Abstract
Microbiological diagnosis of nosocomial candidemia is negatively affected by suboptimal culture yield. Alternative methods are not fully reliable as an aid in candidemia diagnosis. Recently, the detection of (1,3)-β-d-glucan (BG) has been shown to be very promising in this setting. We carried out a prospective study on the clinical usefulness of BG detection in early diagnosis of candidemia. BG detection was performed in patients with fever unresponsive to antibacterial agents and risk factors for candidemia. BG detection was done with the Fungitell test. A total of 152 patients were included in the study; 53 were proven to have candidemia, while in 52 patients candidemia was excluded on microbiological and clinical bases. The remaining 47 patients were considered to have possible candidemia. In summary, 41 of 53 candidemia patients (77.3%), 9 of 52 patients without candidemia (17.3%), and 38 of 47 patients with possible candidemia (80.8%) were positive in the BG assay. With these results, the sensitivity and the specificity of the assay were 77% and 83%, respectively. BG levels of >160 pg/ml were highly predictive of candidemia. In 36 of 41 patients with candidemia and positive BG testing, the BG assay was performed within 48 h from when the first Candida-positive blood sample for culture was drawn, thus allowing a possible earlier start of antifungal therapy. Based on these results, the BG assay may be used as an aid in the diagnosis of nosocomial candidemia. The timing of assay performance is critical for collecting clinically useful information. However, the test results should be associated with clinical data.
INTRODUCTION
Candida species are a growing cause of nosocomial bloodstream infection, particularly in intensive care unit (ICU) and surgical patients. Candida has been reported to be one of the most frequent cause of associated bloodstream infections in different health care settings (16, 26, 28) and carries an attributable mortality ranging from 5 to 71% (8).
Candidemia has been shown to increase both mortality and length of hospital stay for both adult and pediatric patients (29). Several factors affect the risk of candidemia, such as complicated abdominal surgery, long-term use of antibiotics, presence of indwelling central venous catheters, and total parenteral nutrition. Since these risk factors are not easily modifiable, a critical issue is represented by the timing of diagnosis, in consideration of the fact that an early initiation of antifungal therapy can significantly decrease mortality (18). Furthermore, the time to positive blood culture may be significantly delayed in case of infections due to non-C. albicans Candida species, which account for about 50% of all candidemia (9, 11), particularly when fluconazole prophylaxis is used (3).
As an alternative to blood culture, neither antigen detection nor molecular techniques are fully reliable in diagnosing candidemia (4), although the detection of (1,3)-β-d-glucan (BG), a component of the cell walls of several fungal species, has been reported to be a potentially useful aid for diagnosis of invasive fungal infections (IFI) in prospective or retrospective studies involving mainly hematologic patients (13, 21, 23, 25) and in an autopsy-based study (19). BG detection has been applied successfully to Pneumocystis jirovecii pneumonia (PCP) diagnosis in either proven or presumptive cases (5, 6, 27). However, the use of BG as an aid in early diagnosis of candidemia is suboptimal. Currently, data from large prospective studies concerning the diagnostic use of the BG assay for candidemia are not exhaustive. Here we report the results of a prospective study on the clinical use and performance of the BG assay in early diagnosis of candidemia.
MATERIALS AND METHODS
Patients.
Patients admitted to two ICU wards, one abdominal surgery ward, and one infectious disease ward during the period from July 2008 to October 2010 were included in the study. The inclusion criteria were fever lasting for ≥72 h, unresponsive to broad-spectrum antibacterial therapy, and blood cultures negative for bacterial growth, plus one of the following: (i) one risk factor for candidemia (major abdominal surgery, previous antibiotic treatment, total parenteral nutrition, or indwelling central venous catheters) or (ii) Candida colonization in at least two noncontiguous body sites (for example, throat swab, sputum, and tracheobronchial aspirate culture were considered only one site). The exclusion criteria were as follows: risk factors and clinical presentation consistent with PCP, according to the modified 1993 definition of PCP as an AIDS-defining condition (1); risk factors and clinical presentation consistent with invasive aspergillosis (IA), according to the 2008 EORTC/MSG definition of IFI (7); neutropenia (<500 neutrophils/mm3); major surgery performed within 96 h before BG assay; and hemodialysis.
