Abstract
Rationale
Neuroimaging techniques have led to significant advances in our understanding of the neurobiology of drug-taking and the treatment of drug addiction in humans. Neuroimaging approaches provide a powerful translational approach that can link findings from humans and laboratory animals.
Objective
This review describes the utility of neuroimaging toward understanding the neurobiological basis of drug taking, and documents the close concordance that can be achieved among neuroimaging, neurochemical and behavioral endpoints.
Results
The study of drug interactions with dopamine and serotonin transporters in vivo has identified pharmacological mechanisms of action associated with the abuse liability of stimulants. Neuroimaging has identified the extended limbic system, including the prefrontal cortex and anterior cingulate, as important neuronal circuitry that underlies drug taking. The ability to conduct within-subject, longitudinal assessments of brain chemistry and neuronal function has enhanced our efforts to document long-term changes in dopamine D2 receptors, monoamine transporters, and prefrontal metabolism due to chronic drug exposure. Dysregulation of dopamine function and brain metabolic changes in areas involved in reward circuitry have been linked to drug-taking behavior, cognitive impairment and treatment response.
Conclusions
Experimental designs employing neuroimaging should consider well-documented determinants of drug taking, including pharmacokinetic considerations, subject history and environmental variables. Methodological issues to consider include limited molecular probes, lack of neurochemical specificity in brain activation studies, and the potential influence of anesthetics in animal studies. Nevertheless, these integrative approaches should have important implications for understanding drug-taking behavior and the treatment of drug addiction.
Keywords: PET imaging, fMRI, self-administration, cerebral blood flow, cerebral metabolism, dopamine, serotonin, stimulants, cocaine, nonhuman primates
Introduction
It has been known for over 50 years that drug-taking behavior can be maintained in laboratory animals. Early studies examined the effects of morphine in opiate-dependent primates (Laties 1986; Spragg 1940; Thompson and Schuster 1964). At that time, it was assumed that physical dependence was required to maintain drug taking in laboratory animals, and that drug taking was maintained by the alleviation of aversive withdrawal symptoms. However, in a pioneering study, Deneau, Yanagita, and Seevers (1969) documented that nondrug-dependent rhesus monkeys would acquire persistent self-administration of a variety of compounds, including morphine, cocaine, ethanol, and codeine. This initial demonstration has been subsequently confirmed with a large variety of drugs over a wide range of conditions. Furthermore, it revolutionized the conceptualization of drug-taking behavior in laboratory animals, as it demonstrated that the reduction or elimination of aversive withdrawal symptoms was not necessary for maintenance of drug taking. In concert with the results of studies examining the maintenance of behavior by food delivery or termination of aversive stimuli (Kelleher and Morse 1968), drug self-administration became conceptualized as deriving from reinforcing effects. It is now well recognized that drug-taking behavior is determined by an array of variables including the dose, pharmacokinetics, and neurochemistry of the drug, the history of the organism, environmental variables, and drug-elicited subjective effects.
In combination with behavioral pharmacology, non-invasive neuroimaging techniques have led to significant advances in our understanding of the neurobiology of drug-taking behavior and the treatment of drug addiction in humans. Neuroimaging approaches have the distinct and unusual strength that the same technique can be applied in laboratory animals and human subjects, allowing for a powerful translational approach that can link findings from humans and laboratory animals. Laboratory animal models complement human research by allowing for initially drug-naïve subjects and longitudinal designs, which support characterization of within-subject neurobiological changes associated with chronic drug use. Moreover, the use of laboratory animals provides for high levels of experimental control and well documented drug histories, both of which may not be as widely available in studies of human subjects.
Considerable evidence suggests that nonhuman primate neuroanatomy, drug responses, and behavior offer distinct advantages for the use of neuroimaging in the study of drug taking compared to other laboratory animal models. The organizational structure and connections within brain regions with important roles in drug taking, such as the striatum and prefrontal cortex, may have features unique to primates (Haber 1986; Haber and Fudge 1997; Haber and Knutson 2010; Haber and McFarland 1999). As will be described, dopamine is a key neurotransmitter in drug taking, and there are pronounced differences in cortical innervations by dopamine projections between rodents and primates (Berger et al. 1988; Haber et al. 2006). Compared to rodents, nonhuman primates are more similar to humans in the pharmacokinetics and metabolism of several drug classes including 3,4-methylenedioxymethamphetamine (MDMA) (Banks et al. 2007; Weerts et al. 2007). Furthermore, the brain distribution of imaging probes exhibits some heterogeneity even within primates, suggesting greater differences may be encountered when comparisons are drawn across orders (Yokoyama et al. 2010). Lastly, nonhuman primates exhibit complex social behaviors that provide unique opportunities for examining the influence of the environment on the reinforcing effects of drugs (Morgan et al. 2002; Nader and Czoty 2005; Nader et al. 2008).
With few exceptions, neuroimaging studies in nonhuman primates have employed positron emission tomography (PET), single photon emission tomography (SPECT), or functional magnetic resonance imaging (fMRI). Accordingly, these techniques will be the focus of the current review. Researchers have used nuclear imaging with PET and SPECT to define the behaviorally relevant dose range and pharmacokinetics of drugs with high abuse liability in the human and nonhuman primate brain. With the development of new radiotracers and enhanced resolution of imaging systems, nuclear medicine techniques have also been employed to characterize the in vivo neurochemical effects of abused drugs, including effects at neurotransmitter receptors and transporters. Moreover, documentation of the long-term neurobiological consequences of well characterized drug histories has led to novel insights regarding of the pathology and treatment of drug addiction. Significant progress has been achieved in the study of environmental determinants of drug-taking behavior using nuclear medicine and fMRI techniques. In addition, these techniques have informed our understanding of the neurobiology of drug-elicited subjective effects.
Pharmacological Imaging
Neuroimaging techniques provide a minimally invasive approach toward understanding drug effects on central nervous system (CNS) function and the neural mechanisms underlying drug-taking behavior. In PET imaging, ligands of interest are radiolabeled with unstable atomic isotopes (see Fowler et al. 2007; Phelps and Mazziotta 1985; Senda et al. 2002). Detector arrays and computer algorithms map the source and concentration of the radiotracer. Numerous radiotracers have been developed for use in PET neuroimaging that enable in vivo measurement of effective dose ranges, brain pharmacokinetics, and brain neurochemistry. An important advantage of PET imaging over other approaches is that the chemical properties of the radiotracers do not substantially vary from the unlabeled ligand, allowing for the study of function with minimal alterations in pharmacological properties. SPECT is a related approach that uses different radiotracers that emit a single photon. Due to methodological differences, SPECT imaging has lower sensitivity and resolution compared to PET imaging, and it is used less frequently.
In fMRI, function is typically studied using powerful static magnetic fields, strong and rapidly changing magnetic gradients, and Fourier reconstruction techniques. fMRI studies using blood oxygen level dependent (BOLD) contrast are the most widely utilized and infer changes in neuronal function by physiological changes in hemodynamics and oxygen metabolism (Fox 1988; Kwong 1992; Ogawa 1992). Compared to PET and SPECT, fMRI provides higher temporal and spatial resolution for mapping brain activity, allowing for more precise measurements of certain neurobiological changes that result from drug-taking behavior. Pharmacological imaging is simply defined as the use of imaging techniques in the context of drug administration, and accordingly has been used to study acute effects of drugs of abuse. The vast majority of drug abuse research that has utilized neuroimaging in primates has focused on cocaine and related stimulants. Accordingly, the current review will focus on abused stimulants.
Pharmacokinetics
The pharmacokinetic properties of a drug are an important determinant of drug taking behavior. Because of the rapid pharmacokinetics of this route of administration, drug-taking behavior is typically studied in laboratory animals by intravenous drug infusions contingent upon the behavior of the organism. A simple and straightforward way to regulate the time-course of the drug is to alter the rate at which the drug is infused. In such studies, changes in infusion rate dramatically alter drug-taking behavior. In a highly illustrative example, rhesus monkeys were provided access to cocaine self-administration at different infusion rates across different sessions. Drug-taking behavior monotonically decreased as the infusion rate slowed. Strikingly, at the slowest infusion rate, a dose of cocaine that maintained drug-taking behavior at other infusion rates no longer did so. In other words, at this infusion rate, this dose of cocaine no longer functioned as a reinforcer (Panlilio et al. 1998). Similar findings have been reported in both monkeys (Woolverton and Wang 2004) and human subjects (Abreu et al. 2001; Marsch et al. 2001; Nelson et al. 2006).
In addition to studies examining alterations in infusion rate, pharmacokinetics as a determinant of drug-taking behavior have also been established by comparing the effects of drugs with intrinsically different time-courses of action. To this end, several phenyltropane analogs of cocaine have been developed that vary in terms of their neurochemical and pharmacokinetic effects. When neurochemical effects were matched to cocaine but pharmacokinetic variables varied, monkeys self-administered drugs with slower onsets and longer durations of action than cocaine at lower rates than they self-administered cocaine (Howell et al. 2007; Howell et al. 2000; Lindsey et al. 2004; Wilcox et al. 2002). PET neuroimaging of drug biodistribution and kinetics has contributed to a better understanding the mechanism of action of cocaine and related stimulants. An early study focused on the distribution of cocaine binding in the brain of anesthetized baboons using [11C]-labeled cocaine (Fowler et al. 1989). Cocaine binding was heterogeneous but showed some selectivity for dopamine transporter (DAT) rich striatal regions. Striatal cocaine binding was inhibited by pretreatments with pharmacological doses of cocaine and DAT inhibitors but not by norepinephrine transporter (NET) or serotonin transporter (SERT) inhibitors. Direct comparisons in human subjects showed a similar distribution of binding with the highest concentration in the striatum. A subsequent study documented significant overlap in the distributions of binding of [11C]-labeled cocaine and methylphenidate (Volkow et al. 1995). Importantly, a direct relationship was established between self-reports of “high” on the VAS induced by cocaine and the time-course of striatal uptake (Volkow et al. 1997a). A subsequent study compared the levels of DAT occupancy by cocaine that was administered via different routes (Volkow et al. 2000). Although similar levels of DAT occupancy were obtained across all routes of administration, smoked cocaine with the most rapid onset of action induced significantly greater self-reports of “high” on the VAS than intranasal cocaine, again highlighting the importance of pharmacokinetic factors in the subjective effects of cocaine. Collectively, these studies demonstrate that the brain distribution and kinetics of a drug strongly predict key determinants of drug taking behavior, including neurochemical and subjective effects.
Recently, the brain pharmacokinetics of methamphetamine were compared to cocaine in anesthetized baboons using [11C]-labeled d-methamphetamine and (−) cocaine (Fowler et al. 2007). The results indicated that the slower clearance of methamphetamine compared to cocaine likely contributed to its longer-lasting stimulant effects. Finally, the reinforcing effects of several cocaine analogs were compared to the time-course of uptake of the [11C]-labeled drugs in the putamen of awake rhesus monkeys (Kimmel et al. 2008). The cocaine analogs were reliably self-administered but rates of responding were lower than those maintained by cocaine. Importantly, there was a clear trend towards an inverse relationship between the time to peak uptake of [11C]-labeled drugs in putamen and the peak number of i.v. infusions received, such that the faster-onset drugs produced greater levels of responding relative to the slower-onset drugs. There was also a close correspondence between the time-course of drug uptake in brain and drug-induced increases in extracellular dopamine in caudate (Czoty et al. 2002; Ginsburg et al. 2005; Kimmel et al. 2008; Kimmel et al. 2007). These studies clearly show that PET measures of biodistribution and kinetics directly predict drug taking across drugs and across subjects. However, as these studies exclusively examined the effects of stimulants, it remains to be determined if these techniques will prove to be as useful when applied to other drug classes.
