Abstract
Lipopolysaccharide (LPS), composed of lipid A, core, and O-antigen, is a major virulence factor of Salmonella enterica serovar Typhimurium, with lipid A being a major stimulator to induce the proinflammatory response via the Toll-like receptor 4 (TLR4)-MD2-CD14 pathway. While Salmonella msbB mutants lacking the myristate chain in lipid A were investigated widely as an anticancer vaccine, inclusion of the msbB mutation in a Salmonella vaccine to deliver heterologous antigens has not yet been investigated. We introduced the msbB mutation alone or in combination with mutations in other lipid A acyl chain modification genes encoding PagL, PagP, and LpxR into wild-type S. enterica serovar Typhimurium. The msbB mutation reduced virulence, while the pagL, pagP, and lpxR mutations did not affect virulence in the msbB mutant background when administered orally to BALB/c mice. Also, all mutants exhibited sensitivity to polymyxin B but did not display sensitivity to deoxycholate. LPS derived from msbB mutants induced less inflammatory responses in human Mono Mac 6 and murine macrophage RAW264.7 cells in vitro. However, an msbB mutant did not decrease the induction of inflammatory responses in mice compared to the levels induced by the wild-type strain, whereas an msbB pagP mutant induced less inflammatory responses in vivo. The mutations were moved to an attenuated Salmonella vaccine strain to evaluate their effects on immunogenicity. Lipid A modification caused by the msbB mutation alone and in combination with pagL, pagP, and lpxR mutations led to higher IgA production in the vaginal tract but still retained the same IgG titer level in serum to PspA, a test antigen from Streptococcus pneumoniae, and to outer membrane proteins (OMPs) from Salmonella.
INTRODUCTION
Salmonella spp. can infect both humans and animals, resulting in two primary clinical manifestations: enteric (typhoid) fever and gastroenteritis. Key virulence factors include type III secretion systems (T3SS) that export Salmonella effector proteins into host cells and surface structures such as fimbriae and lipopolysaccharide (LPS) (1, 23, 64). LPS provides Salmonella cells with a protective barrier, shielding them from a number of host defenses, including bile salts, hydrophobic antibiotics, and complement. LPS consists of three covalently linked components: lipid A, core oligosaccharide, and O-antigen polysaccharide. Lipid A anchors LPS into the asymmetric outer membrane and is essential for outer membrane barrier function and cell viability. Lipid A is also the endotoxic component of LPS. Salmonella lipid A is a β-1′,6-linked disaccharide of glucosamine, with phosphates at the 1 and 4′ positions and acylated at the 2, 3, 2′, and 3′ positions with R-3-hydroxymyristic acid (3-OH C14:0) (Fig. 1 A). The structure is further acylated with secondary laurate (C12:0) and myristate (C14:0) chains in acyloxyacyl linkage at the 2′ and 3′ positions by HtrB (LpxL) and MsbB (WaaN, LpxM), respectively (45).
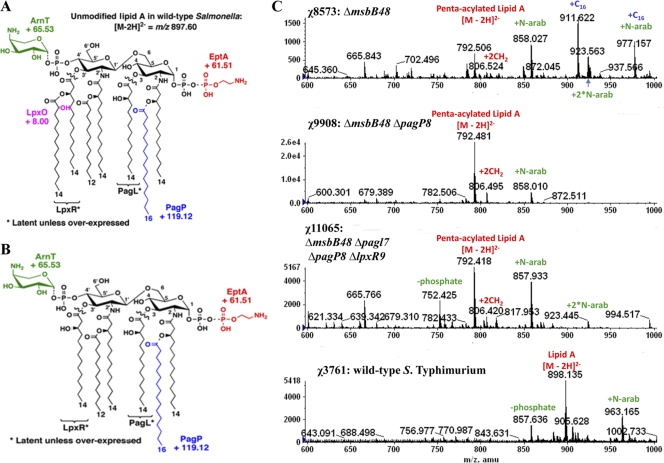
Fig. 1.
Lipid A structures and ESI-MS of the Salmonella enterica serovar Typhimurium wild-type and msbB mutants. (A) Covalent modifications of Salmonella lipid A. LpxR and PagL catalyze the removal of the 3′-acyloxyacyl and the 3-hydroxymyristoyl chains from lipid A, respectively, although these modifications are not seen under laboratory growth conditions. The lipid A species can be identified by ESI-MS in the negative ion mode, with unmodified lipid A as the [M-2H]2− peak at m/z 897.60. Various modifications shift the peak at the indicated m/z values. The addition of palmitate to position 2 of the R-3-hydroxymyristoyl chain catalyzed by PagP is indicated in blue. Other covalent lipid A modifications include the hydroxylation of the 3′ secondary myristoyl chain by LpxO, the addition of l-Ara4N to the 4′ phosphate by ArnT, and the addition of pEtN to the position 1 phosphate by EptA(PmrC). (B) Covalent modifications of lipid A in the msbB mutant. The known covalent modifications of lipid A are indicated. The msbB mutant makes a lipid A species that is fully penta-acylated lipid A, as shown by the [M-2H]2− peak at m/z 792.55. The addition of palmitate (C16) to position 2 of the R-3-hydroxymyristoyl chain catalyzed by PagP shifts the lipid A [M-2H]2− peak by m/z +119.115. The addition of l-Ara4N to the 4′ phosphate catalyzed by ArnT shifts the lipid A [M-2H]2− peak by m/z +65.529. The addition of pEtN to the 1-phosphate, catalyzed by EptA(PmrC), shifts the MS peak by m/z +61.505. (C) Lipid A profiles from ESI-MS analysis of χ8573 (ΔmsbB48), χ9908 (ΔmsbB48 ΔpagP8), χ11065 (ΔmsbB48 ΔpagL7 ΔpagP8 ΔlpxR9), and wild-type χ3761 grown in LB medium at 37°C. N-arab, l-Ara4N.
Lipid A containing two phosphate groups and six acyl chains that are 12 to 14 carbons in length is the most efficient in activating proinflammatory responses through the Toll-like receptor 4 (TLR4)-MD2-CD14 pathway, while lipid As with fewer acyl chains, such as tetra- or penta-acylated lipid A species, have significantly diminished immunostimulatory activity (5). Salmonella has evolved to modify lipid A further by the addition of small molecules, such as phosphoethanolamine (pEtN) moieties or 4-amino-4-deoxy-l-arabinose (l-Ara4N), or variations in the fatty acid chains to modulate host innate immunity and enhance its survival in different microenvironment niches (17, 18, 47, 59). Three enzymes, PagL, PagP, and LpxR, that direct lipid A acyl chain modifications have been described in Salmonella (18, 47, 59). PagL catalyzes the removal of a single R-3-hydroxymyristate chain at position 3 of lipid A (59), PagP catalyzes the addition of a phospholipid-derived palmitate chain to the hydroxyl of the R-3-hydroxymyristate chain at position 2 of lipid A (3, 18), and LpxR is a 3′-O-deacylase that removes the 3′-acyloxyacyl moiety from lipid A (47). The expression of pagL and pagP is regulated by the two-component regulatory system PhoP-PhoQ, while the regulation of lpxR remains to be elucidated (17, 47, 59). Lipid A from wild-type Salmonella growing inside RAW264.7 cells, a mouse-derived macrophage cell line, is heavily modified with l-Ara4N, pEtN, 2-hydroxymyristate, and palmitate (14). However, the structure and activity of lipid A in vivo during host infection is unknown.
Salmonella enterica serovar Typhimurium and other Gram-negative bacteria are capable of releasing LPS during in vitro and in vivo growth. LPS release is significantly enhanced during lysis of S. enterica serovar Typhimurium following exposure to antibiotics or human serum (9, 13). In Escherichia coli or S. enterica serovar Typhimurium infection, lipid A but not other bacterial components (i.e., peptidoglycan, lipopeptides, flagellin, and CpG DNA motifs) is responsible for Gram-negative sepsis and dysregulation of cytokine synthesis in mice (11, 49).
Compared to the wild-type Salmonella strain, an msbB mutant lacking a myristate chain exhibits severe growth defects in LB medium and sensitivity to bile salts (MacConkey medium) and to EGTA-containing medium, but compensatory suppressors such as somA mutation can restore resistance to bile (36); msbB mutants are also more sensitive to CO2, acidic pH, and high osmolarity than wild-type strains (26). The most interesting phenotype conferred by an msbB mutation is the simultaneous reduction in virulence and endotoxic activity, which serves to increase the safety of vaccine candidates for anticancer treatments and other purposes (27, 32, 33, 55). Overexpression of PagL, PagP, or LpxR in the msbB mutant leads to unique lipid A structures which are different from those produced by the wild-type Salmonella and the msbB mutant (Fig. 1B). The classical bisphosphorylated, hexa-acylated lipid A species from Escherichia coli are able to activate the proinflammatory response via the TLR4-MD2-CD14 pathway, while the tetra- or penta-acylated lipid A species significantly diminishes immunostimulatory activity as an antagonist in human cells (5). However, the penta-acylated lipid A species in msbB mutants still has the full ability to stimulate TLR4-MD2-CD14 from the mouse, while the tetra- or tri-acylated lipid As diminish its immunostimulatory activity (51). Whether the enzymes encoded by pagL, pagP, and lpxR are functioning in vivo to modify the lipid A is unknown. If PagL, PagP, and LpxR were functioning in vivo in the msbB mutant, it would produce tri-, tetra-, penta-, or hexa-acylated or mixed lipid A structures, which would display different abilities to activate the TLR4-MD2-CD14 pathway. Each lipid A species would be expected to induce innate immunity to various degrees. Therefore, deletion of pagL, pagP, and/or lpxR in the msbB mutant background will enable us to distinguish the effects of these genes on innate immunity.
