Abstract
Clinical trials with biological modifiers targeting specific inflammatory mediators associated with severe sepsis have shown no or limited survival benefit. The approach taken in studies reported here was to limit the point source of intra-abdominal infection by potentiating innate immune function, thereby lessening the severity of sepsis and improving survival. Soluble beta-glucans, glucose polymers of the fungal cell wall, have been shown to stimulate innate immune host defense in animal and human studies when administered prior to an infectious challenge. We evaluated the effects of poly-(1,6)-β-d-glucopyranosyl-(1,3)-β-d-glucopyranose glucan (PGG glucan) on overall survival when administered intraperitoneally after the onset of polymicrobial infection by cecal ligation and puncture (CLP). Since gender-dependent differences in host immune response to infection have been reported, male and female mice were prospectively stratified for PGG glucan treatment. Outbred CD-1 mice were administered 10 mg/kg of body weight PGG glucan or the polysaccharide control, dextran, 1 h after CLP. Six hours after CLP, blood samples were obtained for cytokine measurements. Surprisingly, a gender-dependent effect on the response to PGG glucan was revealed. PGG glucan enhanced survival in female mice over a 10-day period, but survival in males was improved for only 24 h. In female mice, PGG glucan reduced interleukin-6 (IL-6) and IL-10 levels and reduced the bacterial burden in the liver. Ovariectomy abrogated the response to PGG glucan. Together, the translational potential of these findings is the indicated use of PGG glucan given locally, rather than intravenously, for improved source control during the management of sepsis. This therapy does not require prophylactic beta-glucan administration.
INTRODUCTION
Despite advances in patient care, it is estimated that 750,000 people will become septic in a given year in the United States and 28.6% will die as a result (4, 14). Sepsis is defined as the presence of the systemic inflammatory response syndrome (SIRS) in response to bacterial infection (33). SIRS is considered the early proinflammatory phase of the disease and is followed by an intermediate phase, mixed anti-inflammatory response syndrome (MARS), which consists of mixed pro- and anti-inflammatory mediators. The final phase, compensatory anti-inflammatory response syndrome (CARS), is predominated by anti-inflammatory mediators (29).
Severe sepsis remains a leading cause of death, in spite of treatments, including fluid resuscitation, low-dose steroids, antibiotics, tight control of blood glucose, and activated protein C (APC), which has a modest effect (8). Efforts to meet a clinical need for additional therapeutic modalities focused on development of biological response modifiers directed at ameliorating the exuberant production of individual proinflammatory mediators such as interleukin-1 (IL-1) and tumor necrosis factor alpha (TNF-α), but they offered no survival benefit (33). Therefore, there remains a need for new and better means of managing life-threatening sepsis.
Although the innate immune system has been studied extensively for many years, drugs able to prime immune cell function sufficiently to show clinical benefit are still unavailable. This is so because the favorable aspects of immune stimulation needed to improve cellular host defense, including antimicrobial and antitumor activities, also include excessive production of proinflammatory cytokines. The ideal therapeutic would be able to prime host defense without eliciting or contributing to a systemic inflammatory response.
Beta-glucan, a structural component of the fungal cell wall, is a class of long-chain polymers of glucose consisting of beta-(1,3)-linked β-d-glucopyranosyl units with occasional beta (1,6)-linked side chains of various lengths (10, 15). Poly-(1,6)-β-d-glucopyranosyl-(1,3)-β-d-glucopyranose glucan (PGG glucan; Betafectin and Imprime PGG) is a highly purified, pharmaceutical-grade neutral soluble beta-glucan. It has been evaluated in randomized phase I/II clinical trials, where it was well tolerated at therapeutic doses (5, 6). So far, it is the only beta-glucan preparation that has been tested in humans, where it showed anti-infective properties without serious side effects in a phase III study of patients scheduled to undergo noncolorectal operations (13). This preparation of PGG glucan does not itself elicit proinflammatory cytokines but does augment host immune defense (1, 12, 21, 24, 25, 28, 31, 40).
Animal studies have been conducted to evaluate the effects of commonly used soluble beta-glucan preparations on the mortality induced by various experimental models of sepsis and infection (2, 3, 9, 11, 20, 23, 24, 25, 26, 30, 32, 36, 38, 44, 45). Taken together, these studies demonstrate that beta-glucans can be effective in stimulating the innate immune system and in providing protection against microbial infection when administered prior to challenge. It is not known whether pharmaceutical-grade PGG glucan can have a significant effect on host defense without prophylactic delivery and thereby be appropriate for treatment of an ongoing acute infection.
Cecal ligation and puncture (CLP) in mice is considered to provide a clinically relevant animal model with which to study the inflammatory and clinical effects of sepsis (14, 37, 41, 43, 44). Studies reported here were designed to determine if PGG glucan can be of benefit to septic mice in the absence of other means of source control by utilizing the CLP protocol without supportive antibiotic therapy or removal of the damaged bowel. Moreover, we took into account that surgical drainage and irrigation of the peritoneal cavity are mainstays of the management of abdominal sepsis and that biological response support may be best applied locally during laparotomy (27). We therefore performed intraperitoneal, rather than intravenous, administration of PGG glucan as a means of controlling infection.