Neurochemistry
Another important determinant of drug-taking behavior is the underlying neurochemistry of the drug under study. Generally, reinforcement of behavior by a variety of stimuli has been associated with the neurotransmitter dopamine. In one of the most widely cited studies of the effects of psychomotor-stimulants, a significant correlation was shown between the potency of a series of cocaine analogs to be self-administered and their affinities at the DAT (Ritz et al. 1989). This study has been supported by other research showing that selective DAT inhibitors function as positive reinforcers (Wilcox et al. 2002). Importantly, these data lie in stark contrast to the effects of selective inhibitors of the SERT or the NET, as these compounds have not been found to be self-administered by laboratory animals nor do they exhibit appreciable abuse liability (Howell 2008; Howell and Byrd 1995).
In human subjects, consistent with the data derived from nonhuman primates, dopamine has also been linked to drug taking. These studies have primarily been conducted using neuroimaging, and many of the results are in close agreement with preclinical studies conducted in nonhuman primates. For example, across subjects, the subjective effects of cocaine (Volkow et al. 1997a) or methylphenidate (Volkow et al. 1999b) correlate with occupancy of the DAT. Accordingly, in both nonhuman and human primates, the dopaminergic system has been closely tied to drug-taking behavior. However, it is important to note that other systems, particularly the serotonergic and glutamatergic systems, may also play key roles in drug-taking behavior (Bubar and Cunningham 2006; Howell and Murnane 2008; Kalivas and O'Brien 2008; Kalivas and Volkow 2005).
PET neuroimaging has been used most frequently to characterize drug interactions with protein targets that can be related to their behavioral effects. For example, PET imaging in rhesus monkeys using the [18F]-FECNT, a DAT selective radioligand, showed that FECNT labels a cocaine-sensitive binding site. Moreover, consistent with the importance of drug dose in drug taking, doses of cocaine that produced high levels of DAT occupancy were required for behavioral effects to emerge (Votaw et al. 2002). Similarly, the relationship between doses of local anesthetics that engender significant DAT occupancies and reinforcing effects were evaluated in rhesus monkeys (Wilcox et al. 2005). Doses of dimethocaine that maintained peak response rates under a second-order schedule of i.v. drug delivery produced DAT occupancies between 66–82%. These values are highly concordant with results from human PET imaging studies, which found that DAT occupancies were between 60–77% for cocaine doses that subjects reported as rewarding (Volkow et al., 1997). They are also concordant with PET imaging data in rhesus monkeys, which revealed that cocaine DAT occupancies between 65–76% maintain peak response rates (Wilcox et al. 2002).
Unlike dimethocaine, and consistent with previous reports of marginal reinforcing effects (Ford and Balster 1977; Johanson 1980; Wilcox et al. 1999; Woolverton 1995), procaine was ineffective in maintaining self-administration and resulted in DAT occupancies between 10–41% (Wilcox et al. 2005). However, irrespective of the drug, in vivo microdialysis showed that reinforcing effects and DAT occupancy were closely related to drug-induced increases in extracellular dopamine. These studies illustrate the power of PET imaging to unmask the mechanisms underlying drug-taking behavior, particularly as it relates to monoamine transporters, and they highlight the utility of the translational nature of PET imaging in nonhuman primates. For an illustration of the relationship between known determinants of drug taking and the findings of neuroimaging studies see Table 1. Consistent with these findings, it has been shown recently that DAT occupancy by methylphenidate is highly concordant between rhesus monkeys and humans when blood levels of the drug are matched (Wilcox et al. 2008).
Table 1.
Relationship between known determinants of drug-taking behavior and the results of neuroimaging studies in nonhuman primates and humans
| Determinant | Neuroimaging modality | Relationship |
|---|---|---|
| Dose | PET | Cocaine-elicited changes in CBF are dose dependent |
| fMRI | Never tested | |
|
| ||
| Pharmacokinetics | PET | Brain pharmacokinetics correlate with interoceptive and reinforcing effects |
| PET | Route of administration determines protein occupancy | |
| fMRI | Never tested | |
|
| ||
| Neurochemistry | PET | Distribution of stimulant binding selective for DAT regions |
| PET | Stimulants displace D2 receptor radioligand binding | |
| PET | Drug-induced changes in CBF are attenuated by appropriate pretreatments | |
| fMRI | Never tested | |
|
| ||
| Subject history | PET | Determines distribution of metabolic activation |
| fMRI | Never tested | |
|
| ||
| Environment | PET | Dominance rank determines D2 receptor availability and drug taking |
| PET | Cocaine self-administration under distinct schedules of reinforcement produces distinct changes in CBF | |
| fMRI | Drug-associated stimuli elicit similar brain activational effects to the drug itself | |
|
| ||
| Subjective effects | PET | Correlates with regional uptake |
| fMRI | Correlates with temporal dynamics | |
PET neuroimaging has also been used to study protein occupancy by other stimulants. For example, until recently, the role of the DAT in the behavioral effects of the wake promoting drug modafinil was not well documented. Consistent with the importance of subjective effects in drug taking, a number of clinical studies suggest that modafinil may improve clinical outcomes for treatment of cocaine dependence by reducing self reports of craving and cocaine-induced euphoria (Anderson et al. 2009; Dackis et al. 2005; Dackis et al. 2003; Hart et al. 2008) through a possible DAT-mediated mechanism (Volkow et al. 2009; Zolkowska et al. 2009). To this end, a recent study in rhesus monkeys demonstrated that the in vivo effects of modafinil at the DAT are similar to other stimulants, such as cocaine (Andersen et al. 2010). Modafinil induced nocturnal locomotor-stimulant effects and reinstated extinguished responding previously maintained by cocaine. An effective dose of modafinil resulted in approximately 60% DAT occupancy in the striatum and significantly increased extracellular dopamine levels, comparable to effects observed following cocaine doses that reliably maintain self-administration (Ito et al. 2002; Votaw et al. 2002; Wilcox et al. 2005; Wilcox et al. 2002). Consistent with these findings, Madras and colleagues (2006) found that modafinil (8.0 mg/kg) resulted in approximately 54% DAT occupancy in the striatum of baboons. Similarly, clinically relevant doses of modafinil significantly increased extracellular levels of dopamine through blockade of DAT in the human brain (Volkow et al. 2009).
The results obtained with neuroimaging provide important information about the mechanism of action of modafinil and show low potency DAT-related effects in nonhuman primates that may be relevant for its abuse in humans. Indeed, doses of modafinil that are considerably higher than the clinically-relevant dose range maintain drug taking in rhesus monkeys (Gold and Balster 1996) and humans self-administer modafinil at greater rates than placebo under certain laboratory conditions (Stoops et al. 2005). However, its low potency at the DAT appears to limit modafinil self-administration in nonhuman primates (Gold and Balster 1996) and its abuse liability in humans (Jasinski 2000; Vosburg et al. 2010). These studies collectively demonstrate the power of PET imaging to characterize the transporter-related effects of stimulants and their relationship to drug-taking behavior.
Despite extensive efforts directed toward the development of medications to treat cocaine abuse, no effective pharmacotherapy is currently in clinical use. Given the important role of the DAT in drug taking, the development of compounds that target the DAT represents a reasonable approach for the pharmacological treatment of cocaine abuse. A series of studies was conducted in nonhuman primates that evaluated the effectiveness of DAT inhibitors in reducing cocaine self-administration. PET neuroimaging quantified DAT occupancy at behaviorally-relevant doses, characterized the time-course of drug uptake in brain, and documented drug-induced changes in cerebral blood flow as a model of brain activation. Selective DAT inhibitors were effective in reducing cocaine taking, but only at high (>70%) levels of DAT occupancy. For example, effective doses of the DAT-selective inhibitor RTI-113, which dose-dependently reduced cocaine-maintained responding, produced DAT occupancies between 72–84% (Wilcox et al., 2002). Similar results were observed with other DAT-selective inhibitors, including the phenyltropane RTI-177 and the phenylpiperazine GBR 12909 (Lindsey et al. 2004).
Selective serotonin transporter (SERT) inhibitors were also effective in reducing cocaine taking and blocked cocaine-induced brain activation and increases in extracellular dopamine (Czoty et al. 2002; Howell et al. 2002; Howell and Wilcox 2002). Similarly, a mixed-action inhibitor of the DAT and the SERT, RTI-112, significantly reduced cocaine self-administration by rhesus monkeys at doses producing levels of DAT occupancy below the limit of detection (Lindsey et al., 2004). Furthermore, co-administrations of the selective SERT inhibitors fluoxetine or citalopram and the selective DAT inhibitor RTI-336 produced more robust reductions in cocaine self-administration than RTI-336 alone, even at comparable levels of DAT occupancy by RTI-336 (Howell et al. 2007). Similar to its suppression of stimulant-induced increases in operant responding, and consistent with the importance of neurochemistry in determining drug-taking behavior, it appears that serotonergic effects enhance suppression of cocaine self-administration by DAT inhibitors.
Competition between radiolabeled ligands and endogenous neurotransmitters provides an alternative means of evaluating neurochemical determinants of drug taking. Specifically, this technique supplies an effective means of evaluating drug-induced changes in extracellular neurotransmitter concentrations in vivo (see Laruelle 2000). For example, SPECT imaging with the dopamine D2 receptor ligand [123I]-labeled iodobenzamide (IBZM) in baboons and rhesus monkeys documented amphetamine-induced displacement of binding, ostensibly due to drug-induced elevations in extracelluar dopamine (Innis et al. 1992). After methamphetamine administration, there were positive correlations between reductions in D2 receptor binding in baboons and peak dopamine release measured with microdialysis in vervet monkeys (Laruelle et al. 1997). Moreover, pretreatment with the dopamine synthesis inhibitor, alpha-methyl-paratyrosine, attenuated amphetamine-induced increases in extracellular dopamine and displacement of D2 receptor binding, confirming that the latter effect was mediated through dopamine release.
PET neuroimaging with [18F]-labeled fluoroclebopride (FCP) as a reversible D2 receptor ligand characterized stimulant-induced dopamine release in rhesus monkeys (Mach et al. 1997). Intravenous administration of cocaine, amphetamine, methylphenidate and methamphetamine each increased rates of FCP washout from the basal ganglia, consistent with the capacity of each drug to elevate extracellular dopamine. [11C]-labeled raclopride studies in baboons (Dewey et al. 1992; Villemagne et al. 1998; Volkow et al. 1999a) and [18F]-labeled fallypride studies in rhesus monkeys (Mukherjee et al. 1997) documented that these effects can be demonstrated with several radioligands and in multiple primate species. As such, drug-induced displacement of radioligand binding is an important tool for studying the role of in vivo neurochemistry in drug-taking behavior. However, it is important to note that drug mechanism of action, the relative affinity of the radioligand and the endogenous neurotransmitter, protein density in specific brain regions, and direct interactions between the drug and its metabolites with the protein target are all important considerations that may influence the outcome and interpretation of in vivo displacement studies.
PET imaging has also been used in nonhuman primates to examine the receptor pharmacology influencing dopamine release. In one study, pretreatment with the mGluR1 receptor antagonist 2-methyl-6-(phenylethynyl)pyridine (MPEP) attenuated dopamine release by methamphetamine, as measured by [11C]-labeled MNPA (Tokunaga et al. 2009). Similarly, the mGluR2 agonist LY354740 potentiated amphetamine-elicited dopamine release, as measured by [11C]-labeled raclopride (van Berckel et al. 2006). Similar to biodistribution studies, displacement of radiotracers by drug-induced increases in neurotransmitter levels can be used to study the time-course of drug action (Narendran et al. 2007). In addition, the importance of the intrinsic efficacy of PET radioligands has recently become recognized. For example, the D2 receptor agonist radioligand MNPA is more sensitive than the D2 antagonist radioligand raclopride to amphetamine-elicited increases in dopamine levels (Seneca et al. 2006). This is consistent with prior in vitro competition binding work showing that agonists have a higher apparent affinity for receptors labeled with agonist radioligands than antagonist radioligands (Sleight et al. 1996). Moving forward, an enhanced understanding of these pharmacodynamic and pharmacokinetic factors is likely to yield new insights into the neural mechanisms that mediate drug-taking behavior.
