Abstract
MDX-1303 (Valortim) is a fully human monoclonal antibody (hMAb) with a high affinity for Bacillus anthracis protective antigen (PA). MDX-1303 binds to PA and interferes with the activity of the anthrax toxin; it was selected based on its superior functional activity in the toxin neutralization activity (TNA) assay. MDX-1303 has demonstrated efficacy in the postexposure and therapeutic settings in New Zealand White rabbits, cynomolgus monkeys, and African green monkeys. This phase I study sought to characterize the safety, tolerability, immunogenicity, and pharmacokinetics (PK)/pharmacodynamics (PD) of MDX-1303 in healthy human subjects. Cohorts of 3 to 10 subjects were administered MDX-1303 as either a single intravenous (i.v.) dose at dose levels of 0.3, 1, 3, 10, and 20 mg/kg of body weight or as a single intramuscular (i.m.) dose at 100 mg. Forty-six subjects were enrolled, and 16 (35%) of these subjects experienced one or more grade 1 adverse events considered to be related to treatment with MDX-1303. There were no grade 2 to 4 adverse events or serious adverse events (SAEs) considered to be related to treatment. The mean half-life of MDX-1303 ranged from 22 to 33 days across the i.v. administration cohorts and was approximately 32 days following i.m. administration. Systemic exposure following 100-mg i.m. administration was within the range of exposure following 1-mg/kg i.v. administration with a relative bioavailability of approximately 65%. MDX-1303 was generally well tolerated, and no anti-MDX-1303 antibodies were detected following a single dose.
INTRODUCTION
Bacillus anthracis is a Gram-positive, nonmotile, spore-forming bacillus that is found naturally in soil, often in grazing pastures, and can cause acute anthrax infection in both animals and humans. In its spore form, it can persist in nature for prolonged periods, possibly years. Infection can occur through a break in the skin (cutaneous), by ingestion (gastrointestinal), or by inhalation (inhalational) (3, 7, 9, 10, 14). Ninety-five percent of cases reported in developed countries are cutaneous, while 5% are inhalational; outbreaks of gastrointestinal anthrax are most often reported in developing countries (12). While mortality is only about 20% in cases of untreated cutaneous anthrax, mortality in the gastrointestinal and inhalational forms, if untreated, approaches 100%.
The pathogenesis of anthrax is mediated by toxin produced by germinating spores of B. anthracis. The toxin is composed of three proteins: protective antigen (PA), lethal factor (LF), and edema factor (EF). Individually, the proteins are nontoxic; however, when administered together, they exhibit potent toxicity often leading to cell death. Because macrophages and dendritic cells are commonly targeted by the toxins, immune system dysfunction results in rapid spread of the bacteria and, typically, death. Successful treatment of inhalational anthrax will likely require antibiotics to kill the bacteria and an antitoxin to counter the adverse effects of the lethal and edema toxins (18).
It is well known that B. anthracis spores have been developed into biowarfare agents by several countries, including the United States, Japan, Iraq, the United Kingdom, and the former Soviet Union (2). Several characteristics of B. anthracis spores make them particularly suitable for use as a bioweapon: low visibility, high potency, accessibility, and relative ease of delivery. A millionth of a gram of B. anthracis spores constitutes a lethal dose; a kilogram, depending on meteorological conditions and means of delivery, has the potential to kill thousands of people in a metropolitan area (12). Although a vaccine exists primarily for veterinary and military personnel, its use has been controversial due to perceived undesirable side effects. Thus, the majority of U.S. citizens are unprotected against anthrax, making it a serious threat to both civilian and military populations.
Current therapy for anthrax disease consists of antibiotics and aggressive supportive care, particularly for those hospitalized with the inhalational form of the disease. This approach was tested in late 2001 when B. anthracis spores were intentionally delivered in letters and packages to several sites in the United States. Prior to this experience, the largest documented epidemic of inhalational anthrax occurred in the former Soviet Union in 1979, when persons working in, or living near, a bioweapon facility were exposed to B. anthracis spores released accidentally into the air (1). At least 77 cases of exposure and 66 deaths occurred during this outbreak, resulting in a mortality rate of approximately 85%. During the 2001 U.S. anthrax attacks, 11 cases of inhalational anthrax were confirmed; 5 of those individuals died, resulting in a mortality rate of approximately 45% (11). Thus, while the use of antibiotics and aggressive supportive care resulted in improved survival in the 2001 outbreak, death is still likely, and additional therapeutic options are needed. Further, there is a growing concern that bioterrorists may engineer antibiotic-resistant strains of anthrax, which could render existing antibiotics ineffective. This has led to a search for treatments that will target the toxin and provide other options for treatment that do not rely on antibiotic sensitivity.
