Abstract
The obligately intracellular bacterium Ehrlichia chaffeensis that resides in mononuclear phagocytes is the etiologic agent of human monocytotropic ehrlichiosis (HME). HME is an emerging and often life-threatening, tick-transmitted infectious disease in the United States. Effective primary immune responses against Ehrlichia infection involve generation of Ehrlichia-specific gamma interferon (IFN-γ)-producing CD4+ T cells and cytotoxic CD8+ T cells, activation of macrophages by IFN-γ, and production of Ehrlichia-specific antibodies of the Th1 isotype. Currently, there are no vaccines available against HME. We evaluated the ability of 28-kDa outer membrane proteins (P28-OMP-1) of the closely related Ehrlichia muris to stimulate long-term protective memory T and B cell responses and confer protection in mice. The spleens of mice vaccinated with E. muris P28-9, P28-12, P28-19, or a mixture of these three P28 proteins (P28s) using a DNA prime-protein boost regimen and challenged with E. muris had significantly lower bacterial loads than the spleens of mock-vaccinated mice. Mice immunized with P28-9, P28-12, P28-19, or the mixture induced Ehrlichia-specific CD4+ Th1 cells. Interestingly, mice immunized with P28-14, orthologs of which in E. chaffeensis and E. canis are primarily expressed in tick cells, failed to lower the ehrlichial burden in the spleen. Immunization with the recombinant P28-19 protein alone also significantly decreased the bacterial load in the spleen and liver compared to those of the controls. Our study reports, for the first time, the protective roles of the Ehrlichia P28-9 and P28-12 proteins in addition to confirming previous reports of the protective ability of P28-19. Partial protection induced by immunization with P28-9, P28-12, and P28-19 against Ehrlichia was associated with the generation of Ehrlichia-specific cell-mediated and humoral immune responses.
INTRODUCTION
Human monocytotropic ehrlichiosis (HME) is an important emerging tick-transmitted disease (24). HME in immunocompetent individuals can present as a flu-like illness that may develop into a severe disease with manifestations of toxic shock-like syndrome. Death occurs in 3% of cases and is preceded by lymphocytopenia, thrombocytopenia, and liver injury (8). HME is caused by Ehrlichia chaffeensis, one of the most prevalent life-threatening tick-borne pathogens in North America (22). This unusual pathogen, which infects monocytes and macrophages, is an obligately intracellular, Gram-negative bacterium that lacks the inflammatory pathogen-associated molecular pattern molecules, lipopolysaccharide and peptidoglycan, but contains several antigenic proteins (14, 27, 32). Lack of early diagnosis and treatment of HME and being immunocompromised are the main risk factors that lead to severe and fatal disease. Ehrlichia chaffeensis causes a transient subclinical infection with no reported pathology in immunocompetent mice and does not offer the best opportunity to study a model of disease resembling HME (35). However, murine models of systemic infection associated with the mildly virulent Ehrlichia muris or the highly virulent IOE (Ixodes ovatus Ehrlichia) in C57BL/6 mice have provided knowledge of immunological mechanisms involved in host defenses against ehrlichial infection (7, 11, 23, 29). Protective immunity against E. muris or IOE in the mouse models of mild or fatal ehrlichiosis, respectively, correlates with induction of strong cell-mediated CD4 and CD8 type 1 responses and humoral immunity (11). T cell-independent humoral immunity has also been reported to be sufficient for protection against fatal intracellular ehrlichial infection (2).
The development of effective vaccines for pathogens requiring cellular and humoral immunity has been impeded by a lack of understanding of the factors required for generation of long-term effective and optimal memory responses. Understanding the factors required for the generation of vaccination-induced long-term memory immune responses that are sufficient to control an intracellular Ehrlichia infection is equally important as the identification of protective ehrlichial antigens is. The P28-19 (OMP-1g) outer membrane protein of E. chaffeensis has been identified as an effective target mediating clearance of the bacteria (14, 22). Recombinant P28-19 of IOE also elicited strong humoral and CD4 T cell responses in C57BL/6 mice and induced significant protection against lethal challenge (17). Additionally, a p28 gene-based naked-DNA vaccine (MAP1) was found to protect mice against challenge with a lethal dose of Ehrlichia ruminantium (19). Several vaccination strategies including regimens of pathogen DNA priming followed by administration of homologous recombinant proteins have demonstrated enhanced immune responses compared with vaccines using DNA vaccination alone (6, 18). A significant constraint to vaccine development for Ehrlichia is the high antigenic diversity that is present in outer membrane protein genes among different isolates of a particular species including E. chaffeensis. In nature, there appear to be three stable variant E. chaffeensis lineages that can be identified based on their p28 alleles (3, 15). This antigenic diversity among strains of E. chaffeensis must be considered in the development of broadly effective vaccines. In this study, we examined whether DNA gene priming immunization followed by recombinant protein booster immunization would induce improved protection against Ehrlichia. We chose several P28 paralogs representative of different regions of the multigene p28 locus of E. muris, all of which are known to be transcriptionally active in mice (4), as experimental immunogens. P28-19 was also chosen because an epitope within the amino terminus of the first hypervariable region of the E. chaffeensis ortholog was identified as an effective target of ehrlichial clearance by an IgG2c (reported as IgG2a) monoclonal antibody (MAb) with high avidity (13). Predominant expression of P28-14 orthologs of E. chaffeensis and Ehrlichia canis in tick cells, but not in mammalian cells (27, 28, 33), suggests that it might be required for colonization and survival within the tick environment, and considering it as a potential vaccine in comparison with the other P28 paralogs was worth investigating. In this paper, we demonstrate that P28-9, P28-12, and P28-19, but not P28-14, confer partial protection against Ehrlichia in a mouse model by inducing T cell and antibody responses.
MATERIALS AND METHODS
Plasmid DNA constructs.