Neurocircuitry
The noninvasive measurement of cerebral blood flow with PET neuroimaging and [15O] water provides a useful means to characterize acute drug-induced changes in brain activity. For example, functional changes in cerebral blood flow using PET imaging have been determined in awake, drug-naïve rhesus monkeys following acute i.v. cocaine administration (Howell et al. 2001; Howell et al. 2002). In these studies, brain activation maps normalized to global flow showed prominent cocaine-induced activation of prefrontal cortex, especially dorsolaterally. Importantly, the same dose of the selective SERT inhibitor alaproclate attenuated the brain activational effects, drug-induced increases in striatal dopamine, and self-administration of cocaine (Czoty et al. 2002; Howell et al. 2002). Hence, there was close concordance between in vivo measures of drug taking, neurochemistry and functional imaging.
A more recent study was the first to use PET imaging with [15O] water to document acute cocaine-induced changes in brain activity during cocaine self-administration in nonhuman primates (Howell et al. 2010). The area of major activation included anterior cingulate cortex, a region associated with the extended limbic system. Furthermore, similar to studies reporting conditioned responses to drug-associated environmental stimuli in humans, drug-associated stimuli increased regional cerebral blood flow in the dorsomedial prefrontal cortex, indicating robust cortical activation. Consistent with a well-established literature reporting both quantitative and qualitative differences in the response to cocaine depending on whether the drug is administered passively or self-administered (Dworkin et al. 1995; Hemby et al. 1997), these results document qualitative differences in the pattern of brain activation induced by cocaine during contingent versus non-contingent drug administration. Also consistent with this literature, the brain metabolic effects of self-administered cocaine in rhesus monkeys determined with 2DG autoradiography (Porrino et al. 2002) differed qualitatively from results obtained in previous experiments utilizing non-contingent drug administration in drug naïve monkeys (Lyons et al. 1996). These studies highlight the importance of preclinical models that incorporate voluntary drug taking and provide important insights into the neurocircuitry of drug-taking behavior.
Recently, there has been some success in implementing pharmacological fMRI to study the neurocircuitry of drug taking in nonhuman primates (Brevard et al. 2006; Jenkins et al. 2004; Murnane and Howell 2010). Experiments in anesthetized cynomolgus monkeys used an iron oxide nanoparticle (IRON) technique to measure changes in relative cerebral blood volume (rCBV) following acute intravenous administration of amphetamine (Jenkins et al. 2004). Amphetamine caused marked changes in rCBV in areas with high dopamine receptor density as well as associated circuitry. The largest increases in rCBV were observed in the parafascicular thalamus, nucleus accumbens, putamen, caudate, substantia nigra and ventral tegmental area.
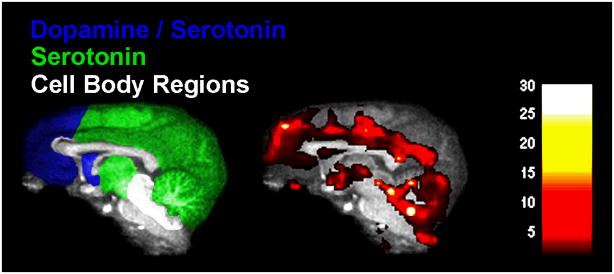
To eliminate the confounding effects of anesthetics, other ambitious work has attempted to extend these findings by determining the neurocircuity of drug taking in awake nonhuman primates. However, there are significant challenges associated with the conduct of fMRI imaging in awake animals as it is inherently more sensitive to subject motion than PET imaging and requires restraint equipment built entirely from non-ferrous materials. Despite these challenges, fMRI should prove to be highly effective in characterizing drug-induced changes in brain activity at a systems level. Indeed, similar to its neurochemical effects (Baumann et al. 2008; Murnane et al. 2010), a recent study found that MDMA-activated brain regions matched the innervation patterns of the mesolimbic and mesocortical dopamine pathways and the raphe-originated serotonergic pathways (Brevard et al. 2006). In further support of these findings, preliminary data from our laboratory using fMRI in rhesus monkeys showed a comparable complex pattern of brain activational effects engendered by MDMA, with elements of dopaminergic and serotonergic activation (Figure 1). Collectively, the results indicate that stimulants with varied mechanisms of action may each induce a unique profile of effects on brain activity. Comparing these unique profiles to differences in drug self-administration would provide a better conceptualization of the neurocircuitry of drug-taking behavior.
Figure 1.
The left panel shows regions that that receive innervations by both dopamine and serotonin (blue), regions that receive innervations by serotonin but little dopamine innervation (green), and regions that contain the cell bodies of dopamine and serotonin neurons (white). The right panel shows the brain activational effects of intravenous administration of S,R(+/−)-MDMA at 1.0 mg/kg (n = 3) expressed as t-values. Both panels are presented in sagital section to illustrate the mixture of dopaminergic and serotonergic effects induced by S,R(+/−)-MDMA. The magnitude of the t-value is color coded as shown by the key inset.
The acute effects of stimulants on cerebral blood flow and metabolism have been examined in human subjects, often by those seeking to define neuronal bases for drug-induced euphoria as a determinant of drug taking. Activation of the anterior cingulate has been observed in response to acute administration of cocaine and related stimulants (Breiter et al. 1997; Volkow et al. 1999c) and cocaine-related environmental cues (Childress et al. 1999; Kilts et al. 2001; Maas et al. 1998; Wexler et al. 2001). Moreover, activation of the dorsolateral prefrontal cortex has also been observed in response to cocaine (Kufahl et al. 2005) and cocaine cues (Grant et al. 1996; Maas et al. 1998). Intravenous administration of methylphenidate in normal subjects caused variable changes in brain metabolism (Volkow et al. 1997b). Subjects with higher dopamine D2 receptor availability tended to show increased metabolism whereas those with lower D2 availability tended to show decreased metabolism. Similar results were observed in cocaine users, in whom methylphenidate-induced increases in metabolism in the right orbitofrontal cortex and right striatum were associated with drug craving (Volkow et al. 1999c). Other investigators have reported that acute cocaine administration increases cerebral blood flow mainly in the frontal and parietal regions (Mathew et al. 1996).
These regional effects highlight the important role of an integrated circuitry in the context of cocaine addiction. The anterior cingulate, part of the extended limbic system, is anatomically linked to the prefrontal cortex and nucleus accumbens, and serves diverse functions including the integration of mood and cognition (Devinsky et al. 1995; Vogt et al. 1992). The dorsolateral and dorsomedial prefrontal cortices are activated during the performance of a variety of cognitive tasks that require working memory or goal-directed behavior (Fuster 1997). Hence, it is apparent that the effects of cocaine extend beyond the limbic system to engage brain areas underlying complex cognitive processes.
It is well documented that environmental variables such as cocaine-associated cues can effectively elicit physiological responses and self reports of cocaine craving and withdrawal (Ehrman et al. 1992). One potential mechanism for this finding is cue-induced dopamine release in the dorsal striatum (Volkow et al. 2006). In support of this contention, others have reported conditioned dopamine release in the ventral striatum in response to amphetamine cues (Boileau et al. 2007). Interestingly, oral methylphenidate administration in cocaine abusers significantly increased dopamine in the striatum as measured by displacement of C11 raclopride, but failed to induce craving unless subjects were concomitantly exposed to cocaine cues (Volkow et al. 2008). Similarly, drug-associated cues have been shown to modulate the brain metabolic effects of stimulants in cocaine abusers. In one study, the brain metabolic effects of methylphenidate were enhanced in cocaine abusers when methylphenidate was administered in the presence of methylphenidate-associated cues (Volkow et al. 2003). Drug-induced increases in self reports of drug “high” were also greater when subjects received methylphenidate in the presence of methylphenidate-associated cues, and self-report measures were significantly correlated with brain metabolic effects. Similar results have been reported for subjects who had minimal experience with stimulant drugs (Volkow et al. 2006). Accordingly, neuroimaging provides an important means of evaluating the mechanisms that mediate the modulation of drug taking by environmental stimuli.
In other work evaluating the role of environmental variables in drug taking, researchers have used fMRI to compare the neural circuitry activated by presentation of cocaine-associated versus neutral stimuli in humans with a history of crack cocaine abuse. Cocaine-associated stimuli activated the anterior cingulate and prefrontal cortex, and activity levels in these regions predicted self reports of craving (Maas et al. 1998). A more tightly controlled study compared the effects of viewing cocaine-associated stimuli, outdoor nature scenes, and sexually explicit content in both cocaine abusers and normal control subjects (Garavan et al. 2000). Brain regions specifically relevant for mediating drug cue processing and cue-elicited craving were operationally defined as those showing significantly greater activation when cocaine abusers viewed cocaine-associated stimuli than when they viewed nature scenes or sexually explicit scenes, and significantly greater activation when cocaine abusers viewed cocaine-associated stimuli than when normal control subjects viewed cocaine-associated stimuli. Across the entire brain, the anterior cingulate, parietal lobe, and caudate were the only regions identified using these criteria as specifically involved in processing cocaine-associated cues and possibly mediated cue-elicited craving. Importantly, the anterior cingulate has been implicated in cognition, including decision making (Walton et al. 2007). Later work examined the relationship between cue-elicited brain activation in cocaine dependent but abstinent subjects and subsequent relapse to cocaine abuse (Kosten et al. 2006). In this study, brain activation in sensory, motor, and cognitive-emotional processing areas was highly predictive of subsequent relapse, and was significantly more predictive of relapse than subjective reports of craving, supporting the use of functional neuroimaging as a tool for medications development.
Long-Term Consequences of Drug Taking
Neurochemistry
A major advantage of functional neuroimaging is the ability to employ longitudinal designs that involve repeated measures over extended periods of time. This approach has been used effectively in nonhuman primates to characterize both transient and long-lasting changes in brain chemistry that are associated with drug taking. For example, PET imaging studies have been conducted in socially housed cynomolgus monkeys to characterize the effects of chronic cocaine exposure in dominant and subordinate individuals. Although dominant monkeys initially exhibit higher D2 receptor availability (Grant et al. 1998; Morgan et al. 2002), chronic exposure to self-administered cocaine resulted in D2 levels that did not differ significantly from those found in subordinate monkeys (Czoty et al. 2004). The authors concluded that chronic exposure to cocaine reduced dopamine receptor availability. A subsequent study examined D2 receptor availability during extended abstinence from cocaine (Nader et al. 2006). In three subjects exposed to cocaine for only one week, D2 receptor availability returned to baseline, pre-drug levels within three weeks. Five subjects that self-administered cocaine for twelve months were studied during cocaine abstinence. Three of the five subjects showed complete recovery of D2 receptor availability within three months of abstinence, whereas the other two subjects did not recover after one year of abstinence. Rate of recovery was not related to total drug intake over the twelve months of cocaine self-administration. It is interesting to note that individual differences in rate of recovery of D2 receptor availability have also been observed following drug-induced increases by the D2 receptor antagonist raclopride (Czoty et al. 2005). Despite any discrepancies, these studies demonstrate that monkeys with long-term histories of cocaine use reliably display lower D2 receptor densities in ways that correlate with cocaine dose and duration of exposure (Moore et al. 1998; Nader et al. 2002).
It has become well accepted that drug-taking behavior can be readily influenced by environmental conditions as well as drug history. Neuroimaging approaches have been used to identify neurobiological mechanisms underlying the influence of environmental determinants of drug-taking behavior. As previously described, cocaine can reliably function as a reinforcer in subordinate monkeys yet fail to maintain self-administration in dominant monkeys. Similarly, subordinate animals were more sensitive to the reinforcing effects of cocaine evaluated with a choice procedure, such that they would choose a lower dose of cocaine over food compared to dominant animals (Czoty et al. 2005). These differences in the dominance rank among socially-housed nonhuman primates have been associated with differential levels of dopamine D2 receptors as measured with [18F]-labeled FCP (see Nader and Czoty 2005). Social housing of male cynomolgus monkeys increased the availability of D2 receptors in dominant animals without producing any changes in subordinate group members, and these changes appeared to exert significant effects on cocaine self-administration (Morgan et al., 2002).
