical coculture of polarized epithelial monolayers with 2 or 4 × 107 parasites of ST-7 (B) resulted in significant inhibition of apical NO release by the enterocytes (P = 0.01) (Fig. 2). Interestingly, no change in NO release was observed in ST-4 (WR-1)-cocultured epithelium (Fig. 2) at the same doses. The ability of ST-7 (B) to suppress host antiparasiticidal NO response might provide it with a survival advantage over other strains in colonization of the hostile intestinal lumen.
Cytokine stimulation of the epithelium was provided from the basolateral side. The cytokines IL-1α, TNF-α, and IFN-γ used for these studies are physiologically produced by cells such as T cells, NK cells, and macrophages, found in the intestinal lamina propria underlying the epithelium. Apical coculture with ST-7 (B) significantly reduced cytokine-stimulated epithelial NO production (P < 0.05) (Fig. 3). This suggests that Blastocystis ST-7 (B) not only inhibits baseline NO production but also prevents NO production by other proinflammatory stimuli.
Blastocystis ST-7 (B) downregulates epithelial iNOS.
NO production in epithelial and other cells is controlled by iNOS, the critical rate-limiting enzyme for the conversion of l-arginine to NO. To identify the role of iNOS in the Blastocystis-induced inhibition of epithelial NO production, we quantified the expression of iNOS mRNA in ST-7 (B)- and ST-4 (WR-1)-cocultured Caco-2 cells (Fig. 4). ST-7 (B) coculture significantly inhibited epithelial iNOS expression at the 6- and 12-h postincubation time points (P = 0.01) (Fig. 4). ST-4 (WR-1) did not have any effect on epithelial iNOS expression.
ST-7 (B) and ST-4 (WR-1) isolates exhibit variation in arginase activity.
Arginase-deficient H. pylori strains do not inhibit iNOS in macrophages, making them more susceptible to host NO defense (10). In our experiments, l-arginine degradation by ST-7 (B) was found to be significantly higher than that of ST-4 (WR-1) (Fig. 5). In the previous section, we found that ST-7 and not ST-4 inhibits epithelial NO production. These findings suggest that inhibition of Caco-2 NO production by Blastocystis might be associated with its arginase activity, as seen in H. pylori and Entamoeba infections (6, 10). Although more experiments are needed, perhaps the higher arginase activity of ST-7 (B) might help the parasite evade the host's NO defense response. Potential mechanisms of NO response inhibition by Blastocystis ST-7 (B) are shown in Fig. 6.
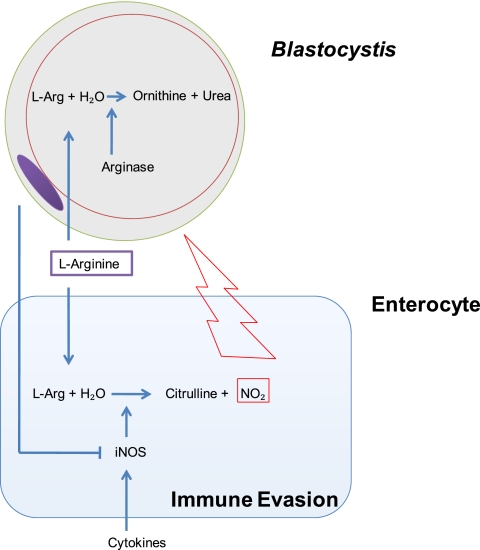
Fig. 6.
Illustration summarizing potential mechanisms of enterocyte NO response inhibition by Blastocystis ST-7 (B). The parasite evades host nitric oxide antiparasitic response by competing with l-arginine, a crucial substrate for epithelial iNOS, to produce nitric oxide by directly inhibiting iNOS.