The open reading frames (ORFs) for Ehrlichia muris p28-9, p28-12, p28-14, and p28-19 (GenBank accession number DQ335244) were directionally cloned into pcDNA 3.1/CT-GFP-TOPO (GFP stands for green fluorescent protein) designed for high-level expression in mammalian hosts under the control of the cytomegalovirus (CMV) promoter and into pET102/D-TOPO, which allows cloning the gene of interest as a fusion with His-Patch thioredoxin (Invitrogen, Carlsbad, CA) (Table 1). Sequence analysis using an ABI Prism 377 DNA sequencer (Perkin Elmer Applied Biosystems, Foster City, CA) was performed on all constructs to verify the proper orientation and frame of the insert. pWRG/mIL-12 is a pBluescript plasmid that contains the two subunits of murine interleukin-12 (mIL-12), p40 and p35. Both subunits are under separate CMV promoter regions and contain bovine growth hormone poly(A) signal. pWRG/mIL-12 was kindly provided by Hua Yu, Moffitt Cancer Center, Tampa, FL. For vaccine purposes, the DNA used was purified utilizing an endotoxin-free mega plasmid prep kit from Qiagen (Valencia, CA) following the manufacturer's instructions. Plasmid DNA expressing E. muris p28-9, p28-12, p28-14, and p28-19 and control constructs used in the vaccine were dissolved in normal saline (0.9% NaCl) to a concentration of 2 μg/ml.
Table 1.
Primers used for cloning and expressing P28 constructs of E. muris
| P28 paralog | Sequences of primer pairs for PCR and cloning into the following vector: |
|
|---|---|---|
| pET102/D expression vector | pcDNA3.1CT/GFP vector | |
| P28-9 | 5′-CACCATGGGAGGGCAATACAG-3 | 5′-GCCATGGGAGGGCAATACAG-3′ |
| 5′-AACCTCGCCACCAAAGTATCC-3′ | 5′-CCTCGCCACCAAAGTATCC-3′ | |
| P28-12 | 5′-CACCTCTGGATTATACATTAGCGGACAA-3′ | 5′-GCCATGGGATTATACATTAGCGGACAA-3′ |
| 5′-ACCAAAGTATCCAACATCAAGTG-3′ | 5′-CAAAGTATCCAACATCAAGTG-3′ | |
| P28-14 | 5′-CACCGTAGGGAGCGCATTAAT-3′ | 5′-GCCATGGTAGGGAGCGCA-3′ |
| 5′-TCCAATTTCTCCACCAAAGTAT-3′ | 5′-CTCCAATTTCTCCACCAAAGTATC-3′ | |
| P28-19 | 5′-CACCGCTTCTCACTTTGGAGTATTTTCAGC-3′ | 5′-GCCATGGCTTCTCACTTTGGAGTATTTTCAGC-3′ |
| 5′-CCTTCCTCCAAGTTCTATACCAAAATG-3′ | 5′-TTCCTCCAAGTTCTATACCAAAATG-3′ | |
Mice.
Six- to 8-week-old female C57BL/6 mice were used in all experiments. Mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and housed and cared for in the Animal Research Center at the University of Texas Medical Branch in accordance with the Institutional Animal Care and Use Committee guidelines under whose review and approval the experiments were conducted.
Immunizations and Ehrlichia muris challenge.
C57BL/6 mice were divided into 6 groups with 8 mice per group. The mice in groups 1 to 4 were inoculated with 100 μg of p28-9, p28-12, p28-14, or p28-19 endotoxin-free plasmid DNA mixed with 100 μg of pWRG/mIL-12 in saline. Group 5 was inoculated with a 100-μg mixture of all four p28 constructs (25 μg each), mixed with 100 μg of pWRG/mIL-12, and group 6 was inoculated with 100 μg of pcDNA 3.1/CT-GFP vector only, which was also mixed with 100 μg of pWRG/mIL-12. All DNA immunizations were administered intramuscularly in the tibialis anterior muscle. As stated above, all mice immunized with DNA received 100 μg of purified pWRG/mIL-12 in saline to enhance a Th1-type cell-mediated immune response. A second DNA immunization identical to the first was administered 4 weeks later. Four weeks after the last DNA inoculation, 100 μg of purified P28-9, P28-12, P28-14, and P28-19 recombinant proteins or a mixture of the four proteins (25 μg each) in Freund's complete adjuvant were administered subcutaneously to mice in groups 1 to 5, respectively. Similarly, mice in group 6 received His-Patch thioredoxin protein as a negative control. A booster immunization of His-Patch thioredoxin was given subcutaneously at the same dosage as described above for all groups except that the recombinant proteins were prepared in Freund's incomplete adjuvant.
One month after the last inoculation, immunized mice were challenged intraperitoneally (i.p.) with a high dose of E. muris (∼1 × 104 bacterial genomes) and observed daily. Three mice from each group were sacrificed 1 day prior to challenge, and the sera were collected. Seven days after challenge, the remaining mice were sacrificed, and their spleens and sera were harvested. Part of the spleen was used to determine the frequency of antigen-specific gamma interferon (IFN-γ)-producing CD4+ T cells, and the other part was used to determine the ehrlichial load by real-time PCR. Sera were assayed for determination of antibody titer.
In the experiments involving immunization with the recombinant P28-19 alone (see Fig. 4, 5, and 6), 50 μg of the protein was mixed with Freund's complete adjuvant and administered by the i.p. route (3 animals per group). The second dose of immunization was administered on day 14 after the first immunization in Freund's incomplete adjuvant. The mice were challenged 14 days after the second immunization with E. muris (∼1 × 104 bacterial genomes) by the i.p. route.
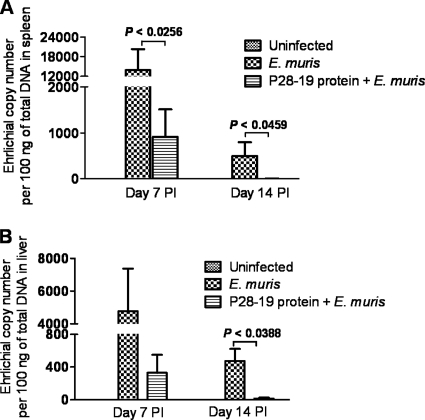
Fig. 4.
