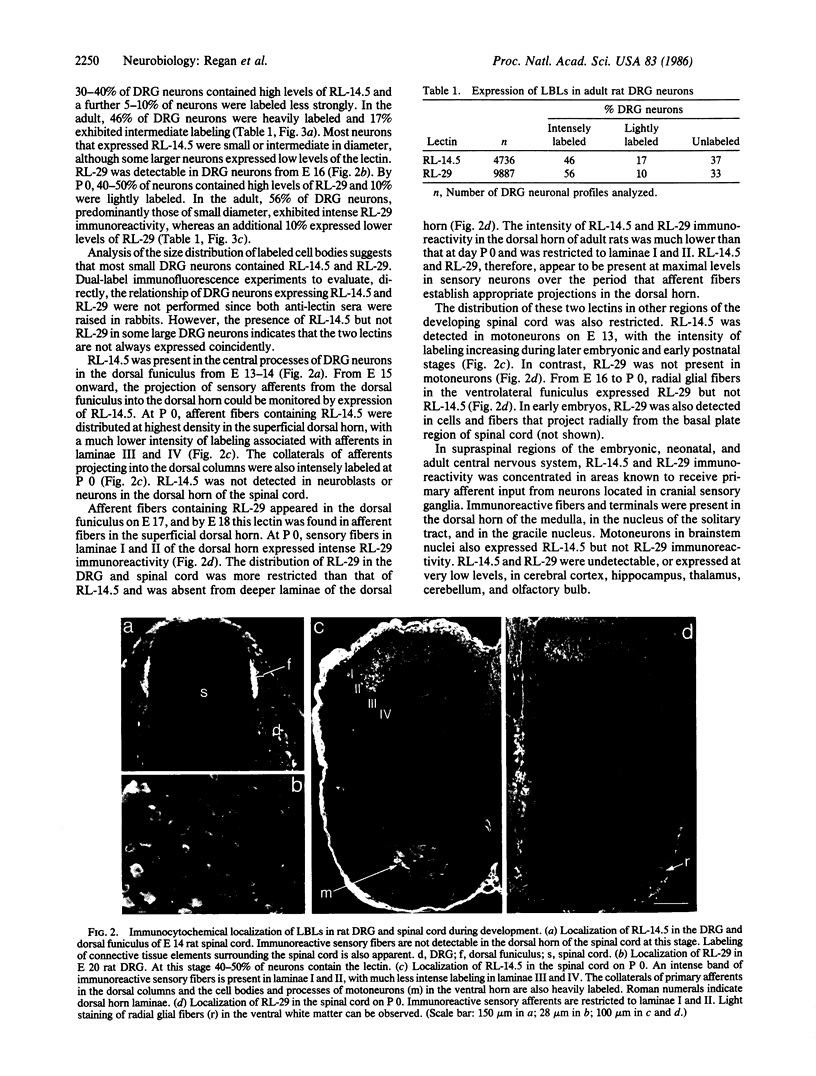
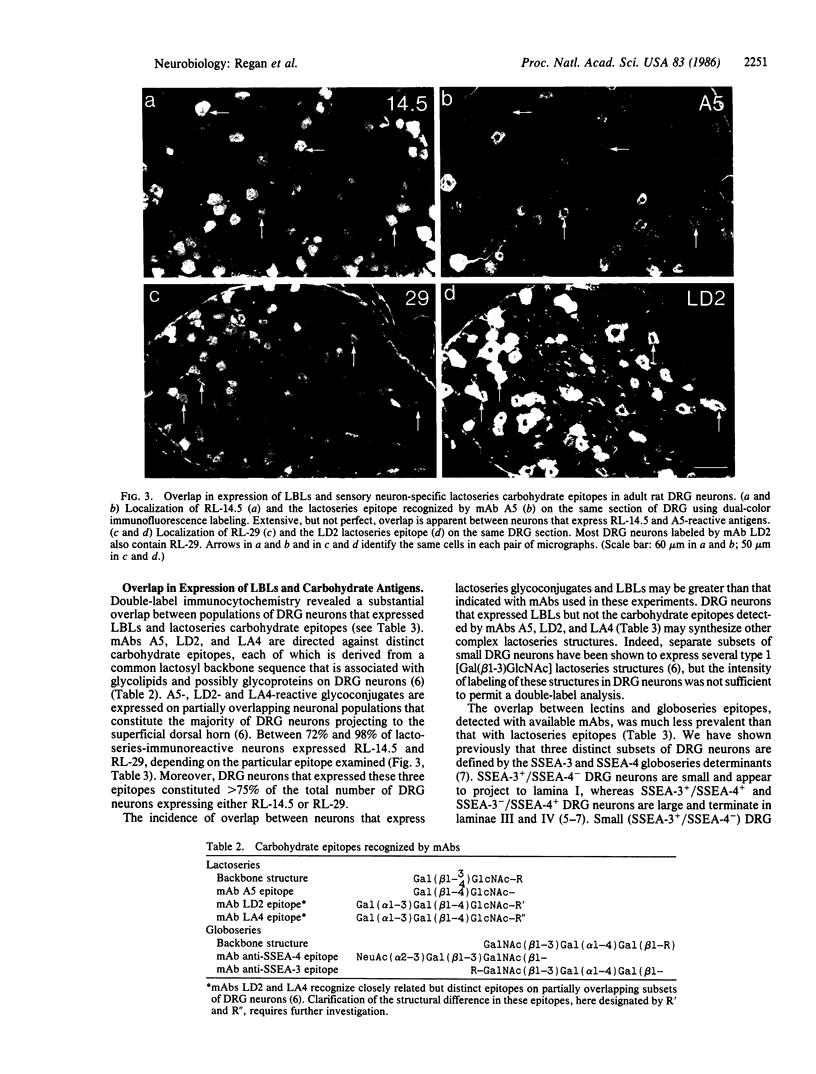
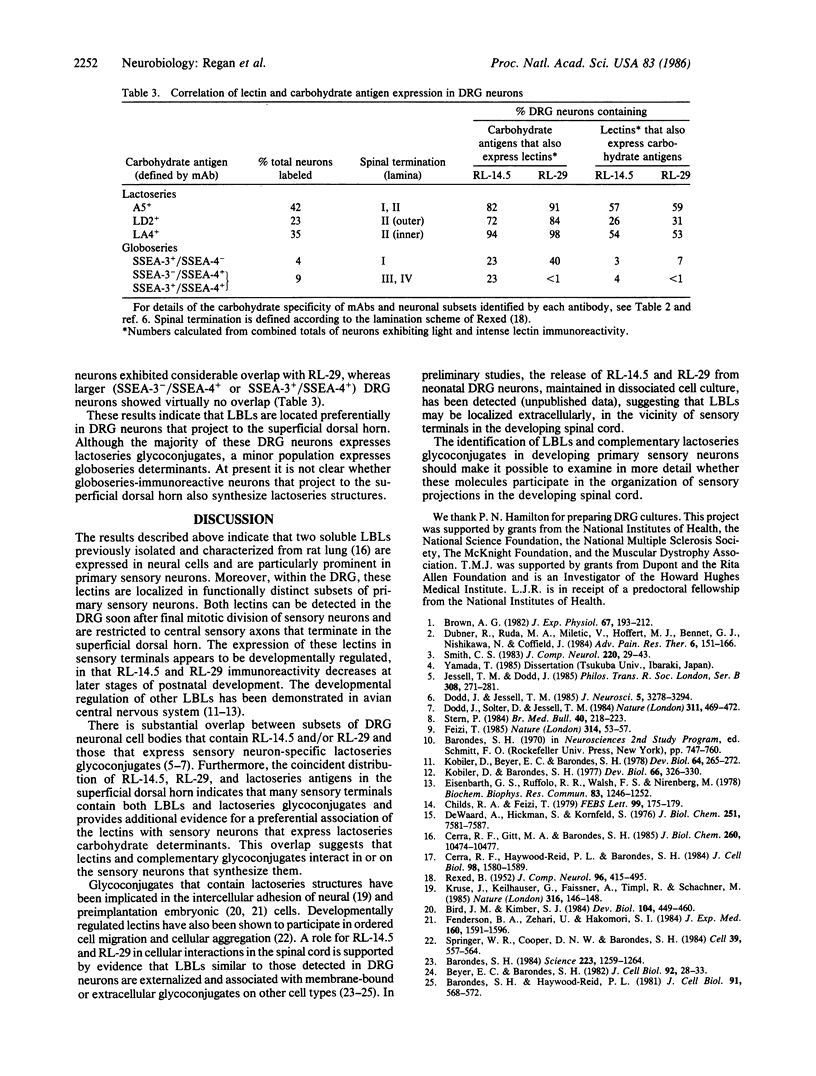
Abstract
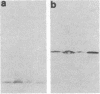
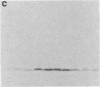
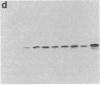
Cell-surface glycoconjugates and endogenous lectins have been implicated in cellular interactions that contribute to embryonic development. Functional subsets of primary sensory neurons in mammalian dorsal root ganglia (DRG) have been shown recently to express specific cell-surface oligosaccharide structures. We report here that endogenous lectins with affinity for sensory neuron glycoconjugates are also synthesized by subsets of DRG neurons and are present in the dorsal horn of the developing spinal cord. The distribution of two endogenous lactose-binding lectins, RL-14.5 and RL-29 (subunit Mrs of 14,500 and 29,000, respectively), was examined by immunoblotting and by immunocytochemistry in embryonic and postnatal rat DRG and spinal cord. The two lectins appear soon after the formation of the DRG and are present in the cell bodies and terminals of subsets of DRG neurons that also express cytoplasmic and cell-surface lactoseries glycoconjugates. RL-14.5 and RL-29 are present in overlapping, but not coincident, subsets of DRG neurons that project to the superficial dorsal horn of the spinal cord. In addition, RL-14.5, but not RL-29, is expressed in spinal motoneurons from embryonic day 14. The preferential localization of lactoseries glycoconjugates and lactose-binding lectins in the DRG and the dorsal horn of the spinal cord suggests that these complementary molecules contribute to the development and function of primary sensory neurons.