Abstract
γ-Aminobutyric acid (GABA) neurons in the medulla oblongata help regulate homeostasis, in part through interactions with the medullary serotonergic (5-HT) system. Previously, we reported abnormalities in multiple 5-HT markers in the medullary 5-HT system of infants dying from sudden infant death syndrome (SIDS), suggesting that 5-HT dysfunction is involved in its pathogenesis. Here, we tested the hypothesis that markers of GABAA receptors are decreased in the medullary 5-HT system in SIDS cases compared to controls. Using tissue receptor autoradiography with the radioligand 3H-GABA, we found 25–52% reductions in GABAA receptor binding density in 7 of 10 key nuclei sampled of the medullary 5-HT system in the SIDS cases (postconceptional age [PCA] = 51.7 ± 8.3, n = 28) vs. age-adjusted controls (PCA = 55.3 ± 13.5, n = 8) (p ≤ 0.04). By Western blotting there was 46.2% reduction in GABAAα3 subunit levels in the gigantocellularis (component of the medullary 5-HT system) of SIDS cases (PCA = 53.9 ± 8.4, n = 24) vs. controls (PCA = 55.3 ± 8.3, n = 8) (56.8% standard in SIDS cases vs. 99.35% in controls; p = 0.026). These data suggest that medullary GABAA receptors are abnormal in SIDS infants and that SIDS is a complex disorder of a homeostatic network in the medulla that involves deficits of the GABAergic and 5-HT systems.
Keywords: Autoradiography, Arcuate nucleus, Autoradiography, Homeostasis, Hypoxia, Raphé
INTRODUCTION
The sudden infant death syndrome (SIDS) is the sudden unexpected death of an infant 1 year of age, with onset of the fatal episode apparently occurring during sleep, and that remains unexplained after a thorough investigation, including performance of a complete autopsy and review of the circumstances of death and clinical history (1). SIDS is the leading cause of post-neonatal infant mortality in the United States, with an overall incidence of 0.53/1000 live births (2). A leading hypothesis in SIDS research is that SIDS is due to impairments in brainstem-mediated responses to homeostatic stressors during sleep (3–5). This hypothesis has focused multiple studies in SIDS research on neurotransmitter and neuromodulator profiles in regions of the brainstem that govern respiration, autonomic function, temperature, and arousal (6). Over the last 2 decades, our group has reported abnormalities in tissue markers of serotonergic (5-HT) function in regions of the medulla oblongata that comprise the so-called medullary 5-HT system that helps modulate homeostatic responses in a state-dependent manner (7–10). This system includes 1) 5-HT source neurons in the raphé, extra-raphé, and ventral surface embedded in the arcuate nucleus, and 2) their projection nuclei that control the upper airway (hypoglossal nucleus), visceral sensory input (nucleus of the solitary tract), diaphragmatic drive (phrenic nucleus), respiratory rhythm generation (region of the paragigantocellularis lateralis, the putative human homologue of the pre-Bötzinger complex) (11), parasympathetic outflow (dorsal motor nucleus of the vagus), and sympathetic outflow (ventrolateral medulla and intermediolateral column of the spinal cord) (6, 12). Yet, like 5-HT, γ-aminobutyric acid (GABA), the major neurotransmitter of inhibitory synaptic neurotransmission, affects virtually every homeostatic function mediated by the medulla. GABA influences respiration (13, 14), chemosensitivity to carbon dioxide (15–19), blood pressure regulation (20–22) and the laryngeal chemoreflex (23, 24). Thus, the GABAergic and 5-HT systems appear to act upon a shared set of neurons that comprises a common homeostatic network involving the medulla. Previously, we reported that GABAergic interneurons in the normal human infant medulla are intermingled among the 5-HT and non-5-HT neurons of the medullary 5-HT system (25). Moreover, 5-HT neurons express GABAA receptors; GABAergic neurons express 5-HT receptors, and a small subpopulation of 5-HT neurons in the caudal raphé co-express GABA. Together, these findings suggest important interactions between the GABAergic and 5-HT systems (25). Given the extensive 5-HT/GABA inter-relationships in the medullary 5-HT system of the human infant, we reasoned that a combined lesion of 5-HT and GABA parameters in SIDS infants may be particularly deleterious. Virtually nothing is known, however, about the GABAergic system in the brainstem of SIDS infants.
Here, we undertook an analysis of GABAergic receptors in the medullary 5-HT system of SIDS cases and controls as a first step towards the elucidation of the role of the GABAergic system in SIDS brainstem. We tested the hypothesis that markers of GABAA receptors are decreased in the medullary 5-HT system in SIDS cases vs. controls. We focused on GABAA receptors because they are critical markers of GABAergic function and are strongly implicated in medullary homeostatic functions (17, 18, 21–23, 26, 27). GABAA receptors are composed of at least 15 subunits (α1–6, β1–3, γ1–3, δ, and ρ1–2) encoded by distinct genes (28, 29). The pentameric arrangement of the GABAA subunits allows for a vast number of channels with various subunit compositions (30, 31), and the subunit composition that determines the functional and pharmacological properties of the receptor is developmentally controlled (30, 32).
We first determined the regional distribution and density of GABAA receptor binding with tissue receptor autoradiography in the medullary 5-HT system. This study was performed in 3 stages. Stage 1 involved 6 SIDS and 5 controls in which brainstems were collected between 1985 and 2000 and in which radioligands for 5-HT (7), muscarinic cholinergic (33), nicotinic cholinergic (34), kainate (35), opioid (36), and α2-adrenergic (37) receptors were analyzed in alternate sections in an expanded cohort from this time frame. A complete GABAergic receptor study could not be performed in this full cohort because medullary tissue sections were no longer available after the analysis of the multiple other receptors in the same specimens. Stage 1 was thus regarded as a pilot study for Stage 2. Stage 2 was a complete and independent study of the medulla of 28 SIDS and 8 controls in which tissues had been collected from 2004 to 2008. The tissue samples in this study came from the same dataset of SIDS cases and controls in the laboratory in which we identified several abnormalities in the medullary 5-HT system in SIDS (10). Of note, medullary tissues from 7 SIDS were no longer available, and tissues from 3 chronic controls were added. The use of this dataset for GABAA receptor binding analysis allowed us to determine if GABAA receptor binding abnormalities co-exist and/or correlate with those in 5-HT markers, including binding levels of the 5-HT1A receptor (tissue receptor autoradiography), levels of tryptophan hydroxylase (TPH2), the key biosynthetic enzyme for 5-HT (Western blotting), and 5-HT levels (high performance liquid chromatography [HPLC]) (10). Stage 3 was an analysis of GABAA receptor binding in which the data from the SIDS and controls in Stages 1 and 2 were combined for a total of 34 SIDS and 13 controls. This analysis was based on the rationale that a combination of datasets was technically feasible because the autoradiography protocols used were similar and such a combination would increase the sample size of the SIDS and control groups. In the dataset of Stage 2, we also measured protein levels of the GABAAα3 receptor subunit by Western blotting in tissue homogenates of the gigantocellularis of the medial reticular formation of the rostral medulla. This nucleus is a major component of 5-HT source neurons in the medullary 5-HT system (12), and we have found that the pattern of 5-HT receptor binding is consistently altered within this nucleus in SIDS cases compared to controls, including in the Stage 2 dataset (7–10). Finally, we determined if known risk factors for SIDS in the Stage 2 dataset were associated with the putative GABAA receptor abnormalities in the SIDS cases Such associations, if present, would suggest interactions between risk factors and GABAergic neurotransmission in the pathogenesis of altered homeostasis and sudden death.
MATERIALS AND METHODS
Clinical Database
The GABAA receptor studies involved medullary tissue from SIDS cases and controls in Stages 1 and 2. All specimens were obtained under the auspices of the Office of the Chief Medical Examiner system, San Diego, CA, in accordance to California law Chapter 955, Statutes of 1989 (SB1069), and the San Diego Research Project. This law permits the use of autopsy tissues from infants with sudden death for research without direct parental permission.
All SIDS cases were defined as described above (1). Acute controls were infants less than 12 months of age who died unexpectedly (in some instances with a minor or acute illness within 48 hours of death), and in whom an autopsy and death scene investigation established a cause of death (7–10). Chronic controls were infants less than 12 months of age that had clinical chronic illnesses but who nevertheless died suddenly and unexpectedly; a complete autopsy confirmed the chronic illness. Analyses were performed blinded by the investigator to diagnosis, age, and all other clinicopathologic variables recorded upon review of autopsy and death scene investigation reports. The diagnoses in all cases were made without knowledge of the biochemical data, and all biochemical data were likewise analyzed without knowledge of diagnosis, age, or other clinical factors. This study was approved by the Committee on Clinical Investigation at Children's Hospital Boston.