Immunization with the recombinant E. muris P28-19 protein induced protection. Bacterial burdens in the spleens and livers of mice immunized with the recombinant P28-19 protein were determined by quantitative real-time PCR after E. muris challenge. (A) The spleens of rP28-19-vaccinated mice had significantly lower bacterial loads on day 7 after E. muris challenge than those of unvaccinated mice. The bacterial levels in the spleens (A) and livers (B) of vaccinated mice were undetectable by day 14. The data were expressed as means plus standard deviations, and three mice from each group were used for this analysis. PI, postinfection.
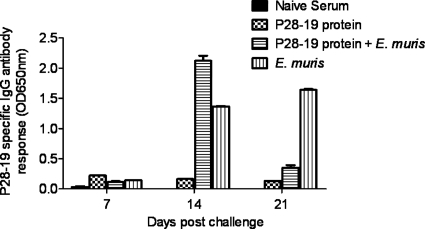
Fig. 5.
Protection induced by the recombinant P28-19 protein was associated with the development of antigen-specific IgG responses. P28-19-specific antibody responses were measured by an ELISA using a 22-amino-acid synthetic P28-19 peptide as the antigen. Mice immunized with the recombinant E. muris P28-19 protein had higher concentrations of P28-19-specific IgG antibodies on day 14 after E. muris challenge than mice in the unvaccinated control group. Antibody responses in mice immunized with the recombinant P28-19 alone are presented for comparison. The data were expressed as means plus standard deviations, and three mice from each group were used for this analysis. OD650nm, optical density at 650 nm.
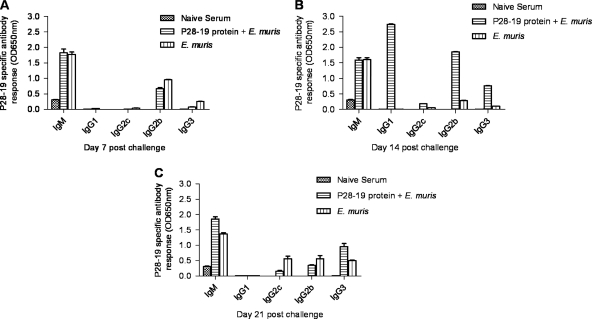
Fig. 6.
Induction of Ehrlichia-specific IgG1, IgG2b, and IgG3 antibody isotypes was associated with protection in mice immunized with P28-19 protein. P28-19-specific antibody responses in mice immunized with the P28-19 protein on days 7, 14, and 21 after E. muris challenge were measured by ELISAs. (A) Comparable concentrations of P28-19-specific IgM and IgG2b antibodies were found in sera from mice immunized with P28-19 protein and unimmunized mice on day 7 after E. muris challenge. (B) Mice immunized with the recombinant P28-19 protein had higher concentrations of P28-19-specific IgG1, IgG2b, and IgG3 antibodies on day 14 after E. muris challenge than the controls. (C) In contrast, unimmunized mice challenged with E. muris developed P28-19-specific IgG2c, IgG2b, and IgG3 antibodies by day 21 postchallenge. The data were expressed as means plus standard deviations, and three mice from each group were used for this analysis.
Assessment of ehrlichial load in organs by quantitative real-time PCR.
The bacterial burdens in the organs were determined by quantitative real-time PCR. The Ehrlichia-specific dsb gene, which encodes a disulfide bond-forming protein (GenBank accession numbers AY236484 and AY236485) was selected as the target gene for amplification of E. muris DNA. The sequences of the primers and probes and thermal cycle conditions were described previously (30). PCR analyses were considered negative for ehrlichial DNA if the critical threshold values (CT) exceeded 40 cycles. The ehrlichial load in organs was normalized relative to the total DNA. Each sample was run in triplicate.
Antibody responses.
One day before challenge and on day 7 after E. muris challenge, serum samples from vaccinated mice were collected for measurement of antibody titers against E. muris by indirect immunofluorescence assay (IFA) as described previously (see Fig. 3) (23). Antigen slides were prepared from E. muris-infected DH82 cells. Serum samples were diluted, and 10 μl was applied to each well on a slide and incubated for 30 min at 37°C in a humidified chamber. The antigen slides were washed and then incubated with 10 μl of fluorescein isothiocyanate (FITC)-labeled anti-mouse IgG (Kirkegaard & Perry Laboratories, Gaithersburg, MD) at a 1:100 dilution. The slides were then washed, counterstained with Evans blue, and examined under a Nikon Labphoto UV microscope. Serological endpoint titers were expressed as the reciprocal of the highest dilution at which specific fluorescence was detected.
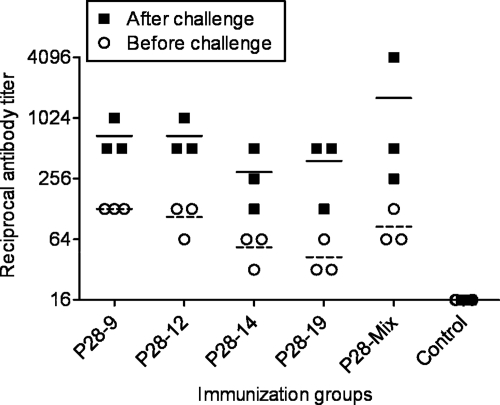
Fig. 3.
Ehrlichia-specific IgG antibody responses develop in mice immunized with the P28 outer membrane proteins. Ehrlichia muris-specific IgG antibody titers in all vaccinated groups before and after challenge were measured by IFA using slides prepared from E. muris cultured in DH82 cells. Higher titers of E. muris-specific IgG antibodies were observed in mice vaccinated with P28-9, P28-12, P28-14, P28-19, and the P28 mixture on day 28 after the last booster immunization before challenge than in the controls. The titers of E. muris-specific IgG antibodies measured after challenge were higher than the titers of antibodies in vaccinated mice before challenge, indicating that infection boosted the antibody response. Each symbol shows the value for an individual mouse. The short horizontal lines represent the means for the groups of mice before challenge (short broken lines) and after challenge (short black lines).