Several studies have compared the responses to sepsis in male and female mice and noted various effects. Some results demonstrated that female mice experience less immune cell dysfunction than male mice following the induction of sepsis (18, 46); however, it has also been indicated that female mice may have a lower rate of survival than male mice following CLP (22). Because of these reported differences in mortality between male and female mice in response to CLP, we evaluated the effects of PGG glucan on CLP-induced sepsis by stratifying male and female mice. We report an extended therapeutic opportunity for PGG glucan administered by intraperitoneal injection after CLP but that the effects are sex dependent. Ovariectomy abolishes the response of female septic mice to PGG glucan. These results support the development of PGG glucan as a broad-spectrum therapeutic agent that can improve survival when administered intraperitoneally after the onset of polymicrobial infection. It fulfills the therapeutic goal of immune potentiation without contributing further to SIRS and the associated morbidity.
MATERIALS AND METHODS
Mice.
Male, female, and ovariectomized female CD-1 mice (weight, 25 to 28 g) were obtained from Charles River Laboratories (Wilmington, MA) at 6 to 8 weeks of age and used within 2 to 3 weeks in all experiments. Animals were housed in the Central Research Facilities at Rhode Island Hospital and fed mouse chow and water ad libitum. Mice were certified to be free of common pathogens by the supplier and were monitored by Brown University/Rhode Island Hospital veterinary personnel. Animal protocols were approved by the Animal Care Committee at Rhode Island Hospital.
CLP/sepsis induction.
CLP was conducted on the basis of a previously described protocol (7). Animals were anesthetized using isoflurane. Abdominal fur was shaved, and the area was cleaned and prepared for surgery using Betadine. A midline incision was made, and the two layers were separated. A second incision was made, exposing the peritoneal cavity and the cecum. The cecum was ligated by tying nylon just below the ileocecal valve. Cecal contents were milked to one end of the cecum, and that end was punctured with an 18-gauge needle. A second puncture was made on the side of the cecum. Pressure was applied to extrude a single droplet of fecal material from each puncture site. Lidocaine was used to treat the site of incision. The abdomen was sutured in two layers using nylon. The animals were resuscitated with 500 μl normal saline given subcutaneously and returned to their cages, which were placed under warming lamps. Animals were provided food and water ad lib. Sham-operated mice, mice that were subjected to midline and cavity incisions but did not undergo cecal ligation or puncture, were included as a control group. The number of animals undergoing CLP operations in any one session ranged from 3 to 10. Data from female animals were obtained from eight separate surgical sessions, while data from male mice were from seven surgical sessions.
Beta-glucan and dextran treatment.
The pharmaceutical-grade beta-glucan PGG glucan (Imprime-PGG) was provided by Biothera (Eagan, MN) as a 1-mg/ml stock solution. Mice were treated with 500 μl of 10 mg/kg body weight PGG glucan or dextran (molecular mass, 80 to 120 kDa; Sigma), a glucose polymer of similar molecular mass and solubility to PGG glucan, by intraperitoneal injection 1 h after CLP. Because experiments evaluating the effect of glucan phosphate on CLP-induced mortality involved administering 50 mg/kg to septic animals (44) and the randomized phase I/II clinical trials evaluating the effects of PGG glucan on patients who underwent major abdominal or thoracic surgery involved administering doses up to 2.25 mg/kg (5, 6), we chose to administer 10 mg/kg PGG glucan to septic animals as an intermediate dose. PGG glucan and dextran were diluted in sterile citrate buffer, also provided by Biothera, prior to injections.
Estradiol administration.
Immediately before fluid resuscitation, a subset of ovariectomized female mice received one subcutaneous injection of 4 mg/kg body weight of 17β-estradiol dissolved in 200 μl sesame oil (Sigma), on the basis of methods published by Knöferl et al. (19).
Plasma collection.
Six hours after CLP, a small incision was made on the tails of a subset of mice. Pressure was applied to the tail to allow collection of approximately 50 to 100 μl of blood. A separate subset of mice was subjected to cardiac puncture using a heparinized needle. The blood obtained by each method was centrifuged to remove cells, and plasma was removed and frozen until used for enzyme-linked immunosorbent assay (ELISA).
IL-6 and IL-10 ELISAs.
IL-6 and IL-10 were assayed by ELISA using mouse antibody kits (BD Biosciences, San Diego, CA) according to the manufacturer's instructions. A 1:200 dilution of plasma was used to assay for IL-6, and a 1:4 dilution of serum was used to assay for IL-10.
Liver colonization.
Mice were euthanized using CO2. Liver tissue samples were isolated from the mice 24 h after CLP, and 260 to 270 mg (wet weight) was homogenized in 400 μl saline and serially diluted in sterile phosphate-buffered saline (PBS). Forty microliters of the liver samples was plated on tryptic soy agar plates with 5% sheep's blood and MacConkey agar, and the plates were incubated aerobically for 24 h at 37°C.
Statistics.
Figures were constructed and statistical analyses were conducted using GraphPad Prism (version 5) software. Results from the survival studies were compared using the Gehan-Breslow-Wilcoxon test and considered significant if P was ≤0.05. The distribution of cytokine values was positively skewed, and a logarithmic transformation was applied prior to analysis. The logarithmically transformed data were compared using Student's t test. The means of the logarithmically transformed data were back-transformed to produce geometric means and confidence intervals. Results from the bacterial clearance studies were analyzed by the Mann-Whitney U test. Results were considered significant if P was ≤0.05.
RESULTS
PGG glucan improved 10-day survival in female but not in male mice.