Importantly, the protective effects associated with high D2 receptor density in dominant animals can be attenuated with prolonged exposure to cocaine (Czoty et al. 2004), suggesting that there is a pronounced interaction between environmental and drug history on drug taking. Furthermore, the observation that female cynomolgus monkeys show significant changes in D2 binding potential associated with menstrual cycle phase indicates that sex differences warrants study as an additional determinant of drug-taking behavior (Czoty et al. 2009). For an overview of the anatomical localization of changes that have been measured in primates as a consequence of drug history see Figure 2. Collectively, these studies demonstrate drug history as a determinant of drug-taking behavior may, in some cases, be mediated by plasticity of monoamine systems.
Figure 2.
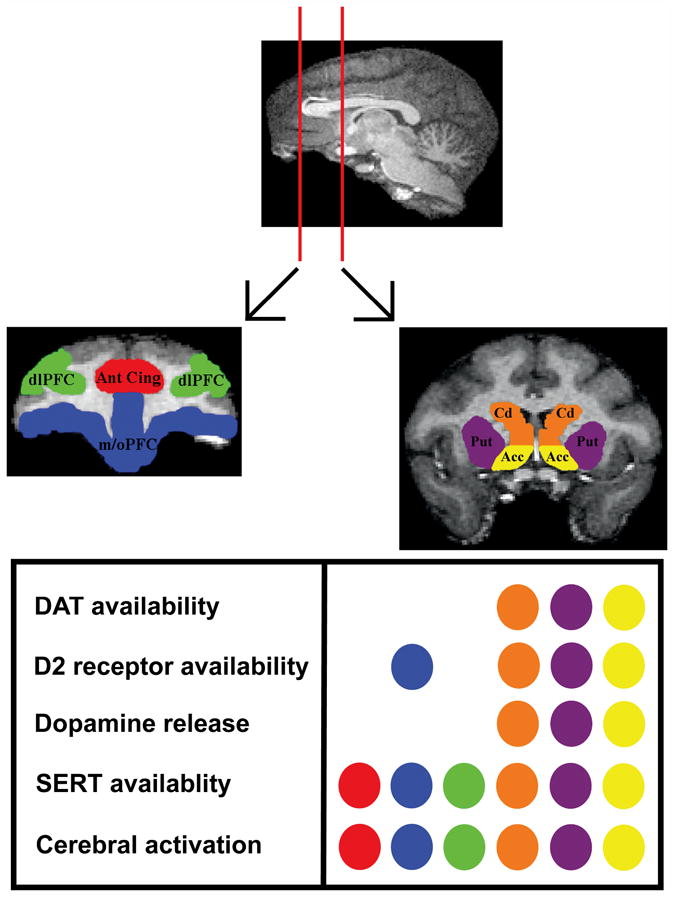
Anatomical localization of long-term changes that have been measured in nonhuman primates as a consequence of exposure to drugs of abuse. The image on the top is a sagittal section from a representative rhesus monkey brain with overlaid cross sections of the subsequently presented coronal sections. The images in the middle represent coronal sections of the prefrontal cortex (left) and the striatum (right) that have been color coded and labeled to indicate brain regions in which changes resulting from exposure to drugs of abuse have been measured. The table on the bottom indicates which changes have occurred and, through the corresponding color coding, the brain regions in which they have been measured. dl PFC = dorsolateral prefrontal cortex; Ant Cing = anterior cingulate; m/o PFC = medial / orbital prefrontal cortex; Cd = caudate; Put = putamen; Acc = nucleus accumbens
Clinical studies that have used functional imaging to characterize the effects of drug history have focused primarily on long-term changes in individuals with a complex history of multidrug use. Similarly to nonhuman primates, chronic exposure to stimulant drugs in humans may also lead to significant reductions in neuronal markers of dopaminergic function. PET studies characterizing dopamine D2 receptors have reliably documented long-lasting decreases in D2 receptor density in stimulant abusers (Volkow and Fowler 2000). The reduction in D2 receptor function may further decrease sensitivity of reward circuits to stimulation by natural rewards and increase the risk for drug-taking (Volkow et al. 2004). Interestingly, no difference in D1 receptor density was observed between cocaine dependent subjects and matched controls as determined with [11C]-labeled NNC 112 (Martinez et al. 2009).
The density of the DAT has also been evaluated with PET imaging studies. In cocaine abusers, dopamine transporter density appears to be elevated shortly after cocaine abstinence but then to normalize with long-term detoxification (Malison et al. 1998). Similarly, methamphetamine-induced reductions in the density of brain dopamine markers have been observed in human users (McCann et al., 1998; Sekine et al., 2001; Volkow et al., 2001b; Volkow et al., 2001d; Johanson et al., 2006). The reduced DAT availability correlated with the duration of drug use and the severity of persistent psychiatric symptoms. Impaired psychomotor and episodic memory functioning was associated with a reduction in DAT availability in the striatum and prefrontal cortex of methamphetamine users (Volkow et al., 2001d). PET imaging using [11C]labeled d-threo-methylphenidate to quantify DAT availability identified partial recovery of DAT binding in methamphetamine abusers during protracted abstinence (Volkow et al. 2001). The correlation persisted in abstinence, as evidenced in a recent study, which found that deficits in memory in abstinent methamphetamine users were associated with decreases in striatal DAT binding potentials (McCann et al., 2008).
Consistent with acute activation of the anterior cingulate by cocaine (Henry et al. 2010; Howell et al. 2010; Murnane and Howell 2010), long-term consumption of cocaine disrupts white matter integrity in this brain region (Lane et al. 2010). Moreover, deficits in white matter integrity have an inverse relationship with the length of abstinence from cocaine abuse in cocaine dependent patients (Xu et al. 2010). Collectively, these studies show that a history of drug taking may influence dopaminergic systems in humans and possibly associated white matter connections. For a comparison of the long-term consequences of exposure to drugs of abuse in nonhuman primates and humans see Table 2.
Table 2.
Long-term consequences of exposure to drugs of abuse in nonhuman primates and humans as measured by microdialysis, autoradiography, or neuroimaging
| Effect | Drug | Nonhuman primates | Humans |
|---|---|---|---|
| DAT Availability | Cocaine | ↑10–3 | ↑16 |
| MDMA/amphetamine | ↓4,28 | ||
| Methamphetamine | ↓5,6 | ↓17,18,19,20,21,27 | |
| Morphine | ↓11 | ||
| D1 receptor availability | Methamphetamine | –5,24 | |
| D2 receptor availability | Cocaine | ↓8,9,13,14 | ↓15 |
| Methamphetamine | –24 | ↓19 | |
| Dopamine release | Cocaine | ↓44 | ↓42 |
| SERT Availability | Cocaine | ↑1 | |
| MDMA | ↓2–1 | ↓25 | |
| vMAT levels | MDMA | –7 | |
| Methamphetamine | –24 | ||
| Distribution of cerebral activation | Cocaine | ↑12,32,33,34 | |
| Cerebral perfusion | Cocaine | ↓35,36,37,38,39 | |
| Cerebral metabolism | Cocaine | ↑12↓32,33,34 | ↑43 ↓40,41 |
| White matter integrity | Cocaine | ↓22,23 | |
| MDMA | ↓25 | ||
| MRS markers | MDMA | ↓29 30,31 |
Czoty et al., 2007,
Czoty et al., 2007,
Letchworth et al., 2001,
Xiao et al., 2006,
McCann et al., 2008,
Sekine et al., 2001,
Johanson et al., 2006,
de Win et al., 2008,
Drug history has also been proposed to compromise CNS function in a manner consistent with “neurotoxic” effects. In this context, drug history effects have been primarily associated with amphetamine derivatives, such as methamphetamine and MDMA. Under a variety of conditions, MDMA has selective and enduring effects on markers of brain serotonin systems. Indeed, one of the most widely cited studies of these neurotoxic effects showed MDMA depleted tissue content of serotonin in the squirrel monkey (Ricaurte et al. 1988). However, early studies were limited by biochemical and histological analyses that required between-subject comparisons. An early PET imaging study in a baboon characterized the effects of MDMA on in vivo SERT availability using [11C]-labeled McN5652 (Scheffel et al. 1998). Following treatment with MDMA twice daily for four consecutive days, PET scans showed reductions in SERT availability in all brain regions analyzed at 13–40 days post-drug treatment but regional differences in its apparent recovery at 9 and 13 months. Similarly, methamphetamine has been shown to reduce DAT availability in baboons (Villemagne et al. 1998) and rhesus monkeys (Hashimoto et al. 2007). However, other studies provided more ambiguous results (Melega et al. 2008), including small and transient changes in D1 receptor availability using [11C]-labeled SCH23390 (Hashimoto et al. 2007). Furthermore, behavioral decrements resulting from neurochemical changes induced by exposure to amphetamine derivatives have been much more difficult to establish (Saadat et al. 2006; Winsauer et al. 2002).
It is critical to note that studies reporting neurotoxic effects of amphetamine derivatives in laboratory animals have relied on non-contingent drug administration rather than models that incorporate drug-taking behavior and have typically administered large and repeated doses. In one of the first studies to characterize the neurochemical effects of self-administered MDMA in nonhuman primates, rhesus monkeys self-administered MDMA for approximately 18 months. PET neuroimaging with [11C]-labeled DTBZ was used to quantify vesicular monoamine transporter (VMAT) availability following at least two months of drug abstinence (Fantegrossi et al. 2004). The reinforcing effects of MDMA were selectively attenuated by chronic MDMA self-administration, perhaps through neurotoxic effects of MDMA. However, there was no significant change in VMAT binding potential and no significant changes in serotonin or dopamine levels in postmortem brains.
A more recent study found a similar lack of significant SERT availability changes following MDMA self-administration in rhesus monkeys using [11C]-labeled DASB (Banks et al. 2008). Hence, non-contingent drug administration has yielded neurochemical changes in the absence of behavioral correlates whereas drug self-administration has yield behavioral alterations in the absence of any significant neurochemical correlates. As such, given the important public health implications of drug-induced neurotoxicity, further study is clearly warranted. In this regard, PET imaging in rhesus monkeys has shown that pre- or post-exposure treatment with the antibiotic minocycline prevents methamphetamine-elicited reductions in DAT availability (Hashimoto et al. 2007). Approaches such as these are likely to be highly beneficial in the prevention or treatment of any neurotoxic effects of amphetamine derivatives.
Studies in human MDMA users have reported enduring decrements in global brain SERT binding which were correlated with the extent of prior MDMA use (Ricaurte et al. 2000). These human studies are consistent with findings in nonhuman primates reported by the same research group. Likewise, humans with histories of methamphetamine use who were imaged after approximately three years of abstinence displayed reduced DAT availability in the caudate and putamen, based on C-11 WIN-35,428 PET studies (McCann et al. 1998). A preliminary study of amphetamine use by recreational users of MDMA also reported reduced striatal DAT binding, as determined by SPECT imaging using [123I]-labeled B-CIT (Reneman et al. 2002). However, similar to studies in laboratory animals, human studies have sometimes produced equivocal findings. For example, recent studies using longitudinal designs did not find a significant correlation between reductions in SERT availability and extent of MDMA abuse. Furthermore, there were no improvements in markers for SERT during periods of drug abstinence (Buchert et al. 2006; Thomasius et al. 2006).
In addition to PET and SPECT neuroimaging, magnetic resonance spectroscopy (MRS) has been effectively applied to the study of subjects with a history of exposure to amphetamine derivatives. This technique allows for quantifying neurochemicals and their metabolites, and putative biochemical markers for gliosis and cell death in discrete brain regions in vivo (see Minati et al. 2007 for a basic description). Similar to PET imaging, this approach has provided mixed results in human MDMA abusers. In one study, decreased ratios of N-acetyl-aspartate to creatine were associated with memory deficits in MDMA users (Reneman et al. 2001). Other studies, however, have reported no differences in biochemical markers between MDMA users and control subjects (Cowan et al. 2007; Daumann et al. 2004). It should be recognized that low magnetic field strength or a limited number of neuroanatomical regions of interest could lead to reduced sensitivity and potential false-negative results. Nonhuman primate research using more tightly controlled subject populations, high field strength magnets, and sufficient access to subjects to provide replication across many brain regions should allow us to address these issues.