In this work, we systematically analyzed the effect of an ΔmsbB deletion mutation alone or in combination with ΔpagL, ΔpagP, and ΔlpxR mutations on virulence and immunogenicity in mice. Our findings indicate that the lipid A structures affected innate and adaptive immunity and provide information useful for developing new attenuated Salmonella vaccines.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. S. enterica serovar Typhimurium cultures were routinely grown at 37°C in LB broth (2) or in N minimal medium (54), supplemented with 0.1% Casamino Acids, 38 mM glycerol, pH 5.8, 10 μM MgCl2 or pH 7.7, 10 mM MgCl2, or on LB agar. LB-0 medium consists of LB without NaCl and 6.5 mM EGTA (Sigma, St. Louis). A 350 mM stock of EGTA at pH 8.0 (adjusted with NaOH) was dissolved and then autoclaved. EGTA was added to the LB-0 medium after autoclaving. MacConkey agar base (Difco) was used to prepare galactose MacConkey agar containing 1% galactose. Diaminopimelic acid (DAP) was added (50 μg/ml) for the growth of Δasd strains (38). LB agar containing 5% sucrose was used for sacB gene-based counterselection in allelic exchange experiments. Streptococcus pneumoniae WU2 was cultured on brain heart infusion agar containing 5% sheep blood or in Todd-Hewitt broth plus 0.5% yeast extract. Morpholinepropanesulfonic acid (MOPS) minimal medium (40) with/without 10 μg/ml p-aminobenzoic acid was used to confirm the phenotype of ΔpabA ΔpabB mutants.
Table 1.
Strains and plasmids used in this work
| Strain or plasmid | Description | Source (reference) |
|---|---|---|
| Plasmids | ||
| pRE112 | sacB mobRP4 R6K ori Cmr | (10) |
| pYA4284 | pagL7 deletion | pRE112 |
| pYA4288 | pagP8 deletion | pRE112 |
| pYA4287 | lpxR9 deletion | pRE112 |
| pYA4876 | PagP promoter inserted into pGOA1193 | pGOA1193 (42) |
| pYA3493 | Plasmid Asd+; pBRori β-lactamase signal sequence-based periplasmic secretion plasmid | (25) |
| pYA4088 | 852-bp DNA encoding the α-helical region of PspA from amino acid 3 to 285 in pYA3493 | (62) |
| S. enterica serovar Typhimurium strains | ||
| χ3761 | Wild type, UK-1 | (19) |
| χ8573 | ΔmsbB48 | χ3761 (15) |
| χ11165 | ΔmsbB48ΔpagL7 | χ8573 |
| χ9908 | ΔmsbB48 ΔpagP8 | χ8573 |
| χ9949 | ΔmsbB48 ΔlpxR9 | χ8573 |
| χ11065 | ΔmsbB48 ΔpagL7 ΔpagP8 ΔlpxR9 | χ3761 |
| χ11164 | ΔmsbB48 ΔarnT6 | χ8573 |
| Vaccine strains | ||
| χ9241 | ΔpabA1516 ΔpabB232 ΔasdA16 ΔaraBAD23 ΔrelA198::araC PBADlacI TT | (62) |
| χ9278 | ΔpabA1516ΔpabB232 ΔasdA16 ΔaraBAD23 ΔrelA198::araC PBADlacI TT ΔmsbB48 | χ9241 |
| χ11318 | ΔpagL7 | χ9278 |
| χ9848 | ΔpagP8 | χ9278 |
| χ9850 | ΔlpxR9 | χ9278 |
| χ11088 | ΔpagL7 ΔpagP8 ΔlpxR9 | χ9278 |
| E. coli strains | ||
| χ7232 (DH5α λ pir, MGN-026e) | endA1 hsdR17 (rK− mK+) glnV44 thi-1 recA1 gyrA relA1Δ(lacZYA-argF)U169λpirdeoR [φ80dlac Δ(lacZ)M15] | (50) |
| χ7213 (MGN-617) | thi-1 thr-1 leuB6 glnV44 fhuA21 lacY1 recA1 RP4-2-Tc::Mu λpir ΔasdA4 Δzhf-2::Tn10 | (50) |
| S. pneumoniae WU2 | Wild type, virulent, encapsulated type 3 | (6) |
Mutant strain construction.
DNA manipulations were carried out as described previously (52). Transformation of E. coli and Salmonella enterica was performed by electroporation. Transformants were selected on LB agar plates containing appropriate antibiotics. Selection for Asd+ plasmids was done on LB agar plates.
S. enterica serovar Typhimurium ΔmsbB48 mutant χ8573 (15) was conjugated with E. coli strain χ7213 harboring suicide vector pYA4284, pYA4288, or pYA4287 (30) to generate double mutant strain χ11165 (ΔmsbB48 ΔpagL7), χ9908 (ΔmsbB48 ΔpagP8), or χ9949 (ΔmsbB48 ΔlpxR9), respectively. Strain χ11065 (ΔmsbB48 ΔpagL7 ΔpagP8 ΔlpxR9) was generated by the sequential introduction of each mutation. All mutations were confirmed by PCR and DNA sequence analysis. Mutations were introduced into S. enterica serovar Typhimurium strain χ9241 using the same strategy and methods.
The presence of the ΔpabA1516 and ΔpabB232 mutations in strain χ9241 and its derivatives was verified by the inability of the strains to grow in MOPS minimal medium without p-aminobenzoate. The presence of the ΔasdA16 mutation was confirmed by inability to grow in medium without DAP and by PCR. The ΔaraBAD23 mutation was verified by a white colony phenotype when the strains were streaked onto MacConkey agar supplemented with 1% arabinose and also by PCR. LPS profiles of Salmonella strains were examined on silver-stained SDS-PAGE gels using previously described methods (22).
Extraction of lipid A from Salmonella.
Each strain was grown, harvested, and washed in LB or N minimal medium as described above. Each cell pellet was extracted with 120 ml of a single-phase Bligh-Dyer mixture (4). After 60 min at room temperature, the mixture was subjected to centrifugation (4,000 × g for 20 min). The resulting cell debris pellet was extracted two times with 120 ml of a single-phase Bligh-Dyer mixture. The final insoluble residue, which contains lipopolysaccharide, was subjected to hydrolysis at 100°C in 25 mM sodium acetate buffer, pH 4.5, in the presence of 1% SDS to cleave the 3-deoxy-d-manno-oct-2-ulosonic acid (Kdo)-lipid A linkage (7). The released lipid A molecular species were extracted with a two-phase Bligh-Dyer system (4) by adding appropriate amounts of chloroform and methanol. The lower phase was saved, and the upper phase was washed once with a pre-equilibrated acidic lower phase. The pooled lower phases were dried under a stream of N2. The isolated lipid A was redissolved in chloroform-methanol (4:1, vol/vol). A portion of the sample was subjected to mass spectrometry (MS) analysis (30).
ESI-MS of lipid preparations.
All mass spectra were acquired on a QSTAR XL quadrupole time-of-flight tandem mass spectrometer (ABI/MDS-Sciex, Toronto, Canada) equipped with an electrospray ionization (ESI) source. Spectra were acquired in the negative ion mode and typically were the summation of 60 scans from 200 to 2,000 atomic mass units. For MS analysis, the lipid A or mild alkali-stable lipid preparations were dissolved in 200 μl of chloroform-methanol (4:1, vol/vol) or chloroform-methanol (2:1, vol/vol). Typically, 20 μl of this material was further diluted into 200 μl of chloroform-methanol (1:1, vol/vol) containing 1% piperidine and immediately infused into the ion source at 5 to 10 μl/min. The negative ion ESI-MS was carried out at −4,200 V. In the tandem MS (MS-MS) mode, collision-induced dissociation tandem mass spectra were obtained using collision energy of −80 V (laboratory frame of energy). Nitrogen was used as the collision gas. Data acquisition and analysis were performed using Analyst QS software (30).
MIC test.
The MICs of deoxycholate (DOC) and polymyxin B for various Salmonella strains were determined in 96-well tissue culture plates (61). Two-fold serial dilutions of the bile salt deoxycholate (0.1 to 50 mg/ml) and of polymyxin B (0.1 to 10 μg/ml) were made across the plates. Bacteria were grown to an optical density at 600 nm (OD600) of 0.8 to 0.9 in LB medium and washed in phosphate-buffered saline (PBS). Cells were diluted to 1 × 105 to 1 × 106 CFU in LB medium, 100-μl amounts of the cell suspensions were added to each well containing the proper antimicrobial substance, and the plate was incubated overnight at 37°C. The optical density of each culture was determined with a conventional enzyme-linked immunosorbent assay plate reader (model SpectraMax M2e; Molecular Devices, Sunnyvale, CA). The threshold of inhibition was 0.1 at OD600. Assays were repeated three times.
β-Galactosidase assay.
Strains were statically cultured overnight at 37°C in medium at pH 5.8 or pH 7.7 with 10 μM or 10 mM Mg2+. The next day, bacteria were diluted 1:10 into the same medium and cultured at 180 rpm and 37°C for about 5 h. The levels of β-galactosidase were determined in triplicate using the method described by Miller (35). The means and standard deviations of the results for the triplicate samples were determined.
LPS purification and concentration determination.
For tissue culture experiments, LPS was prepared from 20 ml of bacterial culture with TRI (total RNA isolation) reagent (Sigma) as described previously (63). To remove trace protein, the samples were repurified using the deoxycholate-phenol method (21). LPS preparations were quantitated using the Kdo method according to reference 41.
Cell line culture and LPS stimulation.
The murine macrophage cell line RAW264.7 (ATCC, Rockville, MD) was maintained in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, San Diego, CA) supplemented with 10% fetal bovine serum (FBS), 100 μg/ml gentamicin, and 100 μg/ml penicillin. The human monocytic leukemia cell line Mono Mac 6 (MM6) (Lonza, Braunschweig, Germany) was cultured in RPMI 1640 containing sodium bicarbonate (2 g/liter), insulin (10 μg/ml), oxalacetic acid (1 mM), 100 μg/ml gentamicin, 100 μg/ml penicillin, 2 mM glutamine, nonessential amino acids for minimum essential medium (MEM; 1%, vol/vol), sodium pyruvate (1 mM), folic acid (40 μg/ml), and 15% FBS. Cells were seeded in 96-well microtiter plates (2.5 × 105/well) in 150 μl of the above-described medium and incubated at 37°C with 5% CO2. After 6 h, various dilutions of LPS to be tested for pyrogenicity were added in a 16-μl volume and the incubation continued. After 24 h, culture supernatants were collected, freed of contaminating cells by centrifugation, and stored at −80°C until determination of cytokine content. All experiments were performed three times.