Tissue Section Autoradiography of GABAA Receptor Binding
Unfixed brainstem sections were stored frozen at −70°C at the time of autopsy, and subsequently sectioned at 20 µm on a Leitz motorized cryostat (7, 38). The tissue sections were pre-incubated in 50 nM Tris-HCL (pH 7.4) (Invitrogen, Carlsbad, CA) containing 100 µm Baclofen (GABAB agonist) (Sigma-Aldrich, St. Louis, MO) for 45 to 60 minutes at room temperature. Total GABAA receptor binding was determined by incubation of unfixed slide-mounted tissue sections in 50 nM 3H-GABA (PerkinElmer Inc, Wellesley, MA) for 20 minutes at room temperature (25, 39). Nonspecific binding was determined by addition of 100 µm isoguvacine (Sigma-Aldrich) to the solution. Following the incubation, the sections were washed in 50 nM Tris-HCL (pH 7.4) (Invitrogen) and then rinsed in ice-cold water before drying in a warm stream of air. Tissue sections were placed in cassettes and exposed to 3H-Hyperfilm (Amersham GE Healthcare Bioscience Corp., Piscataway, NJ) (Stage 1), or phosphoimaging plates (Fujifilm; BAS-TR2025) (Stage 2) for 4 weeks at 4°C. A set of tissue equivalent (µCi/g) 3H standards (GE Healthcare Bioscience) were used for conversion of optical density of silver grains to fmol/mg of tissue to determine GABAA binding. In Stage 1, film autoradiograms were generated according to standard laboratory procedure for development of light sensitive film (38); in Stage 2, imaging plates were read and imaged using a BAS-5000 Phosphoimager (Fuji Biosystems), then exported to a computerized system (MCID Elite 6; Imaging Research Inc, Mississauga, ONT, Canada) for densitometry quantification (10). For each specimen, receptor-binding density was analyzed in 10 medullary nuclei at defined levels (see below) of the brainstem (2 autoradiograms/nucleus), as previously described (7–10, 38, 40).
Analysis of GABAA Receptor Binding in the Medullary 5-HT System
We define the medullary or caudal 5-HT system as the regions of the medulla that contain 5-HT cell bodies and effector nuclei that receive 5-HT projections (6, 12). The effector nuclei include the hypoglossal nucleus, nucleus of the solitary tract, and dorsal motor nucleus of the vagus (12). The 5-HT source cell bodies are present in the raphé (raphé obscurus, raphé pallidus, and raphé magnus), extra-raphé (paragigantocellularis lateralis, gigantocellularis, intermediate reticular zone, subtrigeminalis, and lateral reticular nucleus), and ventral surface (embedded within the arcuate nucleus) (12). The medulla was analyzed at standardized levels based upon the human brainstem atlas of Olszewski and Baxter (41), according to our previously published protocols (7–10, 38, 40). The brainstem atlas of Paxinos and Huang (42) was used for modern nomenclature of the brainstem raphé nuclei. We analyzed the mid-medulla at the level of the nucleus of Roller, as depicted in Plate XII of (41), and the rostral medulla at the level of the nucleus prepositus, paragigantocellularis lateralis, and gigantocellularis, as depicted in Plate XIV of (41).
Western Blot Analysis of GABAAα3 Receptor Subunits in the Gigantocellularis
Tissue samples in Stage 2 were collected from the gigantocellularis of each frozen (unfixed) medulla using a 2-mm micropunch (Harris Uni-core, Electron Microscopy Sciences, Hatfield, PA) (10). One micropunch (wet weight ≈ 20 mg) from each specimen was homogenized in standard 1X RIPA buffer (Thermo Scientific, Rockford, IL) with protease inhibitors (Roche Diagnostics, Indianapolis, IN). Tissue samples were homogenized to a final concentration of 10% weight per volume; a modified Lowry method was used for protein quantification (10). After separation with sodium dodecyl sulfate polyacrylamide (SDS-PAGE), proteins were transferred electrophoretically to an Immunology-P membrane (Millipore, Bedford, MA) overnight and incubated with a subunit specific antibody that recognizes the GABAAα3 subunit of the GABAA receptor (Sigma-Aldrich). Bands at 58 kDa were detected using a goat anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody followed by Chemiluminescence ECL development (Perkin Elmer). For quantification, we used the MCID 6 computerized densitometry system standardized to an adult brainstem control run on the same gel (10). Values were expressed as a percentage of the human adult standard of the gigantocellularis; a micropunch of this standard was run on every gel analyzed. A loading control for the housekeeping protein glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Santa Cruz Biotechnology, Santa Cruz, CA) was also run with each case/control gel.
Statistical Analysis
In Stages 1 and 2, t-tests were used to compare age and postmortem interval between SIDS and control cases, with Fisher exact tests used to compare sex and race. Analysis of covariance was used to test for differences in GABA receptor parameters between SIDS cases and controls while controlling for the potential effects of postconceptional age. Postmortem intervals were included in models when significant. Analysis to determine potential significant differences in measures between the acute and chronic controls generally was negative; therefore, the acute and chronic values were therefore combined into a single control group. The one exception was found in the dorsal accessory olive in the Stage 2 dataset in which the binding density was significantly lower in the acute vs., the chronic controls. Therefore, for this nucleus, we did not combine the acute and chronic values for comparison with the SIDS values in Stages 2 or 3. In Stage 2, GABAA receptor binding was correlated with measures of previously determined 5-HT markers (5-HT1A, 5-HT, and TPH2) (10); the correlations were controlled for postconceptional age (partial correlation). In this correlative analysis, correlations were determined for the 2 control groups combined in comparison to the SIDS cases because there were no differences in the measurements between acute and chronic controls. In Stage 2, t-tests were used to test for differences by risk factors in SIDS cases. In Stage 3, values from the SIDS groups were combined from Stages 1 and 2 and values from the control groups were likewise combined for analysis of both stages together. A p < 0.05 was considered statistically significant in all analyses.
RESULTS
Stage 1 (Dataset from 1985–2000)
Clinicopathologic Information
In Stage 1, we analyzed medullary tissues in 6 SIDS cases and 5 controls. Because of the small sample size of the control group, we did not separate it into acute and chronic subgroups. The causes of death in the controls were as follows: 1) acute pneumonia (n = 1); 2) sudden unexplained death in an infant with mild idiopathic arthrogryposis multiplex congenita (n = 1); and congenital heart disease (n = 3). There was no significant difference in postconceptional age between SIDS cases and controls (46.3 ± 6.4 vs. 42.6 ± 3.6 postconceptional weeks, respectively, p = 0.28). The SIDS cases had a significantly longer postmortem interval compared to controls (19.6 ± 4.0 hours vs. 9.8 ± 8.4 hours, respectively, p = 0.046). Therefore, adjustments for postmortem interval in all statistical analyses were performed.
Tissue Autoradiography for GABAA Receptor Binding in SIDS cases versus Controls
In Stage 1, the components of the medullary 5-HT system that contain 5-HT cell bodies (i.e. raphé obscurus, gigantocellularis, paragigantocellularis lateralis, and intermediate reticular zone) showed significant (39–64%) reductions in binding levels (p ≤ 0.04) in the SIDS cases (n = 6) vs. the controls (n = 5) (Table 1). There were also significant reductions in 2 nuclei that receive projections from medullary 5-HT neurons: the nucleus of the solitary tract (46% decrease) and the dorsal accessory olive (61% decrease) (p < 0.008) (Table 1).
Table 1.
Medullary GABAA Receptor Binding Density in SIDS cases and Controls in Stage 1
| SIDS Cases (n = 6) (fmol/mg tissue) Mean (SE) |
Control Cases (n = 5) (fmol/mg tissue) Mean (SE) |
p value | % Decrease | |
|---|---|---|---|---|
| Medullary 5-HT source nuclei | ||||
| Raphé obscurus | 29.1 (9.8) | 80.7 (10.8) | 0.001 | 63% |
| Paragigantocellularis lateralis | 31.8 (6.6) | 62.4 (7.3) | 0.020 | 49% |
| Gigantocellularis | 41.1 (8.6) | 78.6 (9.5) | 0.020 | 48% |
| Intermediate reticular zone | 43.7 (7.6) | 71.8 (8.4) | 0.040 | 39% |
| Arcuate nucleus | 35.0 (9.1) | 50.4 (10.1) | 0.300 | 31% |
| Medullary 5-HT projection nuclei | ||||
| Medial accessory olive | 39.9 (6.4) | 42.1 (7.0) | 0.830 | 5% |
| Nucleus of the solitary tract | 43.4 (5.5) | 80.8 (6.1) | 0.002 | 46% |
| Hypoglossal nucleus | 51.1 (8.3) | 77.6 (9.2) | 0.070 | 34% |
| Dorsal accessory olive | 29.8 (8.8) | 76.6 (9.7) | 0.008 | 61% |
| Principal inferior olive | 36.9 (6.4) | 49.9 (6.8) | 0.200 | 26% |
Abbreviations: SIDS, sudden infant death syndrome; 5-HT, serotonergic system.
Stage 2 (Dataset from 2004–2008)
Clinicopathologic Information
In Stage 2, we analyzed medullary tissues in 28 SIDS and 8 controls (5 acute and 3 chronic) (Table 2). The SIDS cases were the same cases that had previously analyzed for multiple 5-HT markers (10), except for 7 cases that could not be used due to the lack of available tissue. The acute group was comprised of infants with subclinical congenital heart disease (n = 3) and accidents (n = 2). These were the same acute controls previously analyzed for multiple 5-HT markers (10). The chronic group was comprised of infants with congenital myopathy (n = 1), acute intestinal obstruction due to duodenal stricture complicating a congenital syndrome with absent thumbs, hypospadias, and plagiocephaly (n = 1), and acute infantile febrile illness complicating neonatal repair of gastroschisis and failure to thrive (n = 1). These chronic cases were the only additional controls accrued since the closure of the 5-HT dataset (10). There was no significant difference in postconceptional age between SIDS cases and acute and chronic controls (51.7 ± 8.3 vs. 48.3 ± 11.4 weeks and 58.3 ± 15.7 weeks, respectively, p = 0.35) (Table 2). There was no significant difference in postmortem interval between the groups (SIDS, 17.9 ± 4.8 hours, acute controls, 13.8 ± 3.6 hours, chronic controls, 20.2 ± 2.3 hours, p = 0.11). In the Western blot study, the postconceptional age was not different among the 3 groups (Table 2). The postmortem interval was marginally shorter in the acute controls (n = 6, 12.3 ± 4 hours) vs. the SIDS group (n = 25; 17.1 ± 5.2 hours) and chronic controls (n = 3, 20.2 ± 2.3 hours, p = 0.06). Therefore, adjustment for postmortem interval was made in the Western blot analysis.