DISCUSSION
Our findings suggest that Blastocystisis is susceptible to NO, and signs of both necrosis and apoptosis like cell death were observed in the parasite as early as 3 h after exposure to nitrosative stress. The cytotoxicity and therapeutic potential of NO against a range of microbial agents are well established (20, 30, 33). NO prevents growth as well as encystation of Giardia (3), while it induces apoptosis-like features in Entamoeba (33). One report suggests that Blastocystis, when exposed to NaNO2, undergoes apoptosis-like cell death (4). Our study provides a detailed cytotoxicity analysis of NO donors against two clinically significant Blastocystis subtypes. The Mzr clinical strain of Blastocystis ST-7 (B) and Mzs ST-4 (WR-1) (27) were found to be susceptible to most of the NO donors tested at concentrations reported for other parasites. Interestingly, compared to ST-4 (WR-1), Mzr strain ST-7 (B) was found to be 2 and 3 times more susceptible to GSNO and SNAP, respectively, and NaNO2 was effective against ST-7 (B) alone at the concentrations tested. These findings suggest a superior NO-scavenging ability of Mzs Blastocystis strain ST-4 (WR-1), which might help its survival in NO-rich intestinal lumen. Mzr ST-7 (B), on the other hand, was able to escape Mz chemotherapy due to defective oxygen-scavenging mechanisms, as seen in Mzr Trichomonas (21, 34), but this might have compromised its ability to cope with nitrosative stress.
Although the higher NO susceptibility of Blastocystis ST-7 (B) makes its survival difficult in the gut lumen, our present study suggests that this isolate has developed an interesting survival mechanism. In this study, Blastocystis ST-7 (B) inhibited epithelial NO production by downregulating epithelial iNOS expression. Several bacteria and parasites have evolved mechanisms to thrive in the gut lumen (3, 19). Parasites such as Entamoeba (6) and bacteria like H. pylori (19) significantly inhibit macrophage NO production. Since Blastocystis is noninvasive, it does not come in direct contact with macrophages. Similar to Giardia, Blastocystis also inhibited host epithelial NO production (3). Epithelial NO inhibition might help ST-7 (B) to colonize and induce host pathology and assist it to overcome the disadvantage of increased susceptibility to nitrosative stress. Molecular mechanisms linking NO susceptibility and inhibition of host NO production, as observed in ST-7 (B), are not known and would be interesting to investigate. Also, an association between metronidazole resistance and the ability to evade host immunity in a pathogen has not been observed. These findings are important to report because chronic Blastocystis infections that are nonresponsive to chemotherapy are frequently encountered clinically (40), and this study may explain Blastocystis persistence during infections.
H. pylori, Giardia, and Entamoeba inhibit the host's NO response by competing with the host cell for the crucial substrate l-arginine (3, 6, 19). Bacterial and parasitic arginases are known to modulate epithelial NO production by limiting l-arginine bioavailability (6, 10, 19). Arginase-deficient strains of H. pylori were not able to inhibit NO production by macrophages (10). Similarly, the Blastocystis ST-7 (B) isolate, with 3-times-higher arginase activity, inhibited NO production, while ST-4 (WR-1), with limited arginase activity, did not. A strain-to-strain variation in Blastocystis pathogenesis is frequently reported (12, 26, 39). These findings suggest that apart from cysteine protease activity (a potential virulence factor) (26) and Mz susceptibility (27) observed in previous studies, ST-7 (B) also differs from ST-4 (WR-1) in its ability to inhibit epithelial NO production, and this might be arginase dependent. Studies suggest that reduction of l-arginine bioavailability in the microenvironment does not inhibit iNOS mRNA levels (3, 19). In fact l-arginine bioavailability controls nitric oxide production without affecting iNOS mRNA levels in the cell (18).On the contrary, we observed a downregulation of iNOS mRNA expression in live enterocytes cocultured with Blastocystis ST-7 (B), as seen in Toxoplasma (35)- and Leishmania (25)-infected macrophages. Although iNOS-inhibiting Blastocystis strain ST-7 (B) possesses much higher arginase activity than ST-4 (WR-1), the inhibition of Caco-2 NO production by ST-7 (B) (without altering host cell viability) is primarily due to the downregulation of epithelial iNOS mRNA expression, as reported for Toxoplasma- and Leishmania-infected macrophages (25, 35). A secondary role of Blastocystis arginase in inhibiting epithelial NO production (as seen in cases of H. pylori and Giardia infections) cannot be ruled out. The characterization of Blastocystis arginases as well as further dissection of host epithelial pathways modulated by the parasite will give us a better idea of how Blastocystis escapes epithelial NO response.
An earlier study reported increased NO concentration in various tissues of mice infected with Blastocystis (4), as opposed to the iNOS inhibition observed in our study. Host specificity has been reported in Blastocystis (13), and since mice are not considered a natural host for the parasite (41), an increase in NO in mouse tissues is not surprising. NO is an indiscriminate, innate immune response, and introduction of a new organism to the gut flora may lead to NO upregulation in the host. Moreover, an increase in NO was observed in mouse cecum and ileum, but that study (4) did not report the NO concentration in the colon, the part of the gut colonized by the parasite. Our infection model is more physiologically relevant since the experiments were performed on the colonic epithelium of a natural Blastocystis host.