Cytokine assay.
Cytokine concentrations were determined using the Bio-Plex protein array system (Bio-Rad) according to the manufacturer's recommendations. Cytokine-specific-antibody-coated beads were combined with serum samples diluted with specific serum dilution buffer (Bio-Rad) or cell culture supernates diluted with cell culture medium for 30 min with continuous shaking. The beads were washed 3 times with 100 μl wash buffer to remove unbound protein and then incubated with biotinylated cytokine-specific detection antibody for 30 min with continuous shaking. The beads were washed three times and incubated with streptavidin-phycoerythrin for 10 min. The beads were washed three times in washing buffer and resuspended in 125 μl assay buffer, and the constituents of each well of the microtiter plate were drawn up into the flow-based Bio-Plex suspension array system. Cytokine concentrations were automatically calculated by Bio-Plex Manager software by using a standard curve derived from a recombinant cytokine standard. Two readings were made on each bead set.
Determination of virulence in mice.
The Arizona State University Institutional Animal Care and Use Committee approved all animal procedures. Seven-week-old female BALB/c mice were obtained from the Charles River Laboratories (Wilmington, MA). Mice were acclimated for 7 days after arrival before the experiments were started.
For determination of the 50% lethality dose (LD50), bacteria were grown statically overnight at 37°C in LB, diluted 1:50 into fresh LB medium, and grown with aeration (180 rpm) at 37°C. When the cultures reached an OD600 of 0.8 to 0.9, they were harvested by centrifugation at 3,452 × g at room temperature, washed once, and normalized to the required inoculum density in buffered saline with gelatin (BSG) by adjusting the suspension to the appropriate OD600 value. Groups of five mice each were infected orally with 20 μl containing various doses of S. enterica serovar Typhimurium χ3761 or its derivatives, ranging from 1 × 103 CFU to 1 × 109 CFU. Oral infections were performed using a 20-μl pipette. Animals were observed for 4 weeks postinfection, and deaths were recorded daily. Surviving mice in some groups were challenged with 1 × 109 CFU of wild-type strain χ3761. Animals were observed for 4 weeks postinfection and deaths were recorded daily.
To evaluate colonization, mice were orally inoculated with 20 μl of BSG containing 1 × 109 CFU of each strain. At days 3 and 6 after inoculation, 3 to 11 animals per group were euthanized. Spleen and liver samples were collected. Each sample was homogenized in BSG at a final volume of 1 ml. Dilutions of 10−1 to 10−6 (depending on the tissue) were plated onto MacConkey and LB agar to determine the number of viable bacteria. Twenty colonies from each animal were randomly selected to confirm genotypic markers by PCR.
Immunogenicity of vaccine strains in mice.
Recombinant attenuated Salmonella vaccine (RASV) strains were grown statically overnight in LB broth with 0.1% arabinose at 37°C. The following day, 2 ml of the overnight culture was inoculated into 100 ml of LB broth with 0.1% arabinose and grown with aeration at 37°C to an OD600 of 0.8 to 0.9. Cells were harvested by centrifugation at 3,452 × g for 15 min at room temperature, and the pellet was resuspended in 1 ml of BSG. Mice were orally inoculated with 20 μl of BSG containing 1 × 109 CFU of each strain on day 0 and boosted 5 weeks later with the same dose of the same strain. Blood was obtained by mandibular vein puncture at biweekly intervals. Following centrifugation, the serum was removed from the whole blood and stored at −20°C. This experiment was performed twice; 5 mice per group were involved in the first experiment, and 5 to 8 mice per group were used in the second experiment. The results from both experiments were similar and have been pooled for analysis.
ELISA.
Recombinant PspA (rPspA) protein was purified as described previously (25). S. enterica serovar Typhimurium LPS was obtained from Sigma. The rPspA clone was a kind gift from Susan Hollingshead at the University of Alabama at Birmingham. Enzyme-linked immunosorbent assay (ELISA) was used to assay serum antibodies against S. enterica serovar Typhimurium LPS, rPspA, and whole-cell bacterial suspensions (1 × 109 CFU/ml) as previously described (31). Color development (absorbance) was recorded at 405 nm using an automated ELISA plate reader (model SpectraMax M2e; Molecular Devices, Sunnyvale, CA). Absorbance readings 0.1 higher than PBS control values were considered positive reactions.
Pneumococcal challenge.
We assessed the protective efficacy of attenuated Salmonella strains expressing pspA at week 8 by intraperitoneal challenge of immunized mice with 2 × 104 CFU of S. pneumoniae WU2 in 200 μl of BSG (39). The LD50 of S. pneumoniae WU2 in BALB/c mice was 2 × 102 CFU by intraperitoneal administration (data not shown). Challenged mice were monitored daily for 30 days.
Statistical analysis.
Numerical data are expressed as means ± standard errors of the means (SEM). Two-way analysis of variance (ANOVA) followed by Bonferroni's multiple comparison test were used to evaluate differences in antibody titer data. One-way ANOVA followed by Dunnett's multiple comparison test was used to evaluate serum resistance levels, the proliferation of mutants, cytokine levels, and colonization for multiple comparisons among groups. The LD50 was estimated using a probit analysis based on the XLSTAT. The Kaplan-Meier method was used for survival, and differences were analyzed by the log-rank sum test. All analyses were performed using GraphPad PRISM 5.0. A P value of <0.05 was considered statistically significant.
RESULTS
Lipid A structures in the msbB mutant and its derivatives.
To investigate the lipid A structures from the ΔmsbB mutant and the ΔmsbB mutant coupled with other mutations, we isolated lipid A from the ΔmsbB mutant strain χ8573, mutant χ9908 (ΔmsbB ΔpagP), mutant χ11065 (ΔmsbB48 ΔpagL7 ΔpagP8 ΔlpxR9), and the wild-type strain χ3761 grown in LB broth and in minimal medium at pH 5.8 and 10 μM Mg2+, conditions that activate the PhoPQ and PmrAB systems, and deduced their structures from the ESI-MS spectra.
In LB medium or in pH 7.7 minimal medium with 10 mM Mg2+, the lipid A from the wild-type strain contains predominantly hexa-acylated lipid A (Fig. 1C, [M-2H]2− species with m/z 897.60), and minor lipid A species, including hexa-acylated lipid A with l-Ara4N (m/z 963) and with C16 (m/z 1,016.7), consistent with a previous report (30). The lipid A for the msbB mutant strain χ8573 contained two major peaks, the penta-acylated lipid A (Fig. 1C, [M-2H]2− species with m/z 792.5) and the penta-acylated lipid A with C16 (m/z 911.5), and some minor peaks, including the penta-acylated lipid A with a single l-Ara4N (m/z 858), a double l-Ara4N (m/z 923.5), and C16 combined with l-Ara4N (m/z 977); the penta-acylated lipid A with a pEtN addition was not observed in the ΔmsbB mutant in LB medium. PagP adds a C16 group to penta-acylated lipid A (Fig. 1B and C), although typically not all the lipid A molecules in the population are modified. To evaluate the effect of these proteins in a ΔmsbB genetic background, we introduced a ΔpagP deletion mutation into χ8573, yielding strain χ9908 (Table 1). The lipid A from this msbB pagP double mutant was evaluated by ESI-MS, and we found that the peak for C16 addition disappeared when pagP was deleted, as expected (Fig. 1C).
When cells were grown in minimal medium at pH 5.8 with 10 μM Mg2+, the lipid A extracted from the msbB mutant χ8573 was heavily decorated with C16, l-Ara4N, and pEtN. The lipid As isolated from the msbB pagP double mutant were similarly decorated with l-Ara4N and pEtN (see Fig. S1 in the supplemental material). As expected, the msbB pagP double mutant lost the ability to add C16 to penta-acylated lipid A in low-pH and Mg2+ minimal medium (see Fig. S1 in the supplemental material), consistent with the results in LB medium.
PagL and LpxR are deacylation enzymes, both of which have the potential to deacylate the acyl chains from lipid A (Fig. 1A and B). We did not observe peaks consistent with the action of PagL or LpxR in χ11165 (ΔmsbB48 ΔpagL7) and χ9949 (ΔmsbB48 ΔlpxR9) under conditions of either LB medium or N medium with low pH and Mg2+ (data not shown). χ11065 (ΔmsbB48 ΔpagL7 ΔpagP8 ΔlpxR9) generated the same lipid A structures as χ9908 (ΔmsbB48 ΔpagP8) in N medium or LB broth (Fig. 1C).
Phenotypic evaluation of the msbB mutant strain and its derivatives.
All of the mutant strains' growth rates were similar to that of the UK-1 parent strain in LB broth and in N medium (data not shown). The O-antigen phenotypes from the different mutants were determined by silver staining (Fig. 2 A). The wild-type and mutant strains exhibited differences in O-antigen patterns and intensity of staining. These differences were probably the result of differences in lipid A structure due to the lack of the myristated acyl chain or the addition of a palmitoylated acyl chain. The wild-type strain χ3761 produced an O-antigen ladder with a series of double bands, due to the production of two prominent lipid A structures: hepta-acylated (modified by PagP) and hexa-acylated (not modified by PagP) lipid A in LB medium. The ΔmsbB mutant, lacking one myristate group on its lipid A, produced an LPS pattern reflecting a mixture of faster-migrating penta-acylated and hexa-acylated species. The LPS profiles of the wild-type χ3761, the msbB mutant χ8573, and the msbB pagP mutant χ9908 are consistent with the mass spectrometry results obtained from the lipid A (Fig. 1C and 2A).
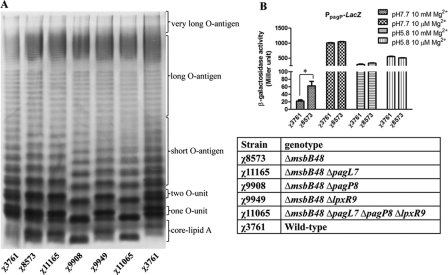
Fig. 2.