Table 2.
Comparison of Clinicopathologic Features between SIDS Cases and Acutely ill and Chronically ill Controls in the Stage 2 Dataset
| GABAA Receptor Binding Study | GABAAα3 Western Blot Study | |||||||
|---|---|---|---|---|---|---|---|---|
| SIDS (n=28) |
Acute Controls (n=5) |
Chronic Illness (n = 3) |
p value* | SIDS (n=25) |
Acute Controls (n=6) |
Chronic Illness (n=3) |
p value* | |
| Postconceptional Age (weeks) | 51.7±8.3 | 48.3±11.4 | 58.3±15.7 | 0.35 | 53.9±8.4 | 52.3±0.9 | 58.3±15.7 | 0.67 |
| Gestational Age (weeks) | 37.6±3.7 | 39.4±1.5 | 39.3±16.0 | 0.40 | 37.0±6.5 | 39.0±0.0 | 39.3±1.2 | 0.65 |
| Postnatal Age (weeks) | 14.1±8.8 | 8.9±10.5 | 19.0±14.9 | 0.34 | 15.7±9.2 | 13.4±11.1 | 19.0±14.9 | 0.72 |
| Postmortem Interval (hours) | 17.9±.8 | 13.8±3.6 | 20.2±2.3 | 0.11 | 17.1±5.2 | 12.3±4.0 | 20.2±2.3 | 0.06 |
| Male gender: | 13 (46%) | 3 (67%) | 2 (67%) | 0.73 | 15 (60%) | (50%) | 2 (67%) | 0.86 |
Mean ± SD or percent,
t-tests were used to compare age and postmortem interval between SIDS cases and controls. Fisher exact tests were used to compare sex between the SIDS cases and controls.
The risk factors in the SIDS cases in Stage 2 were determined based upon their division into “extrinsic” and “intrinsic” categories (9, 10). We considered extrinsic factors to be physical stressors (e.g. prone sleep position and bed sharing) that may place a vulnerable infant at risk for asphyxia or other homeostatic derangements at the time of death. We considered intrinsic factors as events that influence the underlying pathophysiological process (vulnerability) in the infant and include such factors as prematurity and male gender. In the Stage 2 dataset used for GABAA receptor analysis, 96% (27/28) of the SIDS cases had 1 or more risk factors in either category and 86% (24/28) had 2 or more risk factors. In the SIDS cases, 89% (25/28) had at least 1 extrinsic stressor, i.e. prone (54.1%), face-down (40.9%), bed sharing (21.4%), and trivial illness within 1 week of death (37%) (Table 3); 64% (18/28) of the SIDS cases had at least 1 intrinsic (vulnerability) risk factor. The intrinsic risk factors were prematurity (32.1%), male gender (46%), and African American race (13%) (Table 3).
Table 3.
Known SIDS Risk Factors Associated with SIDS Cases in Dataset of Stage 2.
| Variable | SIDS (n = 28) |
|---|---|
| Male gender | 46.0% (13/28) |
| African American | 13.0% (3/22) |
| Preterm Birth* | 32.1% (9/28) |
| Prone sleep position** | 54.1% (13/24) |
| Side sleep position** | 12.5% (3/24) |
| Found face-down** | 40.9% (9/22) |
| Bed sharing | 21.4% (6/28) |
| Adult bed | 52.6% (10/19) |
| Minor illness within week of death | 37.0% (10/27) |
Preterm birth: <37 gestational wks at birth;
Position found at time of discovery of death. Of note, 20.8% (5/24) of SIDS infants were found in the supine (non-risky) sleep position at discovery.
Tissue Autoradiography for GABAA Receptor Binding in SIDS cases versus Controls
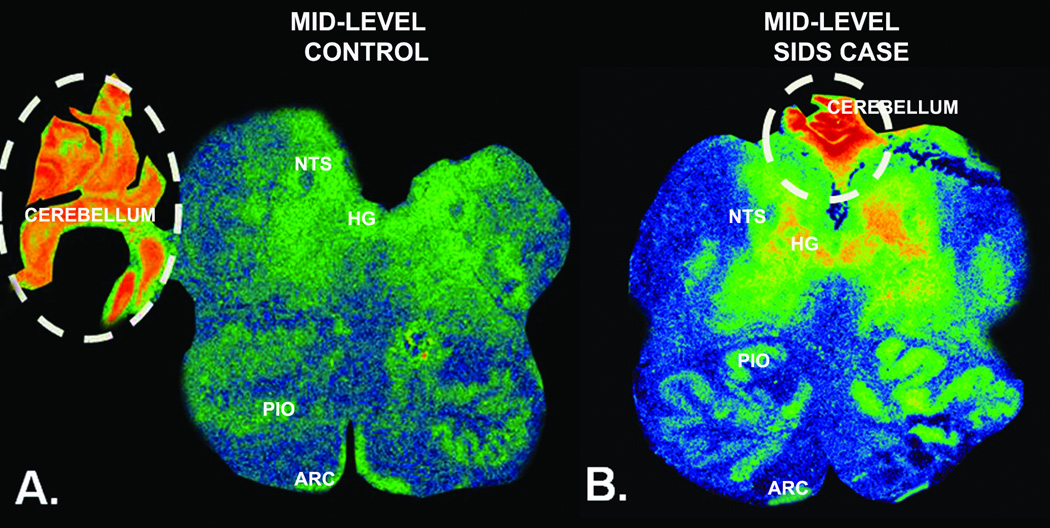
The binding levels in the SIDS cases in Stage 2 were significantly reduced in 7 of the 10 nuclei sampled (Table 4). These reduced levels were generally obvious on visual inspection of the tissue autoradiograms (Fig. 1). There were significant reductions (25–52%) in GABAA receptor binding in the 7 nuclei in the SIDS cases (n = 28) vs. acute and chronic controls combined (n = 8) (p ≤ 0.04) (Table 4). There was, however, no significant difference in binding density between SIDS and combined controls in the principal inferior olive and arcuate nucleus (Table 4). The binding values were significantly lower in the acute vs. chronic controls in the dorsal accessory olive; therefore, these were not combined (Table 4). Differences in the binding values between Stage 1 and Stage 2 (i.e. with more SIDS and control cases) were found: in the hypoglossal nucleus, the marginal reduction in SIDS cases in Stage 1 (p = 0.07) became significant in stage 2 (p < 0.001), and the difference changed from non-significant (p = 0.83) to significant (p = 0.04) in the medial accessory olive (Tables 1, 4). In the sections of 7 SIDS and 2 control cases, fragments of cerebellar tissue were fortuitously present thereby allowing the cerebellum to serve as an internal binding control. There was no difference in GABAA receptor binding in the cerebellar tissues between the SIDS and control cases (Fig. 2). In the acute controls, the GABAA receptor binding was 2.3-fold higher in the cerebellar cortex (217.3 ± 55.9 fmol/mg tissue) than in the medullary nucleus with the highest binding, i.e. the hypoglossal nucleus (85.5 ± 11.0 fmol/mg tissue) (Fig. 2). In the SIDS cases, GABAA receptor binding was 4.3-fold higher in the cerebellar cortex (193 ± 51.84 fmol/mg tissue) than in the medullary nucleus with the highest binding, i.e. the hypoglossal nucleus (44.17 ± 3.6 fmol/mg tissue) (Fig. 2).
Table 4.
Medullary GABAA Receptor Binding Density (fmol/mg tissue) in SIDS Cases and Controls in Stage 2.
| Age- Adjusted |
Mean (SE) | ||||||
|---|---|---|---|---|---|---|---|
| SIDS Cases (n=28) |
Acute Controls (n=5) |
Chronic Control (n=3) |
3–way p value |
Combined Controls (n=8) |
% Decrease |
p value* |
|
| Medullary 5-HT source nuclei | |||||||
| Raphé obscurus | 25.4(2.8) | 41.9(6.4) | 54.9(9.9) | 0.006 | 45.7(5.4) | 44% | 0.003 |
| Paragigantocellularis lateralis | 26.9(3.4) | 48.5(7.7) | 73.8(12.0) | <0.001 | 55.8(6.7) | 52% | <0.001 |
| Gigantocellularis | 32.7(3.1) | 60.1(7.0) | 56.0(11.0) | 0.002 | 58.9(5.9) | 44% | <0.001 |
| Intermediate reticular zone | 32.7(3.1) | 60.6(6.9) | 63.6(10.9) | <0.001 | 61.5(5.8) | 47% | <0.001 |
| Arcuate nucleus | 27.3(3.0) | 22.8(7.0) | 35.7(11.0) | 0.610 | 26.5(5.9) | - | 0.910 |
| Medullary 5-HT projection nuclei | |||||||
| Medial accessory olive | 38.1(2.5) | 53.6(7.5) | 48.0(7.6) | 0.100 | 50.8(5.3) | 25% | 0.040 |
| Nucleus of the solitary tract | 41.9(2.7) | 77.2(7.9) | 81.9(8.1) | <0.001 | 79.5(5.6) | 47% | <0.001 |
| Hypoglossal nucleus | 44.2(3.7) | 78.3(11.0) | 85.5(11.2) | <0.001 | 81.8(7.8) | 46% | <0.001 |
| Dorsal accessory olive | 34.6(2.7) | 36.8(6.2) | 64.6(9.6) | 0.020 | NP | NP | NP |
| Principal inferior olive | 28.9(2.4) | 38.9(5.7) | 39.0(8.9) | 0.200 | 38.9(4.8) | 26% | 0.070 |
p value, comparison of binding values between SIDS and combined acute and chronic groups; NP, Not performed because the binding values for the acute and chronic controls were significantly different from each other.