To conclude, this is the first study highlighting the ability of Blastcystis to modulate antiparasitic host NO defense. This is the first study highlighting a possible association of NO susceptibility with Mz resistance in an anaerobic organism. Blastocystis ST-7 (B), although more susceptible to nitrosative stress, exhibited significantly higher arginase activity than ST-4 (WR-1) and inhibited epithelial NO production. These findings make Blastocystis the only organism known to inhibit an intestinal epithelial NO response apart from Giardia. Blastocystis is the first known extracellular organism shown to downregulate host cell iNOS mRNA expression independent of host cell death. We posit that a lowered NO concentration not only helps ST-7 (B) to escape host defenses, it might also assist other pathogens to evade nitrosative stress and promotes colonization of the gut lumen. Further understanding of NO-suppressive and -coping mechanisms employed by Blastocystis would help in the development of host immune-modulating and chemotherapeutic strategies, respectively, against Blastocystis infections.
ACKNOWLEDGMENT
This work was supported by a generous grant from the National Medical Research Council (NMRC/1011/2006).
Footnotes
Published ahead of print on 19 September 2011.
REFERENCES
- 1. Chandramathi S., Suresh K. G., Mahmood A. A., Kuppusamy U. R. 2010. Urinary hyaluronidase activity in rats infected with Blastocystis hominis—evidence for invasion? Parasitol. Res. 106:1459–1463 [DOI] [PubMed] [Google Scholar]
- 2. Chen X. Q., et al. 1997. A survey of Blastocystis sp. in rodents. Lab. Anim. Sci. 47:91–94 [PubMed] [Google Scholar]
- 3. Eckmann L., et al. 2000. Nitric oxide production by human intestinal epithelial cells and competition for arginine as potential determinants of host defense against the lumen-dwelling pathogen Giardia lamblia. J. Immunol. 164:1478–1487 [DOI] [PubMed] [Google Scholar]
- 4. Eida O. M., Hussein E. M., Eida A. M., El-Moamly A. A., Salem A. M. 2008. Evaluation of the nitric oxide activity against Blastocystis hominis in vitro and in vivo. J. Egypt. Soc. Parasitol. 38:521–536 [PubMed] [Google Scholar]
- 5. Ellis J. E., Wingfield J. M., Cole D., Boreham P. F., Lloyd D. 1993. Oxygen affinities of metronidazole-resistant and -sensitive stocks of Giardia intestinalis. Int. J. Parasitol. 23:35–39 [DOI] [PubMed] [Google Scholar]
- 6. Elnekave K., Siman-Tov R., Ankri S. 2003. Consumption of L-arginine mediated by Entamoeba histolytica L-arginase (EhArg) inhibits amoebicidal activity and nitric oxide production by activated macrophages. Parasite Immunol. 25:597–608 [DOI] [PubMed] [Google Scholar]
- 7. Eroglu F., Genc A., Elgun G., Koltas I. S. 2009. Identification of Blastocystis hominis isolates from asymptomatic and symptomatic patients by PCR. Parasitol. Res. 105:1589–1592 [DOI] [PubMed] [Google Scholar]
- 8. Fernandes P. D., Assreuy J. 1997. Role of nitric oxide and superoxide in Giardia lamblia killing. Braz. J. Med. Biol. Res. 30:93–99 [DOI] [PubMed] [Google Scholar]
- 9. Gkaliagkousi E., Ferro A. 2011. Nitric oxide signalling in the regulation of cardiovascular and platelet function. Front. Biosci. 16:1873–1897 [DOI] [PubMed] [Google Scholar]
- 10. Gobert A. P., et al. 2001. Helicobacter pylori arginase inhibits nitric oxide production by eukaryotic cells: a strategy for bacterial survival. Proc. Natl. Acad. Sci. U. S. A. 98:13844–13849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harris K. M., Goldberg B., Biagini G. A., Lloyd D. 2006. Trichomonas vaginalis and Giardia intestinalis produce nitric oxide and display NO-synthase activity. J. Eukaryot. Microbiol. 53(Suppl. 1):S182–S183 [DOI] [PubMed] [Google Scholar]
- 12. Hussein E. M., Hussein A. M., Eida M. M., Atwa M. M. 2008. Pathophysiological variability of different genotypes of human Blastocystis hominis Egyptian isolates in experimentally infected rats. Parasitol. Res. 102:853–860 [DOI] [PubMed] [Google Scholar]