MDX-1303 (Valortim) is a fully human monoclonal antibody (MAb) that binds to B. anthracis PA (17). Key in vitro features of MDX-1303 include high-affinity binding to both the uncleaved and cleaved forms of PA (PA83 and PA63), corresponding to the unbound and cell-associated forms of PA, which likely results in the potent protection of cells both before and after they are exposed to toxin in a toxin neutralization activity (TNA) assay. MDX-1303 was chosen from a group of fully human MAbs generated against PA based on its superior functional activity in the TNA assay. It recognizes a neutralizing epitope of PA that remains accessible after toxin is bound to cells, compared to other MAbs that block only the initial binding of PA to the cellular receptor. This is similar to high-potency neutralizing polyclonal antibodies in human sera obtained after vaccination with Anthrax Vaccine Absorbed (AVA) (17). In addition, early data suggest that MDX-1303 may augment the immune system's ability to kill B. anthracis (data not shown). Nonclinical in vivo studies demonstrated potent postexposure protection in rabbits and in nonhuman primates, as well as promising therapeutic activity when administered to animals demonstrating signs of infection or evidence of circulating bacterial toxin (data not shown). Advantages of fully human MAbs like MDX-1303 include a half-life that is similar to that of human IgG in circulation (14 to 21 days), enabling long-term protection, and an absence of apparent immunogenicity that might otherwise prevent repeated administration. Based on this information, MDX-1303 appears to have utility for the pre- and postexposure prophylaxis of individuals exposed to, or at risk of exposure to, B. anthracis as well as for the treatment of individuals displaying symptoms and/or signs of inhalation anthrax.
Because it is unethical and infeasible to evaluate the efficacy of an anthrax antitoxin in humans, the Food and Drug Administration (FDA) will evaluate MDX-1303 based on the “Animal Rule” (21 CFR 601.90). Efficacy will be based on survival in appropriate therapeutic animal models, while safety will be based primarily on data generated in clinical trials. A final human dose will be determined through pharmacokinetic (PK) modeling of efficacious animal doses in conjunction with data derived from animal and human PK studies. This study provides the first safety and PK evaluation of MDX-1303 in healthy human volunteers.
MATERIALS AND METHODS
Basic study design.
A phase I, single-center, open-label, single-dose, dose escalation study was conducted in 46 healthy adult volunteers. Cohorts of 3 to 10 subjects meeting study eligibility criteria were administered MDX-1303 (PharmAthene, Inc./Medarex/Bristol Myers Squibb [BMS]) as either a single intravenous (i.v.) dose at 1 of 5 escalating dose levels (0.3, 1, 3, 10, and 20 mg/kg) or as a single intramuscular (i.m.) dose of 100 mg. The total duration of subject participation was approximately 3 months.
This study was conducted between October 2005 and May 2006 at the Quintiles phase I unit (Lenexa, KS) in compliance with standards for good clinical practice and in accordance with the “Recommendations Guiding Physicians in Biomedical Research Involving Human Patients” contained in the 2004 version of the Declaration of Helsinki. Subjects or their legally acceptable representatives provided their written consent to participate in the study after having been informed about the nature and purpose of the study, participation/termination conditions, and risks and benefits of treatment. Informed consent was obtained at screening, prior to the completion of any study-specific procedures.
Subjects.
Healthy male or female volunteers 18 to 60 years of age with no evidence of clinically significant medical or psychiatric conditions were enrolled into the study. Prior known or suspected exposure to B. anthracis, prior vaccination for B. anthracis, detectable antibodies to B. anthracis PA, or evidence of ongoing/previous infection with HIV, hepatitis B, or hepatitis C, as determined at study screening, were all significant exclusionary criteria.
Sexually active women of childbearing potential were required to have a negative urine pregnancy test at study screening, to be using effective contraception for at least 1 month prior to study entry, to have maintained a normal menstrual pattern for the 3 months prior to study entry, and to have agreed to continue contraception for the duration of their participation in the study. Sexually active male subjects were required to use a barrier method of contraception during the course of the study.
The use of systemic immunosuppressive agents was excluded during the course of the study, as was the use of elective vaccinations.
Study agent administration.
MDX-1303, an IgG1 (kappa) isotype, fully human MAb that binds to the PA of B. anthracis, is manufactured in Chinese hamster ovary cells by using standard mammalian cell cultivation and chromatographic purification technologies (17).
MDX-1303 was provided as a liquid formulation and was stored in sterile single-use vials. It was administered as a single 95-min i.v. infusion, using a volumetric pump with a 1.2 μM in-line filter at 1 of 5 escalating dose levels (0.3, 1, 3, 10, and 20 mg/kg) or as an i.m. dose of 100 mg. For i.v. infusion, the total dose was to be diluted to a total volume of 90 ml in 0.9% sodium chloride. In cases where the total dose volume exceeded 90 ml, no dilution was required. For i.m. injection, MDX-1303 was administered as two 2-ml injections (one in each gluteus maximus muscle).
Safety evaluation.
Evaluation of safety and tolerability included assessment of adverse events and drug-induced toxicity, physical examination including vital sign measurements, performance of ECGs, and blood sampling for clinical laboratory parameters.
Adverse events were assessed using the adult toxicity table established by the Division of Microbiology and Infectious Disease (DMID), National Institutes of Health (draft, May 2001), and dose-limiting toxicity (DLT) criteria were established for this study based on this table as well. Assessments of DLTs were made by the Safety Monitoring Committee (SMC), and escalation to the next cohort could not proceed until after selected safety and adverse event data reported through day 15 were reviewed and found acceptable by the SMC. A DLT was defined as any toxicity assessed by the investigator as possibly, probably, or definitely related to MDX-1303 that met predefined criteria based on the DMID adult toxicity table. The DMID grade and number of subjects required to prevent escalation to the subsequent cohort were defined for each abnormality. Abnormalities not found in the DMID tables were evaluated by the SMC on a case-by-case basis. Additionally, safety and tolerability were assessed through day 71 after dosing, and delayed toxicities that occurred in earlier cohorts were included in the 15-day safety report that was reviewed by the SMC prior to dose escalation.