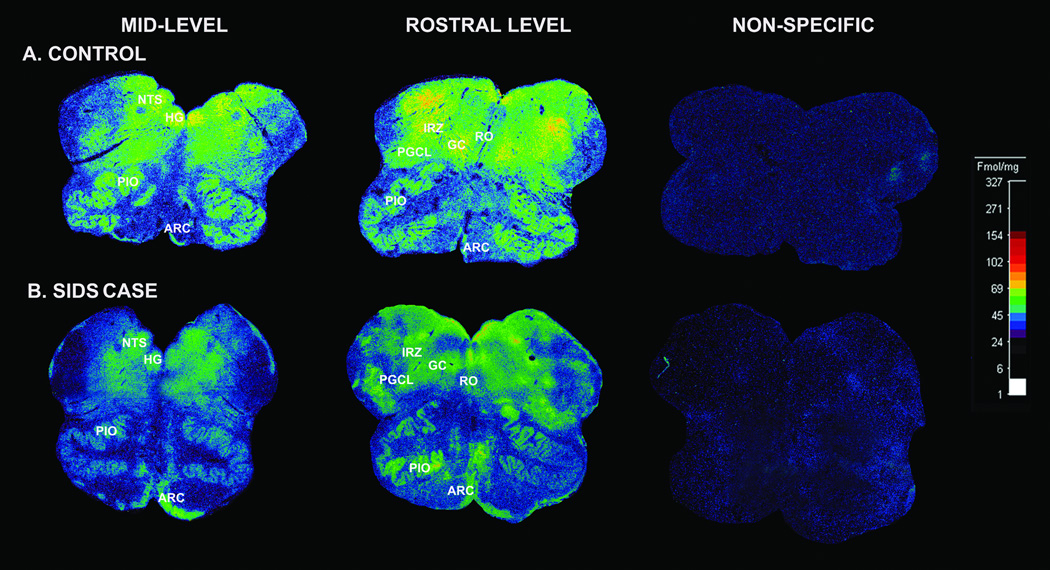
Figure 1.
γ-Aminobutyric acidA (GABAA) receptor binding in a SIDS case and control. (A, B) Illustrative autoradiograms displaying 3H-GABA binding to GABAA receptors in tissue sections at mid- (level of hypoglossal nucleus) and rostral (level of the prepositus nucleus) sections of the medulla from a SIDS infant at 44 postconceptional weeks (B), and a control infant with an acute cause of death at 40 postconceptional weeks (A). The density of GABAA receptor binding in the medullary 5-HT system is lower in the SIDS case, except for the arcuate nucleus in which the binding levels are similar. Abbreviations: ARC, arcuate nucleus; HG, hypoglossal nucleus; NTS, nucleus of the solitary tract; GC, gigantocellularis; PGCL, paragigantocellularis; PIO, principal inferior olive; RO, raphé obscurus.
Figure 2.
γ-Aminobutyric acidA (GABAA) receptor binding in the medulla and cerebellum. (A, B) Binding in the medullary regions is lower in the 44 postconceptional weeks SIDS case (B) vs. the 44 postconceptional weeks control (A), except in the arcuate nucleus in which the binding levels are similar. Fragments of cerebellar tissue (dotted lines) serve as an internal control of receptor binding levels. There is no difference between the cases in binding levels in the cerebellar tissue. ARC, arcuate nucleus; HG, hypoglossal nucleus; NTS, nucleus of the solitary tract; PIO, principal inferior olive; RO, raphé obscurus.
GABAAα3 Receptor Subunit Levels by Western Blotting
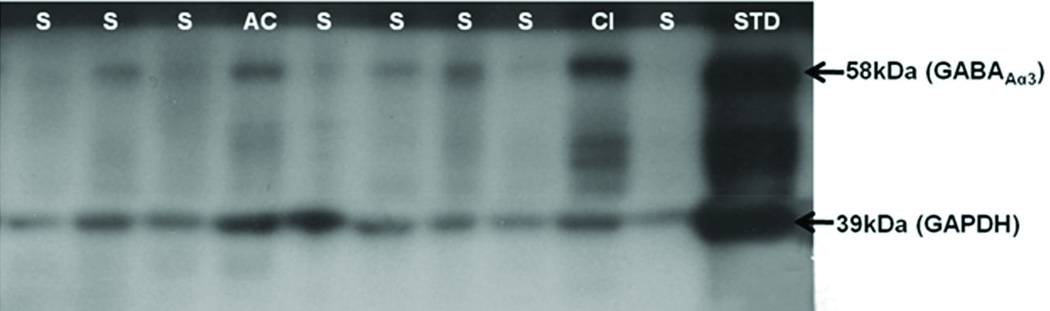
We next sought to measure levels of the GABAAα3 subunit utilizing Western blotting in the dataset of Stage 2 in the gigantocellularis (Table 2). The levels were expressed as a percentage of a human micropunched gigantocellularis (adult standard), co-analyzed on every SDS-PAGE gel (Fig. 3). There were reductions in the GABAAα3 subunit in the SIDS cases (n = 24, 56.9 ± 7% of adult standard) vs. the acute controls (n = 6, 98.0 ± 14.4%) and the chronic controls (n = 2, 100.7 ± 25.1%) (3-way p value = 0.026) (Fig. 3). The expression levels in the SIDS cases (n = 24) vs. acute and chronic controls combined (n = 8) were decreased by 43% in the SIDS cases (p = 0.006).
Figure 3.
Western blot analysis of levels of γ-Aminobutyric acidAa3 (GABAAa3) receptor subunit in the gigantocellularis. A representative Western blot of the GABAAα3 receptor subunit demonstrates differences among SIDS cases, an acute control (AC), and a control with chronic illness (CI). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is used as a loading control and shows little or no difference in abundance levels among the SIDS cases and controls.
Correlations between GABAA Receptor Binding and GABAAα3 Receptor Subunit
Data for GABAA receptor binding based upon tissue receptor autoradiography and for the expression levels of the GABAAα3 receptor subunit based upon Western blotting were available in 13 SIDS cases and 6 combined controls in the stage 2 dataset. There were no significant correlations between the GABAA receptor binding and GABAAα3 levels in either the SIDS (r2 = −0.42, p = 0.18) or control group (r2 = 0.75, p = 0.14). There was also no significant difference between the SIDS and control groups.
Correlations between GABAA Receptor Markers and 5-HT Markers
Data on GABAA receptor and 5-HT markers (based on analyses of alternate tissue blocks from the same medullae) were available in variable numbers of the SIDS and control cases in the Stage 2 dataset (Table 5). The 5-HT markers included 5-HT1A receptor binding in 9 of the same medullary nuclei studied for GABAA receptor binding based upon tissue receptor autoradiography, 5-HT levels in the raphé obscurus and paragigantocellularis lateralis based upon HPLC, and TPH2 levels in the raphé obscurus based upon Western blotting (Table 5) (10). Binding data for 5-HT1A receptors were not available for the principal inferior olive (10). Both GABAA and 5-HT1A receptor binding was abnormal in the following same 5 nuclei: hypoglossal nucleus, nucleus of the solitary tract, paragigantocellularis lateralis, gigantocellularis, and intermediate reticular zone. Binding levels for both receptors were not altered in the arcuate nucleus in this dataset. Levels for GABAA receptor binding but not 5-HT1A receptors were decreased in the raphé obscurus and medial accessory olive, although previous datasets demonstrated 5-HT receptor abnormalities in the raphé obscurus in the SIDS cases (7–10). Moreover, the raphé obscurus in this same dataset (Stage 2) demonstrated abnormalities in 5-HT and TPH2 levels (10). Thus, 6 of the same 9 nuclei (67%) sampled for GABAA receptor and 5-HT1A receptor binding demonstrated abnormalities in both receptor types. Parenthetically, 5-HT1A binding was significantly altered in the SIDS cases in the dorsal accessory olive in the dataset of Stage 2 (10), but, as noted, GABAA receptor binding was not different between SIDS and acute controls (Table 4).
Table 5.