Measurement of P28-19-specific antibody responses.
An enzyme-linked immunosorbent assay (ELISA) was performed to measure the concentration of E. muris-specific IgG subclass antibodies as described previously (11, 16). Briefly, each well in a 96-well ELISA plate was coated with 100 μl of a 22-amino-acid synthetic peptide corresponding to a previously identified dominant B cell epitope in the amino terminus of the first hypervariable region of the P28-19 protein (see Fig. 5 and 6) (13) at a concentration of 4 μg/ml in 50 mM sodium bicarbonate buffer, pH 9.6. Serum samples were diluted 1:100, and 100 μl of each sample was added to each antigen-coated well and incubated at 25°C for 2 h. Alkaline phosphatase-conjugated goat anti-mouse IgG or IgG1, IgG2c, IgG2b, IgG3, and IgM antibodies (Southern Biotech, Birmingham, AL) were added at a dilution of 1:300, and color was developed using Blue Phos phosphatase substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD). Optical densities were measured using an ELISA plate reader (Molecular Devices, Sunnyvale, CA) at 650 nm after 30 min of incubation at room temperature. The wells (on microtiter plates) coated with an unrelated control 22-amino-acid peptide were used to determine the background level for the ELISA. All assays were performed in duplicate wells, and the average values were calculated for analysis after subtraction of the background absorbance.
Splenocyte cultures and in vitro recall cellular immune responses.
The frequencies of antigen-specific IFN-γ-producing T cells in the spleens were determined by flow cytometric analysis. Splenocytes, either pooled from 3 mice/group (see Fig. 2) or from individual mice (see Fig. 7), were cultured in vitro in a 12-well plate at a concentration of 5 × 106 cells per well in complete medium (RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum, 10 mM HEPES buffer, 50 μM 2-mercaptoethanol, and antibiotics [100 units/ml penicillin and 100 μg/ml streptomycin]) in the presence or absence of E. muris antigen (5 μg/ml) prepared from E. muris cultured in DH82 cells (see Fig. 2) or the recombinant P28-19 protein (see Fig. 7). Irradiated naïve syngeneic spleen cells (5 × 106 per well) were used as antigen-presenting cells (APCs). Positive- and negative-control wells contained concanavalin A at a concentration of 5 μg/ml or medium alone, respectively. The cells were harvested after 18 h of in vitro antigen stimulation followed by 4 h of incubation with brefeldin A (BD GolgiPlug; BD Biosciences, San Diego, CA) and stained with specific antibodies as described below.
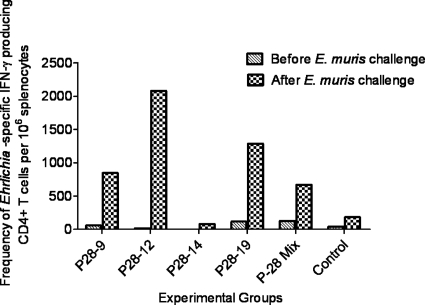
Fig. 2.
Immunization with P28-9, P28-12, and P28-19 induces a strong antigen-specific type 1 immune response. The frequencies of antigen-specific IFN-γ-producing CD4+ Th1 cells in the spleens of different groups of immunized mice were measured before and after E. muris challenge by flow cytometry. Splenocytes from 3 mice per group were pooled and stimulated in vitro for 18 h with the whole-cell lysate antigen prepared from E. muris cultured in DH82 cells. The frequencies of antigen-specific T cells producing IFN-γ were determined after the background staining of nonstimulated cells in wells containing medium only was subtracted. Immunization with P28-9, P28-12, or P28-19, or the P28 mixture resulted in the expansion of antigen-specific IFN-γ-producing CD4+ Th1 cells on day 7 following challenge with E. muris. The data are representative of two independent experiments (the experiments gave similar results), and each bar in the graph represents the frequency of Ehrlichia-specific IFN-γ-producing CD4+ T cells per million splenocytes pooled from 3 mice from each group.
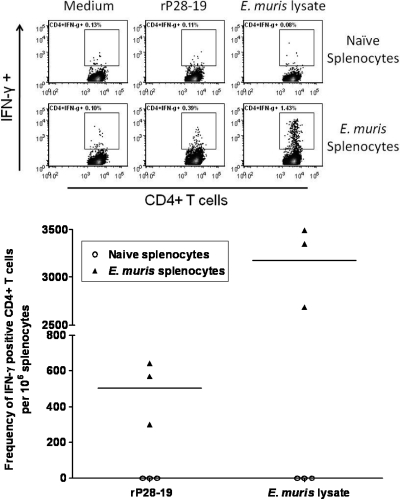
Fig. 7.
Outer membrane protein P28-19-specific CD4+ T cells develop during E. muris infection. We determined the frequencies of P28-19-specific IFN-γ-producing CD4+ T cells in the spleens of mice infected with E. muris by flow cytometry. The spleens of mice infected with E. muris had higher frequencies of P28-19-specific IFN-γ-producing CD4+ T cells on day 45 after infection than the spleens of naïve uninfected mice. The frequencies of E. muris-specific IFN-γ-producing CD4+ T cells in the spleens of the same mice detected following in vitro stimulation with the E. muris whole-cell lysate were shown for comparison. In the graph at the bottom of the figure, each symbol represents the value for an individual mouse, and the short horizontal lines represent the mean for the group.