Lipopolysaccharide profiles and β-galactosidase activities driven by PpagP of the wild-type χ3761 and its isogenic mutants. (A) Lipopolysaccharide profiles of χ3761 (wild-type UK-1, 1st and 7th lanes) and χ8573 (ΔmsbB48), χ11165 (ΔmsbB48 ΔpagL7), χ9908 (ΔmsbB48 ΔpagP8), χ9949 (ΔmsbB48 ΔlpxR9), and χ11065 (ΔmsbB48 ΔpagL7 ΔpagP8 ΔlpxR9). (B) β-Galactosidase activities were determined in the wild-type (χ3761) and the msbB mutant (χ8573). Bacteria were grown for 6 h in N medium under the conditions indicated in the key. *, P < 0.05 compared with χ3761. β-Galactosidase activities for the wild-type and the ΔmsbB mutant strains without the lacZ reporter plasmid were <0.8.
Deletion of msbB was reported to result in a 2-fold increase in the palmitoylated (C16) lipid A molecules compared to the lipid As of the wild-type MsbB+ strain (27). We observed a similar increase in our ΔmsbB mutant (Fig. 1C). To investigate this result further, we constructed a PpagP::lacZ fusion to determine whether the increase in palmitoylation in the ΔmsbB mutants correlated with an increase in pagP expression (see Fig. S3 in the supplemental material). We measured the levels of β-galactosidase activity in both wild-type and ΔmsbB backgrounds under a variety of growth conditions (Fig. 2B). Our results showed a 3-fold increase in expression from the pagP promoter when cells were grown under non-phoPQ-inducing conditions (pH 7.7, 10 mM Mg2+). Interestingly, under any conditions associated with phoPQ activation, there was no difference in β-galactosidase expression from PpagP between the two strains.
The ΔpagL ΔmsbB and ΔlpxR ΔmsbB double mutants produced the same LPS profile as the single ΔmsbB mutant (Fig. 2A, 2nd lane). The ΔmsbB ΔpagP double mutant produced only a single penta-acylated lipid A structure, consistent with the fact that PagP is responsible for the observed double banding pattern in the PagP+ strains. Furthermore, the msbB mutant was susceptible to infection with bacteriophage P22, which requires O-antigen for binding to the bacterial surface (data not shown), confirming that the alteration to lipid A had not affected its ability to synthesize LPS molecules, consistent with the LPS pattern on the SDS-PAGE gel (Fig. 2A).
A previous report indicated that msbB mutants exhibited marked sensitivity to EGTA and MacConkey medium and grew more slowly than the wild-type parent (36). We tested our mutants and did not observe any growth defect or sensitivity to EGTA or MacConkey medium (data not shown). In addition, we evaluated the sensitivities of the ΔmsbB mutants to deoxycholate (DOC), a representative bile salt, and polymyxin B by measuring the MIC to each compound (Table 2). The msbB mutants were highly sensitive to polymyxin B, consistent with previous reports (37, 58). The msbB mutants exhibited the same sensitivity to bile as wild-type UK-1, which is consistent with the results on MacConkey plates. LPS structure can also affect motility (56), which could affect bacterial virulence. However, we found no difference in swimming motility among our mutant strains (Table 2).
Table 2.
MICs of antibiotic substances and swimming motility and virulence of S. enterica serovar Typhimurium strain χ3761 and its derivatives
| Strain | Genotype | MIC (mg/ml) |
Motility (mm)b | LD50 (CFU)c | |
|---|---|---|---|---|---|
| DOCa | Polymyxin B | ||||
| χ8573 | ΔmsbB48 | 6.25 | <0.032 | 41.5 ± 2.5 | 4.5 × 105 |
| χ11165 | ΔmsbB48 ΔpagL7 | 6.25 | <0.032 | 42 ± 2 | 7 × 105 |
| χ9908 | ΔmsbB48 ΔpagP8 | 6.25 | <0.032 | 32 ± 2 | 2.4 × 105 |
| χ9949 | ΔmsbB48 ΔlpxR9 | 3.1 | <0.032 | 38.5 ± 2.5 | 1.4 × 105 |
| χ11065 | ΔmsbB48 ΔpagL7 ΔpagP8 ΔlpxR9 | 6.25 | <0.032 | 36 ± 1 | 1.4 × 105 |
| χ3761 | Wild-type UK1 | 6.25 | 0.59 | 40.5 ± 0.5 | 1.3 × 104 |
DOC, deoxycholate (bile salt).
Colony diameter in mm after 7 h of growth on the LB agar plate.
The data are based on probit analysis.
The msbB mutant and its isogenic derivatives retain high virulence in mice.
To evaluate the virulence of the msbB mutants, we determined their LD50 in BALB/c mice (Table 2). All strains had reduced virulence, with LD50s from 1.4 × 105 to 7 × 105 CFU (Table 2). The wild-type strain χ3761 was the most highly virulent among all the strains, with an LD50 of 1.3 × 104 CFU, which is consistent with our previous observations (28, 29). The msbB mutants had 10-fold higher LD50s than the wild-type strain χ3761 (15), and all mutant strains had oral LD50s no more than 50-fold higher than that of χ3761 (Table 2). The msbB mutation attenuated Salmonella virulence, while other mutations, in pagL, pagP, and lpxR, had only minor effects. The effect of the ΔmsbB mutation on the oral LD50 in mice is consistent with previous reports that ΔmsbB mutations are attenuating. However, we note that while we only observed a 50-fold increase in oral LD50s, others have reported a 100-fold reduction in LD50 when cells were administered by the intraperitoneal route (32) and a 10,000-fold reduction when cells were administered intravenously (33). Some of these differences may be due to the various routes of administration, to strain differences, or to the presence of suppressor mutations in our strains, which may also account for the ability of our mutants to grow as well as the wild-type parent (36).
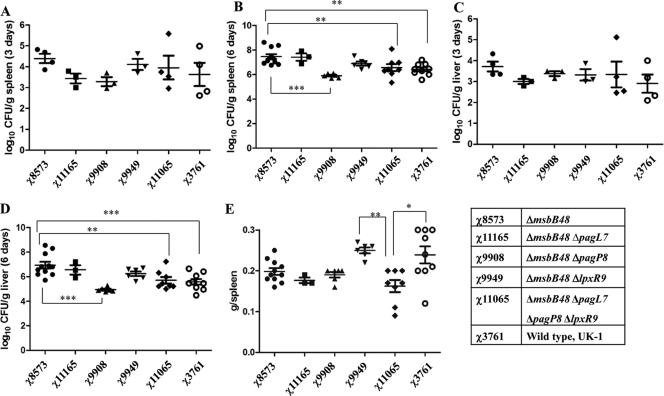
The msbB48 mutant and its derivatives display differences in colonization in the mouse spleen and liver.
To evaluate the systemic distribution of the ΔmsbB mutants in lymphoid tissues, the colonization of the spleen and liver was determined at 3 and 6 days postinoculation (Fig. 3). All strains were able to efficiently colonize these tissues at 3 days after oral administration, with no significant differences between strains. By day 6, the numbers of the msbB mutant χ8573 (ΔmsbB48) in the spleen and liver were significantly greater than the numbers reached by the wild-type strain χ3761. Other mutants with different mutation combinations also can efficiently colonize the spleen and liver; some mutants colonize in smaller numbers than the wild-type, but no significant differences were observed except for the msbB single deletion mutant. By day 6, the numbers of χ9908 (ΔmsbB48 ΔpagP8) in spleen and liver were significantly lower than for the parent strain χ8573 (ΔmsbB48) (P < 0.001). Enlarged spleens (average spleen weight, 0.15 to 0.30 g) were observed in mice inoculated with any of the Salmonella mutants compared to the spleen weight in the BSG group, which was about 0.1 g (data not shown). Inoculation with the wild-type strain χ3761 and the mutant strain χ9949 (ΔmsbB48 ΔlpxR9) resulted in a significant increase in spleen weights compared to the spleen weights following inoculation with χ11065 (ΔmsbB48 ΔpagL7 ΔpagP8 ΔlpxR9) (P < 0.05) (Fig. 3E).
Fig. 3.
Colonization of mouse spleens and livers by χ3761 and its isogenic mutants, and mouse spleen weights. Shown are spleen (A, B) and liver (C, D) colonization by the indicated strains in BALB/c mice at 3 and 6 days postinoculation. (E) Spleen weights following use of the indicated strains in BALB/c mice at 6 days postinoculation. The horizontal lines represent the means, and error bars indicate the means ± SEM. ***, P < 0.001; **, P < 0.01; *, P < 0.05.
The msbB mutation and mutation combinations alter innate immunity in vitro and in vivo.
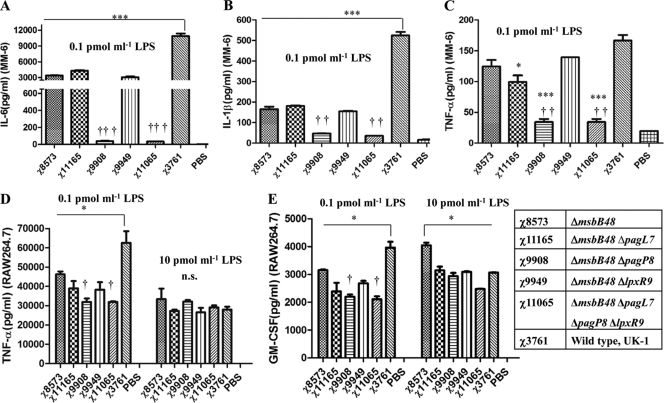
Proinflammatory cytokines have been implicated in the pathogenesis and immunity of Salmonella infections, and msbB mutants induced lower levels of cytokines than the wild-type Salmonella (27, 33). To investigate the impact of LPS purified from the msbB mutants on cytokine induction, we assayed interleukin-6 (IL-6), IL-1β, and tumor necrosis factor alpha (TNF-α) levels in the human monocyte MM6 cell line and TNF-α and granulocyte-macrophage colony-stimulating factor (GM-CSF) levels in the mouse macrophage RAW264.7 cell line after 24 h of stimulation with a final concentration of purified LPS of 0.1 pmol/ml or 10 pmol/ml (Fig. 4). Stimulation of MM6 cells with LPS from the msbB mutant resulted in lower levels of IL-6 production than did wild-type LPS at a concentration of 0.1 pmol/ml (Fig. 4A). The ability to induce IL-6 was further reduced by introduction of the ΔpagP mutation into the ΔmsbB strain, as the LPS from strain χ9908 (ΔmsbB48 ΔpagP8) and strain χ11065 (ΔmsbB48 ΔpagL7 ΔpagP8 ΔlpxR9) induced significantly lower levels of IL-6 than any of the other strains. Similar reductions in IL-1β and TNF-α levels were also observed in the supernatants of MM6 cells (Fig. 4B and C). We observed similar results in RAW264.7 cells with regard to the levels of TNF-α induced by 0.1 pmol/ml of LPS (Fig. 4D), although the effect of ΔpagP was not as dramatic as it was in the MM6 cells. No differences in TNF-α production were observed after induction with 10 pmol/ml LPS from any of the strains.