The patients were divided into three groups, according to clinical and microbiological data: (i) proven candidemia, i.e., patients with at least one blood culture positive for Candida; (ii) noncandidemia, i.e., those with negative blood cultures plus an established, satisfactory alternative clinical diagnosis; and (iii) possible candidemia according to the revised definitions of fungal infections (7), i.e., patients with negative blood cultures but clinical findings consistent with candidemia, including a Candida score (CS) of ≥2.5 (14). In the final analysis, all cases were reviewed according to the revised version of the Candida score described by León et al. (15), and only patients with a CS of ≥3 were considered to have possible candidemia. The response to any antifungal therapy was not a criterion used for defining possible candidemia. All patients were grouped independently of BG results.
Detection of BG.
A single serum BG evaluation was performed, according to the manufacturer's instructions, by means of the Fungitell test (Associates of Cape Cod, Inc., Cape Cod, MA), one of the tests commercially available for the detection of BG and approved in 2003 by the U.S. Food and Drug Administration for the presumptive diagnosis of IFI. BG values of ≥80 pg/ml were considered positive according to the manufacturer's indications.
Statistical methods.
In this descriptive study, data for categorical variables are presented as proportions. Comparisons of proportions use the chi-square test or Fisher's exact test, when appropriate. Continuous variables are reported as medians, and their distributions in the 3 groups of candidemia (proven candidemia, possible candidemia, and noncandidemia) were compared using the Kruskal-Wallis nonparametric test, which is equivalent to a one-way analysis of variance. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were computed with standard methods using all patients included in the study. For an overall assessment of the discriminatory power of the test, a receiver operating characteristic (ROC) curve was plotted. This is a graphical plot of the sensitivity, or true-positive rate, versus the false-positive rate (1 − specificity or 1 − true-negative rate) for a binary classifier system as its discrimination threshold is varied. In our curve, true cases were considered all subjects with proven or possible candidemia, and the relevant area under the curve was computed.
RESULTS
A total of 152 patients were included in the study; 53 were in the candidemia group, 47 in the possible candidemia group, and 52 in the noncandidemia group. Demographic and clinical data, together with BG assay results, are reported in Table 1.
Table 1.
Demographic and clinical characteristics and BG levels of study patients
| Parameter | Value for groupa with: |
P | ||
|---|---|---|---|---|
| Proven candidemia | Possible candidemia | Candidemia excluded | ||
| No. of patients (n = 152) | 53 | 47 | 52 | NS |
| Median age, yr (range) | 72 (35-88) | 64 (18-88) | 59.5 (19-92) | 0.013 |
| Gender (no. male/no. female) | 27/26 | 27/20 | 32/20 | NS |
| Median total hospital stay, days (range) | 60 (11-398) | 56 (9-600) | 45 (10-268) | NS |
| Median days to BG assayb (range) | 28 (4-182) | 21 (3-398) | 15.5 (3-187) | 0.010 |
| Median days to blood culturec (range) | 27 (4-179) | NAf | NA | NA |
| No. (%) of patients with: | ||||
| ICU stayd | 11 (20.7) | 7 (14.9) | 6 (11.5) | NS |
| Abdominal surgery | 17 (32) | 18 (38) | 13 (21) | NS |
| Multifocal colonizatione | 38 (72) | 25 (53) | 19 (52) | <0.001 |
| Positive BG test | 41 (77) | 38 (81) | 9 (17) | <0.001 |
| Median BG value, pg/ml (range) | 324 (6-523) | 162 (6-523) | 27 (6-237) | <0.001 |
See Materials and Methods.
Days elapsed from admission to BG assay performance.
Days elapsed from admission to first blood culture positive for Candida.
Minimum of 48 h.
Isolation of at least 2 Candida spp. in noncontiguous sites.
NA, not applicable.