Immunogenicity testing was performed using a bridging enzyme-linked immunosorbent assay (ELISA) format (data not shown). Serum samples, rabbit anti-MDX-1303 antiserum positive control, and normal human serum negative control samples were added to duplicate wells already containing adherent MDX-1303. After incubation and washing, biotinylated MDX-1303 was added to each well and was measured through the use of an enzyme-conjugated streptavidin detection system. Positives were determined based on signals in excess of 2 standard deviations above the mean of 50 normal human serum samples. Blood samples for immunogenicity testing were drawn on days 1, 8, 15, 29, 57, and 71.
Pharmacokinetic evaluation.
Blood samples for pharmacokinetic analysis were drawn on days 1 (predose, at 30 min, 1.5, 2.5, 3.5, 4.5, 5.5, 7.5, 9.5, 11.5, and 13.5 h), 2, 3, 4, 8, 15, 22, 29, 43, 57, and 71 from subjects in i.v. cohorts and on days 1 (predose, at 30 min, 1, 2, 3, 3.5, 4, 6, 8, 10, and 12 h), 2, 3, 4, 8, 15, 22, 29, 43, 57, and 71 from subjects in the i.m. cohort.
The serum concentration of MDX-1303 was measured using a validated sandwich ELISA methodology (data not shown). Mouse monoclonal anti-PA IgG (Biodesign International, Saco, ME) was coated onto assay plates and incubated with recombinant PA. Samples (including standards) were added, and MDX-1303 was measured with an anti-human IgG antibody-alkaline phosphatase detection system. The serum concentrations of MDX-1303 were then determined from the four-parameter standard curve by use of Softmax Pro version 4.3.
Where feasible, serum concentration-time data for MDX-1303 were analyzed by noncompartmental analysis (NCA) using the software program WinNonlin (WinNonlin Professional version 5.0.1, Pharsight Corp., Mountain View, CA). The area under the concentration-time curve from time zero to the last quantifiable concentration (AUC0-t) was estimated by a combination of linear trapezoidal method on concentrations up and logarithmic trapezoidal methods on concentrations going down (linear up/log down method).
Pharmacodynamic evaluation.
A subset of pharmacokinetic samples, comprised of samples obtained from 3 subjects in the 0.3-mg/kg i.v. group and 7 subjects in the 100-mg i.m. group, were analyzed for activity in the TNA assay. These results were compared to standard curves of TNA for sera from individuals immunized with AVA (Anthrax Vaccine Adsorbed, Emergent BioSolutions) that were obtained from the Centers for Disease Control and Prevention (CDC) and reported as the ratio of MDX-1303 TNA to CDC reference standard TNA.
The TNA assay is a cell-based cytotoxicity assay evaluating the viability of the J774 mouse macrophage cell line after the addition of protective antigen, lethal factor, and samples (as described in reference 17). This assay was not formally validated according to the procedures outlined in the FDA guidance document “Bioanalytical Method Validation” at the time when samples from this study were analyzed and is considered exploratory.
Statistical analyses.
No inferential testing was performed in the analyses of this study. Summary statistics for continuous variables included sample size, mean, median, range, and standard deviation. Summary statistics for categorical variables included frequency count and percentage.
No interim analyses were performed. At the SMC meetings, the SMC reviewed all relevant safety data from each cohort prior to the SMC's deliberations as to whether to allow enrollment of the next study cohort. This was an open-label study, and there was no blinding of study data.
Exclusive of the pharmacokinetic analyses, the only analysis data set was the safety data set, which was defined as all subjects who received at least one dose of study medication.
RESULTS
Subject disposition and demographic characteristics.
Forty-six subjects were randomized to the 6 treatment groups. All 46 randomized subjects were treated, and 43 subjects completed the study. The 3 subjects who discontinued were all lost to follow-up. All 3 completed their day 21 visit, and 2 of the 3 completed their day 57 visit. All 46 treated subjects were included in both the safety population and the pharmacokinetics population. The majority of subjects were female (65.2%) and white (80.4%) with a mean age of 28.5 years (range: 18 to 58 years). Notably, the 3 subjects in the 0.3-mg/kg group were all male, the 3 subjects in the 20-mg/kg group were all female, and 9/10 subjects were female in the 100-mg i.m. group (Table 1).
Table 1.