Correlations of GABAA Receptor Autoradiography with Different 5-HT Markers in SIDS Cases and Controls in Dataset of Stage 2.
| Correlation between GABA autoradiography and |
SIDS | All Controls | |||||
|---|---|---|---|---|---|---|---|
| N | Correlation | p-value | N | Correlation | p-value | ||
| 5-HT1A | |||||||
| ARC | 23 | 0.48 | 0.024 | 7 | −0.53 | 0.280 | |
| NTS | 24 | 0.48 | 0.021 | 6 | 0.66 | 0.220 | |
| HG | 24 | 0.05 | 0.820 | 6 | −0.19 | 0.760 | |
| MAO | 25 | 0.33 | 0.120 | 5 | −0.36 | 0.640 | |
| GC | 24 | 0.18 | 0.420 | 7 | 0.61 | 0.200 | |
| PGCL | 24 | 0.31 | 0.140 | 7 | 0.81 | 0.052 | |
| IRZ | 23 | 0.02 | 0.940 | 7 | 0.46 | 0.350 | |
| DAO | 14 | 0.42 | 0.160 | 5 | 0.93 | 0.066 | |
| ROB | 24 | 0.15 | 0.480 | 7 | 0.94 | 0.005 | |
| 5-HT | |||||||
| ROB | 23 | 0.24 | 0.270 | 7 | 0.003 | 0.990 | |
| GC | 23 | 0.13 | 0.550 | 7 | 0.39 | 0.440 | |
| PGCL | 23 | 0.28 | 0.200 | 7 | 0.05 | 0.920 | |
| TPH2 | |||||||
| ROB | 22 | 0.15 | 0.510 | 7 | −0.51 | 0.300 | |
5-HT1A receptor determined by tissue receptor autoradiography; 5-HT level determined by high performance liquid chromatography; and tryptophan hydroxylase (TPH2) determined by Western blotting (10).
Abbreviations: ARC, arcuate nucleus; NTS, nucleus of the solitary tract; HG, hypoglossal nucleus; MAO, medial accessory olive; GC, gigantocellularis; PGCL, paragigantocellularis lateralis; IRZ, intermediate reticular zone; DAO, dorsal accessory olive; ROB, raphé obscurus
Correlations between 5-HT and GABAA receptor parameters were determined for the SIDS and combined acute and chronic control groups (Table 5). The most statistically robust finding was a positive correlation between 5-HT1A receptor binding with GABAA receptor binding in the raphé obscurus in the controls only (correlation coefficient 0.94; p = 0.005; n = 31) (Table 5). In the controls, 5-HT1A receptor binding increased at this site; the GABAA receptor binding also increased (Table 5), but this correlation was not present in the SIDS cases. The statistically significant correlations in the arcuate nucleus and nucleus of the solitary tract in the SIDS cases were less compelling due to the low correlation coefficients (Table 5). There were no significant correlations in either the SIDS or control groups between GABAA receptor binding and 5-HT or TPH2 levels (Table 5).
Risk Factors in the SIDS Infants
In the SIDS cases in the Stage 2 dataset there were no significant associations between the different risk factors and GABAA receptor binding or GABAAα3 receptor levels. This lack of a significant association is illustrated for prone vs. supine sleep position and GABAA receptor binding (Table 6).
Table 6.
GABAA Receptor Binding and GABAAα3 Subunit Expression Levels in SIDS Cases Found Dead in the Prone vs. Supine Sleep Position
| Prone | Supine | t-test | |||||
|---|---|---|---|---|---|---|---|
| N | Mean | SE | N | Mean | SE | p value | |
| ARC | 13 | 26.1 | 3.64 | 5 | 29.2 | 9.38 | 0.71 |
| NTS | 12 | 41.1 | 4.04 | 5 | 36.6 | 5.54 | 0.54 |
| HG | 13 | 43.4 | 4.66 | 5 | 44.9 | 11.91 | 0.89 |
| PIO | 13 | 28.5 | 3.49 | 5 | 29.3 | 4.06 | 0.90 |
| MAO | 13 | 35.9 | 3.8 | 5 | 42.6 | 6.49 | 0.38 |
| GC | 11 | 36.2 | 4.01 | 5 | 29.3 | 3.23 | 0.30 |
| PGCL | 11 | 28.5 | 2.94 | 5 | 24.9 | 2.39 | 0.45 |
| IRZ | 11 | 33.0 | 4.39 | 5 | 29.7 | 1.96 | 0.50 |
| DAO | 11 | 35.7 | 3.57 | 5 | 29.9 | 1.64 | 0.16 |
| ROB | 11 | 28.6 | 4.48 | 5 | 27.9 | 4.52 | 0.93 |
| Western blot | 6 | 59.6 | 13.72 | 6 | 79.0 | 32.06 | 0.59 |
GABAA receptor binding is expressed as fmol/mg of tissue; GABAAα3 subunit expression levels by Western blot are percent of standard.
Abbreviations: SE; standard error; ARC, arcuate nucleus; NTS, nucleus of the solitary tract; HG, hypoglossal nucleus; PIO, principal inferior olive; MAO, medial accessory olive; GC, gigantocellularis; PGCL, paragigantocellularis lateralis; IRZ, intermediate reticular zone; DAO, dorsal accessory olive; ROB, raphé obscurus.
Tissue Autoradiography for GABAA Receptor Binding in SIDS cases vs. Controls in the Combined Stage 1 and Stage 2 Datasets (Stage 3)
We combined measurements from the 2 datasets accrued over 2 decades to take full advantage of the total number of SIDS and control tissues. These are extraordinarily difficult to collect because of the rarity of sudden infant death, particularly due to known causes (controls). Upon combination of the datasets in Stages 1 and 2, we found highly significant 21–55% reductions (p ≤ 0.02) in GABAA receptor binding between the SIDS (n = 34) and combined acute and chronic control group (n = 13) in all nuclei analyzed except for the arcuate nucleus (Table 7). The dorsal accessory olive was excluded from this analysis. Thus, the binding differences between the SIDS and control groups reached statistical significance in the principal inferior olive with the larger sample size (Table 7). Of note, binding differences between SIDS cases and controls in the datasets of Stages 1, 2, and 3 were present in 5 of the same 10 nuclei sampled, notably the raphé obscurus, paragigantocellularis lateralis, gigantocellularis, intermediate reticular zone, and nucleus of the solitary tract; moreover, no binding difference was found in the arcuate nucleus in all 3 datasets. Thus, the results in the 3 datasets were comparable in 6/10 (60%) of the same nuclei sampled among them. The inconsistent findings between datasets of Stage 1 and 2 were found in components of the olivary complex, i.e., medial and dorsal accessory olives, principal inferior olive, and the hypoglossal nucleus. Of these, the medial accessory olive, principal inferior olive, and hypoglossal nucleus demonstrated significant reductions in binding in the SIDS cases with the largest sample size (Stage 3), likely reflecting increased statistical power.
Table 7.
Medullary GABAA Receptor Binding Density in SIDS Cases and Controls in Stage 3.
| Age-Adjusted | Mean (SE) | ||||||
|---|---|---|---|---|---|---|---|
| SIDS Cases (n=34) |
Acute Controls (n=7) |
Chronic Controls (n=6) |
3 way p value |
Combined Controls (n=13) |
% Decreased |
* p value | |
| Medullary 5-HT source nuclei | |||||||
| Raphé obscurus | 26.5 (3.4) | 55.9 (7.0) | 63.9 (8.5) | <0.001 | 59.2 (5.4) | 55% | <0.001 |
| Paragigantocellularis lateralis | 27.9 (2.8) | 48.8 (5.9) | 72.2 (7.1) | <0.001 | 58.3 (4.9) | 52% | <0.001 |
| Gigantocellularis | 34.5 (2.9) | 60.0 (6.3) | 76.2 (7.6) | <0.001 | 66.5 (5.0 | 48% | <0.001 |
| Intermediate reticular | 34.9 (2.9) | 61.4 (6.0) | 71.5 (7.3) | <0.001 | 65.5 (4.7) | 47% | <0.001 |
| Arcuate nucleus | 28.7 (3.2) | 33.6 (6.9) | 40.2 (8.3) | 0.410 | 36.4 (5.3) | 21% | 0.230 |
| Medullary 5-HT projection nuclei | |||||||
| Medial accessory olive | 37.6 (2.4) | 53.0 (6.2) | 46.2 (5.7) | 0.051 | 49.3 (4.2) | 24% | 0.020 |
| Nucleus of the solitary tract | 42.4 (2.3) | 80.5 (5.9) | 78.3 (5.4) | <0.001 | 79.3 (3.9) | 47% | <0.001 |
| Hypoglossal nucleus | 45.7 (3.2) | 81.2 (8.3) | 77.5 (7.6) | <0.001 | 79.1 (5.6) | 42% | <0.001 |
| Dorsal accessory olive | 33.6 (3.0) | 47.1 (6.4) | 73.5 (7.7) | <0.001 | NP | NP | NP |
| Principal inferior olive | 30.2 (2.3) | 43.3 (5.1) | 44.1 (6.1) | 0.021 | 43.6 (3.9) | 31% | 0.006 |
p value, comparison of SIDS and acute and chronic combined group; NP, not performed because the binding values for the acute and chronic controls were significantly different from each other.