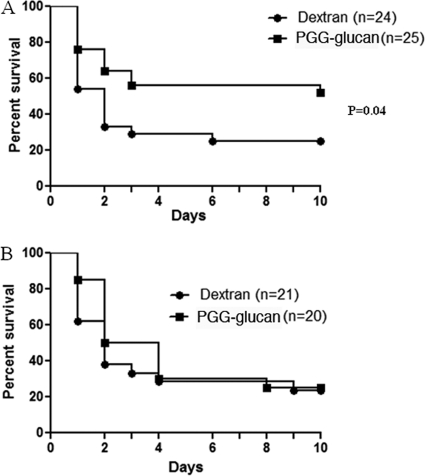
Intra-abdominal sepsis is the result of a loss of source control of infection, allowing systemic spread and multiorgan involvement (27). To date, intravenous delivery of potential therapeutics has uniformly failed to significantly alter the clinical course of the disease after onset. Work performed here considered whether a new avenue of drug delivery might provide a better outcome. To investigate whether PGG glucan had an effect on the mortality induced by sepsis, outbred CD-1 mice of both sexes were subjected to CLP and given a single intraperitoneal dose (10 mg/kg body weight) of PGG glucan or the polysaccharide control dextran 1 h after the induction of sepsis. The results show that PGG glucan significantly enhanced 10-day survival in female mice (52% in PGG glucan-treated mice versus 25% in dextran-treated mice, P < 0.05) (Fig. 1A) and that the protective effect was observed as early as 24 h after CLP. The results also show that PGG glucan induced a significant enhancement on 24-h survival in male mice (87.1% in PGG glucan-treated mice versus 64.5% in dextran-treated mice, P < 0.05) and not thereafter (Fig. 1B).
Fig. 1.
PGG glucan enhanced 10-day survival in female mice and 24-h survival in male mice. Female (A) and male (B) mice were subjected to CLP and administered PGG glucan or dextran (10 mg/kg) 1 h after surgery. Survival was monitored over 10 days.
Correlation of IL-6 with CLP-induced mortality: gender differences and response to immunotherapy.
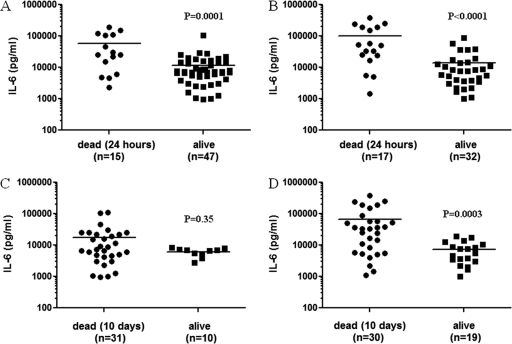
The IL-6 levels measured 6 h after CLP are a predictor of sepsis-induced mortality in mice (35, 39). The IL-6 values of the male and female mice that were dead by 24 h after CLP were significantly higher than the IL-6 levels of mice that survived (male survivors versus nonsurvivors, P < 0.05; female survivors versus nonsurvivors, P < 0.05) (Fig. 2A and B).
Fig. 2.
IL-6 measured 6 h after CLP correlates with early mortality in male and female mice. Six hours after CLP, plasma was obtained from the tails of the mice used to obtain the survival data in Fig. 1 and IL-6 levels were determined by ELISA. The IL-6 values of the male (A) and female (B) mice that were dead by 24 h were compared to the IL-6 values of the mice that survived. The IL-6 values of the male (C) and female (D) mice that were dead by day 10 were compared to the IL-6 values of the mice that survived.
To determine whether IL-6 correlated with long-term survival, the IL-6 values of the mice that were dead by day 10 were compared to the IL-6 values of the mice that survived. Serum samples from mice whose survival is shown in Fig. 1A and B were analyzed. The IL-6 levels of the female mice that were dead by day 10 were significantly higher than the IL-6 values of the female survivors (female survivors versus nonsurvivors, P < 0.05) (Fig. 2D); however, there were no differences between the IL-6 values of the male mice that were dead by day 10 and the IL-6 values of the male survivors (male survivors versus nonsurvivors, P > 0.05) (Fig. 2C). In Fig. 3, data were stratified with treatment and show that PGG glucan treatment correlates with significantly lower levels of IL-6 production in male and female mice (PGG glucan-treated mice versus dextran-treated mice, P < 0.05) (Fig. 3A and B).
Fig. 3.
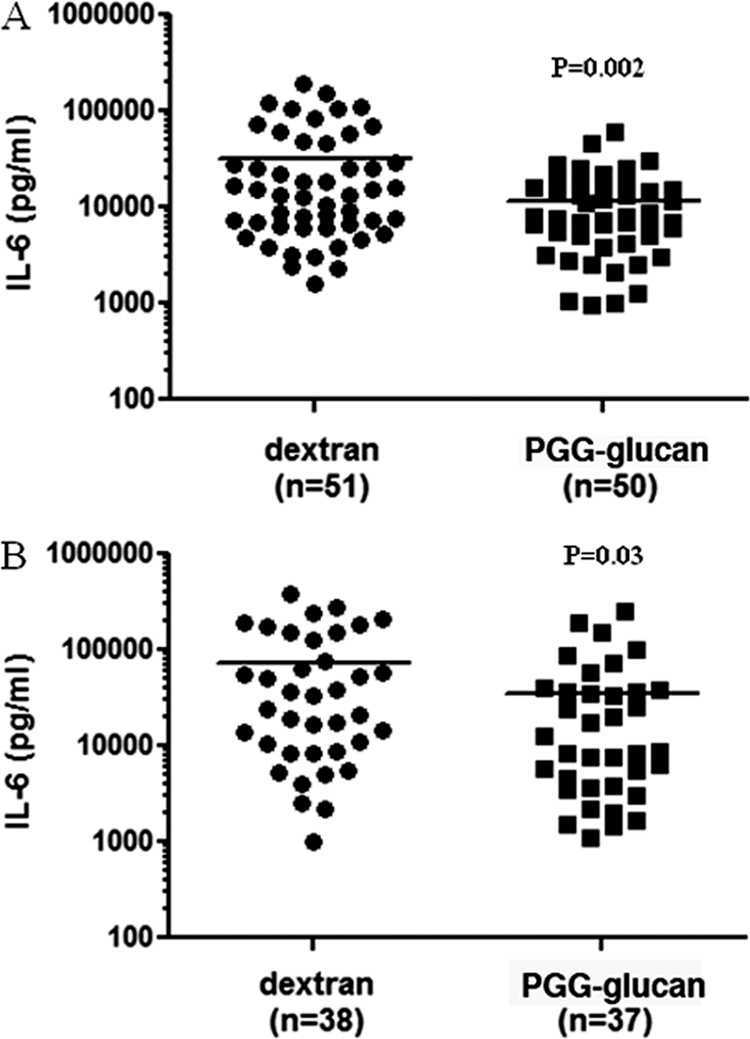
PGG glucan-mediated survival was associated with decreased IL-6 production. Male (A) and female (B) mice were subjected to CLP and administered PGG glucan or dextran as described in the legend to Fig. 1. Plasma was obtained 6 h after CLP, and IL-6 levels were measured by ELISA.