Fig. 4.
Comparison of cytokine levels induced by purified LPS derived from the wild-type strain and its isogenic mutants in human Mono Mac 6 (MM-6) and murine macrophage RAW264.7 cells. (A to C) Mono Mac 6 cells were stimulated for 24 h with LPS of indicated strains at a concentration of 0.1 pmol/ml, and IL-6, IL-1β, and TNF-α levels in supernatants were quantified with the Bio-Plex assay. (A) IL-6 in the supernatants of Mono Mac 6 cells. (B) IL-1β in the supernatants of MM6 cells. (C) TNF-α in the supernatants of Mono Mac 6 cells. (D, E) RAW264.7 cells were stimulated for 24 h with LPS of indicated strains at concentrations of 0.1 pmol/ml and 10 pmol/ml, and TNF-α and GM-CSF levels in supernatants were quantified with the Bio-Plex assay. (D) TNF-α in the supernatants of RAW264.7 cells. (E) GM-CSF in the supernatants of RAW264.7 cells. The results of one experiment representative of two independent experiments are shown. Error bars indicate SEM of triplicates. ***, P < 0.001; **, P < 0.01; *, P < 0.05 compared with χ3761. †††, P < 0.001; ††, P < 0.01; †, P < 0.05 compared with χ8573. n.s., not significant.
We and others have demonstrated that LPS produced by msbB mutants was less able to induce cytokines in human and murine cell lines (Fig. 4) (27, 33). While informative, these in vitro models do not represent the situation in vivo because S. enterica serovar Typhimurium has a complex regulatory network to regulate its own gene expression in different microenvironmental niches. To assess systemic cytokine production in vivo, the inflammatory and immunostimulatory potentials of each msbB mutant were evaluated by comparing cytokine levels in the pooled serum of mice 6 days after oral inoculation with approximately 1 × 109 CFU of each msbB mutant. The cytokines evaluated included hallmarks of inflammation (IL-1α, IL-1β, IL-6, TNF-α, macrophage inflammatory protein [MIP-1β], granulocyte colony-stimulating factor [G-CSF], and GM-CSF), cytokines involved in chemotaxis (keratinocyte-derived chemokine [KC], monocyte chemoattractant protein [MCP-1], and IL-17), and immunomodulatory cytokines [IL-2, IL-4, IL-5, IL-9, IL-10, IL-12(p70), IL-13, and gamma interferon (IFN-γ)]. The levels of all assayed cytokines were significantly elevated in any of the groups inoculated with bacteria compared to their levels in the BSG control group (Tables 3 , 4, and 5). In contrast to our in vitro data, wild-type strain χ3761 and msbB mutant χ8573 induced similar levels of IL-6 and TNF-α in the inoculated mice. In fact, for all assayed cytokines, the two strains induced similar cytokine levels. Notably, the msbB mutant induced lower levels of G-CSF, important for recruiting neutrophils, than the wild-type strain. Strain χ9908 (ΔmsbB48 ΔpagP8) induced less cytokines than wild-type χ3761 and strain χ8573 (ΔmsbB48), indicating that the lipid A from the ΔmsbB48 ΔpagP8 double mutant displays low endotoxic activity in vivo.
Table 3.
Concentrations of inflammation-related cytokines in pooled sera from mice 6 days after inoculationa
| Strain or control | IL-1a | IL-1β | IL-6 | TNF-α | MIP-1β | G-CSF | GM-CSF |
|---|---|---|---|---|---|---|---|
| χ8573 | 66.2 ± 3.7 | 258.3 ± 28.6 | 423.2 ± 49.2 | 1,061.3 ± 119.4 | 44.9 ± 3.9* | 4,683 ± 214*** | 37 ± 1.9 |
| χ11165 | 62 ± 5.9 | 248.1 ± 22.1 | 380.2 ± 35.5 | 1,124.6 ± 42.5 | 48.9 ± 1 | 7,497.7 ± 486.9***†† | 45 ± 4.5 |
| χ9908 | 37.3 ± 0.3**†† | 99.1 ± 4.1*† | 193.8 ± 7**†† | 465.2 ± 4.1**†† | 22.1 ± 2.1***†† | 2,258.8 ± 30.3***†† | 17.8 ± 1.4*† |
| χ9949 | 62.8 ± 0 | 247.1 ± 3.9 | 351.5 ± 18.9 | 1,098.7 ± 43.5 | 45.7 ± 0.8 | 6,475.3 ± 330.8***† | 39.2 ± 0.2 |
| χ11065 | 47.3 ± 0.5*† | 190 ± 4.7 | 417.7 ± 5.6 | 923.4 ± 18.9 | 33 ± 1.2**† | 6,436.3 ± 15.9***† | 25.4 ± 4 |
| χ3761 | 68.4 ± 0.6 | 259.5 ± 41.3 | 493.6 ± 21.5 | 1,052.1 ± 36.4 | 56.8 ± 1.4 | 11,427.1 ± 86.6 | 34.6 ± 0.6 |
| BSG control | 9.6 ± 1.1 | 55 ± 1.5 | 2.3 ± 0.5 | 276.4 ± 30.4 | 15.9 ± 2.7 | 49.8 ± 2.4 | ND |
The Bio-Plex multiple cytokine assay was used to detect and quantitate (pg/ml) the amount of each cytokine in pooled sera collected from mice (n = 3 mice) inoculated with χ3761 and its derivatives 6 days after inoculation. The data represent the means ± SEM. ***, P < 0.001; **, P < 0.01; *, P < 0.05 compared with wild-type UK-1 χ3761. †††, P < 0.001; ††, P < 0.01; †, P < 0.05 compared with χ8573 (ΔmsbB48). ND, value extrapolated beyond standard range.
Table 4.
Concentrations of immunomodulatory cytokines in pooled sera from mice 6 days after inoculationa
| Strain or control | IL-2 | IL-4 | IL-5 | IL-9 | IL-10 | IL-12 (p70) | IL-13 | IFN-γ |
|---|---|---|---|---|---|---|---|---|
| χ8573 | 41 ± 5.4 | 3.12 ± 0.45 | 9 ± 1.1 | 172.4 ± 24.6 | 60.2 ± 3.1 | 30.1 ± 4 | 651.6 ± 91.5 | 143.2 ± 1.5 |
| χ11165 | 41.7 ± 0.2 | 2.61 ± 0.29 | 9 ± 0.12 | 80.8 ± 13.4* | 78.5 ± 8.1 | 22 ± 3.1* | 470.4 ± 18.5 | 92.2 ± 8.8**†† |
| χ9908 | 24.1 ± 1*† | 2.34 ± 0.02 | 5.7 ± 0.08*† | 117.5 ± 9.1 | 29.7 ± 3.2***† | 14.3 ± 0.4**† | 376.5 ± 19.1† | 188.4 ± 3.2*†† |
| χ9949 | 44.1 ± 2.3 | 2.02 ± 0.31 | 8.6 ± 0.05 | 99.3 ± 5.1* | 57.9 ± 0.3 | 22.5 ± 4* | 551.2 ± 17.7 | 104.7 ± 8.3**† |
| χ11065 | 33.2 ± 1.6 | 2.26 ± 0.02 | 8.6 ± 0.13 | 130.7 ± 26.3 | 44.8 ± 1.3** | 25.7 ± 0.5 | 536 ± 21.5 | 171.7 ± 2.9 |
| χ3761 | 40 ± 1.6 | 2.9 ± 0.11 | 8.9 ± 0.14 | 192.4 ± 3 | 79.3 ± 1.4 | 38.8 ± 0 | 577.6 ± 44.1 | 149.3 ± 3 |
| BSG control | 6.8 ± 0.7 | 0.91 ± 0.05 | 2.5 ± 0.47 | ND | ND | 1.63 ± 0.23 | 64.2 ± 11.1 | ND |
The Bio-Plex multiple cytokine assay was used to detect and quantitate (pg/ml) the amount of each cytokine in pooled sera collected from mice (n = 3 mice) inoculated with χ3761 and its derivatives 6 days after inoculation. The data represent the means ± SEM. ***, P < 0.001; **, P < 0.01; *, P < 0.05 compared with wild-type UK-1 χ3761. †††, P < 0.001; ††, P < 0.01; †, P < 0.05 compared with χ8573 (ΔmsbB48). ND, value extrapolated beyond standard range.
Table 5.
