Abstract
Corticobasal syndrome (CBS), once thought to be pathognomonic for corticobasal degeneration pathology, is increasingly reported with various underlying pathologies. Alzheimer’s disease is one such pathology, also once believed to be unique for its clinical syndrome of dementia of the Alzheimer’s type. CBS is believed to result from topography of asymmetric parietofrontal cortical lesion involvement, rather than lesion subtype. However, this topographical pattern is strikingly different to that typically associated with AD for unclear reasons. This article will focus on CBS with underlying AD pathology (CBS-AD), and will review associated clinical, imaging and demographic factors. Predicting AD pathology is of marked interest as disease-modifying therapies loom on the horizon, with biomarkers and imaging research underway. By reviewing the literature for CBS-AD case reports and series and contrasting them with CBS with underlying corticobasal degeneration pathology cases, the article aims to examine factors that may predict AD pathology. How AD pathology may produce this clinical phenotype, rather than the prototype dementia of the Alzheimer’s type, will also be reviewed.
Keywords: alien limb, Alzheimer’s disease, apraxia, corticobasal degeneration, corticobasal syndrome, dementia, myoclonus, rigidity, tau, voxel-based morphometry
Corticobasal syndrome (CBS) is a striking and unusual clinical manifestation of various neurodegenerative pathologies, with tauopathies being the most common. The prototype pathology is corticobasal degeneration (CBD), estimated to account for 50% of cases [1]. Other pathologies reported in the literature include Alzheimer’s disease (AD) [2,3], progressive supranuclear palsy (PSP) [4], frontotemporal dementia with parkinsonism linked to chromosome 17, Pick’s disease with Pick bodies, dementia with Lewy bodies [1,5], neurofilament inclusion body disease [6], Creutzfeldt-Jakob disease [7], frontotemporal degeneration due to progranulin gene mutation [8] and motor neuron inclusion body dementia [9]. Unifying the pathologies that produce CBS is a consistent topographic distribution of cortical damage, involving frontal or frontoparietal cortex in an asymmetric fashion [7].
The focus of this article will be on CBS resulting from underlying AD pathology, hereafter termed CBS-AD. This association is of particular interest as CBD and AD are distinct neurodegenerative pathologies and share relatively little in common apart from abnormal phosphorylated tau protein deposition. Features that set AD apart from CBD include the specific tau protein isoform deposited, the presence of β-amyloid deposition, the clinical hallmark of dementia of the Alzheimer’s type (DAT), and the presence of medial temporal lobe atrophy. To state this latter point further, the brain regions implicated to clinically produce CBS are typically different from those involved in typical AD pathology, hence the conundrum. Ironically, CBD and AD pathology were thought to be pathognomonic for their clinical syndrome, CBS and DAT, respectively. Clinicopathological overlap amongst the tauopathies is now increasingly appreciated, including CBS with AD pathology and dementia with CBD pathology [10]. This article will explore reasons why the topography of AD pathology may vary to produce this distinct syndrome. Factors that may predict underlying AD pathology in cases presenting with CBS will also be examined. Many clinicians have called for further research to look at clinical markers of AD pathology in CBS [3,11,12]. Trying to accurately predict pathology is of great importance, as therapies are typically specific to the underlying disease process. For example, AD may benefit from cholinesterase inhibitors or future AD-modifying therapies that may be ineffective in CBD.
Corticobasal syndrome
This rare clinical syndrome is associated with sporadic disease with typical onset in the sixth to eighth decades. Core clinical features are insidious progressive asymmetric rigidity and apraxia, accompanied by symptoms and signs of cortical (motor, sensory or association cortices) and extrapyramidal dysfunction [13]. Cortical signs include cortical sensory loss, alien limb phenomena, myoclonus, apraxia, pyramidal motor signs, agrammatic aphasia, apraxia of speech and visuospatial impairment. Extrapyramidal involvement includes dystonia and levodopa nonresponsive Parkinsonism (rigidity, tremor and bradykinesia). Typical mean survival is 7 years from symptom onset. Asymmetry is emphasized, although symmetric presentations have been described [14]. Thus clinical findings may be explained by the topographical distribution of cortical damage: cortical sensory loss and apraxia occur with parietal lesions, and occasionally posterior frontal lesions; frontal or parietal lesions can produce alien limb syndrome [15], mirror movements [16], pyramidal tact signs or apraxia; and posterior inferior frontal lesions can produce agrammatic aphasia [17]. The lack of a marked response to levodopa is postulated to reflect postsynaptic nigrostriatal dysfunction rather than degeneration of the substantia nigra [7]. However, severe rigidity, dystonia and tremor have been reported in the absence of basal ganglia or substantia nigra damage, and are postulated to reflect damage to the sensorimotor cortex and its extrapyramidal projections [7,18].
The neuropathological substrate of CBS is most commonly a tauopathy, with a subtype of four microtubule-binding repeats (4R-tau) most common, as seen in CBD and PSP pathology. CBS is also associated with TDP-43 pathology, mostly observed in progranulin gene mutation carriers [19,20], although sporadic cases are also reported [21]. Small clinicopathological series have shown a significant proportion of CBS cases with AD pathology: a mixed 3R/4R tauopathy [22]. In one study of 12 CBS cases followed to autopsy, AD pathology represented 50% of CBS and the remaining 50% were CBD [3]. Increasing numbers of case reports [12,23–33] or small case series comprising two to six patients [2,3,7,12,34,35] of CBS-AD have emerged over the past 20 years in the literature, totaling approximately 42 cases. Litvan et al. proposed criteria to accurately predict underlying CBD pathology, including limb dystonia, ideomotor apraxia, myoclonus and asymmetric akinetic–rigid syndrome with late-onset gait or balance impairment [36]. However, these clinical features have also been reported with AD pathology [2,3].
Corticobasal pathology compared with AD pathology
The first cases of CBS described by Rebeiz et al. in 1968 were associated with a unique underlying pathology, now termed corticobasal degeneration [18]. Current pathologic diagnostic criteria specify tau-positive neuronal and glial lesions in the gray and white cortex and basal ganglia. The abnormal tau protein is hyperphosphorylated and deposited as various inclusions termed astrocytic plaques, thread-like lesions, corticobasal bodies and coiled bodies; swollen or achromatic ballooned neurons are usually present [37]. Frontal or parietal cortices and subcortical regions are typically involved, with relative sparing of the temporal cortex, including the medial temporal cortex. Interestingly, the temporal cortices are the first sites involved in AD, which raises the questions of why and how AD pathology can produce CBS, if topography rather than lesion type is responsible. However, this theory of location of lesion deposition is thought to explain why CBD pathology may produce a variety of clinical phenotypes apart from CBS (e.g., progressive supranuclear palsy syndrome, behavioral variant frontotemporal dementia and apraxia of speech) [7,14].