Taken together, these results indicate that IL-6 correlated with survival in male and female mice regardless of whether animals were treated with PGG glucan or dextran.
PGG glucan attenuated CLP-induced IL-10 production.
To test whether attenuation of cytokine production is limited to a proinflammatory cytokine such as IL-6, we tested whether PGG glucan treatment correlated with production of an anti-inflammatory cytokine, IL-10, using male and female mice 6 h after CLP. It was determined that PGG glucan treatment significantly attenuated the CLP-induced IL-10 production in male and female mice (PGG glucan-treated mice versus dextran-treated mice, P < 0.05) (Fig. 4A and B).
Fig. 4.
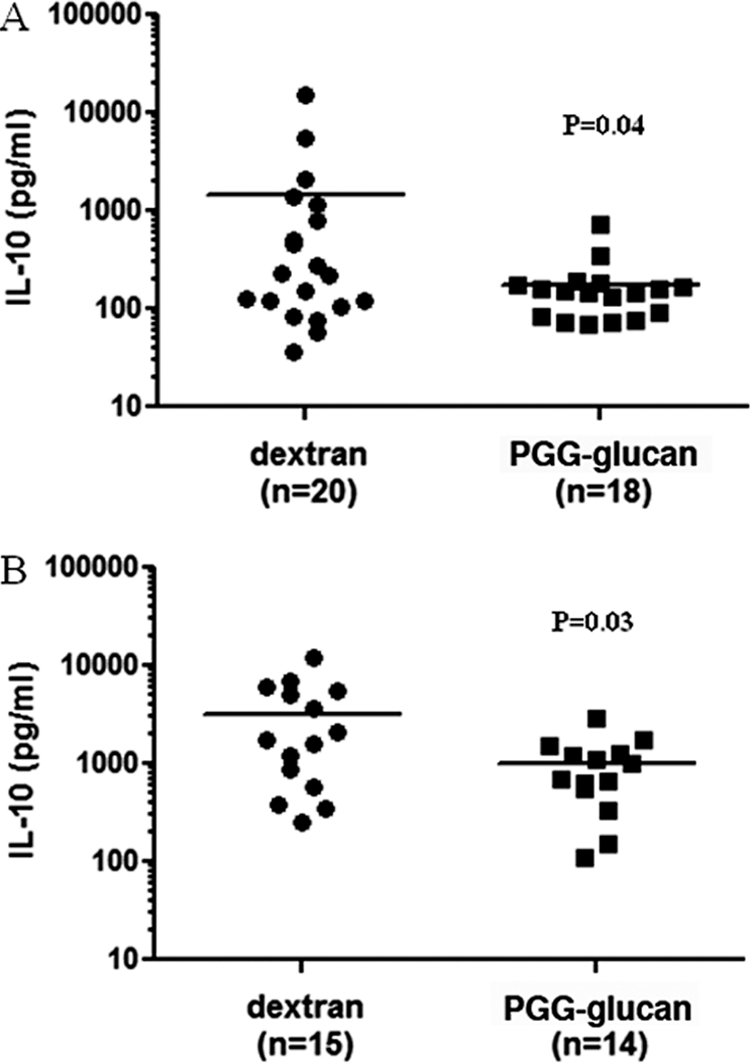
PGG glucan-mediated survival was associated with decreased IL-10 production. Male (A) and female (B) mice were subjected to CLP and administered PGG glucan or dextran as described in the legend to Fig. 1. Six hours after CLP, plasma was isolated and IL-10 levels were measured by ELISA.
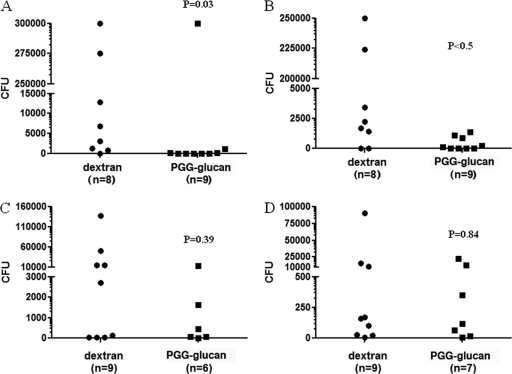
PGG glucan decreased liver colonization in female mice.