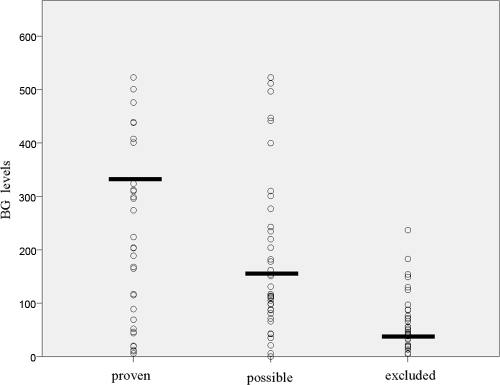
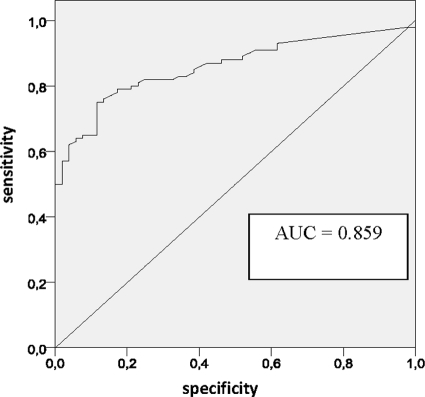
In the candidemia group, the median age was higher than in the other groups, while in the noncandidemia group, the median hospital stay and the time to BG assay (that is, the time range between the admission and BG assay performance) were shorter than in the other groups. Abdominal surgery was more frequent in the possible candidemia group, while an ICU stay was prevalent in proven candidemia group. However, no significant differences were found in regard to the underlying diseases of the study patients. Multifocal colonization was more frequent (P = <0.001) in the proven and possible candidemia groups than in the noncandidemia group (72%, 57%, and 37%, respectively). In regard to BG assay results, 41 out of 53 candidemia patients were positive, 38 out of 47 patients with possible candidemia were positive, and 9 out of 52 patients in the noncandidemia group were positive. With these results, the sensitivity and the specificity of the assay were 77% (±11.3%) and 83% (±10.2%), respectively. Figure 1 shows the distribution of BG values within the three clinical groups. When we grouped together proven and possible candidemia, under the assumption that all possible candidemia cases were proven, we found a slight increase in sensitivity (79%). Table 2 shows the sensitivity, specificity, positive predictive value, and negative predictive value using various cutoffs. The currently used cutoff of 80 pg/ml appears to be an acceptable compromise (sensitivity, 79% ± 6.5%; specificity, 83% ± 10.2%; PPV, 90% ± 6.3%; and NPV, 67% ± 11.5%). However, different balances between sensitivity and specificity could be attained by moving the cutoff value up or down. In particular, values above 160 pg/ml led to a PPV of 98.4%. Conversely, any modification in the cutoffs did not significantly change the NPV. The area under the ROC curve was 0.86 (Fig. 2).
Fig. 1.
BG levels (expressed in pg/ml) in study patients, divided into groups with proven, possible, and excluded candidemia. Horizontal bars represent median levels.
Table 2.
Sensitivity, specificity, PPV, and NPV of the BG assay at different cutoff levels
| Cutoff (pg/ml) | No./totala |
Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|
| Above cutoff (cases) | Below cutoff (controls) | |||||
| >10 | 92/100 | 11/52 | 92 | 21 | 69.2 | 57.9 |
| >20 | 89/100 | 25/52 | 89 | 48 | 76.7 | 69.4 |
| >40 | 87/100 | 30/52 | 87 | 58 | 79.8 | 69.8 |
| >60 | 82/100 | 39/52 | 82 | 75 | 86.3 | 68.4 |
| >80 | 79/100 | 43/52 | 79 | 83 | 89.8 | 67.2 |
| >100 | 73/100 | 46/52 | 73 | 88 | 92.4 | 63.0 |
| >120 | 65/100 | 46/52 | 65 | 88 | 91.5 | 56.8 |
| >140 | 64/100 | 48/52 | 64 | 92 | 94.1 | 57.1 |
| >160 | 62/100 | 51/52 | 62 | 98 | 98.4 | 57.3 |
Cases, patients with proven or possible candidemia; controls, patients with candidemia excluded.
Fig. 2.
Receiver operating characteristic (ROC) curve for performance of the β-d-glucan (BG) assay in the early diagnosis of nosocomial candidemia for the entire cohort (152 patients). The diagnostic performances for different cutoff levels are summarized in Table 2. AUC, area under the curve.
In 36 out of 41 cases of proven candidemia with positive BG results, the BG assay was performed within 48 h from the day when blood for the first positive culture for Candida was drawn, thus potentially allowing for an earlier initiation of antifungal treatment. The timing of BG assay performance in these 36 cases was as follows: in 7 cases before day 0 (day of blood culture sampling), in 12 on day 0, and in 17 between days 1 and 2.
Patients with candidemia due to C. albicans were more frequently BG positive than patients with candidemia due to non-C. albicans species (81% versus 72%; P = not significant [NS]). This difference is mainly due to the reduced sensitivity of BG assay to Candida parapsilosis (65% positivity; 11/17). However, no differences were found when comparing median BG values (309.7 and 306.6 pg/ml for C. albicans and non-C. albicans, respectively).