- 13. Iguchi A., et al. 2007. Infectivity of different genotypes of human Blastocystis hominis isolates in chickens and rats. Parasitol. Int. 56:107–112 [DOI] [PubMed] [Google Scholar]
- 14. Iguchi A., Yoshikawa H., Yamada M., Kimata I., Arizono N. 2009. Expression of interferon gamma and proinflammatory cytokines in the cecal mucosa of rats experimentally infected with Blastocystis sp. strain RN94-9. Parasitol. Res. 105:135–140 [DOI] [PubMed] [Google Scholar]
- 15. Katsarou-Katsari A., et al. 2008. Acute urticaria associated with amoeboid forms of Blastocystis sp. subtype 3. Acta Derm. Venereol. 88:80–81 [DOI] [PubMed] [Google Scholar]
- 16. Kuebler W. M., Yang Y., Samapati R., Uhlig S. 2010. Vascular barrier regulation by PAF, ceramide, caveolae, and NO—an intricate signaling network with discrepant effects in the pulmonary and systemic vasculature. Cell Physiol. Biochem. 26:29–40 [DOI] [PubMed] [Google Scholar]
- 17. Kurniawan A., et al. 2009. Intestinal parasitic infections in HIV/AIDS patients presenting with diarrhoea in Jakarta, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 103:892–898 [DOI] [PubMed] [Google Scholar]
- 18. Lee J., Ryu H., Ferrante R. J., Morris S. M., Ratan R. R. 2003. Translational control of inducible nitric oxide synthase expression by arginine can explain the arginine paradox. Proc. Natl. Acad. Sci. U. S. A. 100:4843–4848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lewis N. D., et al. 2010. Arginase II restricts host defense to Helicobacter pylori by attenuating inducible nitric oxide synthase translation in macrophages. J. Immunol. 184:2572–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lloyd D., et al. 2003. Nitrosative stress induced cytotoxicity in Giardia intestinalis. J. Appl. Microbiol. 95:576–583 [DOI] [PubMed] [Google Scholar]
- 21. Lloyd D., Pedersen J. Z. 1985. Metronidazole radical anion generation in vivo in Trichomonas vaginalis: oxygen quenching is enhanced in a drug-resistant strain. J. Gen. Microbiol. 131:87–92 [DOI] [PubMed] [Google Scholar]
- 22. Long H. Y., Handschack A., König W., Ambrosch A. 2001. Blastocystis hominis modulates immune responses and cytokine release in colonic epithelial cells. Parasitol. Res. 87:1029–1030 [DOI] [PubMed] [Google Scholar]
- 23. Mahmoud M. S., Saleh W. A. 2003. Secretory and humoral antibody responses to Blastocystis hominis in symptomatic and asymptomatic human infections. J. Egypt. Soc. Parasitol. 33:13–30 [PubMed] [Google Scholar]
- 24. Major T. A., et al. 2010. Sodium nitrite-mediated killing of the major cystic fibrosis pathogens Pseudomonas aeruginosa, Staphylococcus aureus, and Burkholderia cepacia under anaerobic planktonic and biofilm conditions. Antimicrob. Agents Chemother. 54:4671–4677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matte C., Descoteaux A. 2010. Leishmania donovani amastigotes impair gamma interferon-induced STAT1alpha nuclear translocation by blocking the interaction between STAT1alpha and importin-alpha5. Infect. Immun. 78:3736–3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mirza H., Tan K. S. 2009. Blastocystis exhibits inter- and intra-subtype variation in cysteine protease activity. Parasitol. Res. 104:355–361 [DOI] [PubMed] [Google Scholar]
- 27. Mirza H., Teo J. D., Upcroft J., Tan K. S. 2011. A rapid, high-throughput viability assay for Blastocystis spp. reveals metronidazole resistance and extensive subtype-dependent variations in drug susceptibilities. Antimicrob. Agents Chemother. 55:637–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Noël C., et al. 2005. Molecular phylogenies of Blastocystis isolates from different hosts: implications for genetic diversity, identification of species, and zoonosis. J. Clin. Microbiol. 43:348–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Noureldin M. S., Shaltout A. A., El Hamshary E. M., Ali M. E. 1999. Opportunistic intestinal protozoal infections in immunocompromised children. J. Egypt. Soc. Parasitol. 29:951–961 [PubMed] [Google Scholar]