Briefly, Fc receptors were blocked with anti-Fc II/III receptor monoclonal antibodies (MAbs) (BD PharMingen, San Diego, CA) in fluorescence-activated cell sorting (FACS) buffer (Dulbecco's phosphate-buffered saline [PBS] without Mg2+ or Ca2+ but with 1% fetal calf serum and 0.09% sodium azide) at 4°C for 15 min. The cells were then labeled with fluorochrome-conjugated MAbs (BD Biosciences Pharmingen, San Diego, CA) specific for mouse CD3 (allophycocyanin [APC]; clone 17A2) and CD4 (FITC; clone RM4-5) surface molecules. The cells were then fixed, permeabilized, and stained for intracellular IFN-γ (phycoerythrin [PE]; clone XMG1.2) using a BD Cytofix/Cytoperm fixation/permeabilization kit following the manufacturer's instructions. Flow cytometric data were collected using FACSCanto (BD Immunocytometry Systems, San Jose, CA). Live cells were gated based on vital dye staining (near-infrared [near-IR] Live/Dead fixable dead cell stain; Invitrogen, Carlsbad, CA), and a total of 200,000 events were collected. Data were analyzed using Flow Jo software (Tree Star, Inc., Ashland, OR). The frequencies of antigen-specific IFN-γ-producing T cells in the spleens were determined after the background staining of unstimulated cells in wells containing medium only was subtracted.
Statistical analysis.
All data presented are representative of two or three independent experiments (the experiments gave similar results). When indicated, unpaired two-tailed t test was used for comparison of two groups using GraphPad Prism (GraphPad Software Inc., La Jolla, CA). One-way analysis of variance (ANOVA) with Dunnett's posttest was used for comparison of multiple test groups to the control group (see Fig. 1). Statistical significance was determined at 95% (P < 0.05), and asterisks indicate levels of statistical significance (*, P = 0.01 to 0.05; **, P = 0.001 to 0.01).
Fig. 1.
Immunization with the outer membrane proteins P28-9, P28-12, and P28-19 confers protection against Ehrlichia muris infection in mice. Quantitative real-time PCR data show that the spleens of mice vaccinated with P28-9, P28-12, P28-19, and the P28 mixture using the DNA prime/protein boost regimen had significantly lower bacterial loads compared with the vector/protein control group. Bacterial loads in the spleens of mice vaccinated with P28-14 were not significantly different from the control group. The data presented are representative of two independent experiments (the experiments gave similar results). The data were expressed as means plus standard deviations (error bars), and three mice from each group were used for this analysis. The values that are significantly different from the values for the control group are indicated by asterisks as follows: *, P < 0.05; **, P < 0.01.
RESULTS
Immunization with the outer membrane proteins P28-9, P28-12, and P28-19 confers partial protection against E. muris infection.
The protective capacity of the memory immune response induced by different genes of the E. muris P28 family was investigated in C57BL/6 mice. To this end, C57BL/6 mice were immunized with recombinant DNA plasmids carrying the p28-9, p28-12, p28-14, and p28-19 genes or a mixture of all four in combination with IL-12 DNA on days 0 and 28 followed by two homologous recombinant protein booster immunizations on days 56 and 84. Mock-immunized mice were immunized similarly with empty vector/unrelated protein (His-Patch thioredoxin) and served as controls. Immunized mice were challenged intraperitoneally with a high dose (∼1 × 104 bacterial genomes) of E. muris 28 days after the last booster immunization, and the bacterial burden in the spleen was determined on day 7 after challenge. A high bacterial burden was detected in the spleens of mice immunized with empty vector/protein (mock-vaccinated mice) (Fig. 1). In contrast, the spleens of mice vaccinated with P28-9, P28-12, P28-19, and the P28 mixture exhibited significantly reduced bacterial loads on day 7 postinfection than those of the mock-vaccinated control group (Fig. 1). Interestingly, our data indicated that immunization of mice with P28-14, orthologs of which in E. chaffeensis and E. canis are primarily expressed in tick cells, failed to lower the E. muris burden in the spleen.
Outer membrane proteins P28-9, P28-12, and P28-19 induce strong antigen-specific type 1 immune responses.
Previous studies have shown that infection of C57BL/6 mice with E. muris, a mildly virulent Ehrlichia species, elicits a potent effector CD4+ and CD8+ type 1 T cell response in the spleen (11, 17). In addition, recently we showed that heterologous protection against lethal ehrlichial infection following immunization with live E. muris organisms is associated with expansion of effector memory CD4+ and CD8+ T cells producing IFN-γ as well as a substantial antibody response (31). To determine the induction of memory T cells in mice vaccinated with different P28 proteins (P28s), we measured Ehrlichia-specific CD4+ T cell responses in these mice before and after E. muris challenge. Compared to the vector/protein control group, the animals vaccinated with P28-9, P28-12, and P28-19 and those immunized with the mixture had higher frequencies of Ehrlichia-specific IFN-γ-producing CD4+ Th1 cells 7 days after E. muris challenge (Fig. 2). We did not detect higher frequencies of Ehrlichia-specific CD4+ Th1 cells after in vitro antigenic stimulation in the spleens of mice vaccinated with P28-14, which failed to induce protective immunity, compared to the other P28-vaccinated groups and the vector/protein controls. No difference in the number of antigen-specific IFN-γ-producing CD8+ memory T cells in the vaccinated groups compared to vector controls was observed (data not shown). These results indicate that vaccination with P28-9, P28-12, or P28-19 effectively increased the magnitude of protective antigen-specific CD4+ Th1 memory cells, which correlated with lower bacterial burdens in the vaccinated mice.
Ehrlichia-specific IgG antibody production is associated with protection in vaccinated mice.
To address the roles of antibodies in the control of Ehrlichia infection in vaccinated mice, we determined the titers of anti-E. muris antibodies in the sera of all vaccinated and control mice 28 days after the last booster immunization and on day 7 after challenge infection. A higher concentration of E. muris-specific IgG antibodies was detected in the sera of all vaccinated animals, including P28-14-immunized mice, compared to mice vaccinated with the vector/protein control (Fig. 3). Furthermore, the infection boosted the antibody response as indicated by an increase in antibody titers in the sera from vaccinated and subsequently challenged mice (Fig. 3).
Recombinant P28-19 protein induces a strong antibody response and confers protection.