The study design required a single BG evaluation per patient. However, in 24 cases, due to reasons unrelated to the study design, two consecutive BG test evaluations were performed within 72 h. A discrepancy in BG assay results (first positive and second negative) between two consecutive samples was found in one case only, in a patient who had a positive methicillin-resistant Staphylococcus aureus (MRSA) blood culture the same day as the positive BG assay.
In the group where candidemia was excluded, patients with multifocal colonization were more frequently BG positive than those without multifocal colonization: 26% (5/19) and 12% (4/33), respectively.
DISCUSSION
Despite the improved sensitivity of microbiological methods, a significant percentage of candidemia goes undetected due to the low yield of traditional blood cultures and the still-unresolved issues about alternative diagnostic methods, although a recent meta-analysis showed interesting data on the clinical performance of PCR (2). Furthermore, the timing for initiating antifungal therapy is critical for the disease outcome. The availability of a reliable diagnostic surrogate marker should be particularly useful in the clinical management of patients at risk of fungal infections, since it may allow for less empirical antifungal therapy and earlier targeted treatment. Among these patients, those benefiting most from the surrogate marker are probably those with a compatible clinical presentation but negative blood cultures. In our study, we prospectively tested the clinical usefulness of the BG assay in early detection of candidemia in order to collect information about the possible use of this test in clinical practice.
Very recently, a meta-analysis showed that the BG assay had a sensitivity and a specificity of 76.8% and 85.3%, respectively (12). This meta-analysis consisted of 16 studies, 11 of which included mainly hematologic patients diagnosed with IFI due to both Candida and Aspergillus. In our study, we chose to exclude neutropenic patients or those patients with risk factors and clinical presentation consistent with PCP or invasive aspergillosis, in order to avoid any positive BG assay results due to Aspergillus or P. jirovecii infections and thus focus on candidemia only. We divided the patients into three groups, with the first (proven candidemia) including those with one or more blood cultures positive for candida, the second including patients in whom candidemia had been reasonably excluded, and the third including patients in whom candidemia, despite negative blood cultures, was considered possible, according to internationally accepted criteria. For patients included in the last group, we did not have any evidence other than an accurate clinical judgment, according to risk factors and clinical presentation as outlined by León et al. (14, 15). However, in our opinion our definition of possible candidemia was quite reliable, minimizing the possibility of a wrong diagnosis. Within the boundaries of this approach, the BG assay was shown to be a fairly reliable test, with a sensitivity and a specificity of 77% and 83%, respectively, which were very close to the results found in the above-mentioned meta-analysis. The PPV was 90% with a cutoff set to 80 pg/ml.
The clinical group of possible candidemia should benefit most from a reliable diagnostic surrogate marker which may support an early initiation of an antifungal medication. The BG assay performance within this group, which was very close to that for the candidemia group, emphasizes that these patients might have true Candida bloodstream infection. In this light, BG assay results may be used similarly to those of the galactomannan assay for invasive aspergillosis, where a positive galactomannan test allows the diagnosis of IA to be shifted from possible to probable (10, 24).
The BG test can allow an earlier start of antifungal therapy. Indeed, in our study, 19 candidemia patients had a positive BG test performed no later than the day when blood for the first positive Candida culture was drawn. This implies a considerable advantage in terms of diagnosis, since at least 2 days usually elapse from blood drawing to growth and report. The same finding was not observed in our recently published study: the BG test used with a small population of allogeneic hematopoietic stem cell transplant (HSCT) patients failed to give clinical information earlier than blood culture, probably due to the peculiar clinical characteristics of the patients studied (17). We found that patients with proven candidemia had higher BG levels than those with possible candidemia, perhaps reflecting a heavier fungal burden and therefore explaining the positivity of blood cultures.
Despite the fact that we did not find any differences in median BG levels between C. albicans and non-C. albicans species, we found the BG assay to be more frequently positive (although not reaching statistical significance) in infections due to C. albicans than in those due to non-C. albicans, particularly C. parapsilosis, in line with what other authors have previously found (21). In our study, the numbers are too small to support the conclusion that C. parapsilosis has a smaller amount of BG. Nonetheless, this finding, if confirmed in a larger study, may help to explain the higher MICs of echinocandins (antifungals blocking BG synthesis) for C. parapsilosis. However, such differences were not reported by Odabasi et al. in an in vitro study, where all Candida species, with the exception of C. lusitaniae, had comparable BG levels in culture supernatants (20).