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barondes S. H., Haywood-Reid P. L. Externalization of an endogenous chicken muscle lectin with in vivo development. J Cell Biol. 1981 Nov;91(2 Pt 1):568–572. doi: 10.1083/jcb.91.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barondes S. H. Soluble lectins: a new class of extracellular proteins. Science. 1984 Mar 23;223(4642):1259–1264. doi: 10.1126/science.6367039. [DOI] [PubMed] [Google Scholar]
- Beyer E. C., Barondes S. H. Secretion of endogenous lectin by chicken intestinal goblet cells. J Cell Biol. 1982 Jan;92(1):28–33. doi: 10.1083/jcb.92.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird J. M., Kimber S. J. Oligosaccharides containing fucose linked alpha(1-3) and alpha(1-4) to N-acetylglucosamine cause decompaction of mouse morulae. Dev Biol. 1984 Aug;104(2):449–460. doi: 10.1016/0012-1606(84)90101-5. [DOI] [PubMed] [Google Scholar]
- Brown A. G. The dorsal horn of the spinal cord. Q J Exp Physiol. 1982 Apr;67(2):193–212. doi: 10.1113/expphysiol.1982.sp002630. [DOI] [PubMed] [Google Scholar]
- Cerra R. F., Gitt M. A., Barondes S. H. Three soluble rat beta-galactoside-binding lectins. J Biol Chem. 1985 Sep 5;260(19):10474–10477. [PubMed] [Google Scholar]
- Cerra R. F., Haywood-Reid P. L., Barondes S. H. Endogenous mammalian lectin localized extracellularly in lung elastic fibers. J Cell Biol. 1984 Apr;98(4):1580–1589. doi: 10.1083/jcb.98.4.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs R. A., Feizi T. Calf heart lectin reacts with blood group Ii antigens and other precursor chains of the major blood group antigens. FEBS Lett. 1979 Mar 1;99(1):175–179. doi: 10.1016/0014-5793(79)80273-2. [DOI] [PubMed] [Google Scholar]