Subject baseline and demographic information (safety population)
| Characteristic | Value for indicated MDX-1303 dose |
||||||
|---|---|---|---|---|---|---|---|
| 0.3 mg/kg i.v. (n = 3) | 1 mg/kg i.v. (n = 10) | 3 mg/kg i.v. (n = 10) | 10 mg/kg i.v. (n = 10) | 20 mg/kg i.v. (n = 3) | 100 mg i.m. (n = 10) | All subjects (n = 46) | |
| Age (yr) | |||||||
| Mean (SD) | 33.3 (9.9) | 30.7 (10.9) | 24.6 (7.1) | 30.9 (11.6) | 20.0 (1.0) | 29.1 (10.3) | 28.5 (9.9) |
| Median | 38.0 | 29.5 | 23.0 | 26.5 | 20.0 | 25.0 | 24.5 |
| Range | 22–40 | 18–46 | 8–38 | 20–58 | 9–21 | 18–46 | 18–58 |
| Gender [no. (%)] | |||||||
| Male | 3 (100.0) | 5 (50.0) | 3 (30.0) | 4 (40.0) | 0 (0.0) | 1 (10.0) | 16 (34.8) |
| Female | 0 (0.0) | 5 (50.0) | 7 (70.0) | 6 (60.0) | 3 (100.0) | 9 (90.0) | 30 (65.2) |
| Race [no. (%)] | |||||||
| White | 1 (33.3) | 7 (70.0) | 10 (100.0) | 9 (90.0) | 3 (100.0) | 7 (70.0) | 37 (80.4) |
| Black | 1 (33.3) | 3 (30.0) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 2 (20.0) | 7 (15.2) |
| Hispanic | 1 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (10.0) | 2 (4.3) |
| Asian | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Weight (kg) | |||||||
| Mean (SD) | 83.3 (10.7) | 79.7 (15.9) | 67.8 (18.1) | 75.4 (12.4) | 60.5 (12.6) | 66.4 (10.7) | 72.3 (15.0) |
| Median | 79.9 | 76.9 | 63.6 | 76.4 | 61.0 | 68.8 | 72.8 |
| Range | 74.8–95.3 | 60.1–109.8 | 47.7–93.0 | 53.0–98.6 | 47.7–72.9 | 52.0–87.1 | 47.7–109.8 |
| Height (cm) | |||||||
| Mean (SD) | 180.3 (7.5) | 173.7 (11.7) | 167.2 (9.9) | 172.8 (9.3) | 159.7 (9.3) | 165.0 (10.7) | 169.7 (10.9) |
| Median | 176.0 | 170.0 | 163.5 | 171.0 | 157.0 | 164.5 | 168.0 |
| Range | 176–189 | 159–196 | 152–180 | 160–190 | 152–170 | 148–186 | 148–196 |
Safety.
MDX-1303 was generally safe, well tolerated, and nonimmunogenic when administered as single 0.3-mg/kg to 20-mg/kg i.v. infusions and single-dose 100-mg i.m. injections. There were no subjects with grade 5 adverse events (death), and none who discontinued due to adverse events. There were no subjects that had a dose adjustment or temporary interruption of dosing due to an adverse event. There was 1 serious adverse event (SAE) that was judged not related to study treatment. This subject had a spontaneous abortion on study day 73, which was followed by a dilation and curettage procedure. Pathology was unrevealing as to an etiology; tests for syphilis, hepatitis B virus (HBV), and HIV were negative, and antibody titers to herpes simplex 2 and rubella were consistent with past infection. No additional testing was performed.
There were no additional serious or grade 3/4 adverse events in the study.
Overall, 112 adverse events were experienced by 40 subjects (87.0%) in this study. Upper respiratory tract infection was the most common adverse event (23.9% overall incidence) with the highest incidence (40%) in the 3-mg/kg i.v. group. Headache was the second most frequent adverse event with a 17.4% overall incidence, followed by injection site pain (15.2% incidence). Of the 8 subjects reporting headache, 4 reported headache on the day of dosing and 4 reported headache on days 6, 24, 43, and 54 following dosing, respectively; the investigator recorded 3 of the 4 headaches on the day of dosing as being “possibly” related to the study drug. All but 2 of the headaches were grade 1 (mild); the remaining 2 were recorded to be grade 2. Three subjects took over-the-counter medication for their headaches; the others had resolution without medication. All headaches resolved within 24 h of onset. There was no relationship between incidence of adverse events and dose group, with the exception of injection site pain, which occurred almost exclusively in the 100-mg i.m. treatment group (incidence of 60% and all classified as grade 1 with spontaneous resolution) (Table 2). Overall, 16 subjects (34.8%) experienced 19 grade 1, treatment-related adverse events during this study. Other than injection site pain, the only other treatment-related adverse event that occurred with a >5% overall incidence was headache.
Table 2.
Most frequenta treatment-emergent adverse events (all causalities) (safety population)
| Adverse event | No. (%) of patients experiencing adverse event at indicated MDX-1303 dose |
||||||
|---|---|---|---|---|---|---|---|
| 0.3 mg/kg i.v. (n = 3) | 1 mg/kg i.v. (n = 10) | 3 mg/kg i.v. (n = 10) | 10 mg/kg i.v. (n = 10) | 20 mg/kg i.v. (n = 3) | 100 mg i.m. (n = 10) | All subjects (n = 46) | |
| Gastrointestinal disorders | |||||||
| Diarrhea | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (20.0) | 2 (4.3) |
| Toothache | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (20.0) | 0 (0.0) | 0 (0.0) | 2 (4.3) |
| General disorders and administration site conditions | |||||||
| Injection site pain | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 6 (60.0) | 7 (15.2) |
| Infections and infestations | |||||||
| Gastroenteritis | 0 (0.0) | 1 (10.0) | 0 (0.0) | 2 (20.0) | 0 (0.0) | 0 (0.0) | 3 (6.5) |
| Upper respiratory tract infection | 0 (0.0) | 3 (30.0) | 4 (40.0) | 1 (10.0) | 0 (0.0) | 3 (30.0) | 11 (23.9) |
| Viral infection | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (66.7) | 1 (10.0) | 3 (6.5) |
| Nervous system disorders | |||||||
| Headache | 0 (0.0) | 2 (20.0) | 1 (10.0) | 3 (30.0) | 0 (0.0) | 2 (20.0) | 8 (17.4) |
| Respiratory, thoracic, and mediastinal disorders | |||||||
| Nasal congestion | 1 (33.3) | 1 (10.0) | 1 (10.0) | 0 (0.0) | 1 (33.3) | 0 (0.0) | 4 (8.7) |
| Pharyngolaryngeal pain | 0 (0.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 1 (10.0) | 3 (6.5) |
| Skin and subcutaneous tissue disorders | |||||||
| Dermatitis | 0 (0.0) | 1 (10.0) | 0 (0.0) | 2 (20.0) | 0 (0.0) | 0 (0.0) | 3 (6.5) |
| Dermatitis contact | 0 (0.0) | 2 (20.0) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 0 (0.0) | 3 (6.5) |
“Most frequent” is defined as occurring in at least 20% and at least 2 subjects in any treatment group.