Neurocircuitry
The influence of drug history on changes in protein binding in vivo is complemented by a recent study that documented cocaine-induced changes in brain metabolic activity as a function of cocaine self-administration history (Henry et al. 2010). Experimentally naive rhesus monkeys were given increasing access to cocaine self-administration. PET neuroimaging with [18F]-labeled FDG was used to measure acute cocaine-induced changes in brain metabolism in the cocaine-naïve state, and following limited- and extended-access conditions. In the cocaine-naïve state, cocaine-induced increases in brain metabolism were restricted to the anterior cingulate and medial prefrontal cortex. Increased cocaine exposure from limited through extended access recruited cocaine-induced metabolic effects in additional frontal cortical areas and within the striatum. In apparent contrast, tolerance to cocaine-induced elevations of dopamine in the striatum was observed in these same animals under both access conditions (Kirkland Henry et al. 2009).
The progressive involvement of cortical and striatal domains as a function of cocaine exposure has also been demonstrated in macaque monkeys utilizing the 2-[14C]deoxyglucose (2-DG) method (Lyons et al. 1996; Porrino et al. 2004; Porrino et al. 2002). In a series of studies, different groups of subjects were evaluated for changes in neurobiological responses to cocaine as assessed by autoradiography following different durations of cocaine self-administration (Porrino et al. 2002; Porrino et al. 2004). Initial exposures to cocaine resulted in metabolic effects of cocaine contained primarily in the ventral medial regions of the prefrontal cortex compared to saline treated subjects. Changes in activity were also noted in the ventral striatum and small areas of the dorsal striatum. Following chronic exposure to cocaine self-administration, activity expanded within the striatum to encompass both dorsal and ventral regions.
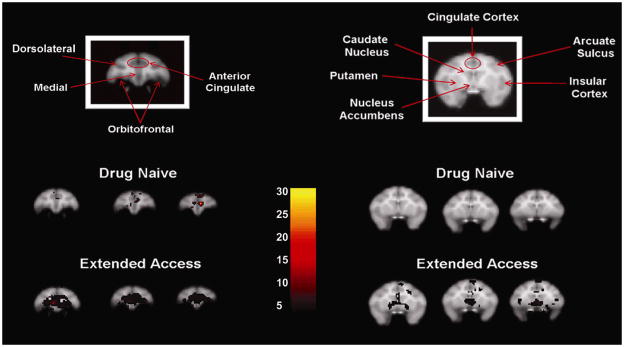
The gradual expansion of the metabolic effects of cocaine is similar to findings reported by Henry and colleagues (2010), which also showed recruitment of metabolic activity in the cortex and striatum in response to cocaine, following a history of taking cocaine. The main difference between these studies is that a history of taking cocaine expanded a pattern of drug-elicited decreases in glucose utilization measured using the 2-DG method whereas a history of taking cocaine expanded a pattern of cocaine-elicited increases in glucose utilization measured using the FDG method. This discrepancy could be attributed to a number of procedural differences, including self-administration versus non-contingent drug administration, multiple within-session doses versus a single dose, total dose administered, and differences between autoradiography and FDG PET neuroimaging. Furthermore, it is important to note that the comparison condition in the 2-DG study was glucose utilization when separate subjects were responding under an operant schedule that resulted in delivery of food, whereas in the FDG study the comparison condition was glucose utilization when the same subjects were administered saline. It is possible that a history of food-reinforced responding may produce distinct and independent effects or there are differences in brain activation when cocaine effects are compared to food reinforced or saline administration. Nevertheless, despite the differences in the direction of cocaine-induced effects on brain activity, there is an obvious pattern in the recruitment of cortical and subcortical regions as a consequence of a drug history. For a summary of this expanded brain activation pattern elicited by acute bolus of cocaine see Figure 3. This finding may explain why a history of drug taking generally increases the sensitivity of subjects to the reinforcing effects of drugs of abuse.
Figure 3.
Increased distribution of metabolic activation by an acute bolus of cocaine as a consequence of cocaine self-administration history. The coronal images on the top designate brain regions at the level of the prefrontal cortex (left) and the striatum (right). The images on the bottom represent cocaine-induced (1.0 mg/kg, IM) metabolic activation in rhesus monkey that were drug naïve (top) or had a history of taking cocaine (bottom) at the level of the prefrontal cortex (left) or the striatum (right). The inset color scale represents the degree of statistical significance expressed in multiples of the t statistic value.
PET imaging has documented decreased blood flow in the prefrontal cortices of chronic cocaine users (Volkow et al. 1988). Additional studies with PET and SPECT imaging have confirmed those results, demonstrating that brain perfusion deficits occur with high frequency (Holman et al. 1991; Holman et al. 1993; Levin et al. 1994; Strickland et al. 1993; Volkow et al. 1991). Local perfusion deficits have been linked closely to changes in cerebral metabolism. Measures of brain glucose metabolism with FDG in chronic users documented transient increases in metabolic activity in dopamine-associated brain regions during cocaine withdrawal (Volkow et al. 1991). Decreases in frontal brain metabolism persisted after months of detoxification. The same pattern of decreased glucose metabolism (Reivich et al. 1985) and perfusion deficits (Volkow et al. 1988) was observed in the prefrontal cortices of a subset of cocaine users who were imaged on multiple occasions. More recently, mood disturbances have been linked to regional cerebral metabolic abnormalities in methamphetamine abusers. Moreover, detoxified cocaine abusers had a marked decrease in dopamine release as measured by methylphenidate-induced decreases in striatal [11C] raclopride binding (Volkow et al., 1997). Self-reports of “high” on the VAS induced by methylphenidate were also less intense in cocaine abusers. Consistent with impaired dopamine function, amphetamine-induced release of striatal dopamine is blunted in cocaine-dependent subjects and this blunted effect is predictive of choice to self-administer cocaine (Martinez et al. 2007). A recent study using fMRI during a working memory task in cocaine-dependent subjects showed impaired activation in frontal, striatal and thalamic brain regions (Moeller et al. 2010). Importantly, thalamic activation significantly correlated with treatment response. Lastly, regional brain glucose metabolism measured by FDG uptake has been characterized in conjunction with dopamine D2 receptors (Volkow et al., 1993, 2001a). Reductions in striatal D2 receptors were associated with decreased metabolic activity in the orbital frontal cortex and anterior cingulate cortex in detoxified individuals. In contrast, the orbital frontal cortex was hypermetabolic in active cocaine abusers (Volkow et al. 1991). In addition, chronic methamphetamine users showed reduced striatal D2 receptors, the loss of which was related to the function of the orbitofrontal cortex (Volkow et al., 2001a), a region important for executive functions. Methamphetamine users also exhibited abnormal brain activity as determined by PET studies to measure cerebral glucose metabolism, with higher activity in the parietal cortex and lower activity in the thalamus and striatum (Volkow et al., 2001c). Collectively, these findings observed in stimulant abusers document significant dysregulation of dopamine systems that are reflected in brain metabolic changes in areas involved in reward circuitry.
Conclusions
Non-invasive neuroimaging techniques have lead to significant advances in our current understanding of the neurobiology of drug-taking behavior and the treatment of drug addiction in humans. The ability to study drug interactions with specific protein targets in vivo has identified pharmacological mechanisms of action associated with the abuse liability of drugs, and supported medications development efforts that have focused primarily on behavioral models of drug abuse. The reinforcing effects of abused stimulants are closely linked to DAT occupancy, and the DAT has been identified as a potential target for medications development. Neuroimaging of cerebral blood flow changes coupled to cerebral metabolism measured with PET and fMRI is particularly well suited to define the neuronal circuitry that underlies drug effects on behavior. It is apparent that the reinforcing effects of abused stimulants extend beyond the limbic system and include the prefrontal cortex and integrated circuitry. The ability to conduct within-subject, longitudinal assessments of brain chemistry and neuronal function should enhance our efforts to document long-term changes due to chronic drug exposure and to elucidate recovery during prolonged abstinence or during treatment interventions. Specifically, dysregulation of dopamine function and brain metabolic changes in areas involved in reward circuitry have been linked to drug-taking behavior, cognitive impairment and treatment response. This review documents the close concordance that can be achieved among functional measures of neuroimaging, neurochemistry and behavior. Importantly, the clinical relevance of information derived from nonhuman primates has been established in several instances, when compared to the outcome of functional imaging studies in humans.
There is a clear need to apply neuroimaging techniques to evaluate classes of abused drugs other than psychostimulants. Although the importance of dopamine in drug addiction is well recognized, other neurotransmitter systems known to play a critical role in the pharmacological effects of abused drugs have been largely ignored in primate neuroimaging. Current technology with PET and SPECT radiochemistry should incorporate the quantification of additional protein targets other than dopamine receptors and transporters. These include serotonin, GABA, glutamate, and other neurotransmitters which play important roles in drug addiction. There has been some progress in the development of techniques to study serotonergic and glutamatergic systems, and a comprehensive understanding of the neurobiology underlying drug addiction will likely depend on the continued development of such novel approaches. In vivo PET measures of neurotransmitter release in nonhuman primates have been limited to dopamine displacement of D2 receptor binding in the striatum. However, it remains to be determined whether neurotransmitters other than dopamine reliably displace PET ligand binding at alternative targets in nonhuman primates, and it will be important to validate these displacement studies with direct measures of neurotransmitter levels derived from in vivo microdialysis.
The study of brain activation by PET imaging with [15O] water and FDG has been mostly replaced in humans by fMRI because of the higher temporal and spatial resolution and lack of radiation exposure with this imaging modality. Recently, there has been some success in implementing pharmacological fMRI in awake nonhuman primates (Brevard et al. 2006; Jenkins et al. 2004; Murnane and Howell 2010). However, there are significant challenges associated with the conduct of fMRI imaging in awake nonhuman primates as it is inherently more sensitive to subject motion than PET imaging and requires restraint equipment built entirely from non-ferrous materials. Despite these challenges, fMRI should prove to be highly effective in characterizing drug-induced changes in brain activity at a systems level but appropriate contrast agents need to be developed that can adequately quantify specific protein targets in brain. Finally, experimental designs employing neuroimaging should consider well documented determinants of drug taking, including pharmacokinetic considerations, subject history and environmental variables. Collectively, these complementary and integrative approaches should further our understanding drug-taking behavior and the treatment of drug abuse and addiction.
Acknowledgments
Research from the laboratory of the authors and preparation of the manuscript were supported in part by U.S. Public Health Service Grants DA10344, DA12514, DA16589, DA00517, and RR00165 (Division of Research Resources, National Institutes of Health).