Alzheimer’s disease pathology is the most common cause of dementia in adults. The ascribed clinical picture of DAT is progressive episodic memory loss accompanied by deficits in executive and visuospatial functioning [38]. The key neuropathological findings are loss of neurons and synaptic density, and the deposition of amyloid plaques and neurofibrillary tangles [39]. AD pathology is deposited initially in hippocampal and entorhinal structures, progressing to involve nearby association areas, usually the temporal and parietal association cortices [40]. This histological progression is thought to parallel the clinical progression of DAT. The motor and sensory cortices are almost always spared. However, focal cortical syndromes have been increasingly recognized with AD pathology due to very selective asymmetric disease burden, including CBS, posterior cortical atrophy and primary progressive aphasia [41]. In these cases, AD pathology is highly concentrated in regions typically spared, such as the sensorimotor cortex or parieto–occipital cortex. An interesting and unanswered question is why the pathology is so asymmetric and what drives the tauopathy to selectively target a focal brain site. This is also of interest in CBD pathology, which tends to be very asymmetric, as compared with some other neurodegenerative diseases, such as typical PSP or Lewy body disease, where dramatic asymmetry is not a well-recognized feature [42,43].
Alzheimer’s disease has established risk factors. Age is the strongest risk factor, with exponentially increased risk after 65 years of age [44]. Apoliprotein E (APOE) e4 status [45] and a positive family history of AD [46] are also risk factors. By contrast, CBD pathology is believed to be sporadic. Familial cases of CBS are relatively less common and have only been described for frontotemporal dementia with parkinsonism linked to chromosome 17 pathology, a hereditary form of tauopathy with heterozygous tau mutation [47], familial multisystem tauopathy with presenile dementia [48] and in progranulin gene mutations [19,20].
Abnormal tau deposition occurs in both AD and CBD where tau dysfunction affects axonal transport and microtubule stability in neurons. However, tau isoforms differ between AD and CBD depending on whether exon 10 is spliced in (4R) or spliced out (3R). CBD is a 4R tauopathy, while both 3R and 4R abnormal tau is found in AD [49]. Although both AD and CBD are tauopathies, AD also has coexisting β-amyloid protein deposition; a combination that is unique amongst the dementias. Clinical and pathologic overlap amongst the tauopathies has been explained, in part, by differences in the location of the tau protein deposition.
Comparison of CBS-AD to CBS-CBD
Predicting AD pathology in CBS currently remains challenging, although current research is specifically trying to tackle this issue. A major hurdle in evaluating this clinical entity is its rarity and the lack of autopsy studies. In addition, the nomenclature has only recently evolved in the last 5–10 years, to discriminate between clinical syndromes and pathology; previously CBD could imply the clinical syndrome or pathological diagnosis, or both. Thus while neurocognitive and imaging profiles reportedly differ between ‘CBD’ and ‘AD’, this tends to reflect the prototype clinical syndromes without autopsy confirmation of pathology. This article focuses on clinical and imaging features in studies with autopsy-proven CBS-AD compared with CBS-CBD. Identifying tools to better predict AD pathology is of particular relevance because disease-modifying therapies for AD are undergoing various phase clinical trials.
Demographic factors
Hu et al. noted that CBS-AD cases in their cohort were younger than CBS-CBD cases [2]. Overall, the age of onset in CBS-AD falls under the time frame of an ‘early-onset AD’, rather than ‘late-onset’, which presents at an age of greater than 65 years. Interestingly, it is early-onset AD that tends to show more rapid progression, generalized cortical deficits, cortical atrophy and hypometabolism compared with late-onset patients of a similar stage [50]. Most studies show that early-onset AD is associated with greater attention, language, praxis, visuospatial and executive dysfunction [51], while late-onset AD is associated with greater impairment in episodic memory. Early-onset AD is typically associated with more severe neuroimaging findings, with increased cortical atrophy, hypoperfusion and hypometabolism, especially in parietal and lateral temporal cortices, versus the medial temporal cortex in late-onset AD; supporting the findings in CBS-AD.
Apoliproprotein E e4 confers an increased risk of AD, but does not account for all cases. Unfortunately, APOE e4 has not been well documented in CBS-AD cases to determine any associations. Interestingly, Schneider et al. showed that pathologically proven CBS-CBD cases have a higher frequency of the APOE e4 allele than controls, although not as high as in AD patients [5]. Thus, APOE genotype may not be a useful discriminator of pathology.
MAPT H1 haplotype may be a genetic risk factor for CBD and hence CBS-CBD. The chromosomal region containing the tau gene involves two major haplotypes, H1 and H2, which are defined by linkage disequilibrium between several polymorphisms over the entire gene. There has not been any strong association between the H1 haplotype and AD [52], while an association with CBD was reported [53].
Family history is positive in approximately 25% of AD cases presenting with typical DAT [54]. On the contrary, family history tends to be negative in CBS presentations of AD and in CBS-CBD; therefore, sporadic CBS could indicate either AD or CBD pathology. Unfortunately, family history information was not recorded in some studies.
Several risk factors, including prior head injury, obesity, insulin resistance and vascular risk factors (hypertension, hypercholesterolemia and obstructive sleep apnea), are believed to increase the risk of AD. The role of these risk factors is not known in CBS-AD. More recently, late-life depression, social isolation and lack of physical exercise have been shown to be associated with AD pathology risk, but it is unclear whether these associations also occur in CBS-AD.
Clinical factors
Myoclonus is well established as a feature of AD, but has also been associated with CBD [54]. However, Hu et al. noted that it was more common in CBS-AD (four out of five) than CBS-CBD (two out of 11) cases [2]. Shelley et al. reported two out of six CBS-AD cases with myoclonus, but no CBS-CBD cases [3]. The presence of myoclonus may, therefore, be more suggestive of CBS-AD than CBS-CBD. Limb apraxia has been reported to occur in both pathologies in approximately equal frequencies. Tremor occurred only within the CBD subgroup in the study by Hu et al [2]. Supranuclear gaze palsy was rarely reported in CBS-AD, but was more common in CBS-CBD. Parkinsonism may point away from CBS-AD, as higher Unified Parkinson’s Disease Rating Scale scores were reported in CBS-CBD compared with CBS-AD [55,56].
Neurocognitive factors
Dementia is recognized in CBS, and may be the most common diagnosis in pathologically confirmed CBD, as it is in AD [10]. The cognitive profile probably varies according to the topographical distribution of pathology. For example, left hemisphere involvement may produce a language disorder, while right hemisphere lesion burden produces visuospatial dysfunction. Memory is usually relatively intact in CBS, consistent with relative sparing of the medial temporal cortex in CBD, but as this is the typical location of AD pathology one would anticipate impairment in these cases. Two studies did not report any difference in memory impairment and attentional deficits between CBS-AD and CBS-CBD [2,56]. However, Shelley et al. noted that initial episodic memory loss appeared to predict CBS-AD [3]. This was also noted by others [11,26,28]. Supporting this observation, Borroni et al. recently reported early memory impairment in CBS cases with an AD pathology bioprofile (cerebrospinal fluid [CSF] and SPECT imaging consistent with AD), compared with CBS cases with a non-AD profile [57].