The incidence of clinically notable abnormalities in vital signs and clinical laboratory tests was low. There was an increased incidence of lowered hematocrit values; however, none was considered to be an adverse event within the “blood and lymphatic system disorder” category. The incidence of low hematocrit values was 20% at study day −1, peaking at 37% for all treatment groups on study day 8, and declined to 21% at study end. There were no abnormally low hematocrit values for the 20-mg/kg i.v. group, although there were only 3 individuals in this group. For all treatment groups, the mean hematocrit at baseline was 39.6, and that on study day 8 was 38.6. Additionally, no clinically relevant physical examination or electrocardiographic findings were noted.
Subjects administered single 0.3-mg/kg to 20-mg/kg i.v. infusions and 100-mg i.m. injections of MDX-1303 did not produce detectable anti-MDX-1303 antibodies (as evaluated by immunogenicity testing) during the course of this study.
Pharmacokinetics.
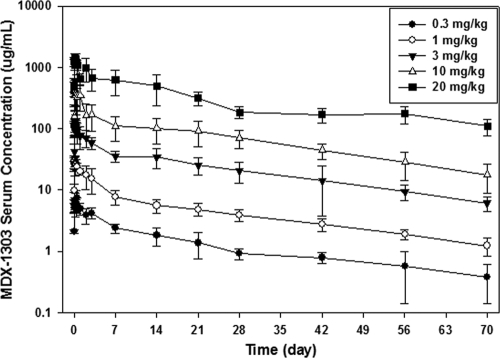
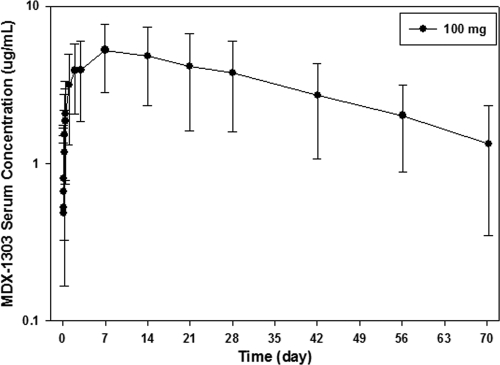
When administered as a 95-min i.v. infusion, MDX-1303 showed variable concentration-time profiles with bimodal pharmacokinetic behavior. When administered via the i.v. route, peak concentrations occurred after the end of infusion. When administered as an i.m. injection, MDX-1303 showed variable absorption, and the peak concentration occurred in 7 days. In all treatment groups, serum concentrations were measurable up to day 71 in the majority of subjects. Mean PK parameters of MDX-1303 administered as an i.v. infusion or as i.m. injections are presented in Table 3 and Fig. 1 and 2.
Table 3.
PK parameters of MDX-1303 given as an i.v. infusion or i.m. injections in healthy volunteers (safety population)
| Route | Group | Dose (mg/kg) | No. | Parameter [mean (SD)]a |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cmax (μg/ml) | Tmax (day) | AUC0-t (μg·day/ml) | AUC∞ (μg·day/ml) | t1/2 (days) | CL (ml/days) | V (ml) | ||||
| i.v. | 1 | 0.3 | 3 | 8.73 (1.76) | 0.15 (0.07–0.48) | 88.2 (21.8) | 103 (34) | 24.2 (6.3) | 263 (90) | 8,633 (1,338) |
| 2 | 1 | 10 | 33.6 (6.6) | 0.17 (0.07–0.57) | 314 (68) | 370 (78) | 26.3 (6.6) | 228 (90) | 8,417 (3,025) | |
| 3 | 3 | 10 | 138 (25) | 0.15 (0.07–0.48) | 1,536 (385) | 1,740 (417) | 22.9 (2.5) | 129 (67) | 4,235 (2,100) | |
| 4 | 10 | 10 | 630 (210) | 0.25 (0.02–1.07) | 4,884 (1,257) | 5,508 (1,460) | 22.1 (7.4) | 145 (43) | 4,451 (1,632) | |
| 5 | 20 | 3b | 1,523 (176) | 0.15 (0.11–0.48) | 20,507 (4,965) | 22,533 (4,606) | 33.3 (1.0) | 53.0 (4.98) | 2,551 (318) | |
| i.m. | 6 | 100 mg | 10c | 5.42 (2.54) | 6.99 (2.00–20.98) | 208 (118) | 325 (163) | 32.0 (12.3) | 369 (148) | 15,824 (6,702) |
Cmax, maximum concentration; Tmax, time to maximum concentration; AUC0-t, area under the concentration-time curve from time zero to the last quantifiable concentration; AUCinf, extrapolation of area under the concentration-time curve to infinity; t1/2, terminal elimination half-life; CL, clearance; V, volume of distribution.
n = 2 for AUCinf, t1/2, CL, and V.
n = 8 for AUCinf, t1/2, CL, and V.