Previous studies suggested a role for the orthologs of P28-19 of E. chaffeensis and IOE in protection (12, 17, 21). In the present study, the P28-19 DNA/protein immunization was found to reduce the ehrlichial burden significantly. Therefore, we further evaluated recombinant P28-19 (rP28-19) as a vaccine candidate. To this end, we immunized mice with two doses of rP28-19 (15 days apart) and challenged them with E. muris (∼1 × 104 bacterial genomes) 15 days after the last dose of immunization. We assayed the bacterial burden in the spleens and livers of mice vaccinated with the P28-19 protein and unvaccinated mice harvested on days 7 and 14 after E. muris challenge by quantitative real-time PCR. The spleens of rP28-19-vaccinated mice had significantly lower bacterial loads on day 7 than the spleens of unvaccinated mice (Fig. 4A). Furthermore, on day 14, there were no detectable bacteria by PCR in either the spleens or livers of the rP28-19-vaccinated mice (Fig. 4A and B).
Analysis of P28-19-specific antibody responses by ELISA on days 7, 14, and 21 after E. muris challenge demonstrated that the IgG response in vaccinated mice challenged with E. muris was highest on day 14 compared to day 7 or 21 (Fig. 5). We further analyzed the isotypes of P28-19-specific antibodies in sera from mice. Both vaccinated and unvaccinated mice challenged with E. muris had higher concentrations of P28-19-specific IgM and IgG2b antibodies on day 7 after the challenge (Fig. 6A). The rP28-19-vaccinated mice challenged with E. muris had substantially higher levels of IgG1, IgG2b, and IgG3 antibodies on day 14 after E. muris challenge than mice infected with E. muris alone (Fig. 6B). In contrast, mice infected with E. muris alone developed substantial levels of P28-19-specific IgG2c, IgG2b, and IgG3 by day 21 postinfection (Fig. 6C).
P28-19-specific CD4+ Th1 responses are induced during E. muris infection.
Finally, we determined by flow cytometry whether P28-19-specific T cells are induced during E. muris infection. Splenocytes from E. muris-infected mice were harvested on day 45 postinfection and stimulated in vitro with the recombinant P28-19 protein for 18 h. Compared to uninfected naïve mice, E. muris-infected mice had significantly higher frequencies of P28-19-specific IFN-γ-producing CD4+ Th1 cells in their spleens (Fig. 7). These results confirm the higher frequencies of antigen-specific IFN-γ-producing CD4+ T cells in the spleens of mice vaccinated with P28-19 DNA/protein (Fig. 2). Additionally, the results indicated that CD4+ T cells target P28-19 during Ehrlichia infection and develop into memory cells.
DISCUSSION
P28 family members are the most studied E. chaffeensis outer membrane proteins (OMPs). They have multiple predicted transmembrane β strands and are encoded by an antigenically variant multigene family composed of 22 paralogous genes clustered in a 27-kb gene locus of the E. chaffeensis genome (20, 21). Although these proteins seem to play a critical role in host-microbe interaction, they have not yet been explored as potential vaccine candidates against monocytotropic Ehrlichia infection. Several studies have shown that these P28 proteins are highly conserved among Ehrlichia species (4, 21, 26, 34), and therefore, we examined whether different P28 paralogs of E. muris are appropriate candidates for vaccines against Ehrlichia. In this study, we analyzed the ability of recombinant DNA vaccines expressing different genes of the E. muris P28 family and the homologous recombinant proteins given in a prime-boost regimen to stimulate long-term protective memory T and B cell responses and protect mice against Ehrlichia infection. Our study indicated, for the first time, the protective roles of P28-9 and P28-12 in addition to confirming the previous reports of the protective role of P28-19 against monocytotropic Ehrlichia (13, 17, 21). Immunization of mice with P28-9, P28-12, and P28-19 provided protection against E. muris as evidenced by reduction in bacterial burden (Fig. 1). A previous study showed that mice immunized with the recombinant E. chaffeensis P28-19 (rP28) enhanced the spontaneous clearance of the infection in BALB/c mice (21). A study by J. S. Li et al. indicated that MAb directed against the E. chaffeensis P28-19 (OMP-1g) reduced the bacterial burden in SCID mice (13). Further, the P28-19 (rOMP-19) of IOE was demonstrated to provide protection against fatal disease caused by IOE in C57BL/6 mice (17).
Our study indicated that mice immunized with P28-14 failed to develop protective immunity against challenge with E. muris cultured in mammalian cells (DH82 cells) administered i.p., which could be due to low levels of expression of P28-14 in the vertebrate host. Although it is not known whether P28 proteins of E. muris are differentially expressed in the vertebrate and tick hosts, our previous study indicated that all 21 p28 genes of E. muris were transcriptionally active in vivo on day 9 postinfection in mice (4). Previous reports suggested that E. chaffeensis and E. canis predominantly express orthologs of P28-19 and P28-20 in mammalian cell lines, compared with expression of orthologs of P28-14 in tick cell lines (27, 28, 33). Further studies indicated that mice infected with E. chaffeensis cultured in tick cells developed prolonged infection with higher bacterial burden than mice infected with E. chaffeensis grown in macrophages (9). Although proteomic analysis indicated the predominant expression of the P28-19 and P28-20 proteins of E. chaffeensis and their orthologs of E. canis in mammalian cells, reverse transcription-PCR (RT-PCR) analysis provided evidence for transcriptional activity of the majority of the genes within the p28 family (3, 15, 20). In addition, Zhang et al. provided indirect evidence for expression of all 22 p28 genes in dogs persistently infected with E. chaffeensis by demonstrating the presence of antibodies to the specific region of the individual P28s (36). These studies not only suggest different levels of expression of P28 proteins in mammalian cells but also reflect differences in the sensitivities of the methods employed. It is interesting to note that Borrelia burgdorferi OspA and OspC, which are primarily expressed in tick and mammalian cells, respectively, induce protective immunity against tick transmission of Borrelia (5, 10). However, unlike OspC, the protective role of antibodies to OspA, which is primarily expressed in tick cells, is restricted to killing of spirochetes in the midgut of engorging ticks (5). Although the Ehrlichia P28-14 protein did not induce protection against i.p. challenge with E. muris cultured in mammalian cells in the present study, it remains to be determined whether P28-14 is effective in blocking tick transmission of Ehrlichia or is effective against i.p. challenge with the bacteria cultured in tick cells.