It has been noted that frontal lobe symptoms (utilization behavior, personality change and frontal release signs) are associated with CBS-CBD [3,5,11]. Neuropsychometric evaluation of frontal lobe involvement and dysexecutive function may thus help identify CBS-CBD [58,59]. Indeed, verbal letter and category fluency testing are abnormal in CBD [60,61]. Agrammatic aphasia and orobuccal apraxia has also been associated with CBS-CBD [3,56,61], although language can remain preserved [23,28]. Conversely, visuospatial impairment may indicate CBS-AD; Alladi et al. reported visuospatial impairment in six out of six cases [11] and Ceccaldi et al. in two out of two [34], although it was found in both CBS-AD and CBS-CBD in the Whitwell et al. series [56]. These findings emphasize that frontal disease distribution and less involvement of the medial temporal lobe are correlated with CBS-CBD, while impairment in parietal lobe function is associated with CBS-AD.
Biochemical markers
Biochemical markers for AD that reflect cortical pathology, including neuronal degeneration, β-amyloid plaque deposition and tau hyperphosphorylation, are currently under extensive research. Serum biomarkers have thus far proved disappointing with no consensus, and verification is waiting [62]. CSF biomarkers appear promising. A decline in Aβ42 increase in total tau (t-tau) and phosphorylated tau (p-tau) and ratio of low Aβ42:high tau is characteristic for AD [63]. The combined sensitivity and specificity is 80–90% when comparing AD to controls, although overlap occurs. Low Aβ42 and ratio of p-tau:Aβ42 in CSF has a high predictive value for AD pathology [64,65]. Therefore, CSF biomarkers may be a useful as amyloid-labeled imaging in predicting AD in patients presenting with CBS.
Neuroimaging markers
Much research has correlated anatomical and functional imaging with CBS and DAT, and found specific patterns of atrophy or altered metabolism, respectively. However, the caveat again is that there are very few studies with autopsy-proven diagnosis of CBD or AD, thus these findings are limited in predicting an underlying pathology for CBS. However, one may surmise that perhaps there may be features of AD on antemortem scans in the setting of CBS that may hint at this pathology. Autopsy-proven pathological case series will be required to confirm this hypothesis.
MRI
Typical MRI findings in CBS are asymmetric cortical atrophy, mainly frontoparietal, with the most severe changes contralateral to the more affected clinical side [66,67]. This pattern of atrophy is sensitive to CBS diagnosis, but not specific to any particular underlying pathology [68]. By comparison, the medial temporal lobes, especially the hippocampus and entorhinal cortex, are amongst the earliest site of pathologic involvement in AD, reflected by reduced hippocampal and entorhinal cortex volumes on brain MRI compared with age-matched controls. Cortical volume loss in AD is usually extensive and involves frontal, temporal and parietal lobes [69,70]. Thus one could postulate that widespread MRI patterns of atrophy may predict AD pathology. Supporting this, diffuse atrophy was noted in several CBS-AD cases [24,28,29].
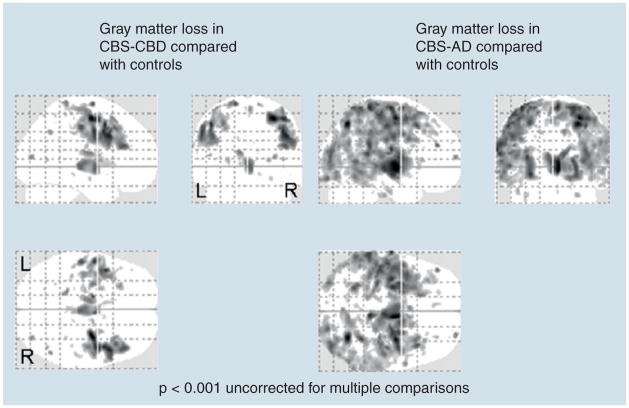
Voxel-based morphology (VBM) uses group analysis to evaluate regional brain atrophy without any a priori assumptions. Whitwell et al. examined CBS cases with various autopsy-proven pathologies, and found the only common site of involvement was the premotor cortex and insula (dominant) and supplementary motor area (dominant and nondominant) [56]. The patterns of atrophy were relatively restricted to inferior and superior posterior frontal regions in CBS-CBD, but were more widespread in CBS-AD, with additional involvement of the temporoparietal lobes [55,56]. Therefore, a more widespread and posterior pattern of atrophy is associated with CBS-AD. Interestingly, both CBS-CBD and CBS-AD showed relative sparing of the hippocampus, typical for CBD pathology but atypical for AD pathology [55,56] (Figure 1). These patterns of atrophy may, therefore, be useful to help predict pathology in subjects with CBS.
Figure 1. Voxel-based morphometry results showing patterns of gray matter loss in six subjects with corticobasal syndrome with corticobasal degeneration and five subjects with corticobasal syndrome with Alzheimer’s disease compared with 20 age- and gender-matched controls.
The CBS-AD subjects were more highly educated and younger than the CBS-CBD subjects. Age and gender were included as covariates in the model. Results are shown on transparent renders of the brain at a statistical threshold of p < 0.001 uncorrected for multiple comparisons. AD: Alzheimer’s disease; CBD: Corticobasal degeneration; CBS: Corticobasal syndrome; L: Left; R: Right.
Temporoparietal atrophy has also been shown to be a sensitive and specific marker of AD pathology in a study assessing autopsy-proven AD and non-AD pathology cases that underwent VBM [71]. Rates of brain atrophy may also be a helpful discriminator for AD pathology versus CBD pathology in CBS, since rates of atrophy in CBD have been shown to be faster than that of AD [72].
Functional imaging with resting state MRI
Resting state functional MRI (fMRI) demonstrates reduced activity in AD in the default mode network, encompassing the posterior cingulate, inferior parietal, inferolateral temporal, ventral anterior cingulate and hippocampus [73,74]. This is consistent with typical structural imaging for AD. There are no published studies as yet assessing resting state fMRI in pathologically confirmed cases of AD, or comparing CBD to AD.
SPECT
SPECT imaging studies appear promising. Asymmetric hypoperfusion in frontoparietal lobes and basal ganglia is reportedly common in CBS [66,75]. By comparison, the AD hypoperfusion pattern in autopsy-proven cases involves the parietal and temporal lobes, precuneus and posterior cingulate cortex [76,77]. SPECT parietal lobe hypoperfusion may be more associated with CBS-AD than CBS-CBD [2]. Furthermore, CBS cases with a CSF AD profile had SPECT images that also resembled AD, with greater hypoperfusion in the precuneus, posterior cingulate and bilateral hippocampi [57].