Fig. 1.
Mean MDX-1303 serum concentration versus time profiles following a single 90-min intravenous infusion in healthy volunteers (groups 1 through 5) (safety population).
Fig. 2.
MDX-1303 serum concentration versus time profile following a single intramuscular administration of 100 mg in healthy volunteers (group 6).
MDX-1303 exhibited nonlinear pharmacokinetic behavior across the range of doses evaluated. The mean maximum concentration of drug in serum (Cmax), AUC0-t, and the extrapolation of area under the concentration-time curve to infinity (AUC∞) increased in a greater-than-dose-proportional manner between the 0.3-mg/kg and 20-mg/kg dose levels. The mean time to maximum concentration of drug in serum (Tmax) was observed between 0.15 and 0.25 days in all subjects across treatment groups following i.v. administration, and for the i.m. administration group, the mean Tmax was 6.99 days after study drug administration. The mean half-life (t1/2) values were similar between dose groups, but the clearance and volume terms changed with changing dose. Systemic exposure following 100-mg i.m. administration was within the range of exposure following 1-mg/kg i.v. administration with a relative bioavailability of approximately 65%.
Pharmacodynamics.
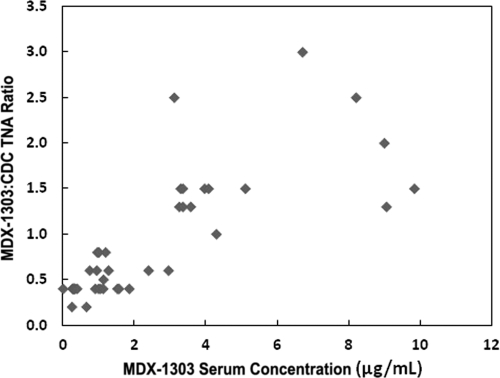
Figure 3 shows the results of the ratio of MDX-1303 TNA to CDC reference standard TNA as a function of MDX-1303 serum concentration. These data show that the TNA of serum samples with concentrations ranging from 3 to 10 μg/ml of MDX-1303 was similar to, or greater than, that of the CDC reference standard derived from pooled sera of individuals immunized with the currently licensed anthrax vaccine. In the 7 subjects who were evaluated between 1 and 29 days post-i.m. injection, all had TNA that was in excess of that seen with the CDC reference standard, as defined by the ratio of sample dilutions to achieve the 50% effective concentration (EC50) (MDX-1303 TNA/CDC TNA ratio range of 1.3 to 2.5). No pharmacokinetic/pharmacodynamic relationship analysis was performed.
Fig. 3.
MDX-1303 TNA/CDC TNA ratios versus MDX-1303 serum concentrations for a subset of individuals from groups 1 and 6.
DISCUSSION
In this initial phase I study of a fully human MAb that binds to B. anthracis PA, MDX-1303 was evaluated alone without the use of antimicrobial agents and was generally safe, well tolerated, and nonimmunogenic when administered as single 0.3-mg/kg to 20-mg/kg i.v. infusions and single-dose 100-mg i.m. injections. The single SAE in this study, a dilation and curettage for spontaneous abortion/retained fetal products, resulted from an unplanned pregnancy resulting from a failure to adhere to protocol-specified contraception in a 40-year-old woman with a history of 2 successful pregnancies and no spontaneous abortions; the estimated date of conception for this pregnancy was approximately 36 days following MDX-1303 dosing. A limited evaluation to determine a possible etiology was performed and was unrevealing. It seems unlikely that MDX-1303 caused the spontaneous abortion. The risk of spontaneous abortion is thought to increase with maternal age (8) and, although MDX-1303 is an IgG1 antibody which may be expected to cross the placenta, preclinical evaluations of MDX-1303 revealed no cross-reactivity to any of multiple human tissues, including placental tissue (fetal tissue was not specifically tested). In addition, there were no clinical or pathological sequelae noted in monkeys dosed repetitively with multiple doses of MDX-1303 as high as 50 mg/kg; formal reproductive toxicology testing in animals has not been performed.
The most notable nonserious clinical adverse reactions were grade 1 injection site reactions for the subjects receiving i.m. injections and an incidence of headache of 17.4%, with an incidence of grade 1 (mild) headache of 6.5%. The overall frequency of headache in this study is lower than that reported in the general population of 30 to 80%, depending upon the study (4, 13). Therefore, this adverse event was not felt to be of clinical significance or to represent a lack of tolerability of MDX-1303.
There was a noticeable increase in the incidence of low-grade postbaseline hematocrit declines, peaking after the first week of study and returning to baseline levels by the end of the study. We speculate that these were caused by blood draws for the pharmacokinetic sampling and clinical laboratory testing, since subjects had approximately 85 ml of blood drawn in the first 4 days of the study. Subsequent sampling was limited to 20 ml per time point (days 8, 15, 57, 71); the incidence of low hematocrit returned to the approximately 20% seen at baseline by day 71. Although the lack of a placebo control group in this study makes this conclusion uncertain, this finding is not inconsistent with the findings of Thavendiranathan et al. (15), in which phlebotomy was highly associated with changes in hematocrit in hospitalized patients.