Literature Cited
- Abreu ME, Bigelow GE, Fleisher L, Walsh SL. Effect of intravenous injection speed on responses to cocaine and hydromorphone in humans. Psychopharmacology (Berl) 2001;154:76–84. doi: 10.1007/s002130000624. [DOI] [PubMed] [Google Scholar]
- Andersen ML, Kessler E, Murnane KS, McClung JC, Tufik S, Howell LL. Dopamine transporter-related effects of modafinil in rhesus monkeys. Psychopharmacology (Berl) 2010;210:439–48. doi: 10.1007/s00213-010-1839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AL, Reid MS, Li SH, Holmes T, Shemanski L, Slee A, Smith EV, Kahn R, Chiang N, Vocci F, Ciraulo D, Dackis C, Roache JD, Salloum IM, Somoza E, Urschel HC, 3rd, Elkashef AM. Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend. 2009;104:133–9. doi: 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Czoty PW, Gage HD, Bounds MC, Garg PK, Garg S, Nader MA. Effects of cocaine and MDMA self-administration on serotonin transporter availability in monkeys. Neuropsychopharmacology. 2008;33:219–25. doi: 10.1038/sj.npp.1301420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Sprague JE, Kisor DF, Czoty PW, Nichols DE, Nader MA. Ambient temperature effects on 3,4-methylenedioxymethamphetamine-induced thermodysregulation and pharmacokinetics in male monkeys. Drug Metab Dispos. 2007;35:1840–5. doi: 10.1124/dmd.107.016261. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Franken FH, Rutter JJ, Rothman RB. Tolerance to 3,4-methylenedioxymethamphetamine in rats exposed to single high-dose binges. Neuroscience. 2008;152:773–84. doi: 10.1016/j.neuroscience.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger B, Trottier S, Verney C, Gaspar P, Alvarez C. Regional and laminar distribution of the dopamine and serotonin innervation in the macaque cerebral cortex: a radioautographic study. J Comp Neurol. 1988;273:99–119. doi: 10.1002/cne.902730109. [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Welfeld K, Booij L, Diksic M, Benkelfat C. Conditioned dopamine release in humans: a positron emission tomography [11C]raclopride study with amphetamine. J Neurosci. 2007;27:3998–4003. doi: 10.1523/JNEUROSCI.4370-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Brevard ME, Meyer JS, Harder JA, Ferris CF. Imaging brain activity in conscious monkeys following oral MDMA ("ecstasy") Magn Reson Imaging. 2006;24:707–14. doi: 10.1016/j.mri.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Serotonin 5-HT2A and 5-HT2C receptors as potential targets for modulation of psychostimulant use and dependence. Curr Top Med Chem. 2006;6:1971–85. doi: 10.2174/156802606778522131. [DOI] [PubMed] [Google Scholar]
- Buchert R, Thomasius R, Petersen K, Wilke F, Obrocki J, Nebeling B, Wartberg L, Zapletalova P, Clausen M. Reversibility of ecstasy-induced reduction in serotonin transporter availability in polydrug ecstasy users. Eur J Nucl Med Mol Imaging. 2006;33:188–99. doi: 10.1007/s00259-005-1850-8. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–8. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan RL, Bolo NR, Dietrich M, Haga E, Lukas SE, Renshaw PF. Occipital cortical proton MRS at 4 Tesla in human moderate MDMA polydrug users. Psychiatry Res. 2007;155:179–88. doi: 10.1016/j.pscychresns.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Gage HD, Nader MA. PET imaging of striatal dopamine D2 receptors in nonhuman primates: increases in availability produced by chronic raclopride treatment. Synapse. 2005;58:215–9. doi: 10.1002/syn.20200. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Ginsburg BC, Howell LL. Serotonergic attenuation of the reinforcing and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 2002;300:831–7. doi: 10.1124/jpet.300.3.831. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Morgan D, Shannon EE, Gage HD, Nader MA. Characterization of dopamine D1 and D2 receptor function in socially housed cynomolgus monkeys self-administering cocaine. Psychopharmacology (Berl) 2004;174:381–8. doi: 10.1007/s00213-003-1752-z. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Riddick NV, Gage HD, Sandridge M, Nader SH, Garg S, Bounds M, Garg PK, Nader MA. Effect of menstrual cycle phase on dopamine D2 receptor availability in female cynomolgus monkeys. Neuropsychopharmacology. 2009;34:548–54. doi: 10.1038/npp.2008.3. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O'Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30:205–11. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Lynch KG, Yu E, Samaha FF, Kampman KM, Cornish JW, Rowan A, Poole S, White L, O'Brien CP. Modafinil and cocaine: a double-blind, placebo-controlled drug interaction study. Drug Alcohol Depend. 2003;70:29–37. doi: 10.1016/s0376-8716(02)00335-6. [DOI] [PubMed] [Google Scholar]
- Daumann J, Fischermann T, Pilatus U, Thron A, Moeller-Hartmann W, Gouzoulis-Mayfrank E. Proton magnetic resonance spectroscopy in ecstasy (MDMA) users. Neurosci Lett. 2004;362:113–6. doi: 10.1016/j.neulet.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Deneau G, Yanagita T, Seevers MH. Self-administration of psychoactive substances by the monkey. Psychopharmacologia. 1969;16:30–48. doi: 10.1007/BF00405254. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118 ( Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dewey SL, Smith GS, Logan J, Brodie JD, Yu DW, Ferrieri RA, King PT, MacGregor RR, Martin TP, Wolf AP, et al. GABAergic inhibition of endogenous dopamine release measured in vivo with 11C-raclopride and positron emission tomography. J Neurosci. 1992;12:3773–80. doi: 10.1523/JNEUROSCI.12-10-03773.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin SI, Mirkis S, Smith JE. Response-dependent versus response-independent presentation of cocaine: differences in the lethal effects of the drug. Psychopharmacology (Berl) 1995;117:262–6. doi: 10.1007/BF02246100. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O'Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107:523–9. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Woolverton WL, Kilbourn M, Sherman P, Yuan J, Hatzidimitriou G, Ricaurte GA, Woods JH, Winger G. Behavioral and neurochemical consequences of long-term intravenous self-administration of MDMA and its enantiomers by rhesus monkeys. Neuropsychopharmacology. 2004;29:1270–81. doi: 10.1038/sj.npp.1300442. [DOI] [PubMed] [Google Scholar]
- Ford RD, Balster RL. Reinforcing properties of intravenous procaine in rhesus monkeys. Pharmacol Biochem Behav. 1977;6:289–96. doi: 10.1016/0091-3057(77)90027-2. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Kroll C, Ferrieri R, Alexoff D, Logan J, Dewey SL, Schiffer W, Schlyer D, Carter P, King P, Shea C, Xu Y, Muench L, Benveniste H, Vaska P, Volkow ND. PET studies of d-methamphetamine pharmacokinetics in primates: comparison with l-methamphetamine and ( --)-cocaine. J Nucl Med. 2007;48:1724–32. doi: 10.2967/jnumed.107.040279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, Macgregor RR, Hitzemann R, Logan J, Bendriem B, Gatley SJ, et al. Mapping cocaine binding sites in human and baboon brain in vivo. Synapse. 1989;4:371–7. doi: 10.1002/syn.890040412. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dense C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Network memory. Trends Neurosci. 1997;20:451–9. doi: 10.1016/s0166-2236(97)01128-4. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–98. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Ginsburg BC, Kimmel HL, Carroll FI, Goodman MM, Howell LL. Interaction of cocaine and dopamine transporter inhibitors on behavior and neurochemistry in monkeys. Pharmacol Biochem Behav. 2005;80:481–91. doi: 10.1016/j.pbb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Gold LH, Balster RL. Evaluation of the cocaine-like discriminative stimulus effects and reinforcing effects of modafinil. Psychopharmacology (Berl) 1996;126:286–92. doi: 10.1007/BF02247379. [DOI] [PubMed] [Google Scholar]
- Grant KA, Shively CA, Nader MA, Ehrenkaufer RL, Line SW, Morton TE, Gage HD, Mach RH. Effect of social status on striatal dopamine D2 receptor binding characteristics in cynomolgus monkeys assessed with positron emission tomography. Synapse. 1998;29:80–3. doi: 10.1002/(SICI)1098-2396(199805)29:1<80::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93:12040–5. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN. Neurotransmitters in the human and nonhuman primate basal ganglia. Hum Neurobiol. 1986;5:159–68. [PubMed] [Google Scholar]
- Haber SN, Fudge JL. The primate substantia nigra and VTA: integrative circuitry and function. Crit Rev Neurobiol. 1997;11:323–42. doi: 10.1615/critrevneurobiol.v11.i4.40. [DOI] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–76. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, McFarland NR. The concept of the ventral striatum in nonhuman primates. Ann N Y Acad Sci. 1999;877:33–48. doi: 10.1111/j.1749-6632.1999.tb09259.x. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. Smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacology. 2008;33:761–8. doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Tsukada H, Nishiyama S, Fukumoto D, Kakiuchi T, Iyo M. Protective effects of minocycline on the reduction of dopamine transporters in the striatum after administration of methamphetamine: a positron emission tomography study in conscious monkeys. Biol Psychiatry. 2007;61:577–81. doi: 10.1016/j.biopsych.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology (Berl) 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Henry PK, Murnane KS, Votaw JR, Howell LL. Acute brain metabolic effects of cocaine in rhesus monkeys with a history of cocaine use. Brain Imaging Behav. 2010a doi: 10.1007/s11682-010-9100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman BL, Carvalho PA, Mendelson J, Teoh SK, Nardin R, Hallgring E, Hebben N, Johnson KA. Brain perfusion is abnormal in cocaine-dependent polydrug users: a study using technetium-99m-HMPAO and ASPECT. J Nucl Med. 1991;32:1206–10. [PubMed] [Google Scholar]
- Holman BL, Mendelson J, Garada B, Teoh SK, Hallgring E, Johnson KA, Mello NK. Regional cerebral blood flow improves with treatment in chronic cocaine polydrug users. J Nucl Med. 1993;34:723–7. [PubMed] [Google Scholar]
- Howell LL. Nonhuman primate neuroimaging and cocaine medication development. Exp Clin Psychopharmacol. 2008;16:446–57. doi: 10.1037/a0014196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Byrd LD. Serotonergic modulation of the behavioral effects of cocaine in the squirrel monkey. J Pharmacol Exp Ther. 1995;275:1551–9. [PubMed] [Google Scholar]
- Howell LL, Carroll FI, Votaw JR, Goodman MM, Kimmel HL. Effects of combined dopamine and serotonin transporter inhibitors on cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320:757–65. doi: 10.1124/jpet.106.108324. [DOI] [PubMed] [Google Scholar]
- Howell LL, Czoty PW, Kuhar MJ, Carrol FI. Comparative behavioral pharmacology of cocaine and the selective dopamine uptake inhibitor RTI-113 in the squirrel monkey. J Pharmacol Exp Ther. 2000;292:521–9. [PubMed] [Google Scholar]
- Howell LL, Hoffman JM, Votaw JR, Landrum AM, Jordan JF. An apparatus and behavioral training protocol to conduct positron emission tomography (PET) neuroimaging in conscious rhesus monkeys. J Neurosci Methods. 2001;106:161–9. doi: 10.1016/s0165-0270(01)00345-4. [DOI] [PubMed] [Google Scholar]
- Howell LL, Hoffman JM, Votaw JR, Landrum AM, Wilcox KM, Lindsey KP. Cocaine-induced brain activation determined by positron emission tomography neuroimaging in conscious rhesus monkeys. Psychopharmacology (Berl) 2002;159:154–60. doi: 10.1007/s002130100911. [DOI] [PubMed] [Google Scholar]
- Howell LL, Murnane KS. Nonhuman primate neuroimaging and the neurobiology of psychostimulant addiction. Ann N Y Acad Sci. 2008;1141:176–94. doi: 10.1196/annals.1441.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Votaw JR, Goodman MM, Lindsey KP. Cortical activation during cocaine use and extinction in rhesus monkeys. Psychopharmacology (Berl) 2010;208:191–9. doi: 10.1007/s00213-009-1720-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Wilcox KM. Functional imaging and neurochemical correlates of stimulant self-administration in primates. Psychopharmacology (Berl) 2002;163:352–61. doi: 10.1007/s00213-002-1207-y. [DOI] [PubMed] [Google Scholar]