Fluorodeoxyglucose-PET
Glucose hypometabolism in the precuneus, posterior cingulate and biparietal regions is observed in AD. This correlates with elevated CSF t-tau and p-tau and reduced metabolism. By comparison, CBS typically has asymmetric hypoperfusion within the parietofrontal cortex and basal ganglia [78,79], although PET findings in pathologically proven CBD are rarely reported. Asymmetric hypometabolism in the contralateral frontoparietal cortex, basal ganglia and cerebellum has been reported [14]. A recent radiological study found that fluorodeoxyglucose-PET scans performed early can predict clinical syndromes that later meet diagnostic criteria. Disease-specific patterns of relatively decreased metabolic activity were found in CBS (contralateral cortical regions) and AD (parietotemporal regions); however, they were not pathologically confirmed [80]. If this was supported by autopsy studies, then early PET in CBS could be helpful to predict the underlying pathology.
Amyloid PET imaging
This powerful new imaging technique uses ligands that attach to fibrillar amyloid, such as Pittsburgh compound B (PiB), flor-betaben and AV-45 [62], and allows the detection of amyloid in the brain during life. Widespread use of this modality is restricted by requirements for an on-site cyclotron and radiochemistry laboratory. However, PiB has been shown to be sensitive and specific for amyloid deposition, and has been reliably demonstrated to differentiate AD from FTD. It can also differentiate AD from normal controls, although some normal elderly patients may have positive findings due to age-related amyloid deposition [81]. No comparisons with CBD have yet been reported.
Literature summary of CBS-AD compared with CBS-CBD cases
To determine whether any of the discussed demographic or clinical factors may be useful predictors of pathology, a literature summary was compiled for all cases published up to 30 May 2011, in the literature of CBS-AD compared with CBS-CBD cases. A PubMed search with the terms “corticobasal syndrome” or “corticobasal degeneration”, cross-matched with “Alzheimer’s disease” was employed to identify all cases of CBS-AD and CBS-CBD. Approximately 42 cases of CBS-AD were either published as case reports or small case series [2,3,7,11,12,23–33,35,55,56,82,83]. Duplicate cases or cases with coexistent pathology with AD were excluded where these could be determined. Cases were included (n = 29) only if there was sufficient detail to include demographic, clinical, imaging and pathologic information to compile a literature summary. These were compared with CBS-CBD cases (n = 24) to identify clinical or imaging differences that could increase the antemortem diagnosis of AD. Given the number of variables assessed, we consider p < 0.01 as significant. Continuous variables were compared using Mann–Whitney U test and categorical variables were assessed using χ2 test.
Comparing 29 CBS-AD to 24 CBS-CBD cases, there were several key findings that appeared to be associated with AD pathology (Table 1): longer disease duration, younger age at onset, hemisensory neglect, memory impairment, visuospatial difficulties, dressing apraxia and myoclonus. Importantly, there was no difference between aphasia, limb apraxia, alien limb phenomenon, fisted hand, parkinsonism, pyramidal motor signs, dystonia or Gerstmann syndrome. This is not surprising given that both subgroups had the same clinical diagnosis of CBS, of which these signs meet inclusion criteria. Perhaps of more interest, there was no difference in change of gait (which is usually normal in typical AD), aphasia (as observed by several small group studies) or personality change or frontal release signs (anticipated for CBD). There were no significant associations with CBS-CBD, although we noted a trend for utilization behavior, rigidity and extraocular dysfunction to be associated with CBS-CBD. These findings are not surprising given that imaging demonstrates focal frontal lobe atrophy in CBS-CBD, compared with more parietal lobe findings in CBS-AD. Family history was recorded in too few cases to compare with confidence. Overall these findings are of interest, and require confirmation in larger prospective studies. It will also be important to identify whether a combination of clinical features is most sensitive and specific for CBS-AD.
Table 1.
Comparison of demographics and clinical features of corticobasal syndrome with underlying Alzheimer’s disease pathology and corticobasal syndrome with underlying corticobasal degeneration.
| Demographic and clinical features | CBS-AD (n = 29), positive cases/total cases recorded (%) | CBS-CBD (n = 24) (%) | p-value |
|---|---|---|---|
| Gender (males) | 12/29 (41) | 10/24 (42) | 0.98 |
| Right-handed | 22/23 (96) | 13/14 (93) | 0.72 |
| Age of onset (years) | 60 (50–75) (n = 29) | 66 (41–73) (n = 24) | 0.003 |
| Disease duration (years)† | 9 (3–22) (n = 27) | 6 (3–11) (n = 24) | 0.0006 |
| Age at death (years)† | 68 (61–88) (n = 26) | 72 (47–84) (n = 24) | 0.23 |
| Left side of onset | 19/26 (73) | 12/24 (50) | 0.18 |
| Cortical | |||
| Apraxia (nonspecified) | 27/27 (100) | 23/24 (96) | 0.22 |
| Alien limb | 9/13 (69) | 7/13 (54) | 0.42 |
| Aphasia | 9/22 (41) | 12/20 (60) | 0.22 |
| Memory impairment | 21/25 (84) | 10/24 (42) | 0.002 |
| Dressing apraxia | 12/12 (100) | 4/7 (57) | 0.008 |
| Personality/behavior change | 7/18 (39) | 8/13 (62) | 0.21 |
| Cortical sensory loss | 12/17 (71) | 8/21 (38) | 0.04 |
| Visuospatial disturbance | 12/20 (60) | 2/13 (15) | 0.009 |
| Utilization behavior | 0/8 (0) | 3/6 (50) | 0.01 |
| Constructional apraxia | 10/10 (100) | 5/7 (71) | 0.04 |
| MMSE on presentation† | 16 (9–23) | 22 (20–27) | 0.04 |
| Gerstmann’s syndrome | 2/3 (67) | 3/7 (43) | 0.49 |
| Hemisensory neglect | 8/8 (100) | 3/7 (43) | 0.005 |
| Frontal release signs | 15/22 (68) | 11/20 (55) | 0.38 |
| Motor | |||
| Extraocular disturbance | 5/17 (29) | 15/24 (63) | 0.04 |
| Parkinsonism | 15/17 (88) | 16/17 (94) | 0.54 |
| Bradykinesia | 5/5 (100) | 10/11 (91) | 0.38 |
| Rigidity | 12/15 (80) | 18/18 (100) | 0.02 |
| Dystonia | 8/21 (38) | 10/20 (50) | 0.44 |
| Tremor | 5/6 (83) | 10/11 (91) | 0.65 |
| Myoclonus | 17/23 (74) | 4/20 (20) | 0.0003 |
| Postural instability/gait change | 9/20 (45) | 16/24 (67) | 0.15 |
| Fisted hand | 3/3 (100) | 5/7 (71) | 0.20 |
| Dysarthria | 4/9 (44) | 5/13 (38) | 0.78 |
| Dysphagia | 4/10 (40) | 3/6 (50) | 0.70 |
| Pyramidal signs | 9/10 (90) | 4/7 (57) | 0.11 |
Data shown as median (range).