DISCUSSION
The first of 3 major findings in this study is a striking (25–52% in Stage 2) reduction in GABAA receptor binding in key cardiorespiratory-related medullary nuclei in SIDS cases vs. controls, suggesting a novel underlying deficit of GABAA receptors in SIDS that may compromise GABA-mediated homeostatic function. The reductions in GABAA receptor binding in SIDS cases were found in 2 independent (non-overlapping) datasets studied in our laboratory over the last decade (Stages 1 and 2), albeit that Stage 1 was a pilot analysis. The second major finding of this study is a reduction in GABAAα3 receptor subunit expression levels in the gigantocellularis in the same site in SIDS cases with reduced GABAA receptor binding. Thus, there is a deficit in GABAA receptors in the same SIDS cases assessed by 2 different methods, i.e. tissue receptor autoradiography and Western blotting. The third major finding is the presence of GABAA receptor abnormalities in SIDS cases from the same dataset (Stage 2), and in the same medullary regions in which there are abnormalities in 5-HT1A receptor binding and 5-HT and TPH2 levels (10). The affected nuclei with decreased GABAA receptor binding in this study are part of the medullary 5-HT system and include nuclei with prominent 5-HT neuronal populations (i.e. the raphé obscurus, paragigantocellularis lateralis, gigantocellularis, and intermediate reticular zone), as well as nuclei that are modulated by 5-HT via projections from the medullary 5-HT source neurons (i.e. the nucleus of the solitary tract and hypoglossal nucleus).
We and others have reported various neurotransmitter abnormalities in SIDS brainstems (6), but we believe that the GABAA receptor binding abnormalities reported here are among the most extensive. Because they parallel most closely the regional distribution and severity of abnormalities in 5-HT receptor binding, the abnormalities in the medullary GABA and 5-HT systems likely interact with each other to compromise homeostasis in SIDS infants. Thus, we now propose that SIDS is due to dysfunction within a multi-neurotransmitter homeostatic network in the medulla that includes the GABAergic and 5-HT systems. In an as yet to be determined manner, a particular stressor/trigger combined with sleep itself may “unmask” the medullary neurotransmitter compromise in affected SIDS infants, resulting in sleep-related death.
The fundamental basis of the GABAA receptor deficit in SIDS appears to involve an absolute reduction of receptor number. Given that tissue receptor autoradiography is a measure of receptor number and binding affinity, we applied Western blotting to assess GABAA receptors in SIDS cases. We analyzed the abundance levels of the GABAAα3 receptor subunit in a representative nucleus that also demonstrated reduced GABAA receptor binding with autoradiography, i.e. the gigantocellularis. We selected the GABAAα3 subunit for analysis in SIDS because of animal data that indicate a developmental change in its expression in the pre-Bötzinger complex and nucleus of the solitary tract (nuclei critical for respiratory control) in a critical period analogous to the human critical period for SIDS (27, 29, 43). The absolute reduction in the expression levels of the GABAAα3 subunit suggests that the reduction in binding demonstrated by tissue receptor autoradiography involves at least in part a reduction in absolute receptor number. Given that α subunits are also critical for optimal binding affinity (44), the reduction of GABAAα3 subunit levels likely contributes to the reduced binding density detected by tissue receptor autoradiography via both impaired binding affinity and number of GABAA receptors. Interestingly, we did not find a correlation between GABAA receptor binding density and GABAAα3 subunit levels in either the SIDS cases or controls in the dataset of Stage 2. This discrepancy may be due to the different subcellular localization of the populations of receptors measured by each assay: autoradiography is a measure of predominantly cell membrane expressed receptors that are accessible to the exogenously applied radioligand, while Western blot measures absolute tissue receptor protein level regardless of localization, i.e. both cell surface and intracellular receptor populations. Under these conditions, receptor binding density and GABAAα3 subunit protein levels may not necessarily correlate.
Whether the GABAA receptor deficit in SIDS is secondary to the 5-HT deficits, or vice-versa, and whether the GABA and 5-HT defects are separate and independent of each other (although still in the same SIDS cases) are not known. Substantial evidence suggests that 5-HT-related mechanisms help regulate GABA function (45–47). For example, in mice with pharmacological blockade of 5-HT1A receptors during the first 3 weeks of life, GABAA receptor subunits are increased in different brain regions, suggesting that early 5-HT1A development is essential for normal GABAA receptor development (46). In addition, knockout mice for the 5-HT1A receptor on the Swiss-Webster background display several GABAA receptor defects, including reduced expression of GABAA α receptor subunits in the cerebral cortex and amygdala (45). These data suggest a pathologic pathway that is initiated by a 5-HT1A receptor deficit that directly leads to abnormalities in GABAA receptor levels; lack of the 5-HT1A receptor may alter its G-coupled signal transduction pathways and cause changes in GABAA receptor subunit gene expression (45). Here, we investigated potential correlations between 5-HT and GABA markers in the same SIDS and control cases to determine if abnormalities in one system were causally linked to those in the other system. There was a significant positive correlation between 5-HT1A and GABAA receptor binding in the raphé obscurus in the controls but not SIDS cases, suggesting a potential “dysregulation” between GABAA and 5-HT1A receptors in the SIDS cases, i.e. the positive correlation was “lost” in SIDS. Yet, we found no other correlations between the GABAA receptor measures and any of the 5-HT measures, suggesting that the GABAA receptor and 5-HT abnormalities are not directly linked to one another in SIDS. We recognize that there are multiple other GABA and 5-HT parameters (e.g. transporters, cell number) for which we do not have information for correlation in this study. Moreover, it is possible that there is an upstream abnormality in a shared, as yet unknown, locus that leads to downstream alterations in both GABAA and 5-HT receptor binding in the same SIDS cases. In addition, combined 5-HT and GABA defects may reflect an abnormality in the well-recognized single population of 5-HT neurons that co-express GABA in the medullary 5-HT system. We have reported that 6% of the 5-HT neurons in the human infant caudal raphé co-express GABA (25). The effect of abnormalities in this small (GABA-expressing) 5-HT subpopulation does not likely account for the widespread and substantial reduction in GABAA receptor binding we found across multiple nuclei in the SIDS medulla.
While the GABA and 5-HT defects in the SIDS medulla may arise independently of each other, they are, nevertheless, likely to interact with each other to compound medullary dysfunction. Indeed, interactions between the GABAergic and 5-HT systems are well-documented, including those in respiration (48, 49), stress-related abnormalities (45–47, 50), autonomic function (51, 52), and thermoregulation (26). Serotonin and GABA either promote or inhibit each other depending on the location and subtype of 5-HT and GABA receptors involved. For example, serotonin stimulates respiration by disinhibition, i.e. the reduction of GABA-mediated inhibition via 5-HT1A and/or 5-HT1B receptors (53), and it inhibits respiration by directly increasing GABA release via post-synaptic 5-HT2A/C receptors (54, 55). Previously, we reported the expression of GABAAα3 receptor subunits on 5-HT neurons in the raphé obscurus and paragigantocellularis lateralis in the normal infant medulla (25). The present study suggests that the loss of GABAA receptor binding in these components of the medullary 5-HT system may lead to altered GABAergic modulation, notably of 5-HT neurons expressing GABAergic receptors. Given that GABA appears to be excitatory in early life, its function in the human brainstem at the peak age of SIDS (infancy) is a question. In a previous study of the medullary GABAergic system in the human fetus and infant, we determined the expression of the chloride transporter KCC2 because its emergence is considered a “marker” of the developmental switch from excitation to inhibition by GABAergic neurons (25, 56–59). We found that KCC2 immunostaining is in place by mid-gestation in the medullary nuclei of the human fetus and does not change through infancy, thereby suggesting that the inhibitory effects of GABA are in place in the human fetal medulla and persist into the period of SIDS risk (25). Thus, the medullary deficit in GABAA receptors in SIDS cases is likely to compromise inhibitory effects in GABAergic neuronal activity.
The cause(s) of the GABAA receptor defects in SIDS is (are) unknown. The possibility that the changes in GABAergic receptors in the SIDS cases are due to hypoxia-ischemia is a major consideration. An important role for hypoxia/asphyxia in SIDS is suggested by reports of subclinical defects in cardiorespiratory control in infants who are studied prospectively and subsequently die of SIDS (3, 6). Hypoxia is known to cause differential changes to GABAergic parameters, depending upon the species, age, timing, and experimental conditions (60–63). Long-term hypoxia, for example, produces a significant reduction in GABAA and GABAB receptor sites in the cerebral cortex and brainstem (60–63). Further research is needed to determine the role of hypoxia in GABAA receptor reductions in SIDS. While risk factors were present in 98% of the SIDS cases (Stage 2), there was no direct correlation between the abnormal GABAA receptor markers and the presence or absence of specific risk factors for SIDS for which we had information. Thus, this analysis suggests that the GABAA receptor defects occur in the SIDS cases irrespective of the presence or absence of specific risk factors, and that the risk factors analyzed here may not play a directly causal role in the GABAA receptor pathology.
In the combined dataset (Stage 3), the only nucleus sampled without a significant difference in GABAA receptor binding between the SIDS cases and combined controls was the arcuate nucleus, which did not demonstrate a significant difference in the smaller datasets of Stage 1 and Stage 2. This finding was unanticipated because of the demonstration of multiple receptor defects in the arcuate nucleus by us in datasets studied over the last two decades. In SIDS cases compared to controls, we have reported isolated decreased binding to muscarinic cholinergic (33) and kainate (35) receptors in the arcuate nucleus, in addition to decreased 5-HT receptor binding in 3 previous datasets (7–9). These abnormalities are of interest because the arcuate nucleus is the putative human homologue of the respiratory chemosensitive fields in the ventral medulla of experimental animals (64). We previously reported GABAergic neurons, receptors, and KCC2 within the arcuate nucleus of the normal human (25), consistent with known role of GABA in the enhancement of chemosensitivity at the ventral surface in animal models (15–17). The basis of the sparing of the arcuate nucleus in the GABAA receptor-binding deficit in SIDS cases is unknown.