During polymicrobial infection induced by cecal ligation and puncture, bacteria are released into the peritoneal cavity and exposed to vicinal macrophages. In addition, bacteria enter the systemic circulation and accumulate in the liver, where they are encountered by phagocytic Kupffer cells (16). We hypothesized that if PGG glucan improved survival due to potentiation of immune host defense, the beneficial effect of PGG glucan observed in female mice would show enhanced microbial clearance and result in diminished colonization of the liver. Indeed, homogenates of liver samples obtained from female animals treated with PGG glucan had less colony growth of Gram-positive and Gram-negative bacteria than those of liver samples obtained from the female animals treated with dextran (PGG glucan-treated mice versus dextran-treated mice, P < 0.05) (Fig. 5A and B). There were no differences in the number of colonies that formed on the plates containing liver samples obtained from the male animals treated with PGG glucan and the plates containing liver samples obtained from the male animals treated with dextran (PGG glucan-treated mice versus dextran-treated mice, P > 0.05) (Fig. 5C and D). These results suggest that a mechanism of action of PGG glucan includes enhanced bacterial clearance in female mice but not in male mice.
Fig. 5.
PGG glucan decreased liver colonization in female mice. Female mice were subjected to CLP and administered PGG glucan or dextran (10 mg/kg) 1 h after surgery. Twenty-four hours after surgery, liver samples from female (A and B) and male (C and D) mice were obtained, diluted in PBS, and incubated on tryptic soy agar with 5% sheep's blood, which allows the growth of a variety of fastidious bacteria (A and C), and MacConkey agar, which allows the growth of Gram-negative bacteria only (B and D), overnight at 37°C.
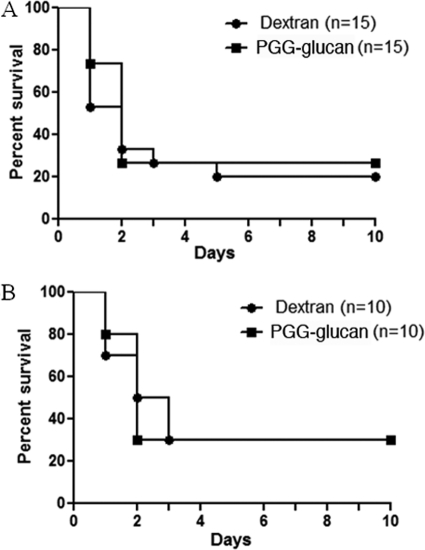
Hormones impacted the PGG glucan-mediated protection in female mice.
Because the response to PGG glucan was seen only in female mice, we determined if hormones influenced the response. CLP was performed on ovariectomized female mice, followed by PGG glucan or dextran administration as described above. There were no significant differences in 10-day survival of the ovariectomized mice that received dextran and the mice that received PGG glucan (PGG glucan-treated mice versus dextran-treated mice, P > 0.05) (Fig. 6A). To determine if estrogen was capable of restoring the protective effect of PGG glucan in female mice, 17β-estradiol was administered at the time of fluid resuscitation but prior to dextran or PGG glucan injection. This 17β-estradiol treatment has been shown to restore the impaired splenocyte proliferation observed in ovariectomized female mice in previous studies (19). The mice that received dextran and PGG glucan had comparable 10-day survival patterns (PGG glucan-treated mice versus dextran-treated mice, P > 0.05), indicating that one dose of 17β-estradiol is unable to restore the ability of PGG glucan to protect ovariectomized female mice from CLP-induced mortality (Fig. 6B). Taken together, these results indicate that female hormones are necessary for protection following PGG glucan administration in female mice.
Fig. 6.
PGG glucan did not enhance 10-day survival in ovariectomized female mice or in ovariectomized female mice given a single dose of estrogen. Ovariectomized female mice (A) and ovariectomized female mice administered β-estradiol at the time of fluid resuscitation (B) were subjected to CLP and administered PGG glucan or dextran (10 mg/kg) 1 h after surgery. Survival was monitored over 10 days.
DISCUSSION
Our data show that PGG glucan is able to improve the ability of female mice to respond to intra-abdominal sepsis through a mechanism that includes potentiation of innate immune host defense. Unlike other therapeutic strategies, such as those seeking to control excessive cytokine production, we show benefit to the host when infection control is exerted at the source. The benefit of PGG glucan to host health is reflected in significant decreases in IL-6 plasma levels and bacterial colonization of the liver. That PGG glucan is effective in the absence of prophylactic delivery extends it potential use to the management of ongoing acute severe sepsis. Finding that PGG glucan affects CLP-induced mortality in females but not males (Fig. 1) suggests that gender may be relevant in the clinical development of this or other drugs indicated to treat severe sepsis. Moreover, it underlies a previously unrecognized mechanism of action of PGG glucan which requires female sex hormones for an effect against intra-abdominal sepsis.
Figure 5 demonstrates that PGG glucan administration reduced the extent of bacterial colonization of the liver in septic female mice, indicating an antimicrobial effect resulting from enhanced host defense. A reasonable explanation is that PGG glucan exerts a systemic potentiation of host innate immune function and clearance is enhanced by augmented activity of peritoneal and liver macrophages in responding mice. PGG glucan has no direct microbiocidal or microbiostatic effect on its own (J. S. Reichner, unpublished observations). It is noteworthy that IL-6 levels in PGG glucan-treated mice were not enhanced and were significantly diminished in responding animals. Taken together, these findings show that PGG glucan does not exacerbate an ongoing proinflammatory response yet is able to stimulate innate immune activity. The ability to augment cellular antimicrobial function without contributing further to excessive cytokine production is the clinical ideal in development of immunotherapeutics. This has been an obstacle in design of a clinical modality, since most immune stimulants that affect cellular antimicrobial activity, such as respiratory burst and phagocytosis, also induce release of proinflammatory mediators. Iatrogenic intervention leading to elevation of these humoral factors beyond levels already induced by the host response to infection may increase the risk of SIRS and organ dysfunction to life-threatening levels.