CBS-AD: Corticobasal syndrome with Alzheimer’s disease pathology; CBS-CBD: Corticobasal syndrome with underlying corticobasal degeneration; MMSE: Mini-Mental State Examination.
The findings from this literature review should be considered hypothesis generating as many studies are limited by the absence of well-designed prospective protocols utilizing standardized and validated tests. This is important since some of the signs reported may not have been properly characterized. Memory impairment, for example, will need to be operationally defined with a verbal serial list-learning test comprised of three elements: very low performance on delay-free recall; random or near random performance on a delayed recognition test; and the production of extra-list intrusion errors. Aphasia should be better defined and the presence or absence of apraxia of speech included. Limb apraxia should also be separated into anterior versus posterior limb apraxia. Therefore, it is important to assess other cognitive domains. Memory impairment in CBS-CBD, for example, is often confined to visual memory [84], a finding that may be confounded by visuospatial impairment, although the underlying nature of the visuospatial impairment is controversial.
It should be noted that since the completion of this literature review another CBS study with pathology has been published with results supporting many of the findings reported in this article [85].
Expert commentary
Clinicopathological studies have now clearly demonstrated that CBS, as currently defined, is nonspecific, with an increasing number of reports showing AD pathology underlying the syndrome. Differentiating CBS-AD from CBS-CBD based solely on clinical features has not been possible. Given that recent evidence from VBM studies showing that CBS-CBD is associated with posterior frontal atrophy while CBS-AD is associated with more widespread atrophy, particularly involving the parietal lobes, symptoms associated with parietal lobe dysfunction should predict CBS-AD (Box 1). However, more promising to differentiate CBS-CBD from CBS-AD, are CSF and amyloid imaging biomarkers, although neither would allow us to predict dual pathologies.
Box 1. Clinical, cerebral spinal fluid and imaging signs suggestive of Alzheimer’s disease pathology in corticobasal syndrome.
Clinical signs
Longer disease duration (compared with CBD pathology)
Limb myoclonus
Dressing apraxia
Memory loss
Lower MMSE on presentation
Cortical sensory loss
Visuospatial difficulties
Hemisensory neglect
Absence of limb rigidity
Cerebral spinal fluid signs
Aβ42 reduction, and elevated total and phosphorylated tau
Imaging signs
MRI: posterior lateral temporoparietal atrophy
FDG-PET: temporoparietal hypometabolism
C11-PiB-PET positive amyloid imaging
Aβ: Amyloid β; CBD: Corticobasal degeneration; FDG: Fluorodeoxyglucose; MMSE: Mini-Mental State Examination; PiB: Pittsburgh compound B ([N-methyl-11C]2-(4′-methylaminophenyl)-6-hydroxybenzothiazole).
Five-year view
One would anticipate that future research will aim to improve pathologic diagnostic accuracy for all neurodegenerative diseases, spurred on by the development of disease-modifying agents. Clinical diagnosis of CBS, coupled with improved imaging techniques and biomarkers, should help to identify underlying AD pathology. To refine the clinicopathological diagnosis of CBS-AD, the gold standard would be prospective recruitment of CBS cases as a multicenter study, collecting CSF biomarkers and performing periodic anatomical, functional and amyloid-labeled imaging, with autopsy follow-up for pathologic confirmation to better define which investigations aid the diagnosis. Borroni et al.’s recently published study is employing some of these very techniques to try to predict AD pathology in CBS cases, although autopsy confirmation is not included [57]. Future imaging developments that could label both 3R and 4R tau in vivo in order to suggest AD pathology, rather than a pure 4R tauopathy, such as CBD, would be useful.
Early diagnosis of CBS-AD would allow therapeutic agents, such as cholinesterase inhibitors or NMDA receptor antagonists to be potentially employed for benefit, although at present there is no evidence that these therapies will be helpful in CBS-AD. CBS cases cannot be treated empirically as therapies have side effects. Of importance, early identification of AD could lead to early utility of disease-modifying agents to slow AD-associated neurodegeneration. For example, reducing amyloid burden via β-secretase or γ-secretase inhibitors, amyloid monoclonal antibodies or amyloid anti-aggregation therapies, or reducing tau deposition or improving stability via tau microtubule stabilizers. Response to therapy could be tracked through these very biomarkers.
Key issues.
Corticobasal syndrome (CBS) is a rare neurodegenerative disorder characterized by asymmetrical cortical and extrapyramidal dysfunction.
Although the CBS was initially thought to be specific to a single pathology, it is increasingly recognized that the syndrome is nonspecific to any one pathology.
Many pathologies underlie the corticobasal syndrome, including corticobasal degeneration (CBD) and Alzheimer’s disease (AD).
Both CBD and AD are considered tauopathies; however, CBD is a four microtubule-binding repeats tauopathy, while AD is a three microtubule-binding repeats and four microtubule-binding repeats tauopathy.
Clinical studies have not been able to differentiate AD from CBD when they underlie the corticobasal syndrome.
Imaging studies have demonstrated that CBS-CBD is characterized by relatively focal posterior frontal (premotor) atrophy, while CBS-AD shows more widespread frontal and parietal atrophy.
This literature review has identified clinical features that appear to be associated with CBS-AD, including myoclonus, visuospatial deficits, memory impairment, dressing apraxia, hemisensory neglect and younger age at onset.
No features were significantly associated with CBS-CBD, although there was some suggestion of association with utilization behavior and ocular motor impairment.