Concentrations of chemokines in pooled sera from mice 6 days after inoculationa
| Strain or control | KC | MCP-1 | IL-17 |
|---|---|---|---|
| χ8573 | 668.6 ± 22.9 | 1349.3 ± 39.4 | 20.8 ± 3.7 |
| χ11165 | 709.4 ± 33.6* | 862 ± 94.2**†† | 28.8 ± 2.9 |
| χ9908 | 370.7 ± 10.2**††† | 491.5 ± 27.3***††† | 63.1 ± 1.3***††† |
| χ9949 | 477 ± 3.9*†† | 924.7 ± 3.7*†† | 36.1 ± 4.7* |
| χ11065 | 647.8 ± 15.5 | 1341.2 ± 31 | 19.4 ± 0.4 |
| χ3761 | 594.8 ± 5.6 | 1,265.7 ± 6.5 | 27.5 ± 0.7 |
| BSG control | 20.6 ± 1.3 | 54.1 ± 6.6 | 11.7 ± 0.7 |
The Bio-Plex multiple cytokine assay was used to detect and quantitate (pg/ml) the amount of each cytokine in pooled sera collected from mice (n = 3 mice) inoculated with χ3761 and its derivatives 6 days after inoculation. The data represent the means ± SEM. ***, P < 0.001; **, P < 0.01; *, P < 0.05 compared with wild-type UK-1 χ3761. †††, P < 0.001; ††, P < 0.01; †, P < 0.05 compared with χ8573 (ΔmsbB48). ND, value extrapolated beyond standard range.
The msbB mutation and mutation combinations alter acquired immune responses to a heterologous antigen and Salmonella antigens in msbB vaccine strains.
The ΔpabA and ΔpabB mutations in strain χ9241 are attenuating mutations. Strain χ9241 is routinely used in our laboratory to evaluate the effects of other mutations on the immunogenicity of an attenuated antigen carrier (28, 29). The msbB48 mutation and other mutation(s) were moved to χ9241 to generate the vaccine strains χ9278(pYA4088) (ΔmsbB48), χ11318(pYA4088) (ΔmsbB48 ΔpagL7), χ9848(pYA4088) (ΔmsbB48 ΔpagP8), χ9850(pYA4088) (ΔmsbB48 ΔlpxR9), and χ11088(pYA4088) (ΔmsbB48 ΔpagL7 ΔpagP8 ΔlpxR9). pYA4088, expressing the pspA antigen, was transformed into each of the strains (28, 29).
Mice were inoculated orally (1.0 × 109 CFU) with each of seven mutant strains harboring pYA4088 or χ9241 harboring the empty vector pYA3493. Mice were boosted with a similar dose of the same strain 5 weeks later. The antibody responses to rPspA, Salmonella LPS, and Salmonella outer membrane protein (SOMP) in the sera of immunized mice were measured as described in Materials and Methods (Fig. 5).
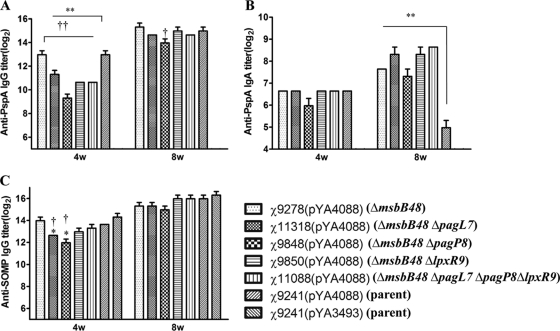
Fig. 5.
Serum IgG responses in orally immunized and control mice. Total serum IgG specific for rPspA (A), IgA specific for rPspA (B), and IgG specific for SOMP (C) were measured by ELISA. The data represent reciprocal anti-IgG antibody levels in pooled sera from mice orally immunized with attenuated Salmonella carrying either pYA4088 (pspA) or pYA3493 (control) at 4 and 8 weeks after immunization (4w and 8w, respectively). The error bars represent variations between triplicate wells. The mice were boosted at week 5. ***, P < 0.001; **, P < 0.01; *, P < 0.05 compared with χ9241(pYA4088). †††, P < 0.001; ††, P < 0.01; †, P < 0.05 compared with χ9278(pYA4088). No immune responses to PspA were detected in mice immunized with χ9241 containing the control plasmid (reciprocal titer of <1:50 for IgG and <1:25 for IgA).
High serum IgG titers against rPspA were observed 4 weeks after the primary immunization in mice inoculated with χ9278(pYA4088) (ΔmsbB48), χ11318(pYA4088) (ΔmsbB48 ΔpagL7), χ9848(pYA4088) (ΔmsbB48 ΔpagP8), χ9850(pYA4088) (ΔmsbB48 ΔlpxR9), χ11088(pYA4088) (ΔmsbB48 ΔpagL7 ΔpagP8 ΔlpxR9), and χ9241(pYA4088). However, there were significant differences between the serum IgG titers from the mice immunized by χ11318(pYA4088), χ9848(pYA4088), χ9850(pYA4088), χ11088(pYA4088), and χ9278(pYA4088) and the titer induced by χ9241(pYA4088) (P < 0.001). The IgA titers against rPspA from vaginal wash samples were low but detectable at 4 weeks. The IgA titer from mice inoculated with χ9848 was lower than those from mice immunized by χ9278(pYA4088) and χ9241(pYA4088), but no significant differences were observed. Anti-OMP IgG titers were high at 4 weeks postinoculation with each of the vaccine strains. However, the anti-OMP titers from mice immunized by χ11318(pYA4088) and χ9848(pYA4088) were significantly lower than those of mice immunized by χ9241(pYA4088) and χ9278(pYA4088) (P < 0.05).
After the second immunization at week 5, anti-rPspA and OMP IgG titers from serum and anti-rPspA IgA from vaginal wash samples reached maximum levels. Anti-rPspA IgG titers from mice immunized by χ9848(pYA4088) were significantly lower than those from mice immunized by χ9278(pYA4088). The anti-rPspA IgA titers from mice immunized by χ9278(pYA4088), χ11318(pYA4088), χ9848(pYA4088), χ9850(pYA4088), and χ11088(pYA4088) were significantly higher than the titer from mice immunized by χ9241(pYA4088). No significant differences were observed for anti-OMP IgG titers from mice immunized with different strains 8 weeks after the first inoculation.
Protection against lethal S. pneumoniae challenge.
To examine the ability of RASV-rPspA vaccines to protect against pneumococcal infection, mice were challenged intraperitoneally with ∼2.0 × 104 CFU (100 LD50) of S. pneumoniae WU2 4 weeks after they were boosted. The groups received either a vaccine strain harboring pYA4088 expressing rPspA, which is cross-reactive with PspA produced by S. pneumoniae WU2 (see Table S1 in the supplemental material), or, for the control group, strain χ9241 containing pYA3493 (empty vector). Immunization with any of the pspA-expressing strains provided significant protection against challenge (P < 0.001) compared with the results for control strain χ9241 containing pYA3493. However, there were no significant differences in the protection rates afforded by χ9278(pYA4088), χ11318(pYA4088), χ9848(pYA4088), χ9850(pYA4088), χ11088(pYA4088), and χ9241(pYA4088). All of the mice that died in these experiments succumbed within 4 days of the challenge.
DISCUSSION
Our ESI-MS data clearly demonstrate that the single msbB mutant lacking the myristate chain is capable of producing two major lipid A structures: the penta-acylated (“3+2 type,” with three fatty acid chains attached to the glucosamine II and two fatty acid chains attached to the glucosamine I) lipid A and the hexa-acylated (3+3 type) lipid A (Fig. 1B and C), which was further confirmed by the LPS profile (Fig. 2). The pagP mutation in the wild type leads to production of the hexa-acylated (4+2 type) lipid A, while the wild-type Salmonella strain produces the hexa-acylated (4+2 type) and hepta-acylated (4+3 type) lipid A in LB medium (30). The msbB pagP double mutant only produces the penta-acylated lipid A (3+2 type) (Fig. 1C), which is an antagonist for human cells (12, 48). The msbB mutant produced more hexa-acylated lipid A (3+3 type) than penta-acylated lipid A, both of which were heavily modified by l-Ara4N or pEtN moieties under pH 5.8, 10 μM Mg2+ minimal medium growth conditions, which activate phoPQ and pmrAB, two regulatory systems. We did not observe severe growth defects for the msbB mutant and its derivatives on the EGTA or bile plate (data not shown) or in the bile sensitivity assay (Table 2). A suppression mutation may be present to confer resistance to the bile in these msbB mutants (36, 37).
We observed that the lipid A of the UK-1 msbB mutant could be greatly modified by the addition of l-Ara4N (Fig. 1C) when grown in LB medium. To confirm our conclusion, we constructed a strain in which the arnT (pqaB or pmrK) gene was deleted (Table 1). arnT encodes l-Ara4N transferase, the enzyme responsible for the addition of l-Ara4N to the phosphate groups of lipid A (16). The peaks associated with l-Ara4N were absent in the lipid A produced by an ΔarnT ΔmsbB mutant (see Fig. S2 in the supplemental material), confirming our assignment of the peaks at m/z 858, m/z 923, and m/z 957 obtained for the ΔmsbB mutant lipid A as l-Ara4N. When our ΔmsbB mutant was grown in N medium at pH 5.8 with 10 μM Mg2+, we observed peaks corresponding to penta-acylated lipid A species decorated with l-Ara4N(s) (m/z 858, 919, 923, and 1,039) (see Fig. S1 in the supplemental material), consistent with a previous report in which the authors reported that, while the amount of l-Ara4N detected on the lipid A of a Salmonella msbB mutant of strain C5 was drastically reduced, they observed a minor peak corresponding to the addition of a single l-Ara4N to the penta-acylated lipid A (58). In contrast, another group using an msbB mutant of S. enterica serovar Typhimurium strain 14028s did not detect any l-Ara4N decorating the lipid A of that strain; however, they detected the addition of pEtN to lipid A conferring resistance to polymyxin B, which required the pmrHFIJKL operon (37). Our unpublished data demonstrate that the addition of pEtN does not contribute to polymyxin B resistance in the msbB mutant, since the pmrC msbB double mutant exhibited higher resistance to polymyxin B than the msbB mutant and the arnT msbB mutant is more sensitive to polymyxin B than the msbB mutant and the pmrC msbB mutant (Q. Kong, unpublished data) in LB with 200 μM Fe2+, which is capable of fully activating the pmrAB regulatory system (20).