Other biologically active beta-glucans commonly used experimentally, such as glucan phosphate, possess biological activity in vivo, including the ability to prime cells for enhanced responses to a secondary challenge (9). Posttreatment experiments during which mice were administered glucan phosphate 15 min after CLP indicated that glucan phosphate can increase long-term survival when given after the infectious onset (44). Although this further supports the clinical potential of beta-glucans in the treatment of sepsis, glucan phosphate is currently not available for administration into humans. Our findings are in support of those for glucan phosphate, in that prophylactic administration was not necessary for a host benefit to manifest. Future studies are currently aimed at determining the breadth of the therapeutic window available for PGG glucan administration.
Motivated by findings from Remick and colleagues that showed the prognostic significance of proinflammatory cytokines in predicting mortality due to severe sepsis, we assayed plasma levels of IL-6 collected 6 h after CLP (29, 34). Our findings are consistent with those reports in the sense that levels of IL-6 in surviving animals were significantly lower than levels in animals that died. This was seen whether animals were grouped in total (Fig. 2) or stratified according to PGG glucan or dextran treatment (Fig. 3). Our studies were not sufficiently powered to identify the threshold levels of IL-6 that predict death. We therefore present our findings conservatively (and in support of the aforementioned studies) as a correlation between IL-6 and survival. It is unlikely that reduction of IL-6 by PGG glucan is itself the cause for increased survival. This is so because studies have shown that IL-6 serves as a predictor of CLP-induced mortality rather than a cause of mortality. There were no differences between the mortality of the IL-6-knockout mice and wild-type mice, demonstrating that a complete lack of IL-6 does not alter CLP-induced mortality (34). Anti-IL-6 monotherapies have also failed to improve survival in mice with IL-6 levels greater than 14,000 pg/ml, demonstrating that targeted therapy directed at IL-6 had no benefit in these animals (39). These results support the predictive role of IL-6 in determining the course of disease rather than suggesting a direct pathological mechanism. Similarly, as shown in Fig. 4, IL-10 was reduced in both male and female mice and demonstrated a response to PGG glucan; however, reduced IL-10 levels per se may not be a biological effector response responsible for determining survival due to treatment.
While antibiotic therapy has been shown to improve the survival of animals and patients with sepsis, antibiotic treatment does not affect the outcome of animals with an IL-6 level identified 6 h after sepsis above the threshold at which animals are destined to die (39). Many studies designed to examine mortality using this model of sepsis incorporate the introduction of antibiotic therapy. We tested the effects of PGG glucan on sepsis when given in the absence of antibiotic therapy to determine if PGG glucan could have an effect on the host response to sepsis when administered as a single agent. Whether PGG glucan acts synergistically with antibiotics in the treatment of sepsis remains to be determined.
Under the conditions of estrogen replacement use in this study, PGG glucan did not increase survival in ovariectomized females. The simple conclusion is that estrogen does not support the protective effect of PGG glucan seen in septic female mice. However, this could be due to the fact that estrogen is necessary but not sufficient to provide protection in the presence of PGG glucan. Another possibility is that estrogen is necessary and sufficient to provide protection in the presence of PGG glucan and that the reason that this was not demonstrated by our experiments is that a large enough dose of estradiol was not administered for an effect to be observed or that our treatment period was too brief. Our studies were originally designed to examine the sex differences of the septic murine response to PGG glucan, and that was furthered by inclusion of ovariectomized females. This work will be extended by subjecting ovariectomized female mice to CLP and administering several doses of estradiol to these mice over time on a regular basis. This would allow us to determine the effects of PGG glucan on sepsis while administering estradiol utilizing a protocol to mimic the estrus cycle of a female mouse.
The receptor-mediated mechanism of action of PGG glucan, while not proven in this study, is most likely to result from ligation of the leukocyte integrin CR3. This hypothesis is supported by multiple studies demonstrating a requirement for CR3 for a therapeutic response to soluble PGG glucan in mice (reviewed in reference 25). Whereas dectin-1 has been shown to be an important beta-glucan receptor for the phagocytosis of beta-glucan-containing particles and Candida species, it binds to but does not mediate a cellular response to soluble beta-glucan (17).
The mechanism by which PGG glucan decreases cytokine production remains speculative. PGG glucan has been shown to induce the formation of a transcriptional heterodimer consisting of C/EBPβ protein with p65-containing NF-κB (1, 40). A similar structure was identified in monocytes made tolerant to high-dose TNF-α stimulation by repeated long-term exposure to the cytokine at low doses (42). As opposed to binding of the canonical C/EBPβ and NF-κB transcription factors, binding of the transcriptional heterodimer to cytokine promoter regions has been shown to have a suppressive effect on cytokine transcription. Although additional work will be needed to determine if this mechanism is operative in the altered cytokine production in septic mice treated with PGG glucan, it may serve to explain the reduction in both a proinflammatory protein such as IL-6 and an anti-inflammatory protein such as IL-10.
Our findings support the potential development of PGG glucan as a therapeutic agent for the treatment of severe intra-abdominal sepsis with negligible side effects. This work also suggests that improved host defense at the point source of infection may be a new avenue of therapy in high-risk patients.