Some authors have claimed the need to perform more than one BG test for the initial diagnosis (25); for 24 patients we had two consecutive BG assays performed within 72 h, and in 23 of the 24 cases we had full concordance between the two results (positive/positive or negative/negative). Therefore, based on these data, we think that evaluation at a single time point can be reliably used for diagnostic purposes.
The BG test was shown to have acceptable rates of both sensitivity and specificity as well as a good PPV. Any modification in the threshold of the test did not significantly change BG assay performance; in fact, increasing the cutoff above 80 pg/ml did not consistently improve either the specificity or the NPV, but it decreased the sensitivity by a significant measure. However, it should be pointed out that values above 160 pg/ml are highly indicative of candidemia.
The BG test is not the solution to all our problems in diagnosing and managing Candida infections in critically ill patients, but it is certainly a major advance. Limitations remain, particularly due to the possibility of false-positive results in the noncandidemia group, i.e., the group for whom, with good probability, we ruled out candidemia on clinical as well as microbiological grounds. Despite the caveats due to the very small numbers, multifocal colonization seems to play a role in this group, since 26% of patients with multifocal colonization were also BG positive, in comparison to 12% of noncolonized patients.
BG is an ubiquitous compound, and several factors, such as nutritional intake, administration of albumin, recent surgery with use of surgical sponges and/or gauze, and hemodialysis, are known to be potential causes of false positivity (22). In fact, surgical sponges, gauze, and cellulose membranes for dialysis can directly leach BG, whereas albumin may result in BG positivity due to BG leaching during the filtration process with cellulose membranes.
Conceivably, critically ill patients with longer hospital stays may have an increased risk of candidemia; however, they may also be more likely to have false-positive BG results due to the above-mentioned causes.
In addition, the BG assay is rather expensive, and not all institutions can afford it. However, considering the economic burden that these patients with complicated illness usually carry, the implementation of a BG-based diagnostic policy may be truly cost-effective.
In conclusion, the BG assay seems to be reliable as an aid in candidemia diagnosis. However, its positivity or negativity should be interpreted very carefully and compared with clinical data, preferably by experienced clinicians. The timing of performance of the assay is critical to allow for an early start of antifungal therapy.
ACKNOWLEDGMENTS
We thank Franca Miletich for her assistance with BG assay performance.
All of the authors declare an absence of conflicting interests regarding this paper.
Footnotes
Published ahead of print on 12 October 2011.
REFERENCES
- 1. Anonymous. 1992. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adult. MMWR Recommend. Rep. 41(RR-17):1–19 [PubMed] [Google Scholar]
- 2. Avni T., Leibovici L., Paul M. 2011. PCR diagnosis of invasive candidiasis: systematic review and meta-analysis. J. Clin. Microbiol. 49:665–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bassetti M., et al. 2009. Incidence of candidaemia and relationship with fluconazole use in an intensive care unit. J. Antimicrob. Chemother. 64:625–629 [DOI] [PubMed] [Google Scholar]
- 4. Bille J. 2010. New nonculture-based methods for the diagnosis of invasive candidiasis. Curr. Opin. Crit. Care 16:460–464 [DOI] [PubMed] [Google Scholar]
- 5. Damiani C., Le Gal S., Lejeune D. 2011. Serum (1-3)-β-d-glucan levels in primary infection and pulmonary colonization with Pneumocystis jirovecii. J. Clin. Microbiol. 49:2000–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Del Bono V., et al. 2009. Clinical evaluation of a (1,3)-β-d-glucan assay for presumptive diagnosis of Pneumocystis jiroveci pneumonia in immunocompromised patients. Clin. Vaccine Immunol. 16:1524–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Pauw B., et al. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycosis Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46:1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Falagas M. E., Apostolou K. E., Pappas V. D. 2006. Attributable mortality of candidemia: a systematic review of matched cohort and case-control studies. Eur. J. Clin. Microbiol. Infect. Dis. 25:419–425 [DOI] [PubMed] [Google Scholar]