Amyloid imaging and cerebrospinal fluid biomarkers show promise as potential biomarkers to differentiate CBS-CBD from CBS-AD.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
Jennifer L Whitwell and Keith A Josephs are supported by NIH grants R01 DC10367, R01 AG37491, R21 AG38736 and the Dana Foundation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Boeve BF. Links between frontotemporal lobar degeneration, corticobasal degeneration, progressive supranuclear palsy, and amyotrophic lateral sclerosis. Alzheimer Dis Assoc Disord. 2007;21(4):S31–S38. doi: 10.1097/WAD.0b013e31815bf454. [DOI] [PubMed] [Google Scholar]
- 2•.Hu WT, Rippon GW, Boeve BF, et al. Alzheimer’s disease and corticobasal degeneration presenting as corticobasal syndrome. Mov Disord. 2009;24(9):1375–1379. doi: 10.1002/mds.22574. Demonstrates that the presence of myoclonus suggests corticobasal syndrome with Alzheimer’s disease (CBS-AD) [DOI] [PubMed] [Google Scholar]
- 3•.Shelley BP, Hodges JR, Kipps CM, Xuereb JH, Bak TH. Is the pathology of corticobasal syndrome predictable in life? Mov Disord. 2009;24(11):1593–1599. doi: 10.1002/mds.22558. Details that the presence of memory impairment suggests CBS-AD. [DOI] [PubMed] [Google Scholar]
- 4.Josephs KA, Petersen RC, Knopman DS, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006;66(1):41–48. doi: 10.1212/01.wnl.0000191307.69661.c3. [DOI] [PubMed] [Google Scholar]
- 5.Schneider JA, Watts RL, Gearing M, Brewer RP, Mirra SS. Corticobasal degeneration: neuropathologic and clinical heterogeneity. Neurology. 1997;48(4):959–969. doi: 10.1212/wnl.48.4.959. [DOI] [PubMed] [Google Scholar]
- 6.Josephs KA, Holton JL, Rossor MN, et al. Neurofilament inclusion body disease: a new proteinopathy? Brain. 2003;126(Pt 10):2291–2303. doi: 10.1093/brain/awg231. [DOI] [PubMed] [Google Scholar]
- 7.Boeve BF, Maraganore DM, Parisi JE, et al. Pathologic heterogeneity in clinically diagnosed corticobasal degeneration. Neurology. 1999;53(4):795–800. doi: 10.1212/wnl.53.4.795. [DOI] [PubMed] [Google Scholar]
- 8.Masellis M, Momeni P, Meschino W, et al. Novel splicing mutation in the progranulin gene causing familial corticobasal syndrome. Brain. 2006;129(Pt 11):3115–3123. doi: 10.1093/brain/awl276. [DOI] [PubMed] [Google Scholar]
- 9.Grimes DA, Bergeron CB, Lang AE. Motor neuron disease-inclusion dementia presenting as cortical-basal ganglionic degeneration. Mov Disord. 1999;14(4):674–680. doi: 10.1002/1531-8257(199907)14:4<674::aid-mds1019>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 10•.Grimes DA, Lang AE, Bergeron CB. Dementia as the most common presentation of cortical-basal ganglionic degeneration. Neurology. 1999;53(9):1969–1974. doi: 10.1212/wnl.53.9.1969. Contrary to previous thinking, this article suggests that dementia is a common presentation of corticobasal degeneration (CBD) [DOI] [PubMed] [Google Scholar]
- 11.Alladi S, Xuereb J, Bak T, et al. Focal cortical presentations of Alzheimer’s disease. Brain. 2007;130(Pt 10):2636–2645. doi: 10.1093/brain/awm213. [DOI] [PubMed] [Google Scholar]
- 12.Doran M, du Plessis DG, Enevoldson TP, Fletcher NA, Ghadiali E, Larner AJ. Pathological heterogeneity of clinically diagnosed corticobasal degeneration. J Neurol Sci. 2003;216(1):127–134. doi: 10.1016/s0022-510x(03)00232-6. [DOI] [PubMed] [Google Scholar]
- 13.Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol. 2003;54(Suppl 5):S15–S19. doi: 10.1002/ana.10570. [DOI] [PubMed] [Google Scholar]
- 14.Hassan A, Whitwell JL, Boeve BF, et al. Symmetric corticobasal degeneration (S-CBD) Parkinsonism Relat Disord. 2010;16(3):208–214. doi: 10.1016/j.parkreldis.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doody RS, Jankovic J. The alien hand and related signs. J Neurol Neurosurg Psychiatry. 1992;55(9):806–810. doi: 10.1136/jnnp.55.9.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottlieb D, Robb K, Day B. Mirror movements in the alien hand syndrome. Case report. Am J Phys Med Rehabil. 1992;71(5):297–300. doi: 10.1097/00002060-199210000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Josephs KA, Duffy JR, Strand EA, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129(Pt 6):1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rebeiz JJ, Kolodny EH, Richardson EP., Jr Corticodentatonigral degeneration with neuronal achromasia. Arch Neurol. 1968;18(1):20–33. doi: 10.1001/archneur.1968.00470310034003. [DOI] [PubMed] [Google Scholar]
- 19.Yu CE, Bird TD, Bekris LM, et al. The spectrum of mutations in progranulin: a collaborative study screening 545 cases of neurodegeneration. Arch Neurol. 2010;67(2):161–170. doi: 10.1001/archneurol.2009.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelley BJ, Haidar W, Boeve BF, et al. Prominent phenotypic variability associated with mutations in Progranulin. Neurobiol Aging. 2009;30(5):739–751. doi: 10.1016/j.neurobiolaging.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Josephs KA, Hodges JR, Snowden JS, et al. Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol. 2011;122(2):137–153. doi: 10.1007/s00401-011-0839-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22••.Ling H, O’Sullivan SS, Holton JL, et al. Does corticobasal degeneration exist? A clinicopathological re-evaluation. Brain. 2010;133(Pt 7):2045–2057. doi: 10.1093/brain/awq123. Details that the pathological diagnosis associated with CBS are heterogeneous. [DOI] [PubMed] [Google Scholar]
- 23.Ball JA, Lantos PL, Jackson M, Marsden CD, Scadding JW, Rossor MN. Alien hand sign in association with Alzheimer’s histopathology. J Neurol Neurosurg Psychiatry. 1993;56(9):1020–1023. doi: 10.1136/jnnp.56.9.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chand P, Grafman J, Dickson D, Ishizawa K, Litvan I. Alzheimer’s disease presenting as corticobasal syndrome. Mov Disord. 2006;21(11):2018–2022. doi: 10.1002/mds.21055. [DOI] [PubMed] [Google Scholar]
- 25.Eberhard DA, Lopes MB, Trugman JM, Brashear HR. Alzheimer’s disease in a case of cortical basal ganglionic degeneration with severe dementia. J Neurol Neurosurg Psychiatry. 1996;60(1):109–110. doi: 10.1136/jnnp.60.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golaz J, Bouras C, Hof PR. Motor cortex involvement in presenile dementia: report of a case. J Geriatr Psychiatry Neurol. 1992;5(2):85–92. doi: 10.1177/002383099200500205. [DOI] [PubMed] [Google Scholar]