Our study indicated that the protection induced by P28-9, P28-12, and P28-19 was associated with the induction of antigen-specific IFN-γ-producing CD4+ T cells. Furthermore, we provided evidence of the induction of P28-19-specific CD4+ Th1 cells in mice infected with E. muris, which suggests that P28-19 is targeted by T cells during the infection (Fig. 7). Previous studies from our laboratory and others have indicated that CD4+ T cells and IFN-γ are required for protection against Ehrlichia (1, 7). Moreover, the cross-protection induced by E. muris against fatal infection with IOE in mice was associated with the induction of Th1 responses (11, 31). These findings are consistent with the increased severity of E. chaffeensis infection observed in HIV-infected patients (25). We did not find higher frequencies of IFN-γ-positive CD4+ T cells in mice immunized with P28-14, which could be due to low levels of P28-14 expression in E. muris cultured in DH82 cells used for in vitro stimulation of T cells.
Partial protection induced by P28-9, P28-12, and P28-19 was correlated with the development of Ehrlichia-specific IgG antibodies. Studies from our laboratory and others have previously demonstrated the importance of antibodies in protection against Ehrlichia. Passive transfer of polyclonal immune sera or MAbs conferred protection in SCID mice against E. muris and E. chaffeensis, respectively (7, 12, 13). Mice immunized with the recombinant P28-19 protein alone had higher concentrations of antigen-specific IgG1, IgG2b, and IgG3 on day 14 after E. muris infection (Fig. 6B), which coincided with the reduction of bacteria to undetectable levels in the spleens and livers of these mice. In contrast, unimmunized mice challenged with E. muris developed IgG2c, IgG2b, and IgG3 antibodies by day 21 postinfection (Fig. 6C). Our previous study indicated that the cross-protection induced by E. muris against fatal IOE challenge in mice was associated with the development of Ehrlichia-specific IgG2c (reported as IgG2a) antibodies (11). Similar to these findings, J. S. Li et al. reported that the majority of highly effective P28-19-specific MAbs recovered from E. chaffeensis-infected C57BL/6 mice were of the IgG2c isotype, but not IgG1 (13). The same group reported the production of all IgG isotypes (IgG1, IgG2c, IgG2b, and IgG3) in C57BL/6 mice immunized with the recombinant IOE P28-19 (OMP-19) (17). These studies suggested that the quality of antibody responses elicited by immunization, which usually involves use of adjuvants, is fundamentally different from infection-induced immune responses. The presence of Ehrlichia-specific antibodies as determined by IFA of sera from mice immunized with P28-14 suggests either possible cross-reactivity with the other P28s or the presence of a low concentration of P28-14 in the antigen preparations obtained from E. muris grown in DH82 cells as discussed above. The induction of antibody responses in mice immunized with P28-14 also suggests that the lack of protection observed in these mice is not due to the failure of the development of immune responses.
In conclusion, our study showed that the Ehrlichia P28 outer membrane proteins exhibit differential roles in protective immunity against Ehrlichia. Our data also indicate for the first time the protective roles of P28-9 and P28-12 in addition to confirming previous findings of the protective role of P28-19 against Ehrlichia. The partial protection induced by the P28 outer membrane proteins was associated with the generation of CD4+ Th1 and Ehrlichia-specific IgG responses. The lack of protection against Ehrlichia by i.p. challenge observed in mice immunized with P28-14 possibly suggests the importance of using a challenge model involving tick transmission of Ehrlichia to identify transmission blocking protective candidate antigens.
ACKNOWLEDGMENTS
This study was supported by grant AI31431 from the National Institute of Allergy and Infectious Diseases.
The excellent secretarial assistance by Doris Baker is gratefully acknowledged.
Footnotes
Published ahead of print on 26 October 2011.
REFERENCES
- 1. Bitsaktsis C., Huntington J., Winslow G. 2004. Production of IFN-gamma by CD4 T cells is essential for resolving ehrlichia infection. J. Immunol. 172:6894–6901 [DOI] [PubMed] [Google Scholar]
- 2. Bitsaktsis C., Nandi B., Racine R., MacNamara K. C., Winslow G. 2007. T-cell-independent humoral immunity is sufficient for protection against fatal intracellular ehrlichia infection. Infect. Immun. 75:4933–4941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheng C., Paddock C. D., Reddy G. R. 2003. Molecular heterogeneity of Ehrlichia chaffeensis isolates determined by sequence analysis of the 28-kilodalton outer membrane protein genes and other regions of the genome. Infect. Immun. 71:187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crocquet-Valdes P. A., et al. 2005. Analysis of ehrlichial p28 gene expression in a murine model of persistent infection. Ann. N. Y. Acad. Sci. 1063:420–424 [DOI] [PubMed] [Google Scholar]
- 5. de Silva A. M., Telford S. R., III, Brunet L. R., Barthold S. W., Fikrig E. 1996. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J. Exp. Med. 183:271–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Diaz-Montero C. M., Feng H. M., Crocquet-Valdes P. A., Walker D. H. 2001. Identification of protective components of two major outer membrane proteins of spotted fever group Rickettsiae. Am. J. Trop. Med. Hyg. 65:371–378 [DOI] [PubMed] [Google Scholar]
- 7. Feng H. M., Walker D. H. 2004. Mechanisms of immunity to Ehrlichia muris: a model of monocytotropic ehrlichiosis. Infect. Immun. 72:966–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fishbein D. B., Dawson J. E., Robinson L. E. 1994. Human ehrlichiosis in the United States, 1985 to 1990. Ann. Intern. Med. 120:736–743 [DOI] [PubMed] [Google Scholar]