ACKNOWLEDGMENTS
We acknowledge contribution to this work from Rye-Ji Kim. We also thank members of the Alfred Ayala laboratory, especially Yaping Chen and Chun-Shiang Chung, for advice on the cecal ligation and puncture model, Virginia Hovanesian for image collection, Jason Machan for assistance with statistical analysis, and members of the laboratories of the Division of Surgical Research at Rhode Island Hospital.
This work was supported by National Institutes of Health grant GM066194 (to J.S.R.), grant GM046354 (to A.A.), the Brown University IMSD Program, which is funded by grants R25GM083270 and F31GM086069 (to C.T.N.), and allocations to the Department of Surgery by Rhode Island Hospital.
Footnotes
Published ahead of print on 5 October 2011.
REFERENCES
- 1. Adams D. S., et al. 1997. PGG glucan activates NF-kappaB-like and NF-IL-6-like transcription factor complexes in a murine monocytic cell line. J. Leukoc. Biol. 62:865–873 [DOI] [PubMed] [Google Scholar]
- 2. Almdahl S. M., Bogwald J., Hoffman J., Giercksky K. E., Seljelid R. 1987. Protection by aminated glucan in experimental endogenous peritonitis. Eur. Surg. Res. 19:78–85 [DOI] [PubMed] [Google Scholar]
- 3. Almdahl S. M., Bogwald J., Hoffman J., Seljelid R. 1987. Treatment of experimental peritonitis in rats by transfer of peritoneal mononuclear cells from rats injected with semisoluble aminated glucan. Acta Chir. Scand. 153:535–539 [PubMed] [Google Scholar]
- 4. Angus D. C., et al. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29:1303–1310 [DOI] [PubMed] [Google Scholar]
- 5. Babineau T. J., et al. 1994. A phase II multicenter, double-blind, randomized, placebo-controlled study of three dosages of an immunomodulator (PGG-glucan) in high-risk surgical patients. Arch. Surg. 129:1204–1210 [DOI] [PubMed] [Google Scholar]
- 6. Babineau T. J., et al. 1994. Randomized phase I/II trial of a macrophage-specific immunomodulator (PGG-glucan) in high-risk surgical patients. Ann. Surg. 220:601–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baker C. C., Chaudry I. H., Gaines H. O., Baue A. E. 1983. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery 94:331–335 [PubMed] [Google Scholar]
- 8. Bernard G. R., et al. 2001. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 344:699–709 [DOI] [PubMed] [Google Scholar]
- 9. Bowers G. J., Patchen M. L., MacVittie T. J., Hirsch E. F., Fink M. P. 1989. Glucan enhances survival in an intraabdominal infection model. J. Surg. Res. 47:183–188 [DOI] [PubMed] [Google Scholar]
- 10. Brown G. D., Gordon S. 2003. Fungal beta-glucans and mammalian immunity. Immunity 19:311–315 [DOI] [PubMed] [Google Scholar]
- 11. Cisneros R. L., Gibson F. C., III, Tzianabos A. O. 1996. Passive transfer of poly-(1-6)-beta-glucotriosyl-(1-3)-beta-glucopyranose glucan protection against lethal infection in an animal model of intra-abdominal sepsis. Infect. Immun. 64:2201–2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cramer D. E., et al. 2008. Mobilization of hematopoietic progenitor cells by yeast-derived beta-glucan requires activation of matrix metalloproteinase-9. Stem Cells 26:1231–1240 [DOI] [PubMed] [Google Scholar]
- 13. Dellinger E. P., et al. 1999. Effect of PGG-glucan on the rate of serious postoperative infection or death observed after high-risk gastrointestinal operations. Betafectin Gastrointestinal Study Group. Arch. Surg. 134:977–983 [DOI] [PubMed] [Google Scholar]
- 14. Ebong S., et al. 1999. Immunopathologic alterations in murine models of sepsis of increasing severity. Infect. Immun. 67:6603–6610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gawronski M., Park J. T., Magee A. S., Conrad H. 1999. Microfibrillar structure of PGG-glucan in aqueous solution as triple-helix aggregates by small angle X-ray scattering. Biopolymers 50:569–578 [DOI] [PubMed] [Google Scholar]
- 16. Godshall C. J., Scott M. J., Burch P. T., Peyton J. C., Cheadle W. G. 2003. Natural killer cells participate in bacterial clearance during septic peritonitis through interactions with macrophages. Shock 19:144–149 [DOI] [PubMed] [Google Scholar]
- 17. Goodridge H. S., et al. 2011. Activation of the innate immune receptor dectin-1 upon formation of a ‘phagocytic synapse.’ Nature 472:471–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hwang T. L., Yang Y. M. 2008. Sex differences in response to immunonutrition in sepsis. Nutrition 24:761–766 [DOI] [PubMed] [Google Scholar]
- 19. Knöferl M. W., Angele M. K., Schwacha M. G., Bland K. I., Chaudry I. H. 2002. Preservation of splenic immune functions by female sex hormones after trauma-hemorrhage. Crit. Care Med. 30:888–893 [DOI] [PubMed] [Google Scholar]
- 20. Kournikakis B., Mandeville R., Brousseau P., Ostroff G. 2003. Anthrax-protective effects of yeast beta 1,3 glucans. Med. Gen. Med. 5:1. [PubMed] [Google Scholar]
- 21. LeBlanc B. W., Albina J. E., Reichner J. S. 2006. The effect of PGG-beta-glucan on neutrophil chemotaxis in vivo. J. Leukoc. Biol. 79:667–675 [DOI] [PubMed] [Google Scholar]
- 22. Leon L. R., White A. A., Kluger M. J. 1998. Role of IL-6 and TNF in thermoregulation and survival during sepsis in mice. Am. J. Physiol. 275:R269–R277 [DOI] [PubMed] [Google Scholar]