- 27.Horoupian DS, Wasserstein PH. Alzheimer’s disease pathology in motor cortex in dementia with lewy bodies clinically mimicking corticobasal degeneration. Acta Neuropathol. 1999;98(3):317–322. doi: 10.1007/s004010051087. [DOI] [PubMed] [Google Scholar]
- 28.Imamura A, Wszolek ZK, Lucas JA, Dickson DW. Corticobasal syndrome with Alzheimer’s disease pathology. Mov Disord. 2009;24(1):152–153. doi: 10.1002/mds.21877. [DOI] [PubMed] [Google Scholar]
- 29.Jagust WJ, Davies P, Tiller-Borcich JK, Reed BR. Focal Alzheimer’s disease. Neurology. 1990;40(1):14–19. doi: 10.1212/wnl.40.1.14. [DOI] [PubMed] [Google Scholar]
- 30.Kaida K, Takeda K, Nagata N, Kamakura K. Alzheimer’s disease with asymmetric parietal lobe atrophy: a case report. J Neurol Sci. 1998;160(1):96–99. doi: 10.1016/s0022-510x(98)00221-4. [DOI] [PubMed] [Google Scholar]
- 31.Lleo A, Rey MJ, Castellvi M, Ferrer I, Blesa R. Asymmetric myoclonic parietal syndrome in a patient with Alzheimer’s disease mimicking corticobasal degeneration. Neurologia. 2002;17(4):223–226. [PubMed] [Google Scholar]
- 32.Osako M, Mochizuki Y, Kugio Y, Mizutani T, Hayashi H. Autopsy case of atypical type of alzheimer’s disease clinically diagnosed as corticobasal degeneration. Rinsho Shinkeigaku. 2007;47(9):581–584. [PubMed] [Google Scholar]
- 33.Pogacar S, Williams RS. Alzheimer’s disease presenting as slowly progressive aphasia. RI Med J. 1984;67(4):181–185. [PubMed] [Google Scholar]
- 34.Ceccaldi M, Poncet M, Gambarelli D, Guinot H, Bille J. Progressive severity of left unilateral apraxia in 2 cases of Alzheimer disease. Rev Neurol (Paris) 1995;151(4):240–246. [PubMed] [Google Scholar]
- 35.Okazaki K, Fu YJ, Nishihira Y, et al. Alzheimer’s disease: report of two autopsy cases with a clinical diagnosis of corticobasal degeneration. Neuropathology. 2010;30(2):140–148. doi: 10.1111/j.1440-1789.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 36.Litvan I, Agid Y, Goetz C, et al. Accuracy of the clinical diagnosis of corticobasal degeneration: a clinicopathologic study. Neurology. 1997;48(1):119–125. doi: 10.1212/wnl.48.1.119. [DOI] [PubMed] [Google Scholar]
- 37.Dickson DW, Bergeron C, Chin SS, et al. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol. 2002;61(11):935–946. doi: 10.1093/jnen/61.11.935. [DOI] [PubMed] [Google Scholar]
- 38.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 39.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 40.Braak H, Braak E. Evolution of the neuropathology of Alzheimer’s disease. Acta Neurol Scand Suppl. 1996;165:3–12. doi: 10.1111/j.1600-0404.1996.tb05866.x. [DOI] [PubMed] [Google Scholar]
- 41.Kramer JH, Miller BL. Alzheimer’s disease and its focal variants. Semin Neurol. 2000;20(4):447–454. doi: 10.1055/s-2000-13177. [DOI] [PubMed] [Google Scholar]
- 42.Whitwell JL, Weigand SD, Shiung MM, et al. Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer’s disease. Brain. 2007;130(Pt 3):708–719. doi: 10.1093/brain/awl388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Josephs KA, Whitwell JL, Dickson DW, et al. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging. 2008;29(2):280–289. doi: 10.1016/j.neurobiolaging.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katzman RKC. In: The Epidemiology of Dementia and Alzheimer’s Disease. Terry RD, Bick KL, editors. Raven Press; New York, NY, USA: 1994. [Google Scholar]
- 45.Weiner MF, Vega G, Risser RC, et al. Apolipoprotein E ε4, other risk factors, and course of Alzheimer’s disease. Biol Psychiatry. 1999;45(5):633–638. doi: 10.1016/s0006-3223(98)00222-4. [DOI] [PubMed] [Google Scholar]
- 46.van Duijn CM, Stijnen T, Hofman A. Risk factors for Alzheimer’s disease: overview of the EURODEM collaborative re-analysis of case–control studies. EURODEM Risk Factors Research Group. Int J Epidemiol. 1991;20(Suppl 2):S4–S12. doi: 10.1093/ije/20.supplement_2.s4. [DOI] [PubMed] [Google Scholar]
- 47.Hutton M, Lendon CL, Rizzu P, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393(6686):702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 48.Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci USA. 1998;95(13):7737–7741. doi: 10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scaravilli T, Tolosa E, Ferrer I. Progressive supranuclear palsy and corticobasal degeneration: lumping versus splitting. Mov Disord. 2005;20(Suppl 12):S21–S28. doi: 10.1002/mds.20536. [DOI] [PubMed] [Google Scholar]
- 50.Rabinovici GD, Furst AJ, Alkalay A, et al. Increased metabolic vulnerability in early-onset Alzheimer’s disease is not related to amyloid burden. Brain. 2010;133(Pt 2):512–528. doi: 10.1093/brain/awp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Licht EA, McMurtray AM, Saul RE, Mendez MF. Cognitive differences between early- and late-onset Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2007;22(3):218–222. doi: 10.1177/1533317506299156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Green EK, Thaker U, McDonagh AM, et al. A polymorphism within intron 11 of the tau gene is not increased in frequency in patients with sporadic Alzheimer’s disease, nor does it influence the extent of tau pathology in the brain. Neurosci Lett. 2002;324(2):113–116. doi: 10.1016/s0304-3940(02)00190-8. [DOI] [PubMed] [Google Scholar]
- 53.Houlden H, Baker M, Morris HR, et al. Corticobasal degeneration and progressive supranuclear palsy share a common tau haplotype. Neurology. 2001;56(12):1702–1706. doi: 10.1212/wnl.56.12.1702. [DOI] [PubMed] [Google Scholar]
- 54.Bird T. Alzheimer disease overview. In: Pagon RA, Bird TE, Dolan CR, et al., editors. Gene Reviews. University of Washington; Seattle, WA, USA: 2003. [Google Scholar]
- 55••.Josephs KA, Whitwell JL, Boeve BF, et al. Anatomical differences between CBS-corticobasal degeneration and CBS-Alzheimer’s disease. Mov Disord. 2010;25(9):1246–1252. doi: 10.1002/mds.23062. CBS-AD compared directly with corticobasal syndrome-CBD (CBS-CBD) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56••.Whitwell JL, Jack CR, Jr, Boeve BF, et al. Imaging correlates of pathology in corticobasal syndrome. Neurology. 2010;75(21):1879–1887. doi: 10.1212/WNL.0b013e3181feb2e8. Different patholgies associated with CBS show different patterns of atrophy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57•.Borroni B, Premi E, Agosti C, et al. CSF Alzheimer’s disease-like pattern in corticobasal syndrome: evidence for a distinct disorder. J Neurol Neurosurg Psychiatry. 2011;82(8):834–838. doi: 10.1136/jnnp.2010.221853. Cerebrospinal fluid amyloid and tau levels may be useful to differentiate CBS-AD from CBS-CBD. [DOI] [PubMed] [Google Scholar]