A potential limitation of this study is the nature of the control population, a problematic issue in all SIDS research studies. Given that SIDS is defined by death and no current biomarker exists that identifies living infants at risk for SIDS, autopsy studies are mandatory. Thus, the control autopsy population is comprised of necessity by infants typically with underlying (lethal) disease processes, and consequently it may not entirely reflect normality. Infants with accidental deaths are extraordinarily rare in pediatric forensic autopsy populations, and the time period necessary to accrue a sufficient number of such cases alone would be prohibitive (and likely not necessary) for advancing SIDS-related pathological studies. Nevertheless, we have found striking and robust differences in GABAA receptor parameters between SIDS cases and infants dying of a variety of causes, including those with acute and chronic disorders. In only 1 of the 10 medullary nuclei sampled (dorsal accessory olive) did we find a significant difference in GABAA receptor binding between the acute and chronic controls. While the explanation for this difference among non-SIDS infants is unknown, it requires verification in future analyzes.
In conclusion, we report widespread and substantial reductions in GABAA receptor binding in the medullary 5-HT system in SIDS cases with 5-HT defects in the same regions; in addition, there is a reduction in the GABAAα3 receptor subunit in a main component of the medullary 5-HT system (gigantocellularis). The GABAA receptor deficits may lead to decreased GABAergic inhibition resulting in hypo- or hyperactivity, depending upon the expression and cellular location of the different GABA receptor subtypes involved. The findings of GABAA receptor and 5-HT abnormalities in the same SIDS cases may help explain why a SIDS death is associated with only a partial 5-HT deficiency, i.e. death may result from combined 5-HT and GABA dysfunction in the same medullary network with the GABAergic defects enhancing the partial 5-HT defects. These human data are hypothesis-generating for determining in animal models the specific mechanistic links among GABA, 5-HT, hypoxia, homeostasis, a critical developmental period, SIDS risk factors, and sudden death. In addition, they suggest that the eventual correction of identified brainstem defects in living infants at risk for SIDS may ultimately need to involve therapeutic agents that target more than one neurotransmitter system.
ACKNOWLEDGMENTS
The authors are grateful for the dedicated assistance of the medical examiners of the San Diego County Medical Examiner’s Office, San Diego, CA, in tissue collection in this study. The authors also thank Drs. Eugene E. Nattie and Robin L. Haynes for critical readings of the manuscript, without compensation. The role of the funding organizations was solely to provide funding support of the project for peer review; these organizations had no role in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript.
This work was supported by a NHLBI Training Grant in Pathobiology (T32 HL 076115-05), Supplement to Promote Diversity in Health-Related Research Program (5U01 HD045991-06, KGB), Deborah Evelyn Barrett Fellowship in SIDS (KGB), CJ Foundation for SIDS (DSP, HFK), First Candle/SIDS Alliance (HFK), Christopher James Murphy Foundation (HCK), Intellectual and Developmental Disabilities Research Center, Children's Hospital of Boston (P30-HD18655), and National Institute of Child Health and Development (R37-H020991, PO1-HD036379) (HCK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Krous HF, Beckwith JB, Byard RW, et al. Sudden infant death syndrome and unclassified sudden infant deaths: a definitional and diagnostic approach. Pediatrics. 2004;114:234–238. doi: 10.1542/peds.114.1.234. [DOI] [PubMed] [Google Scholar]
- 2.Mathews TJ, MacDorman MF. Infant mortality statistics from the 2006 period linked birth/infant death data set. Natl Vital Stat Rep. 2008;58:1–31. [PubMed] [Google Scholar]
- 3.Kinney HC, Thach BT. The sudden infant death syndrome. N Engl J Med. 2009;361:795–805. doi: 10.1056/NEJMra0803836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunt CE, Brouillette RT. Sudden infant death syndrome: 1987 perspective. J Pediatr. 1987;110:669–678. doi: 10.1016/s0022-3476(87)80001-x. [DOI] [PubMed] [Google Scholar]
- 5.Sahni R, Fifer WP, Myers MM. Identifying infants at risk for sudden infant death syndrome. Curr Opin Pediatr. 2007;19:145–149. doi: 10.1097/MOP.0b013e32808373b6. [DOI] [PubMed] [Google Scholar]
- 6.Kinney HC, Richerson GB, Dymecki SM, et al. The brainstem and serotonin in the sudden infant death syndrome. Annu Rev Pathol. 2009;4:517–550. doi: 10.1146/annurev.pathol.4.110807.092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panigrahy A, Filiano J, Sleeper LA, et al. Decreased serotonergic receptor binding in rhombic lip-derived regions of the medulla oblongata in the sudden infant death syndrome. J Neuropathol Exp Neurol. 2000;59:377–384. doi: 10.1093/jnen/59.5.377. [DOI] [PubMed] [Google Scholar]
- 8.Kinney HC, Randall LL, Sleeper LA, et al. Serotonergic brainstem abnormalities in Northern Plains Indians with the sudden infant death syndrome. J Neuropathol Exp Neurol. 2003;62:1178–1191. doi: 10.1093/jnen/62.11.1178. [DOI] [PubMed] [Google Scholar]
- 9.Paterson DS, Trachtenberg FL, Thompson EG, et al. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. Jama. 2006;296:2124–2132. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- 10.Duncan JR, Paterson DS, Hoffman JM, et al. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA. 2010;303:430–437. doi: 10.1001/jama.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwarzacher SW, Rub U, Deller T. Neuroanatomical characteristics of the human pre-Botzinger complex and its involvement in neurodegenerative brainstem diseases. Brain. 2011;134:24–35. doi: 10.1093/brain/awq327. [DOI] [PubMed] [Google Scholar]
- 12.Kinney HC, Belliveau RA, Trachtenberg FL, et al. The development of the medullary serotonergic system in early human life. Auton Neurosci. 2007;132:81–102. doi: 10.1016/j.autneu.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Shao XM, Feldman JL. Respiratory rhythm generation and synaptic inhibition of expiratory neurons in pre-Botzinger complex: differential roles of glycinergic and GABAergic neural transmission. J Neurophysiol. 1997;77:1853–1860. doi: 10.1152/jn.1997.77.4.1853. [DOI] [PubMed] [Google Scholar]
- 14.Pierrefiche O, Schwarzacher SW, Bischoff AM, et al. Blockade of synaptic inhibition within the pre-Botzinger complex in the cat suppresses respiratory rhythm generation in vivo. J Physiol. 1998;509:245–254. doi: 10.1111/j.1469-7793.1998.245bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curran AK, Chen G, Darnall RA, et al. Lesion or muscimol in the rostral ventral medulla reduces ventilatory output and the CO(2) response in decerebrate piglets. Respir Physiol. 2000;123:23–37. doi: 10.1016/s0034-5687(00)00143-2. [DOI] [PubMed] [Google Scholar]
- 16.Curran AK, Darnall RA, Filiano JJ, et al. Muscimol dialysis in the rostral ventral medulla reduced the CO(2) response in awake and sleeping piglets. J Appl Physiol. 2001;90:971–980. doi: 10.1152/jappl.2001.90.3.971. [DOI] [PubMed] [Google Scholar]
- 17.Kuribayashi J, Sakuraba S, Hosokawa Y, et al. CO2-sensitivity of GABAergic neurons in the ventral medullary surface of GAD67-GFP knock-in neonatal mice. Adv Exp Med Biol. 2008;605:338–342. doi: 10.1007/978-0-387-73693-8_59. [DOI] [PubMed] [Google Scholar]
- 18.Curran AK, Peraza D, Elinsky CA, et al. Enhanced baroreflex-mediated inhibition of respiration after muscimol dialysis in the rostroventral medulla. J Appl Physiol. 2002;92:2554–2564. doi: 10.1152/japplphysiol.00895.2001. [DOI] [PubMed] [Google Scholar]
- 19.Kanazawa M, Sugama S, Okada J, et al. Pharmacological properties of the CO2/H+-sensitive area in the ventral medullary surface assessed by the effects of chemical stimulation on respiration. J Auton Nerv Syst. 1998;72:24–33. doi: 10.1016/s0165-1838(98)00085-x. [DOI] [PubMed] [Google Scholar]
- 20.Heesch CM, Laiprasert JD, Kvochina L. RVLM glycine receptors mediate GABAA and GABAB)independent sympathoinhibition from CVLM in rats. Brain Res. 2006;1125:46–59. doi: 10.1016/j.brainres.2006.09.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menezes RC, Fontes MA. Cardiovascular effects produced by activation of GABA receptors in the rostral ventrolateral medulla of conscious rats. Neuroscience. 2007;144:336–343. doi: 10.1016/j.neuroscience.2006.08.062. [DOI] [PubMed] [Google Scholar]
- 22.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- 23.Bohm I, Xia L, Leiter JC, et al. GABAergic processes mediate thermal prolongation of the laryngeal reflex apnea in decerebrate piglets. Respir Physiol Neurobiol. 2007;156:229–233. doi: 10.1016/j.resp.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Van der Velde L, Curran AK, Filiano JJ, et al. Prolongation of the laryngeal chemoreflex after inhibition of the rostral ventral medulla in piglets: a role for SIDS? J Appl Physiol. 2003;94:1883–1895. doi: 10.1152/japplphysiol.01103.2002. [DOI] [PubMed] [Google Scholar]