- Dodd J., Jessell T. M. Lactoseries carbohydrates specify subsets of dorsal root ganglion neurons projecting to the superficial dorsal horn of rat spinal cord. J Neurosci. 1985 Dec;5(12):3278–3294. doi: 10.1523/JNEUROSCI.05-12-03278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J., Solter D., Jessell T. M. Monoclonal antibodies against carbohydrate differentiation antigens identify subsets of primary sensory neurones. Nature. 1984 Oct 4;311(5985):469–472. doi: 10.1038/311469a0. [DOI] [PubMed] [Google Scholar]
- Eisenbarth G. S., Ruffolo R. R., Jr, Walsh F. S., Nirenberg M. Lactose sensitive lectin of chick retina and spinal cord. Biochem Biophys Res Commun. 1978 Aug 29;83(4):1246–1252. doi: 10.1016/0006-291x(78)91355-4. [DOI] [PubMed] [Google Scholar]
- Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature. 1985 Mar 7;314(6006):53–57. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]
- Fenderson B. A., Zehavi U., Hakomori S. A multivalent lacto-N-fucopentaose III-lysyllysine conjugate decompacts preimplantation mouse embryos, while the free oligosaccharide is ineffective. J Exp Med. 1984 Nov 1;160(5):1591–1596. doi: 10.1084/jem.160.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell T. M., Dodd J. Structure and expression of differentiation antigens on functional subclasses of primary sensory neurons. Philos Trans R Soc Lond B Biol Sci. 1985 Feb 19;308(1136):271–281. doi: 10.1098/rstb.1985.0027. [DOI] [PubMed] [Google Scholar]
- Kobiler D., Barondes S. H. Lectin activity from embryonic chick brain, heart, and liver: changes with development. Dev Biol. 1977 Oct 1;60(1):326–330. doi: 10.1016/0012-1606(77)90130-0. [DOI] [PubMed] [Google Scholar]