- 9. Ganta R. R., et al. 2007. Differential clearance and immune responses to tick cell-derived versus macrophage culture-derived Ehrlichia chaffeensis in mice. Infect. Immun. 75:135–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gilmore R. D., Jr., Kappel K. J., Dolan M. C., Burkot T. R., Johnson B. J. 1996. Outer surface protein C (OspC), but not P39, is a protective immunogen against a tick-transmitted Borrelia burgdorferi challenge: evidence for a conformational protective epitope in OspC. Infect. Immun. 64:2234–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ismail N., et al. 2004. Overproduction of TNF-alpha by CD8+ type 1 cells and down-regulation of IFN-gamma production by CD4+ Th1 cells contribute to toxic shock-like syndrome in an animal model of fatal monocytotropic ehrlichiosis. J. Immunol. 172:1786–1800 [DOI] [PubMed] [Google Scholar]
- 12. Li J. S., Chu F., Reilly A., Winslow G. M. 2002. Antibodies highly effective in SCID mice during infection by the intracellular bacterium Ehrlichia chaffeensis are of picomolar affinity and exhibit preferential epitope and isotype utilization. J. Immunol. 169:1419–1425 [DOI] [PubMed] [Google Scholar]
- 13. Li J. S., et al. 2001. Outer membrane protein-specific monoclonal antibodies protect SCID mice from fatal infection by the obligate intracellular bacterial pathogen Ehrlichia chaffeensis. J. Immunol. 166:1855–1862 [DOI] [PubMed] [Google Scholar]
- 14. Lin M., Rikihisa Y. 2003. Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival. Infect. Immun. 71:5324–5331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Long S. W., et al. 2002. Antigenic variation of Ehrlichia chaffeensis resulting from differential expression of the 28-kilodalton protein gene family. Infect. Immun. 70:1824–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McBride J. W., et al. 2003. Kinetics of antibody response to Ehrlichia canis immunoreactive proteins. Infect. Immun. 71:2516–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nandi B., Hogle K., Vitko N., Winslow G. M. 2007. CD4 T-cell epitopes associated with protective immunity induced following vaccination of mice with an ehrlichial variable outer membrane protein. Infect. Immun. 75:5453–5459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nyika A., Barbet A. F., Burridge M. J., Mahan S. M. 2002. DNA vaccination with map1 gene followed by protein boost augments protection against challenge with Cowdria ruminantium, the agent of heartwater. Vaccine 20:1215–1225 [DOI] [PubMed] [Google Scholar]
- 19. Nyika A., et al. 1998. A DNA vaccine protects mice against the rickettsial agent Cowdria ruminantium. Parasite Immunol. 20:111–119 [DOI] [PubMed] [Google Scholar]
- 20. Ohashi N., Rikihisa Y., Unver A. 2001. Analysis of transcriptionally active gene clusters of major outer membrane protein multigene family in Ehrlichia canis and E. chaffeensis. Infect. Immun. 69:2083–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ohashi N., Zhi N., Zhang Y., Rikihisa Y. 1998. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect. Immun. 66:132–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olano J. P., Masters E., Hogrefe W., Walker D. H. 2003. Human monocytotropic ehrlichiosis, Missouri. Emerg. Infect. Dis. 9:1579–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Olano J. P., Wen G., Feng H. M., McBride J. W., Walker D. H. 2004. Histologic, serologic, and molecular analysis of persistent ehrlichiosis in a murine model. Am. J. Pathol. 165:997–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paddock C. D., Childs J. E. 2003. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin. Microbiol. Rev. 16:37–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paddock C. D., et al. 2001. Infections with Ehrlichia chaffeensis and Ehrlichia ewingii in persons coinfected with human immunodeficiency virus. Clin. Infect. Dis. 33:1586–1594 [DOI] [PubMed] [Google Scholar]
- 26. Reddy G. R., et al. 1998. Molecular characterization of a 28 kDa surface antigen gene family of the tribe Ehrlichiae. Biochem. Biophys. Res. Commun. 247:636–643 [DOI] [PubMed] [Google Scholar]
- 27. Singu V., Liu H., Cheng C., Ganta R. R. 2005. Ehrlichia chaffeensis expresses macrophage- and tick cell-specific 28-kilodalton outer membrane proteins. Infect. Immun. 73:79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singu V., et al. 2006. Unique macrophage and tick cell-specific protein expression from the p28/p30-outer membrane protein multigene locus in Ehrlichia chaffeensis and Ehrlichia canis. Cell. Microbiol. 8:1475–1487 [DOI] [PubMed] [Google Scholar]
- 29. Sotomayor E. A., Popov V. L., Feng H. M., Walker D. H., Olano J. P. 2001. Animal model of fatal human monocytotropic ehrlichiosis. Am. J. Pathol. 158:757–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stevenson H. L., et al. 2006. An intradermal environment promotes a protective type 1 response against lethal systemic monocytotropic ehrlichial infection. Infect. Immun. 74:4856–4864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thirumalapura N. R., Stevenson H. L., Walker D. H., Ismail N. 2008. Protective heterologous immunity against fatal ehrlichiosis and lack of protection following homologous challenge. Infect. Immun. 76:1920–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thomas S., Thirumalapura N., Crossley E. C., Ismail N., Walker D. H. 2009. Antigenic protein modifications in Ehrlichia. Parasite Immunol. 31:296–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Unver A., Rikihisa Y., Stich R. W., Ohashi N., Felek S. 2002. The omp-1 major outer membrane multigene family of Ehrlichia chaffeensis is differentially expressed in canine and tick hosts. Infect. Immun. 70:4701–4704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Heerden H., Collins N. E., Brayton K. A., Rademeyer C., Allsopp B. A. 2004. Characterization of a major outer membrane protein multigene family in Ehrlichia ruminantium. Gene 330:159–168 [DOI] [PubMed] [Google Scholar]
- 35. Winslow G. M., Yager E., Shilo K., Collins D. N., Chu F. K. 1998. Infection of the laboratory mouse with the intracellular pathogen Ehrlichia chaffeensis. Infect. Immun. 66:3892–3899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang J. Z., Guo H., Winslow G. M., Yu X. J. 2004. Expression of members of the 28-kilodalton major outer membrane protein family of Ehrlichia chaffeensis during persistent infection. Infect. Immun. 72:4336–4343 [DOI] [PMC free article] [PubMed] [Google Scholar]