- Innis RB, Malison RT, al-Tikriti M, Hoffer PB, Sybirska EH, Seibyl JP, Zoghbi SS, Baldwin RM, Laruelle M, Smith EO, et al. Amphetamine-stimulated dopamine release competes in vivo for [123I]IBZM binding to the D2 receptor in nonhuman primates. Synapse. 1992;10:177–84. doi: 10.1002/syn.890100302. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci. 2002;22:6247–53. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski DR. An evaluation of the abuse potential of modafinil using methylphenidate as a reference. J Psychopharmacol. 2000;14:53–60. doi: 10.1177/026988110001400107. [DOI] [PubMed] [Google Scholar]
- Jenkins BG, Sanchez-Pernaute R, Brownell AL, Chen YC, Isacson O. Mapping dopamine function in primates using pharmacologic magnetic resonance imaging. J Neurosci. 2004;24:9553–60. doi: 10.1523/JNEUROSCI.1558-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE. The reinforcing properties of procaine, chloroprocaine and proparacaine in rhesus monkeys. Psychopharmacology (Berl) 1980;67:189–94. doi: 10.1007/BF00431976. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O'Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–80. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kelleher RT, Morse WH. Determinants of the specificity of behavioral effects of drugs. Ergeb Physiol. 1968;60:1–56. doi: 10.1007/BFb0107250. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–41. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Negus SS, Wilcox KM, Ewing SB, Stehouwer J, Goodman MM, Votaw JR, Mello NK, Carroll FI, Howell LL. Relationship between rate of drug uptake in brain and behavioral pharmacology of monoamine transporter inhibitors in rhesus monkeys. Pharmacol Biochem Behav. 2008;90:453–62. doi: 10.1016/j.pbb.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel HL, O'Connor JA, Carroll FI, Howell LL. Faster onset and dopamine transporter selectivity predict stimulant and reinforcing effects of cocaine analogs in squirrel monkeys. Pharmacol Biochem Behav. 2007;86:45–54. doi: 10.1016/j.pbb.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland Henry P, Davis M, Howell LL. Effects of cocaine self-administration history under limited and extended access conditions on in vivo striatal dopamine neurochemistry and acoustic startle in rhesus monkeys. Psychopharmacology (Berl) 2009;205:237–47. doi: 10.1007/s00213-009-1534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31:644–50. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Li Z, Risinger RC, Rainey CJ, Wu G, Bloom AS, Li SJ. Neural responses to acute cocaine administration in the human brain detected by fMRI. Neuroimage. 2005;28:904–14. doi: 10.1016/j.neuroimage.2005.06.039. [DOI] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, Cheng H-M, Brady TJ, Rosen BR. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci. 1992;89:5675. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SD, Steinberg JL, Ma L, Hasan KM, Kramer LA, Zuniga EA, Narayana PA, Moeller FG. Diffusion tensor imaging and decision making in cocaine dependence. PLoS One. 2010;5:e11591. doi: 10.1371/journal.pone.0011591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–51. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Iyer RN, al-Tikriti MS, Zea-Ponce Y, Malison R, Zoghbi SS, Baldwin RM, Kung HF, Charney DS, Hoffer PB, Innis RB, Bradberry CW. Microdialysis and SPECT measurements of amphetamine-induced dopamine release in nonhuman primates. Synapse. 1997;25:1–14. doi: 10.1002/(SICI)1098-2396(199701)25:1<1::AID-SYN1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Laties VG. Lessons from the History of Behavioral Pharmacology. In: Krasnegor NA, Gray DB, Thompson T, editors. Advances in Behavioral Pharmacology. Lawrence Erlbaum Associates; Hillsdale, NJ: 1986. [Google Scholar]
- Levin JM, Holman BL, Mendelson JH, Teoh SK, Garada B, Johnson KA, Springer S. Gender differences in cerebral perfusion in cocaine abuse: technetium-99m-HMPAO SPECT study of drug-abusing women. J Nucl Med. 1994;35:1902–9. [PubMed] [Google Scholar]
- Lindsey KP, Wilcox KM, Votaw JR, Goodman MM, Plisson C, Carroll FI, Rice KC, Howell LL. Effects of dopamine transporter inhibitors on cocaine self-administration in rhesus monkeys: relationship to transporter occupancy determined by positron emission tomography neuroimaging. J Pharmacol Exp Ther. 2004;309:959–69. doi: 10.1124/jpet.103.060293. [DOI] [PubMed] [Google Scholar]
- Lyons D, Friedman DP, Nader MA, Porrino LJ. Cocaine alters cerebral metabolism within the ventral striatum and limbic cortex of monkeys. J Neurosci. 1996;16:1230–8. doi: 10.1523/JNEUROSCI.16-03-01230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry. 1998;155:124–6. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- Mach RH, Nader MA, Ehrenkaufer RL, Line SW, Smith CR, Gage HD, Morton TE. Use of positron emission tomography to study the dynamics of psychostimulant-induced dopamine release. Pharmacol Biochem Behav. 1997;57:477–86. doi: 10.1016/s0091-3057(96)00449-2. [DOI] [PubMed] [Google Scholar]
- Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L, Johnson R, Livni E, Spencer TJ, Bonab AA, Miller GM, Fischman AJ. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006;319:561–9. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- Malison RT, Best SE, van Dyck CH, McCance EF, Wallace EA, Laruelle M, Baldwin RM, Seibyl JP, Price LH, Kosten TR, Innis RB. Elevated striatal dopamine transporters during acute cocaine abstinence as measured by [123I] beta-CIT SPECT. Am J Psychiatry. 1998;155:832–4. doi: 10.1176/ajp.155.6.832. [DOI] [PubMed] [Google Scholar]
- Marsch LA, Bickel WK, Badger GJ, Rathmell JP, Swedberg MD, Jonzon B, Norsten-Hoog C. Effects of infusion rate of intravenously administered morphine on physiological, psychomotor, and self-reported measures in humans. J Pharmacol Exp Ther. 2001;299:1056–65. [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Narendran R, Foltin RW, Broft A, Hwang DR, Perez A, Abi-Dargham A, Fischman MW, Kleber HD, Laruelle M. Dopamine D1 receptors in cocaine dependence measured with PET and the choice to self-administer cocaine. Neuropsychopharmacology. 2009;34:1774–82. doi: 10.1038/npp.2008.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Lowe JV, Humphries D. Acute changes in cranial blood flow after cocaine hydrochloride. Biol Psychiatry. 1996;40:609–16. doi: 10.1016/0006-3223(95)00033-x. [DOI] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA. Positron emission tomographic evidence of toxic effect of MDMA ("Ecstasy") on brain serotonin neurons in human beings. Lancet. 1998;352:1433–7. doi: 10.1016/s0140-6736(98)04329-3. [DOI] [PubMed] [Google Scholar]
- Melega WP, Jorgensen MJ, Lacan G, Way BM, Pham J, Morton G, Cho AK, Fairbanks LA. Long-term methamphetamine administration in the vervet monkey models aspects of a human exposure: brain neurotoxicity and behavioral profiles. Neuropsychopharmacology. 2008;33:1441–52. doi: 10.1038/sj.npp.1301502. [DOI] [PubMed] [Google Scholar]
- Minati L, Grisoli M, Bruzzone MG. MR spectroscopy, functional MRI, and diffusion-tensor imaging in the aging brain: a conceptual review. J Geriatr Psychiatry Neurol. 2007;20:3–21. doi: 10.1177/0891988706297089. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Steinberg JL, Schmitz JM, Ma L, Liu S, Kjome KL, Rathnayaka N, Kramer LA, Narayana PA. Working memory fMRI activation in cocaine-dependent subjects: association with treatment response. Psychiatry Res. 2010;181:174–82. doi: 10.1016/j.pscychresns.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RJ, Vinsant SL, Nader MA, Porrino LJ, Friedman DP. Effect of cocaine self-administration on dopamine D2 receptors in rhesus monkeys. Synapse. 1998;30:88–96. doi: 10.1002/(SICI)1098-2396(199809)30:1<88::AID-SYN11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–74. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Yang ZY, Lew R, Brown T, Kronmal S, Cooper MD, Seiden LS. Evaluation of d-amphetamine effects on the binding of dopamine D-2 receptor radioligand, 18F-fallypride in nonhuman primates using positron emission tomography. Synapse. 1997;27:1–13. doi: 10.1002/(SICI)1098-2396(199709)27:1<1::AID-SYN1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Murnane KS, Fantegrossi WE, Godfrey JR, Banks ML, Howell LL. Endocrine and neurochemical effects of 3,4-methylenedioxymethamphetamine and its stereoisomers in rhesus monkeys. J Pharmacol Exp Ther. 2010;334:642–50. doi: 10.1124/jpet.110.166595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane KS, Howell LL. Development of an apparatus and methodology for conducting functional magnetic resonance imaging (fMRI) with pharmacological stimuli in conscious rhesus monkeys. J Neurosci Methods. 2010;191:11–20. doi: 10.1016/j.jneumeth.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Czoty PW. PET imaging of dopamine D2 receptors in monkey models of cocaine abuse: genetic predisposition versus environmental modulation. Am J Psychiatry. 2005;162:1473–82. doi: 10.1176/appi.ajp.162.8.1473. [DOI] [PubMed] [Google Scholar]
- Nader MA, Czoty PW, Gould RW, Riddick NV. Review. Positron emission tomography imaging studies of dopamine receptors in primate models of addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3223–32. doi: 10.1098/rstb.2008.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, Friedman DP, Porrino LJ. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;27:35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–6. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Narendran R, Slifstein M, Hwang DR, Hwang Y, Scher E, Reeder S, Martinez D, Laruelle M. Amphetamine-induced dopamine release: duration of action as assessed with the D2/3 receptor agonist radiotracer (-)-N-[(11)C]propyl-norapomorphine ([11C]NPA) in an anesthetized nonhuman primate. Synapse. 2007;61:106–9. doi: 10.1002/syn.20346. [DOI] [PubMed] [Google Scholar]
- Nelson RA, Boyd SJ, Ziegelstein RC, Herning R, Cadet JL, Henningfield JE, Schuster CR, Contoreggi C, Gorelick DA. Effect of rate of administration on subjective and physiological effects of intravenous cocaine in humans. Drug Alcohol Depend. 2006;82:19–24. doi: 10.1016/j.drugalcdep.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim S-G, Merkle H, Ugurbil K. Intrinsic Signal Changes Accompanying Sensory Stimulation: Functional Brain Mapping with Magnetic Resonance Imaging Proc. Natl Acad Sci. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Goldberg SR, Gilman JP, Jufer R, Cone EJ, Schindler CW. Effects of delivery rate and non-contingent infusion of cocaine on cocaine self-administration in rhesus monkeys. Psychopharmacology (Berl) 1998;137:253–8. doi: 10.1007/s002130050618. [DOI] [PubMed] [Google Scholar]
- Phelps ME, Mazziotta JC. Positron emission tomography: human brain function and biochemistry. Science. 1985;228:799–809. doi: 10.1126/science.2860723. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Daunais JB, Smith HR, Nader MA. The expanding effects of cocaine: studies in a nonhuman primate model of cocaine self-administration. Neurosci Biobehav Rev. 2004;27:813–20. doi: 10.1016/j.neubiorev.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Miller MD, Smith HR, Friedman DP, Daunais JB, Nader MA. Metabolic mapping of the effects of cocaine during the initial phases of self-administration in the nonhuman primate. J Neurosci. 2002;22:7687–94. doi: 10.1523/JNEUROSCI.22-17-07687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reivich M, Alavi A, Wolf A, Fowler J, Russell J, Arnett C, MacGregor RR, Shiue CY, Atkins H, Anand A, et al. Glucose metabolic rate kinetic model parameter determination in humans: the lumped constants and rate constants for [18F]fluorodeoxyglucose and [11C]deoxyglucose. J Cereb Blood Flow Metab. 1985;5:179–92. doi: 10.1038/jcbfm.1985.24. [DOI] [PubMed] [Google Scholar]