The msbB mutation in S. enterica serovar Typhimurium lacking the myristate chain leads to reduced proinflammatory cytokines (TNF-α) and nitric oxide production (27, 33). Further research using rabbit and bovine ligated ileal loops has also demonstrated that the msbB mutant induced smaller enteropathogenic responses with respect to levels of fluid secretion, histological damage, inflammation intensity, and polymorphonuclear leukocyte infiltration (15, 60). Our in vitro data herein also demonstrate that the purified LPS derived from msbB mutants exhibit low endotoxic activity for stimulating IL-6, IL-1β, and TNF-α in MM6 cells and TNF-α and MG-CSF in RAW264.7 cells (Fig. 4). However, LPS derived from χ9908 (ΔmsbB48 ΔpagP8) or χ11065 (ΔmsbB48 ΔpagL7 ΔpagP8 ΔlpxR9) induces much less IL-6 or IL-1β in MM6 than LPS derived from the msbB mutant. This is reasonable and consistent with other reports (46, 55) and reflects the diversity of the lipid A structures produced by the msbB mutant and its derivatives (Fig. 1). Deletion of the msbB gene in χ8573 (ΔmsbB48) leads to pagP upregulation, enhancing the amount of hexa-acylated lipid A (3+3 type) in total lipid A, which is an agonist for the human TLR4 receptor (48); in contrast, loss of both msbB and pagP in χ9908 (ΔmsbB48 ΔpagP8) results in the penta-acylated lipid A structure, which induces less IL-6 and IL-1β in MM6 cells. Similar results were also achieved in the murine RAW264.7 cell line when using a low concentration of LPS to stimulate the cells; no differences were observed when the high concentration of LPS was used to stimulate RAW264.7 cells (Fig. 4). This discrepancy between the two cell lines reflects the complicated interaction between the TLR4-MD2 dimer and the diverse LPS (48).
The results from the cytokine assay showed that wild-type S. enterica serovar Typhimurium strain χ3761 and the msbB mutant χ8573 induced the same levels of production of 16 cytokines, except for MIP-1β and G-CSF, in the serum from mice 6 days after oral inoculation. χ11165 (ΔmsbB48 ΔpagL7), χ9949 (ΔmsbB48 ΔlpxR9), and χ11065 (ΔmsbB48 ΔpagL7 ΔpagP8 ΔlpxR9) also induced cytokine production similar to that of χ8573 (ΔmsbB48) (Table 3). However, the levels of proinflammatory cytokines from mice induced by χ9908 (ΔmsbB48 ΔpagP8) were dramatically lower than those from mice induced by the msbB single mutant χ8573 or wild-type χ3761 (Table 3), suggesting that the lipid A structure from χ9908 (ΔmsbB48 ΔpagP8) exhibits less endotoxic activity than those from the msbB mutant and other mutants in vivo (Fig. 1) and that the reduced cytokine induction by χ9908 (ΔmsbB48 ΔpagP8) is mainly attributable to altered lipid A (Fig. 4 and Table 3). In fact, a recombination-based in vivo expression technology was employed to analyze the spatial-temporal patterns of in vivo expression of pagL, pagP, and lpxR. Increased levels of pagP transcription were observed in bacteria isolated from the lumen of the distal ileum, not pagL and lpxR in this position. Bacteria isolated from spleens of orally inoculated mice showed increasing induction of pagL and lpxR and had the highest expression of pagP (34; Q. Kong, unpublished data).
It is not suitable to only use msbB mutations involved in lipid A modification to evaluate their effects on acquired immunity, since mutants still retain high virulence and high ability to colonize lymphoid tissues in the wild-type background (Table 2 and Fig. 3). Therefore, the mutations were moved to an attenuated Salmonella vaccine strain, χ9241, to evaluate their effects on acquired immunity. Mice immunized with two doses of each of the Salmonella vaccines developed systemic (IgG) and mucosal (secretory IgA) antibodies to the heterologous antigen PspA. All vaccine strains except χ9848 (ΔmsbB48 ΔpagP8) induced equally significant IgG antibody responses to PspA 8 weeks after the first oral immunization, which appear to be cytokine mediated (Table 4 and Fig. 5). The low-endotoxicity lipid A from strains with the msbB mutation appears to contribute to mucosal immune responses, since significant differences in IgA antibody titers to PspA induced by msbB vaccine strains were observed compared to the titers induced by the parent strain in vaginal wash samples (Fig. 5). Evidence for this correlation also comes from other findings that a Salmonella vaccine strain with monophosphoryl lipid A, which exhibited low endotoxic activity, also elicited higher IgA titers against PspA than the parent strain (30), and oral vaccination resulted in identical or even significantly higher levels of PspA-specific IgA response in MyD88−/− or MyD88−/− and Trif−/− BALB/c mice, in which the TLR4 pathway was blocked, than in wild-type mice (43). All mutants elicit identical levels of antibodies against Salmonella OMP (Fig. 5), which indicates that full angagement of the TLR4 pathway is not critical for the induction of humoral antibody to OMPs from S. enterica serovar Typhimurium but is essential for the full induction and enhancement of adaptive immunity against heterologous antigens delivered by attenuated Salmonella (Fig. 5) (24, 53).
S. enterica serovar Typhimurium bacteria are regarded as potential weapons against cancer, as these bacteria are capable of both preferentially amplifying within tumors and expressing prodrug-converting enzymes (33, 44). However, their use is limited by the potential induction of inflammatory responses stimulated by lipid A via TLR4-MD2. Genetic modifications targeting alterations of lipid A were investigated for antitumor Salmonella vaccines. The msbB mutation was preferred for incorporation into the tumor-targeting Salmonella since disruption of msbB in Salmonella resulted in reduced TNF-α and virulence (8, 33, 57). In our work, the palmitoylation state of lipid A in the msbB mutant had dramatic impacts on the inflammatory responses via oral administration (Fig. 4 and Table 3). We suggest that it is worth considering inclusion of the pagP mutation in future antitumor vaccine strains. In order to try such an approach, it will be necessary to determine whether the msbB pagP mutant with other mutations retains its tumor-targeting properties, antitumor activity, and sufficient stimulation of inflammation to serve as a safe antitumor vaccine via the use of systemically administered Salmonella vaccine in humans.
Supplementary Material
Acknowledgments
We thank Heather Matthies for her expert technical assistance. We especially thank Ziqiang Guan from the Raetz laboratory at Duke University for making Fig. 1C.
This work was supported by NIH R01 grants AIO56289 and AIO60557 and grant 37863 from the Bill and Melinda Gates Foundation to R.C. and by NIH grant GM-51796 to C.R.H.R. The mass spectrometry facility in the Department of Biochemistry of the Duke University Medical Center is supported by LIPID MAPS Large Scale Collaborative grant number GM-069338 from NIH.
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
Published ahead of print on 19 September 2011.
REFERENCES
- 1. Abrahams G. L., Hensel M. 2006. Manipulating cellular transport and immune responses: dynamic interactions between intracellular Salmonella enterica and its host cells. Cell Microbiol. 8:728–737 [DOI] [PubMed] [Google Scholar]
- 2. Bertani G. 1951. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 62:293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bishop R. E., et al. 2000. Transfer of palmitate from phospholipids to lipid A in outer membranes of Gram-negative bacteria. EMBO J. 19:5071–5080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 37:911–917 [DOI] [PubMed] [Google Scholar]
- 5. Brandenburg K., Wiese A. 2004. Endotoxins: relationships between structure, function, and activity. Curr. Top. Med. Chem. 4:1127–1146 [DOI] [PubMed] [Google Scholar]
- 6. Briles D. E., et al. 1996. PspA, a protection-eliciting pneumococcal protein: immunogenicity of isolated native PspA in mice. Vaccine 14:858–867 [DOI] [PubMed] [Google Scholar]
- 7. Caroff M., Tacken A., Szabo L. 1988. Detergent-accelerated hydrolysis of bacterial endotoxins and determination of the anomeric configuration of the glycosyl phosphate present in the “isolated lipid A” fragment of the Bordetella pertussis endotoxin. Carbohydr. Res. 175:273–282 [DOI] [PubMed] [Google Scholar]
- 8. Clairmont C., et al. 2000. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J. Infect. Dis. 181:1996–2002 [DOI] [PubMed] [Google Scholar]
- 9. Dofferhoff A. S., et al. 1991. Effects of different types and combinations of antimicrobial agents on endotoxin release from gram-negative bacteria: an in vitro and in vivo study. Scand. J. Infect. Dis. 23:745–754 [DOI] [PubMed] [Google Scholar]
- 10. Edwards R. A., Keller L. H., Schifferli D. M. 1998. Improved allelic exchange vectors and their use to analyze 987P fimbria gene expression. Gene 207:149–157 [DOI] [PubMed] [Google Scholar]
- 11. Elson G., Dunn-Siegrist I., Daubeuf B., Pugin J. 2007. Contribution of Toll-like receptors to the innate immune response to Gram-negative and Gram-positive bacteria. Blood 109:1574–1583 [DOI] [PubMed] [Google Scholar]
- 12. Erridge C., Bennett-Guerrero E., Poxton I. R. 2002. Structure and function of lipopolysaccharides. Microbes Infect. 4:837–851 [DOI] [PubMed] [Google Scholar]
- 13. Evans M. E., Pollack M. 1993. Effect of antibiotic class and concentration on the release of lipopolysaccharide from Escherichia coli. J. Infect. Dis. 167:1336–1343 [DOI] [PubMed] [Google Scholar]
- 14. Gibbons H. S., Kalb S. R., Cotter R. J., Raetz C. R. H. 2005. Role of Mg2+ and pH in the modification of Salmonella lipid A after endocytosis by macrophage tumour cells. Mol. Microbiol. 55:425–440 [DOI] [PubMed] [Google Scholar]
- 15. Gunn B. M., Wanda S. Y., Burshell D., Wang C. H., Curtiss III R. 2010. Construction of recombinant attenuated Salmonella enterica serovar Typhimurium vaccine vector strains for safety in newborn and infant mice. Clin. Vaccine Immunol. 17:354–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gunn J. S., Ryan S. S., Van Velkinburgh J. C., Ernst R. K., Miller S. I. 2000. Genetic and functional analysis of a PmrA-PmrB-regulated locus necessary for lipopolysaccharide modification, antimicrobial peptide resistance, and oral virulence of Salmonella enterica serovar Typhimurium. Infect. Immun. 68:6139–6146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guo L., et al. 1997. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science 276:250–253 [DOI] [PubMed] [Google Scholar]