MDX-1303 exhibited nonlinear pharmacokinetic behavior across the range of doses evaluated. The mean Cmax, AUC0-t, and AUC∞ increased in a greater-than-dose-proportional manner between the 0.3-mg/kg and 20-mg/kg i.v. dose levels. Systemic exposure following 100-mg i.m. administration was within the range of exposure following 1-mg/kg i.v. administration with a relative bioavailability of approximately 65%. In all groups, serum concentrations were measurable up to day 70 in the majority of subjects. The dose levels for this clinical trial were selected based on an evaluation of a range of in vivo activity data and toxicology data.
An important measure of the ability of a given concentration of MDX-1303 in sera to neutralize anthrax toxin is the activity of that sera in the TNA assay. While there is no clear definition of what constitutes a TNA result that can be expected to provide protection or enhance survival in the setting of established B. anthracis infection, the CDC reference standard, derived from pooled sera taken from individuals who have been vaccinated using the licensed anthrax vaccine, has become a standard against which to measure the neutralizing activity of anti-PA antibody in serum samples. In this study, the TNA for MDX-1303 was reported as a ratio of MDX-1303 TNA versus the CDC reference standard TNA for a subset of study subjects. A ratio of 1.0 or greater suggests that the neutralizing activity of MDX-1303 in that particular sample is at least as good as that obtained using the CDC reference standard. Based upon this, the data generated in this study suggest that MDX-1303 concentrations of greater than 3.0 μg/ml are sufficient to produce results in the TNA assay that are comparable or better than that seen using the CDC reference standard (Fig. 3). This conclusion should be interpreted with caution, as it is not based upon a robust data set generated using a validated assay, and the results do not necessarily translate directly into therapeutic efficacy in vivo. In order to establish the efficacy of MDX-1303 as a therapeutic, in vivo efficacy must be demonstrated. Since it is unethical and infeasible to evaluate the efficacy of MDX-1303 in humans, its efficacy will be based on survival in appropriate animal models, while safety will be based on safety data in humans (the “Animal Rule” [21 CFR Part 601.90]). A recommended human dose of MDX-1303 will be determined through PK modeling of a variety of animal model efficacy tests and a human PK database. This modeling will include an evaluation of PK in both nonchallenged and challenged animals, establishing whether the pathophysiology of the disease affects the PK (6).
Based on previous nonclinical studies, which were all performed using MDX-1303 alone with no adjunct antimicrobial agents, and the initial clinical safety and PK data reported from this phase I study, MDX-1303 may have utility for the pre- and postexposure prophylaxis of individuals exposed to, or at risk of exposure to, B. anthracis and for the treatment of individuals displaying symptoms and/or signs of inhalational anthrax.
In the therapeutic setting, animals have received MDX-1303 starting at fixed time points after spore exposure (24 or 48 h in the New Zealand White [NZW] rabbit), after first demonstrating PA antigenemia by a positive electrochemiluminescence (ECL) assay (African green monkeys [AGMs]), or after first positive PA ECL or significant increases in body temperature (SIBT) (NZW rabbits). MDX-1303 protected 88% and 42% of NZW rabbits that initiated treatment at 24 and 48 h after spore exposure, respectively (13). When given at the time of first positive PA ECL or after SIBT, MDX-1303 was found to be an efficacious treatment with survival rates exceeding 90% when given at 20 mg/kg i.v. Importantly, survival rates of 75% were seen at very low i.v. doses (2.5 mg/kg) (S. Basu et al., presented at the Keystone Symposium, Banff, Alberta, Canada, 2009). Comparable efficacy has been demonstrated in two separate studies, suggesting the consistency of this animal model. When AGMs were treated with MDX-1303 at the time of first positive PA ECL after spore exposure, up to 70% of the animals survived (L. Pitt et al., presented at the International Bacillus anthracis, B. cereus, and B. thuringiensis Conference, Santa Fe, New Mexico, 2009).
In the postexposure setting, NZW rabbits and cynomolgus monkeys were given MDX-1303 starting 1 h after spore exposure. When treated with doses as low as 1 mg/kg, up to 90% of NZW rabbits and 100% of cynomolgus monkeys were protected by MDX-1303 (17). In this latter study, MDX-1303 doses of 1 and 10 mg/kg i.m. were completely protective (6/6 monkeys in each dosing group), while none of the controls survived. The Cmax for the challenged animals in this study was similar to the Cmax in a nonchallenged PK study in cynomolgus monkeys at both the 1- and 10-mg/kg dosing levels. The mean serum concentration was 3 to 10 μg/ml during the first 2 weeks after exposure in the 1-mg/kg group with a Cmax of 9 μg/ml (13) (data not shown). In the nonchallenged PK study, cynomolgus monkeys dosed with 1-mg/kg i.m. MDX-1303 had a Cmax of 8 μg/ml and an AUC∞ of 218 μg·day/ml (data not shown). This compares favorably with the PK of the 100-mg i.m. dose in the phase I clinical trial. In human subjects dosed with 100 mg of i.m. MDX-1303, the Cmax was 5.4 μg/ml with an AUC∞ of 325 μg·day/ml. In those volunteers with available data (n = 9), a mean MDX-1303 serum concentration of greater than 3 μg/ml was maintained from the Tmax to study day 29. These limited data suggest that a dose slightly higher than the 100-mg i.m. administration in the phase I clinical study could provide drug exposure comparable to the 1-mg/kg i.m. dose that was completely protective in the cynomolgus monkey postexposure study. Additional efficacy and PK information will need to be acquired before formal PK modeling can provide a more accurate “dose translation.”