- 9. Fernandez J., Erstad B. L., Petty W., Nix D. E. 2009. Time to positive culture and identification for Candida bloodstream infections. Diagn. Microbiol. Infect. Dis. 64:402–407 [DOI] [PubMed] [Google Scholar]
- 10. Hope W. W., Walsh T. J., Denning D. W. 2005. Laboratory diagnosis of invasive aspergillosis. Lancet Infect. Dis. 5:609–622 [DOI] [PubMed] [Google Scholar]
- 11. Horn D. L., et al. 2009. Epidemiology and outcomes of candidemia in 2019 patients: data from the Prospective Antifungal Therapy Alliance Registry. Clin. Infect. Dis. 48:1695–1703 [DOI] [PubMed] [Google Scholar]
- 12. Karageorgopoulos D. E., et al. 2011. β-d-Glucan assay for the diagnosis of invasive fungal infections: a meta-analysis. Clin. Infect. Dis. 52:750–770 [DOI] [PubMed] [Google Scholar]
- 13. Koo S., Bryar J. M., Page J. H., Baden L. R., Marty F. M. 2009. Diagnostic performance of the (1→3)-beta-d-glucan assay for invasive fungal disease. Clin. Infect. Dis. 49:1650–1659 [DOI] [PubMed] [Google Scholar]
- 14. León C., et al. 2006. A bedside scoring system (“Candida score”) for early antifungal treatment in nonneutropenic critically ill patients with Candida colonization. Crit. Care Med. 34:730–737 [DOI] [PubMed] [Google Scholar]
- 15. León C., et al. 2009. Usefulness of the “Candida score” for discriminating between Candida colonization and invasive candidiasis in non-neutropenic critically ill patients: a prospective multicenter study. Crit. Care Med. 37:1624–1633 [DOI] [PubMed] [Google Scholar]
- 16. Marchetti O., et al. 2004. Epidemiology of candidemia in Swiss tertiary care hospitals: secular trends, 1991-2000. Clin. Infect. Dis. 38:311–320 [DOI] [PubMed] [Google Scholar]
- 17. Mikulska M., et al. 2011. Persistence of a positive (1,3)-beta-d-glucan test after clearance of candidemia in hematopoietic stem cell transplant recipients. Clin. Vaccine Immunol. 18:518–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morrell M., Fraser V. J., Kollef M. H. 2005. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob. Agents Chemother. 49:3640–3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Obayashi T., Negishi K., Suzuki T., Funata N. 2008. Reappraisal of the serum (1→3)-β-d-glucan assay for the diagnosis of invasive fungal infections—a study based on autopsy cases from 6 years. Clin. Infect. Dis. 46:1864–1870 [DOI] [PubMed] [Google Scholar]
- 20. Odabasi Z., et al. 2006. Differences in beta-glucan levels in culture supernatants of a variety of fungi. Med. Mycol. 44:267–272 [DOI] [PubMed] [Google Scholar]
- 21. Ostrosky-Zeichner L., et al. 2005. Multicenter clinical evaluation of the (1→3)-β-d-glucan assay as an aid to diagnosis of fungal infections in humans. Clin. Infect. Dis. 41:654–659 [DOI] [PubMed] [Google Scholar]
- 22. Ostrosky-Zeichner L., et al. 2011. Early treatment of candidemia in adults: a review. Med. Mycol. 49:113–120 [DOI] [PubMed] [Google Scholar]
- 23. Persat F., et al. 2008. Contribution of the (1→3)-β-d-glucan assay for diagnosis of invasive fungal infections. J. Clin. Microbiol. 46:1009–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Segal B. H. 2009. Aspergillosis. N. Engl. J. Med. 360:1870–1884 [DOI] [PubMed] [Google Scholar]
- 25. Senn L., et al. 2008. 1,3-β-d-Glucan antigenemia for early diagnosis of invasive fungal infections in neutropenic patients with acute leukemia. Clin. Infect. Dis. 46:878–885 [DOI] [PubMed] [Google Scholar]
- 26. Viscoli C., et al. 1999. Candidemia in cancer patients: a prospective, multicenter surveillance study by the Invasive Fungal Infection Group (IFIG) of the European Organization for Research and Treatment of Cancer (EORTC). Clin. Infect. Dis. 28:1071–1079 [DOI] [PubMed] [Google Scholar]
- 27. Watanabe T., Yasuoka A., Tanuma J. 2009. Serum as a noninvasive adjunct marker for the diagnosis of Pneumocystis pneumonia in patients with AIDS. Clin. Infect. Dis. 49:1128–1131 [DOI] [PubMed] [Google Scholar]
- 28. Wisplinghoff H., et al. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309–317 [DOI] [PubMed] [Google Scholar]
- 29. Zaoutis T. E., et al. 2005. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin. Infect. Dis. 41:1232–1239 [DOI] [PubMed] [Google Scholar]