- 25.Broadbelt KG, Paterson DS, Rivera KD, et al. Neuroanatomic relationships between the GABAergic and serotonergic systems in the developing human medulla. Auton Neurosci. 2010;154:30–41. doi: 10.1016/j.autneu.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao WH, Morrison SF. Disinhibition of rostral raphe pallidus neurons increases cardiac sympathetic nerve activity and heart rate. Brain Res. 2003;980:1–10. doi: 10.1016/s0006-8993(03)02981-0. [DOI] [PubMed] [Google Scholar]
- 27.Liu Q, Wong-Riley MT. Developmental changes in the expression of GABAA receptor subunits alpha1, alpha2, and alpha3 in brain stem nuclei of rats. Brain Res. 2006;1098:129–138. doi: 10.1016/j.brainres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 29.Hornung JP, Fritschy JM. Developmental profile of GABAA-receptors in the marmoset monkey: expression of distinct subtypes in pre- and postnatal brain. J Comp Neurol. 1996;367:413–430. doi: 10.1002/(SICI)1096-9861(19960408)367:3<413::AID-CNE7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 30.Sieghart W, Fuchs K, Tretter V, et al. Structure and subunit composition of GABA(A) receptors. Neurochem Int. 1999;34:379–385. doi: 10.1016/s0197-0186(99)00045-5. [DOI] [PubMed] [Google Scholar]
- 31.Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- 33.Kinney HC, Filiano JJ, Sleeper LA, et al. Decreased muscarinic receptor binding in the arcuate nucleus in sudden infant death syndrome. Science. 1995;269:1446–1450. doi: 10.1126/science.7660131. [DOI] [PubMed] [Google Scholar]
- 34.Nachmanoff DB, Panigrahy A, Filiano JJ, et al. Brainstem 3H-nicotine receptor binding in the sudden infant death syndrome. J Neuropathol Exp Neurol. 1998;57:1018–1025. doi: 10.1097/00005072-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Panigrahy A, Filiano JJ, Sleeper LA, et al. Decreased kainate receptor binding in the arcuate nucleus of the sudden infant death syndrome. J Neuropathol Exp Neurol. 1997;56:1253–1261. doi: 10.1097/00005072-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Kinney HC, Filiano JJ, Assmann SF, et al. Tritiated-naloxone binding to brainstem opioid receptors in the sudden infant death syndrome. J Auton Nerv Syst. 1998;69:156–163. doi: 10.1016/s0165-1838(98)00021-6. [DOI] [PubMed] [Google Scholar]
- 37.Mansouri J, Panigrahy A, Filiano JJ, et al. Alpha2 receptor binding in the medulla oblongata in the sudden infant death syndrome. J Neuropathol Exp Neurol. 2001;60:141–146. doi: 10.1093/jnen/60.2.141. [DOI] [PubMed] [Google Scholar]
- 38.Kinney HC, Filiano JJ, White WF. Medullary serotonergic network deficiency in the sudden infant death syndrome: review of a 15-year study of a single dataset. J Neuropathol Exp Neurol. 2001;60:228–247. doi: 10.1093/jnen/60.3.228. [DOI] [PubMed] [Google Scholar]
- 39.Bowery NG, Hudson AL, Price GW. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987;20:365–383. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- 40.Paterson DS, Belliveau RA, Trachtenberg F, et al. Differential development of 5-HT receptor and the serotonin transporter binding in the human infant medulla. J Comp Neurol. 2004;472:221–231. doi: 10.1002/cne.20105. [DOI] [PubMed] [Google Scholar]
- 41.Olszewski J, Baxter D. Cytoarchitecture of the Human Brain Stem. 2nd edition. Karger; 1982. 2nd edition. [Google Scholar]
- 42.Paxinos G, Huang X-F. Atlas of the Human Brainstem. New York: Academic Press; 1995. [Google Scholar]
- 43.Wong-Riley MT, Liu Q. Neurochemical development of brain stem nuclei involved in the control of respiration. Respir Physiol Neurobiol. 2005;149:83–98. doi: 10.1016/j.resp.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 44.Hevers W, Luddens H. diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- 45.Sibille E, Pavlides C, Benke D, et al. Genetic inactivation of the Serotonin(1A) receptor in mice results in downregulation of major GABA(A) receptor alpha subunits, reduction of GABA(A) receptor binding, and benzodiazepine-resistant anxiety. J Neurosci. 2000;20:2758–2765. doi: 10.1523/JNEUROSCI.20-08-02758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vinkers CH, Oosting RS, van Bogaert MJ, et al. Early-life blockade of 5-HT(1A) receptors alters adult anxiety behavior and benzodiazepine sensitivity. Biol Psychiatry. 2010;67:309–316. doi: 10.1016/j.biopsych.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 47.Bruening S, Oh E, Hetzenauer A, et al. The anxiety-like phenotype of 5-HT receptor null mice is associated with genetic background-specific perturbations in the prefrontal cortex GABA-glutamate system. J Neurochem. 2006;99:892–899. doi: 10.1111/j.1471-4159.2006.04129.x. [DOI] [PubMed] [Google Scholar]
- 48.Cao Y, Matsuyama K, Fujito Y, et al. Involvement of medullary GABAergic and serotonergic raphe neurons in respiratory control: electrophysiological and immunohistochemical studies in rats. Neurosci Res. 2006;56:322–331. doi: 10.1016/j.neures.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 49.Abdala AP, Dutschmann M, Bissonnette JM, et al. Correction of respiratory disorders in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A. 2010;107:18208–18213. doi: 10.1073/pnas.1012104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vinkers CH, van Oorschot R, Korte SM, et al. 5-HT1A receptor blockade reverses GABA(A) receptor alpha3 subunit-mediated anxiolytic effects on stress-induced hyperthermia. Psychopharmacology (Berl) 2010;211:123–130. doi: 10.1007/s00213-010-1895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lovick TA. GABA-mediated inhibition in nucleus paragigantocellularis lateralis in the cat. Neurosci Lett. 1988;92:182–186. doi: 10.1016/0304-3940(88)90057-2. [DOI] [PubMed] [Google Scholar]
- 52.Lovick TA. Convergent afferent inputs to neurones in nucleus paragigantocellularis lateralis in the cat. Brain Res. 1988;456:183–187. doi: 10.1016/0006-8993(88)90361-7. [DOI] [PubMed] [Google Scholar]
- 53.Xiao Q, Suguihara C, Hehre D, et al. Effects of GABA receptor blockade on the ventilatory response to hypoxia in hypothermic newborn piglets. Pediatr Res. 2000;47:663–668. doi: 10.1203/00006450-200005000-00018. [DOI] [PubMed] [Google Scholar]
- 54.Liu Q, Wong-Riley MT. Postnatal changes in the expressions of serotonin 1A, 1B, and 2A receptors in ten brain stem nuclei of the rat: implication for a sensitive period. Neuroscience. 2000;165:61–78. doi: 10.1016/j.neuroscience.2009.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Serrats J, Mengod G, Cortes R. Expression of serotonin 5-HT2C receptors in GABAergic cells of the anterior raphe nuclei. J Chem Neuroanat. 2005;29:83–91. doi: 10.1016/j.jchemneu.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 56.Plotkin MD, Snyder EY, Hebert SC, et al. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA's excitatory role in immature brain. J Neurobiol. 1997;33:781–795. doi: 10.1002/(sici)1097-4695(19971120)33:6<781::aid-neu6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 57.Rivera C, Voipio J, Payne JA, et al. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 58.Rivera C, Voipio J, Kaila K. Two developmental switches in GABAergic signalling: the K+-Cl− cotransporter KCC2 and carbonic anhydrase CAVII. J Physiol. 2005;562:27–36. doi: 10.1113/jphysiol.2004.077495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li K, Xu E. The role and the mechanism of gamma-aminobutyric acid during central nervous system development. Neurosci Bull. 2008;24:195–200. doi: 10.1007/s12264-008-0109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wood JD, Watson WJ, Ducker AJ. The effect of hypoxia on brain gamma-aminobutyric acid levels. J Neurochem. 1968;15:603–608. doi: 10.1111/j.1471-4159.1968.tb08959.x. [DOI] [PubMed] [Google Scholar]
- 61.Viapiano MS, Mitridate de Novara AM, Fiszer de Plazas S, et al. Prolonged exposure to hypobaric hypoxia transiently reduces GABA(A) receptor number in mice cerebral cortex. Brain Res. 2001;894:31–36. doi: 10.1016/s0006-8993(00)03194-2. [DOI] [PubMed] [Google Scholar]
- 62.Anju TR, Abraham PM, Antony S, et al. Alterations in cortical GABAB receptors in neonatal rats exposed to hypoxic stress: role of glucose, oxygen, and epinephrine resuscitation. Mol Cell Biochem. 2010;343:1–11. doi: 10.1007/s11010-010-0491-9. [DOI] [PubMed] [Google Scholar]
- 63.Anju TR, Peeyush Kumar T, Paulose CS. Decreased GABAA receptors functional regulation in the cerebral cortex and brainstem of hypoxic neonatal rats: effect of glucose and oxygen supplementation. Cell Mol Neurobiol. 2010;30:599–606. doi: 10.1007/s10571-009-9485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Filiano JJ, Choi JC, Kinney HC. Candidate cell populations for respiratory chemosensitive fields in the human infant medulla. J Comp Neurol. 1990;293:448–465. doi: 10.1002/cne.902930308. [DOI] [PubMed] [Google Scholar]