There is a need for additional interventions against anthrax, as current therapies for inhalational anthrax are limited. Antibiotics do not address the toxemia associated with anthrax infection. Once there is a critical concentration of the toxin within an infected individual, death may ensue even if the replicating bacteria are eradicated through the administration of antibiotics (10). Further, there is concern about bacterial resistance to antibiotics, both natural and genetically engineered. It has been reported that the former Soviet Union successfully created an anthrax strain resistant to multiple antibiotics without a loss of virulence (5). Finally, in the postexposure prophylaxis setting there have been issues with adherence to prolonged antibiotic regimens, as documented among those for whom 60 days of antibiotic prophylaxis was initially recommended in the 2001 anthrax attacks. This could decrease the effectiveness of such therapy (16). An antitoxin MAb such as MDX-1303 could potentially address all of these issues. Ongoing/planned animal efficacy studies and human clinical trials will further evaluate the efficacy and safety of MDX-1303.
ACKNOWLEDGMENTS
This clinical trial was largely supported by NIH grant number 1UC1AI062544-02 with supplemental funding provided by Medarex and PharmAthene.
Sample analysis was performed by Medarex, Inc. Pharmacokinetic analysis was performed by PRI Consulting. We thank the Safety Monitoring Committee (SMC) members who participated in this study. These members included Barbara Jantausch (Chair), Children's National Medical Center, Washington, DC; Alan Cross, University of Maryland School of Medicine; Steven Deeks, University of California School of Medicine; and Philip LaRussa, Columbia University College of Physicians and Surgeons.
I.L. and D.B. were employed full-time by Medarex. M.A., M.M., and V.R. were employed full-time by PharmAthene. P.L. was employed by Quintiles Phase I Services.
Footnotes
Published ahead of print on 5 October 2011.
REFERENCES
- 1. Abramova F. A., Grinberg L. M., Yampolskaya O. V., Walker D. H. 1993. Pathology of inhalational anthrax in 42 cases from the Sverdlosk outbreak of 1979. Proc. Natl. Acad. Sci. U. S. A. 90:2291–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Christopher G. W., Cieslak J., Pavlin J. A., Eitzen E. M., Jr 1997. Biological warfare: a historical perspective. JAMA 278:412–417 [PubMed] [Google Scholar]
- 3. Dixon T. C., Meselson M., Guillemin J., Hanna P. C. 1999. Anthrax. N. Engl. J. Med. 341:815–826 [DOI] [PubMed] [Google Scholar]
- 4. Edmeads J., et al. 1993. Impact of migraine and tension-type headache on lifestyle, consulting behavior, and medication use: a Canadian population survey. Can. J. Neurol. Sci. 20:131–137 [DOI] [PubMed] [Google Scholar]
- 5. Executive Action, LLC 2007. Spores: the threat of a catastrophic anthrax attack on America. Signature Book Printing, Gaithersburg, MD [Google Scholar]
- 6. FDA 2009. Draft guidance for industry: animal models—essential elements to address efficacy under the Animal Rule. http://www.fda.gov/cder/guidance/index.htm Accessed 31 March 2009
- 7. Friedlander A. M. 2000. Anthrax: clinical features, pathogenesis, and potential biological warfare threat. Curr. Clin. Top. Infect. Dis. 20:335–349 [PubMed] [Google Scholar]
- 8. Hansen J. 1986. Older maternal age and pregnancy outcome: a review of the literature. Obstet. Gynecol. Surv. 41:726–742 [DOI] [PubMed] [Google Scholar]
- 9. Inglesby T. V., et al. 1999. Anthrax as a biological weapon: medical and public health management. JAMA 281:1735–1745 [DOI] [PubMed] [Google Scholar]
- 10. Inglesby T. V., et al. 2002. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 287:2236–2252 [DOI] [PubMed] [Google Scholar]
- 11. Jernigan J., et al. 2001. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg. Infect. Dis. 7:933–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mandell G. L., Bennet J. E., Dolin R. (ed.). 2000. Principles and practice of infectious diseases, 5th ed., p. 1887–1888 Churchill-Livingstone, New York, NY [Google Scholar]
- 13. Rasmussen B. K., Jensen R., Schroll M., Olesen J. 1991. Epidemiology of headache in a general population: a prevalence study. J. Clin. Epidemiol. 44:1147–1157 [DOI] [PubMed] [Google Scholar]
- 14. Swartz M. N. 2001. Recognition and management of anthrax—an update. N. Engl. J. Med. 345:1621–1626 [DOI] [PubMed] [Google Scholar]
- 15. Thavendiranathan P., Bagai A., Ebidia A., Detsky A. S., Choudhry N. K. 2005. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J. Gen. Intern. Med. 20:520–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tierney B., et al. 2003. Serious adverse events among participants in the Centers for Disease Control and Prevention's anthrax vaccine and antimicrobial availability program for persons at risk for bioterrorism-related inhalational anthrax. Clin. Infect. Dis. 37:905–911 [DOI] [PubMed] [Google Scholar]
- 17. Vitale L., et al. 2006. Prophylaxis and therapy of inhalational anthrax by a novel monoclonal antibody to protective antigen that mimics vaccine-induced immunity. Infect. Immun. 74:5840–5847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Young J. T., Collier R. J. November 2003. Attacking anthrax. Scientific American Exclusive Online Issue, p. 26–32 [Google Scholar]