- 30. Park G. C., Ryu J. S., Min D. Y. 1997. The role of nitric oxide as an effector of macrophage-mediated cytotoxicity against Trichomonas vaginalis. Korean J. Parasitol. 35:189–195 [DOI] [PubMed] [Google Scholar]
- 31. Puthia M., Vaithilingam A., Lu J., Tan K. S. 2005. Degradation of human secretory immunoglobulin A by Blastocystis. Parasitol. Res. 97:386–389 [DOI] [PubMed] [Google Scholar]
- 32. Puthia M. K., Lu J., Tan K. S. 2008. Blastocystis ratti contains cysteine proteases that mediate interleukin-8 response from human intestinal epithelial cells in an NF-kappaB-dependent manner. Eukaryot. Cell 7:435–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ramos E., et al. 2007. Entamoeba histolytica: apoptosis induced in vitro by nitric oxide species. Exp. Parasitol. 116:257–265 [DOI] [PubMed] [Google Scholar]
- 34. Rasoloson D., Tomková E., Cammack R., Kulda J., Tachezy J. 2001. Metronidazole-resistant strains of Trichomonas vaginalis display increased susceptibility to oxygen. Parasitology 123:45–56 [DOI] [PubMed] [Google Scholar]
- 35. Rozenfeld C., et al. 2005. Toxoplasma gondii prevents neuron degeneration by interferon-gamma-activated microglia in a mechanism involving inhibition of inducible nitric oxide synthase and transforming growth factor-beta1 production by infected microglia. Am. J. Pathol. 167:1021–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Santos H. J., Rivera W. L. 2009. Kinetic analysis of antibody responses to Blastocystis hominis in sera and intestinal secretions of orally infected mice. Parasitol. Res. 105:1303–1310 [DOI] [PubMed] [Google Scholar]
- 37. Sarti P., et al. 2004. Trichomonas vaginalis degrades nitric oxide and expresses a flavorubredoxin-like protein: a new pathogenic mechanism? Cell. Mol. Life Sci. 61:618–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Steinert J. R., Chernova T., Forsythe I. D. 2010. Nitric oxide signaling in brain function, dysfunction, and dementia. Neuroscientist 16:435–452 [DOI] [PubMed] [Google Scholar]
- 39. Stensvold C. R., et al. 2009. Blastocystis: unravelling potential risk factors and clinical significance of a common but neglected parasite. Epidemiol. Infect. 137:1655–1663 [DOI] [PubMed] [Google Scholar]
- 40. Stensvold C. R., Smith H. V., Nagel R., Olsen K. E., Traub R. J. 2010. Eradication of Blastocystis carriage with antimicrobials: reality or delusion? J. Clin. Gastroenterol. 44:85–90 [DOI] [PubMed] [Google Scholar]
- 41. Tan K. S. 2008. New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin. Microbiol. Rev. 21:639–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tan K. S., Mirza H., Teo J. D., Wu B., Macary P. A. 2010. Current views on the clinical relevance of Blastocystis spp. Curr. Infect. Dis. Rep. 12:28–35 [DOI] [PubMed] [Google Scholar]
- 43. Taşova Y., Sahin B., Koltaş S., Paydaş S. 2000. Clinical significance and frequency of Blastocystis hominis in Turkish patients with hematological malignancy. Acta Med. Okayama 54:133–136 [DOI] [PubMed] [Google Scholar]
- 44. Thomas C., Svehla L., Moffett B. S. 2009. Sodium-nitroprusside-induced cyanide toxicity in pediatric patients. Expert Opin. Drug Saf. 8:599–602 [DOI] [PubMed] [Google Scholar]
- 45. Valsecchi R., Leghissa P., Greco V. 2004. Cutaneous lesions in Blastocystis hominis infection. Acta Derm. Venereol. 84:322–323 [DOI] [PubMed] [Google Scholar]
- 46. Zuckerman M. J., Watts M. T., Ho H., Meriano F. V. 1994. Blastocystis hominis infection and intestinal injury. Am. J. Med. Sci. 308:96–101 [DOI] [PubMed] [Google Scholar]