- 58.Pillon B, Blin J, Vidailhet M, et al. The neuropsychological pattern of corticobasal degeneration: comparison with progressive supranuclear palsy and Alzheimer’s disease. Neurology. 1995;45(8):1477–1483. doi: 10.1212/wnl.45.8.1477. [DOI] [PubMed] [Google Scholar]
- 59.Soliveri P, Monza D, Paridi D, et al. Cognitive and magnetic resonance imaging aspects of corticobasal degeneration and progressive supranuclear palsy. Neurology. 1999;53(3):502–507. doi: 10.1212/wnl.53.3.502. [DOI] [PubMed] [Google Scholar]
- 60.Bak TH, Crawford LM, Hearn VC, Mathuranath PS, Hodges JR. Subcortical dementia revisited: similarities and differences in cognitive function between progressive supranuclear palsy (PSP), corticobasal degeneration (CBD) and multiple system atrophy (MSA) Neurocase. 2005;11(4):268–273. doi: 10.1080/13554790590962997. [DOI] [PubMed] [Google Scholar]
- 61.Graham NL, Bak T, Patterson K, Hodges JR. Language function and dysfunction in corticobasal degeneration. Neurology. 2003;61(4):493–499. doi: 10.1212/01.wnl.0000081230.09863.ed. [DOI] [PubMed] [Google Scholar]
- 62.Cummings JL. Biomarkers in Alzheimer’s disease – perspectives for the future. US Neurology. 2010;6(1):23–27. [Google Scholar]
- 63.Blennow K, Hampel H. CSF markers for incipient Alzheimer’s disease. Lancet Neurol. 2003;2(10):605–613. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- 64.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65(4):403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tapiola T, Alafuzoff I, Herukka SK, et al. Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66(3):382–389. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- 66.Koyama M, Yagishita A, Nakata Y, Hayashi M, Bandoh M, Mizutani T. Imaging of corticobasal degeneration syndrome. Neuroradiology. 2007;49(11):905–912. doi: 10.1007/s00234-007-0265-6. [DOI] [PubMed] [Google Scholar]
- 67.Schrag A, Good CD, Miszkiel K, et al. Differentiation of atypical parkinsonian syndromes with routine MRI. Neurology. 2000;54(3):697–702. doi: 10.1212/wnl.54.3.697. [DOI] [PubMed] [Google Scholar]
- 68.Josephs KA, Tang-Wai DF, Edland SD, et al. Correlation between antemortem magnetic resonance imaging findings and pathologically confirmed corticobasal degeneration. Arch Neurol. 2004;61(12):1881–1884. doi: 10.1001/archneur.61.12.1881. [DOI] [PubMed] [Google Scholar]
- 69.Jones BF, Barnes J, Uylings HB, et al. Differential regional atrophy of the cingulate gyrus in Alzheimer disease: a volumetric MRI study. Cereb Cortex. 2006;16(12):1701–1708. doi: 10.1093/cercor/bhj105. [DOI] [PubMed] [Google Scholar]
- 70.Rusinek H, de Leon MJ, George AE, et al. Alzheimer disease: measuring loss of cerebral gray matter with MR imaging. Radiology. 1991;178(1):109–114. doi: 10.1148/radiology.178.1.1984287. [DOI] [PubMed] [Google Scholar]
- 71.Whitwell JL, Jack CR, Jr, Przybelski SA, et al. Temporoparietal atrophy: a marker of AD pathology independent of clinical diagnosis. Neurobiol Aging. 2009;32(9):1531–1541. doi: 10.1016/j.neurobiolaging.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Whitwell JL, Jack CR, Jr, Parisi JE, et al. Rates of cerebral atrophy differ in different degenerative pathologies. Brain. 2007;130(Pt 4):1148–1158. doi: 10.1093/brain/awm021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu X, Li R, Fleisher AS, et al. Altered default mode network connectivity in alzheimer’s disease – a resting functional MRI and bayesian network study. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21153. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang L, Murata Y, Ishida R, Saitoh Y, Mizusawa H, Shibuya H. Differentiating between progressive supranuclear palsy and corticobasal degeneration by brain perfusion SPET. Nucl Med Commun. 2001;22(7):767–772. doi: 10.1097/00006231-200107000-00007. [DOI] [PubMed] [Google Scholar]
- 76.Bonte FJ, Harris TS, Roney CA, Hynan LS. Differential diagnosis between Alzheimer’s and frontotemporal disease by the posterior cingulate sign. J Nucl Med. 2004;45(5):771–774. [PubMed] [Google Scholar]
- 77.McNeill R, Sare GM, Manoharan M, et al. Accuracy of single-photon emission computed tomography in differentiating frontotemporal dementia from Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2007;78(4):350–355. doi: 10.1136/jnnp.2006.106054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Juh R, Pae CU, Kim TS, Lee CU, Choe B, Suh T. Cerebral glucose metabolism in corticobasal degeneration comparison with progressive supranuclear palsy using statistical mapping analysis. Neurosci Lett. 2005;383(1–2):22–27. doi: 10.1016/j.neulet.2005.03.057. [DOI] [PubMed] [Google Scholar]
- 79.Peigneux P, Salmon E, Garraux G, et al. Neural and cognitive bases of upper limb apraxia in corticobasal degeneration. Neurology. 2001;57(7):1259–1268. doi: 10.1212/wnl.57.7.1259. [DOI] [PubMed] [Google Scholar]
- 80.Teune LK, Bartels AL, de Jong BM, et al. Typical cerebral metabolic patterns in neurodegenerative brain diseases. Mov Disord. 2010;25(14):2395–2404. doi: 10.1002/mds.23291. [DOI] [PubMed] [Google Scholar]
- 81.Quigley H, Colloby SJ, O’Brien JT. PET imaging of brain amyloid in dementia: a review. Int J Geriatr Psychiatry. 2011;26(10):991–999. doi: 10.1002/gps.2640. [DOI] [PubMed] [Google Scholar]
- 82.Balasa M, Gelpi E, Antonell A, et al. Clinical features and APOE genotype of pathologically proven early-onset Alzheimer disease. Neurology. 2011;76(20):1720–1725. doi: 10.1212/WNL.0b013e31821a44dd. [DOI] [PubMed] [Google Scholar]
- 83.Crystal HA, Horoupian DS, Katzman R, Jotkowitz S. Biopsy-proved Alzheimer disease presenting as a right parietal lobe syndrome. Ann Neurol. 1982;12(2):186–188. doi: 10.1002/ana.410120210. [DOI] [PubMed] [Google Scholar]
- 84.Libon DJ, Xie SX, Moore P, et al. Patterns of neuropsychological impairment in frontotemporal dementia. Neurology. 2007;68(5):369–375. doi: 10.1212/01.wnl.0000252820.81313.9b. [DOI] [PubMed] [Google Scholar]
- 85.Lee SE, Rabinovici GD, Mayo MC, et al. Clinicopathological correlations in corticobasal degeneration. Ann Neurol. 2011;70(2):327–340. doi: 10.1002/ana.22424. [DOI] [PMC free article] [PubMed] [Google Scholar]