- Kobiler D., Beyer E. C., Barondes S. H. Developmentally regulated lectins from chick muscle, brain, and liver have similar chemical and immunological properties. Dev Biol. 1978 Jun;64(2):265–272. doi: 10.1016/0012-1606(78)90077-5. [DOI] [PubMed] [Google Scholar]
- Kruse J., Keilhauer G., Faissner A., Timpl R., Schachner M. The J1 glycoprotein--a novel nervous system cell adhesion molecule of the L2/HNK-1 family. Nature. 1985 Jul 11;316(6024):146–148. doi: 10.1038/316146a0. [DOI] [PubMed] [Google Scholar]
- REXED B. The cytoarchitectonic organization of the spinal cord in the cat. J Comp Neurol. 1952 Jun;96(3):414–495. doi: 10.1002/cne.900960303. [DOI] [PubMed] [Google Scholar]
- Smith C. L. The development and postnatal organization of primary afferent projections to the rat thoracic spinal cord. J Comp Neurol. 1983 Oct 10;220(1):29–43. doi: 10.1002/cne.902200105. [DOI] [PubMed] [Google Scholar]
- Springer W. R., Cooper D. N., Barondes S. H. Discoidin I is implicated in cell-substratum attachment and ordered cell migration of Dictyostelium discoideum and resembles fibronectin. Cell. 1984 Dec;39(3 Pt 2):557–564. doi: 10.1016/0092-8674(84)90462-8. [DOI] [PubMed] [Google Scholar]
- Stern P. L. Differentiation antigens of teratomas and embryos. Br Med Bull. 1984 Jul;40(3):218–223. doi: 10.1093/oxfordjournals.bmb.a071980. [DOI] [PubMed] [Google Scholar]
- de Waard A., Hickman S., Kornfeld S. Isolation and properties of beta-galactoside binding lectins of calf heart and lung. J Biol Chem. 1976 Dec 10;251(23):7581–7587. [PubMed] [Google Scholar]