- 23. Li B., et al. 2007. Yeast glucan particles activate murine resident macrophages to secrete proinflammatory cytokines via MyD88- and Syk kinase-dependent pathways. Clin. Immunol. 124:170–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liang J., et al. 1998. Enhanced clearance of a multiple antibiotic resistant Staphylococcus aureus in rats treated with PGG-glucan is associated with increased leukocyte counts and increased neutrophil oxidative burst activity. Int. J. Immunopharmacol. 20:595–614 [DOI] [PubMed] [Google Scholar]
- 25. Liu J., Gunn L., Hansen R., Yan J. 2009. Combined yeast-derived beta-glucan with anti-tumor monoclonal antibody for cancer immunotherapy. Exp. Mol. Pathol. 86:208–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lyuksutova O. I., et al. 2005. Glucan phosphate treatment attenuates burn-induced inflammation and improves resistance to Pseudomonas aeruginosa burn wound infection. Shock 23:224–232 [PubMed] [Google Scholar]
- 27. Marshall J. C. 2010. Principles of source control in the early management of sepsis. Curr. Infect. Dis. Rep. 12:345–353 [DOI] [PubMed] [Google Scholar]
- 28. Onderdonk A. B., Cisneros R. L., Hinkson P., Ostroff G. 1992. Anti-infective effect of poly-beta 1-6-glucotriosyl-beta 1-3-glucopyranose glucan in vivo. Infect. Immun. 60:1642–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Osuchowski M. F., Welch K., Siddiqui J., Remick D. G. 2006. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J. Immunol. 177:1967–1974 [DOI] [PubMed] [Google Scholar]
- 30. Patchen M. L., et al. 1998. Mobilization of peripheral blood progenitor cells by Betafectin PGG-glucan alone and in combination with granulocyte colony-stimulating factor. Stem Cells 16:208–217 [DOI] [PubMed] [Google Scholar]
- 31. Poutsiaka D. D., Mengozzi M., Vannier E., Sinha B., Dinarello C. A. 1993. Cross-linking of the beta-glucan receptor on human monocytes results in interleukin-1 receptor antagonist but not interleukin-1 production. Blood 82:3695–3700 [PubMed] [Google Scholar]
- 32. Pretus H. A., et al. 1991. Isolation, physicochemical characterization and preclinical efficacy evaluation of soluble scleroglucan. J. Pharmacol. Exp. Ther. 257:500–510 [PubMed] [Google Scholar]
- 33. Remick D. G. 2007. Pathophysiology of sepsis. Am. J. Pathol. 170:1435–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Remick D. G., Bolgos G., Copeland S., Siddiqui J. 2005. Role of interleukin-6 in mortality from and physiologic response to sepsis. Infect. Immun. 73:2751–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Remick D. G., Bolgos G. R., Siddiqui J., Shin J., Nemzek J. A. 2002. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock 17:463–467 [DOI] [PubMed] [Google Scholar]
- 36. Rice P. J., et al. 2005. Oral delivery and gastrointestinal absorption of soluble glucans stimulate increased resistance to infectious challenge. J. Pharmacol. Exp. Ther. 314:1079–1086 [DOI] [PubMed] [Google Scholar]
- 37. Swan R., et al. 2007. Polymicrobial sepsis enhances clearance of apoptotic immune cells by splenic macrophages. Surgery 142:253–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tzianabos A. O., Gibson F. C., III, Cisneros R. L., Kasper D. L. 1998. Protection against experimental intraabdominal sepsis by two polysaccharide immunomodulators. J. Infect. Dis. 178:200–206 [DOI] [PubMed] [Google Scholar]
- 39. Vyas D., et al. 2005. Early antibiotic administration but not antibody therapy directed against IL-6 improves survival in septic mice predicted to die on basis of high IL-6 levels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289:R1048–R1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wakshull E., et al. 1999. PGG-glucan, a soluble beta-(1,3)-glucan, enhances the oxidative burst response, microbicidal activity, and activates an NF-kappa B-like factor in human PMN: evidence for a glycosphingolipid beta-(1,3)-glucan receptor. Immunopharmacology 41:89–107 [DOI] [PubMed] [Google Scholar]
- 41. Walley K. R., Lukacs N. W., Standiford T. J., Strieter R. M., Kunkel S. L. 1996. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect. Immun. 64:4733–4738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weber M., et al. 2003. Transcriptional inhibition of interleukin-8 expression in tumor necrosis factor-tolerant cells. J. Biol. Chem. 278:23586–23593 [DOI] [PubMed] [Google Scholar]
- 43. Wichterman K. A., Baue A. E., Chaudry I. H. 1980. Sepsis and septic shock—a review of laboratory models and a proposal. J. Surg. Res. 29:189–201 [DOI] [PubMed] [Google Scholar]
- 44. Williams D. L., et al. 1999. Inhibiting early activation of tissue nuclear factor-kappa B and nuclear factor interleukin 6 with (1→3)-beta-d-glucan increases long-term survival in polymicrobial sepsis. Surgery 126:54–65 [DOI] [PubMed] [Google Scholar]
- 45. Williams D. L., et al. 1991. Development, physicochemical characterization and preclinical efficacy evaluation of a water soluble glucan sulfate derived from Saccharomyces cerevisiae. Immunopharmacology 22:139–155 [DOI] [PubMed] [Google Scholar]
- 46. Zellweger R., et al. 1997. Females in proestrus state maintain splenic immune functions and tolerate sepsis better than males. Crit. Care Med. 25:106–110 [DOI] [PubMed] [Google Scholar]