- 18. Guo L., et al. 1998. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell 95:189–198 [DOI] [PubMed] [Google Scholar]
- 19. Hassan J. O., Curtiss R., III 1990. Control of colonization by virulent Salmonella typhimurium by oral immunization of chickens with avirulent Δcya Δcrp Salmonella typhimurium. Res. Microbiol. 141:839–850 [DOI] [PubMed] [Google Scholar]
- 20. Herrera C. M., Hankins J. V., Trent M. S. 2010. Activation of PmrA inhibits LpxT-dependent phosphorylation of lipid A promoting resistance to antimicrobial peptides. Mol. Microbiol. 76:1444–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hirschfeld M., Ma Y., Weis J. H., Vogel S. N., Weis J. J. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J. Immunol. 165:618–622 [DOI] [PubMed] [Google Scholar]
- 22. Hitchcock P. J., Brown T. M. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver stained polyacrylamide gels. J. Bacteriol. 154:269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ibarra J. A., Steele-Mortimer O. 2009. Salmonella—the ultimate insider. Salmonella virulence factors that modulate intracellular survival. Cell Microbiol. 11:1579–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iweala O. I., et al. 2009. Vaccine-induced antibody isotypes are skewed by impaired CD4 T cell and invariant NKT cell effector responses in MyD88-deficient mice. J. Immunol. 183:2252–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kang H. Y., Srinivasan J., Curtiss III R. 2002. Immune responses to recombinant pneumococcal PspA antigen delivered by live attenuated Salmonella enterica serovar Typhimurium vaccine. Infect. Immun. 70:1739–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karsten V., et al. 2009. msbB deletion confers acute sensitivity to CO2 in Salmonella enterica serovar Typhimurium that can be suppressed by a loss-of-function mutation in zwf. BMC Microbiol. 9:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Khan S. A., et al. 1998. A lethal role for lipid A in Salmonella infections. Mol. Microbiol. 29:571–579 [DOI] [PubMed] [Google Scholar]
- 28. Kong Q., Liu Q., Jansen A. M., Curtiss III R. 2010. Regulated delayed expression of rfc enhances the immunogenicity and protective efficacy of a heterologous antigen delivered by live attenuated Salmonella enterica vaccines. Vaccine 28:6094–6103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kong Q., Liu Q., Roland K. L., Curtiss R., III 2009. Regulated delayed expression of rfaH in an attenuated Salmonella enterica serovar Typhimurium vaccine enhances immunogenicity of outer membrane proteins and a heterologous antigen. Infect. Immun. 77:5572–5582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kong Q., et al. 2011. Salmonella synthesizing 1-monophosphorylated lipopolysaccharide exhibits low endotoxic activity while retaining its immunogenicity. J. Immunol. 187:412–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li Y., et al. 2008. A sopB deletion mutation enhances the immunogenicity and protective efficacy of a heterologous antigen delivered by live attenuated Salmonella enterica vaccines. Infect. Immun. 76:5238–5246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu T., et al. 2008. Immunological responses against Salmonella enterica serovar Typhimurium Braun lipoprotein and lipid A mutant strains in Swiss-Webster mice: potential use as live-attenuated vaccines. Microb. Pathog. 44:224–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Low K. B., et al. 1999. Lipid A mutant Salmonella with suppressed virulence and TNF alpha induction retain tumor-targeting in vivo. Nat. Biotechnol. 17:37–41 [DOI] [PubMed] [Google Scholar]
- 34. Merighi M., Ellermeier C. D., Slauch J. M., Gunn J. S. 2005. Resolvase-in vivo expression technology analysis of the Salmonella enterica serovar Typhimurium PhoP and PmrA regulons in BALB/c mice. J. Bacteriol. 187:7407–7416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miller J. 1972. Experiments in molecular genetics, p. 352-355. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 36. Murray S. R., Bermudes D., de Felipe K. S., Low K. B. 2001. Extragenic suppressors of growth defects in msbB Salmonella. J. Bacteriol. 183:5554–5561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murray S. R., Ernst R. K., Bermudes D., Miller S. I., Low K. B. 2007. PmrA(Con) confers pmrHFIJKL-dependent EGTA and polymyxin resistance on msbB Salmonella by decorating lipid A with phosphoethanolamine. J. Bacteriol. 189:5161–5169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nakayama K., Kelly S. M., Curtiss R., III 1988. Construction of an Asd+ expression-cloning vector: stable maintenance and high level expression of cloned genes in a Salmonella vaccine strain. Nat. Biotechnol. 6:693–697 [Google Scholar]
- 39. Nayak A. R., et al. 1998. A live recombinant avirulent oral Salmonella vaccine expressing pneumococcal surface protein A induces protective responses against Streptococcus pneumoniae. Infect. Immun. 66:3744–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Neidhardt F. C., Bloch P. L., Smith D. F. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Osborn M. J. 1963. Studies on gram-negative cell wall. 1. Evidence for role of 2-keto-3-deoxyoctonate in lipopolysaccharide of Salmonella typhimurium. Proc. Natl. Acad. Sci. U. S. A. 50:499–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Osorio C. G., et al. 2005. Second-generation recombination-based in vivo expression technology for large-scale screening for Vibrio cholerae genes induced during infection of the mouse small intestine. Infect. Immun. 73:972–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Park S. M., et al. 2008. MyD88 signaling is not essential for induction of antigen-specific B cell responses but is indispensable for protection against Streptococcus pneumoniae infection following oral vaccination with attenuated Salmonella expressing PspA antigen. J. Immunol. 181:6447–6455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pawelek J. M., Low K. B., Bermudes D. 1997. Tumor-targeted Salmonella as a novel anti-cancer vector. Cancer Res. 57:4537–4544 [PubMed] [Google Scholar]
- 45. Raetz C. R. H., Whitfield C. 2002. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71:635–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ranallo R. T., et al. 2010. Virulence, inflammatory potential, and adaptive immunity induced by Shigella flexneri msbB mutants. Infect. Immun. 78:400–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reynolds C. M., et al. 2006. An outer membrane enzyme encoded by Salmonella typhimurium ipxR that removes the 3′-acyloxyacyl moiety of lipid A. J. Biol. Chem. 281:21974–21987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rietschel E. T. 1994. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 8:217–225 [DOI] [PubMed] [Google Scholar]
- 49. Roger T., et al. 2009. Protection from lethal Gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc. Natl. Acad. Sci. U. S. A. 106:2348–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Roland K., Curtiss III R., Sizemore D. 1999. Construction and evaluation of a Δcya Δcrp Salmonella typhimurium strain expressing avian pathogenic Escherichia coli O78 LPS as a vaccine to prevent airsacculitis in chickens. Avian Dis. 43:429–441 [PubMed] [Google Scholar]
- 51. Rossignol D. P., Lynn M. 2005. TLR4 antagonists for endotoxemia and beyond. Curr. Opin. Investig. Drugs 6:496–502 [PubMed] [Google Scholar]
- 52. Sambrook J., Fritsch E. F., Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 53. Seibert S. A., Mex P., Kohler A., Kaufmann S. H. E., Mittrucker H. W. 2010. TLR2-, TLR4- and Myd88-independent acquired humoral and cellular immunity against Salmonella enterica serovar Typhimurium. Immunol. Lett. 127:126–134 [DOI] [PubMed] [Google Scholar]
- 54. Snavely M. D., Miller C. G., Maguire M. E. 1991. The mgtB Mg2+ transport locus of Salmonella typhimurium encodes a P-type ATPase. J. Biol. Chem. 266:815–823 [PubMed] [Google Scholar]
- 55. Somerville J. E., Cassiano L., Bainbridge B., Cunningham M. D., Darveau R. P. 1996. A novel Escherichia coli lipid A mutant that produces an anti-inflammatory lipopolysaccharide. J. Clin. Invest. 97:359–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Toguchi A., Siano M., Burkart M., Harshey R. M. 2000. Genetics of swarming motility in Salmonella enterica serovar Typhimurium: critical role for lipopolysaccharide. J. Bacteriol. 182:6308–6321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Toso J. F., et al. 2002. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J. Clin. Oncol. 20:142–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tran A. X., et al. 2005. Resistance to the antimicrobial peptide polymyxin requires myristoylation of Escherichia coli and Salmonella typhimurium lipid A. J. Biol. Chem. 280:28186–28194 [DOI] [PubMed] [Google Scholar]
- 59. Trent M. S., Pabich W., Raetz C. R. H., Miller S. I. 2001. A PhoP/PhoQ-induced lipase (PagL) that catalyzes 3-O-deacylation of lipid A precursors in membranes of Salmonella typhimurium. J. Biol. Chem. 276:9083–9092 [DOI] [PubMed] [Google Scholar]
- 60. Watson P. R., et al. 2000. Mutation of waaN reduces Salmonella enterica serovar Typhimurium-induced enteritis and net secretion of type III secretion system 1-dependent proteins. Infect. Immun. 68:3768–3771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wiegand I., Hilpert K., Hancock R. E. W. 2008. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 3:163–175 [DOI] [PubMed] [Google Scholar]
- 62. Xin W., et al. 2008. Analysis of type II secretion of recombinant pneumococcal PspA and PspC in a Salmonella enterica serovar Typhimurium vaccine with regulated delayed antigen synthesis. Infect. Immun. 76:3241–3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yi E. C., Hackett M. 2000. Rapid isolation method for lipopolysaccharide and lipid A from Gram-negative bacteria. Analyst 125:651–656 [DOI] [PubMed] [Google Scholar]
- 64. Zhou D. G., Galan J. 2001. Salmonella entry into host cells: the work in concert of type III secreted effector proteins. Microbes Infect. 3:1293–1298 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.