- Reneman L, Booij J, Lavalaye J, de Bruin K, Reitsma JB, Gunning B, den Heeten GJ, van Den Brink W. Use of amphetamine by recreational users of ecstasy (MDMA) is associated with reduced striatal dopamine transporter densities: a [123I]beta-CIT SPECT study--preliminary report. Psychopharmacology (Berl) 2002;159:335–40. doi: 10.1007/s00213-001-0930-0. [DOI] [PubMed] [Google Scholar]
- Reneman L, Majoie CB, Schmand B, van den Brink W, den Heeten GJ. Prefrontal N-acetylaspartate is strongly associated with memory performance in (abstinent) ecstasy users: preliminary report. Biol Psychiatry. 2001;50:550–4. doi: 10.1016/s0006-3223(01)01177-5. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Forno LS, Wilson MA, DeLanney LE, Irwin I, Molliver ME, Langston JW. (+/-)3,4-Methylenedioxymethamphetamine selectively damages central serotonergic neurons in nonhuman primates. Jama. 1988;260:51–5. [PubMed] [Google Scholar]
- Ricaurte GA, McCann UD, Szabo Z, Scheffel U. Toxicodynamics and long-term toxicity of the recreational drug, 3, 4-methylenedioxymethamphetamine (MDMA, 'Ecstasy') Toxicol Lett. 2000;112-113:143–6. doi: 10.1016/s0378-4274(99)00216-7. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Boja JW, George FR, Kuhar MJ. Cocaine binding sites related to drug self-administration. NIDA Res Monogr. 1989;95:239–46. [PubMed] [Google Scholar]
- Saadat KS, Elliott JM, Green AR, Moran PM. High-dose MDMA does not result in long-term changes in impulsivity in the rat. Psychopharmacology (Berl) 2006;188:75–83. doi: 10.1007/s00213-006-0470-8. [DOI] [PubMed] [Google Scholar]
- Scheffel U, Szabo Z, Mathews WB, Finley PA, Dannals RF, Ravert HT, Szabo K, Yuan J, Ricaurte GA. In vivo detection of short- and long-term MDMA neurotoxicity--a positron emission tomography study in the living baboon brain. Synapse. 1998;29:183–92. doi: 10.1002/(SICI)1098-2396(199806)29:2<183::AID-SYN9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Senda M, Kimura Y, Herscovitch P. Brain Imaging using PET. Academic Press; 2002. [Google Scholar]
- Seneca N, Finnema SJ, Farde L, Gulyas B, Wikstrom HV, Halldin C, Innis RB. Effect of amphetamine on dopamine D2 receptor binding in nonhuman primate brain: a comparison of the agonist radioligand [11C]MNPA and antagonist [11C]raclopride. Synapse. 2006;59:260–9. doi: 10.1002/syn.20238. [DOI] [PubMed] [Google Scholar]
- Sleight AJ, Stam NJ, Mutel V, Vanderheyden PM. Radiolabelling of the human 5-HT2A receptor with an agonist, a partial agonist and an antagonist: effects on apparent agonist affinities. Biochem Pharmacol. 1996;51:71–6. doi: 10.1016/0006-2952(95)02122-1. [DOI] [PubMed] [Google Scholar]
- Spragg SDS. Morphine Addiction in Chimpanzees. Comparative Psychology Monographs. 1940;15:1–132. [Google Scholar]
- Stoops WW, Lile JA, Fillmore MT, Glaser PE, Rush CR. Reinforcing effects of modafinil: influence of dose and behavioral demands following drug administration. Psychopharmacology (Berl) 2005;182:186–93. doi: 10.1007/s00213-005-0044-1. [DOI] [PubMed] [Google Scholar]
- Strickland TL, Mena I, Villanueva-Meyer J, Miller BL, Cummings J, Mehringer CM, Satz P, Myers H. Cerebral perfusion and neuropsychological consequences of chronic cocaine use. J Neuropsychiatry Clin Neurosci. 1993;5:419–27. doi: 10.1176/jnp.5.4.419. [DOI] [PubMed] [Google Scholar]
- Thomasius R, Zapletalova P, Petersen K, Buchert R, Andresen B, Wartberg L, Nebeling B, Schmoldt A. Mood, cognition and serotonin transporter availability in current and former ecstasy (MDMA) users: the longitudinal perspective. J Psychopharmacol. 2006;20:211–25. doi: 10.1177/0269881106059486. [DOI] [PubMed] [Google Scholar]
- Thompson T, Schuster CR. Morphine Self-Administration, Food-Reinforced, and Avoidance Behaviors in Rhesus Monkeys. Psychopharmacologia. 1964;5:87–94. doi: 10.1007/BF00413045. [DOI] [PubMed] [Google Scholar]
- Tokunaga M, Seneca N, Shin RM, Maeda J, Obayashi S, Okauchi T, Nagai Y, Zhang MR, Nakao R, Ito H, Innis RB, Halldin C, Suzuki K, Higuchi M, Suhara T. Neuroimaging and physiological evidence for involvement of glutamatergic transmission in regulation of the striatal dopaminergic system. J Neurosci. 2009;29:1887–96. doi: 10.1523/JNEUROSCI.2559-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berckel BN, Kegeles LS, Waterhouse R, Guo N, Hwang DR, Huang Y, Narendran R, Van Heertum R, Laruelle M. Modulation of amphetamine-induced dopamine release by group II metabotropic glutamate receptor agonist LY354740 in non-human primates studied with positron emission tomography. Neuropsychopharmacology. 2006;31:967–77. doi: 10.1038/sj.npp.1300902. [DOI] [PubMed] [Google Scholar]
- Villemagne V, Yuan J, Wong DF, Dannals RF, Hatzidimitriou G, Mathews WB, Ravert HT, Musachio J, McCann UD, Ricaurte GA. Brain dopamine neurotoxicity in baboons treated with doses of methamphetamine comparable to those recreationally abused by humans: evidence from [11C]WIN-35,428 positron emission tomography studies and direct in vitro determinations. J Neurosci. 1998;18:419–27. doi: 10.1523/JNEUROSCI.18-01-00419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–43. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21:9414–8. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley JS, Dewey S, Ashby C, Liebermann J, Hitzemann R, et al. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry. 1995;52:456–63. doi: 10.1001/archpsyc.1995.03950180042006. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–25. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Gatley SJ, Dewey SL, Wang GJ, Logan J, Ding YS, Franceschi D, Gifford A, Morgan A, Pappas N, King P. Comparable changes in synaptic dopamine induced by methylphenidate and by cocaine in the baboon brain. Synapse. 1999a;31:59–66. doi: 10.1002/(SICI)1098-2396(199901)31:1<59::AID-SYN8>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F, Wang GJ, Jayne M, Hooker JM, Wong C, Hubbard B, Carter P, Warner D, King P, Shea C, Xu Y, Muench L, Apelskog-Torres K. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. Jama. 2009;301:1148–54. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–69. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, Alpert R, Hoff A. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry. 1991;148:621–6. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Mullani N, Gould KL, Adler S, Krajewski K. Cerebral blood flow in chronic cocaine users: a study with positron emission tomography. Br J Psychiatry. 1988;152:641–8. doi: 10.1192/bjp.152.5.641. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin R, Fowler JS, Franceschi D, Franceschi M, Logan J, Gatley SJ, Wong C, Ding YS, Hitzemann R, Pappas N. Effects of route of administration on cocaine induced dopamine transporter blockade in the human brain. Life Sci. 2000;67:1507–15. doi: 10.1016/s0024-3205(00)00731-1. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, Vitkun S, Logan J, Gatley SJ, Pappas N, Hitzemann R, Shea CE. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997a;386:827–30. doi: 10.1038/386827a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, Dewey SL, Hitzemann R, Gifford AN, Pappas NR. Blockade of striatal dopamine transporters by intravenous methylphenidate is not sufficient to induce self-reports of "high". J Pharmacol Exp Ther. 1999b;288:14–20. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Hitzemann R, Angrist B, Gatley SJ, Logan J, Ding YS, Pappas N. Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: implications in addiction. Am J Psychiatry. 1999c;156:19–26. doi: 10.1176/ajp.156.1.19. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Angrist B, Hitzemann R, Lieberman J, Pappas N. Effects of methylphenidate on regional brain glucose metabolism in humans: relationship to dopamine D2 receptors. Am J Psychiatry. 1997b;154:50–5. doi: 10.1176/ajp.154.1.50. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Jayne M, Telang F, Swanson JM. Effects of expectation on the brain metabolic responses to methylphenidate and to its placebo in non-drug abusing subjects. Neuroimage. 2006;32:1782–92. doi: 10.1016/j.neuroimage.2006.04.192. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Zhu W, Maynard L, Telang F, Vaska P, Ding YS, Wong C, Swanson JM. Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. J Neurosci. 2003;23:11461–8. doi: 10.1523/JNEUROSCI.23-36-11461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage. 2008;39:1266–73. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosburg SK, Hart CL, Haney M, Rubin E, Foltin RW. Modafinil does not serve as a reinforcer in cocaine abusers. Drug Alcohol Depend. 2010;106:233–6. doi: 10.1016/j.drugalcdep.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votaw JR, Howell LL, Martarello L, Hoffman JM, Kilts CD, Lindsey KP, Goodman MM. Measurement of dopamine transporter occupancy for multiple injections of cocaine using a single injection of [F-18]FECNT. Synapse. 2002;44:203–10. doi: 10.1002/syn.10068. [DOI] [PubMed] [Google Scholar]
- Walton ME, Croxson PL, Behrens TE, Kennerley SW, Rushworth MF. Adaptive decision making and value in the anterior cingulate cortex. Neuroimage. 2007;36(Suppl 2):T142–54. doi: 10.1016/j.neuroimage.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerts EM, Fantegrossi WE, Goodwin AK. The value of nonhuman primates in drug abuse research. Exp Clin Psychopharmacol. 2007;15:309–27. doi: 10.1037/1064-1297.15.4.309. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Kimmel HL, Lindsey KP, Votaw JR, Goodman MM, Howell LL. In vivo comparison of the reinforcing and dopamine transporter effects of local anesthetics in rhesus monkeys. Synapse. 2005;58:220–8. doi: 10.1002/syn.20199. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Lindsey KP, Votaw JR, Goodman MM, Martarello L, Carroll FI, Howell LL. Self-administration of cocaine and the cocaine analog RTI-113: relationship to dopamine transporter occupancy determined by PET neuroimaging in rhesus monkeys. Synapse. 2002;43:78–85. doi: 10.1002/syn.10018. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Paul IA, Woolverton WL. Comparison between dopamine transporter affinity and self-administration potency of local anesthetics in rhesus monkeys. Eur J Pharmacol. 1999;367:175–81. doi: 10.1016/s0014-2999(98)00967-4. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Zhou Y, Wong DF, Alexander M, Rahmim A, Hilton J, Weed MR. Blood levels and DA transporter occupancy of orally administered methylphenidate in juvenile rhesus monkeys measured by high resolution PET. Synapse. 2008;62:950–2. doi: 10.1002/syn.20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsauer PJ, McCann UD, Yuan J, Delatte MS, Stevenson MW, Ricaurte GA, Moerschbaecher JM. Effects of fenfluramine, m-CPP and triazolam on repeated-acquisition in squirrel monkeys before and after neurotoxic MDMA administration. Psychopharmacology (Berl) 2002;159:388–96. doi: 10.1007/s00213-001-0942-9. [DOI] [PubMed] [Google Scholar]
- Woolverton WL. Comparison of the reinforcing efficacy of cocaine and procaine in rhesus monkeys responding under a progressive-ratio schedule. Psychopharmacology (Berl) 1995;120:296–302. doi: 10.1007/BF02311177. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Wang Z. Relationship between injection duration, transporter occupancy and reinforcing strength of cocaine. Eur J Pharmacol. 2004;486:251–7. doi: 10.1016/j.ejphar.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Xu J, DeVito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. White matter integrity is associated with treatment outcome measures in cocaine dependence. Neuropsychopharmacology. 2010;35:1541–9. doi: 10.1038/npp.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama C, Yamanaka H, Onoe K, Kawasaki A, Nagata H, Shirakami K, Doi H, Onoe H. Mapping of serotonin transporters by positron emission tomography with [(11)C]DASB in conscious common marmosets: comparison with rhesus monkeys. Synapse. 2010;64:594–601. doi: 10.1002/syn.20766. [DOI] [PubMed] [Google Scholar]
- Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, Prisinzano TE, Baumann MH. Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J Pharmacol Exp Ther. 2009;329:738–46. doi: 10.1124/jpet.108.146142. [DOI] [PMC free article] [PubMed] [Google Scholar]